Abstract
Western blots are used to estimate the relative concentrations of proteins of interest based on staining by specific antibodies. Quantitative measurements are often subject to error due to overloading of the loading control and over-reliance on normalization. We have found that at the protein concentrations normally used to quantify most low-abundance proteins of interest, frequently used single-protein loading controls, such as GAPDH and β-actin, do not accurately reflect differences in protein concentration. Two total protein stains, SYPRO® Ruby and Amido Black, were compared and found to be acceptable alternatives to single protein controls. Although we cannot prove that high-abundance loading controls are inaccurate under all possible conditions, we conclude that the burden of proof should lie with the researcher to demonstrate that their loading control is reflective of quantitative differences in protein concentration.
Introduction
The Western, or immunoblot, is widely used for determining the presence or absence of a protein within a cellular homogenate, limited only by the availability of a specific antibody. Demonstrating absence requires proof that protein is in each lane of the gel. A control antibody, or loading control (LC) often serves this purpose. Antibodies against β-actin and glyceraldehyde 3-phosphate dehydrogenase (GAPDH), along with other high-abundance housekeeping proteins, are used most often because they bind to proteins in nearly any sample.
Increasingly, investigators are utilizing measurements of antibody binding such as fluorescence intensity to quantify differences between samples of interest. In these cases each sample must contain the same amount of total protein. Protein levels are first measured with colorimetric assays, such as the Bicinchoninic Acid (BCA) assay. However, gels relying on these tests may still be subject to differential protein transfer or human loading error, and thus journal reviewers usually require a second control. After measuring the protein of interest (POI) with a specific antibody (marked by a chemiluminescent reaction), a second set of antibodies is used to quantify the protein defined as the LC. The ratio of the POI to the LC is used by many laboratories to compare different samples, under the assumption that both measures vary to the same degree with concentration, and thus dividing or “normalizing” by the LC will correct for any loading errors or differential blot transfer (eg: Asaka et al., 2006; Vasudevan et al., 2004; Wagner et al., 2006). For qualitative studies, the loading control is often just compared visually or displayed in the figure to provide evidence of even loading.
Two issues arise from the use of normalization. First, using a single protein LC changes the fundamental hypothesis being addressed. A difference between two samples could be the result of an actual difference in the POI, or a difference in the abundance of the LC. Instead of quantifying protein relative to cell number, tissue volume, or total protein, one has reformulated the hypothesis to ask how much protein there is relative to, for example, β-actin concentration. For this reason, most loading controls are high abundance housekeeping proteins whose levels are thought not to change under most circumstances. This assumption, however, appears imprudent. In the field of RT-PCR, (a technique used to measure levels of mRNA), the use of these loading controls is also being questioned (Huggett et al., 2005; Yperman et al., 2004). In the case of each traditionally used loading control, circumstances have been described where the levels of the protein (or mRNA, eg: Nahlik et al., 2003), differ between experimental groups. For example, it was observed that when cells of the rat spinal cord were exposed to traumatic injury, levels of β-actin were significantly altered (Liu and Xu, 2006). GAPDH and Tubulin levels have been found to change over the course of development (Alexander et al., 1985; Moskowitz and Oblinger, 1995), and it seems dubious to assume that no other experimental manipulation would affect the expression of other commonly used housekeeping proteins.
The second issue, and the focus of this study, stems directly from the use of high abundance loading controls. Many proteins of interest, such as PSD-95 and pERK, are low-abundance compared with ubiquitous housekeeping or structural proteins. Unfortunately, this discrepancy in protein abundance between POI and the LC means that homogenate concentrations that allow the POI to be in the linear range of detection on a polyacrylamide gel, necessarily put the LC outside the linear range of detection. Recently it was shown that β-actin is a poor control for many Western blot analyses because at the protein concentrations most often used, optical density values are not only outside the linear range, but they become essentially uncorrelated with protein concentration (Dittmer and Dittmer, 2006). This second issue is pertinent even in the case of qualitative studies, where loading controls are just compared visually, because such studies often assume that if the protein bands appear to be equal, they must be very nearly so.
Given these issues, we decided to determine the feasibility of total protein alternatives to single-protein loading controls. We tested two total protein stains. SYPRO® Ruby is a commercially available protein stain that is used prior to antibody staining, thus circumventing a potential problem of extraneous antibody adhering to the blot and falsely elevating the measured protein concentration. Amido black is a commonly used permanent post-antibody stain, and low-cost in comparison to SYPRO® Ruby. We compared linearity of these stains to the loading control GAPDH, and in some cases β-actin. We used a serial dilution of cell homogenate spanning concentrations commonly used for post-synaptic density protein-95 (PSD-95) and pERK, two proteins that are of broad interest in neuroscience. PSD-95 is a synaptic scaffolding molecule and ERK is a ubiquitous signaling protein that is regulated by phosphorylation. In this study we show that total protein stains are acceptable alternatives to single-protein loading controls, and include additional cautionary notes about the use of semi-quantitative Western blotting.
METHODS
Samples and Blotting
Nuclear and cytoplasmic fractions extracted from pooled mouse cortex (adapted from Kitchener et al., (2004)) were recombined and aliquoted to create a standard protein homogenate that we have found remains stable over time. Protein concentration was determined using a Bicinchoninic Acid Solution (BCA) protein assay test (Sigma). 10% serial dilutions ranging from 21μg to 41μg per well were loaded in duplicate based on our experience that the optimal range for most low-abundance proteins of interest is 30μg. Different dilutions served as intentional “loading errors”, by which we could test the ability of a particular loading control to normalize differences due to loaded amount. Samples were denatured at 90°C for 7min in Laemmli’s sample buffer plus 0.5M DTT (2% SDS, 10% glycerol, 5% β-mercaptoethanol, 62.5mM Tris, pH 6.8, 0.008% bromophenol blue) and run at randomly assigned positions or in order on a 10% polyacrylamide gel and transferred to a nitrocellulose membrane. Blots were stained with SYPRO® Ruby (Invitrogen) according to the manufacturer’s instructions (before any other stain or blocker, as milk was observed to block SYPRO staining). A FluorChem ™ 8900 Alpha Imager (Alpha Innotech) was used to capture digitally the emitted light of SYPRO® Ruby staining excited by 302nm UV light, as well as chemiluminescence from the reaction of HRP-linked secondary antibody and SuperSignal West Pico (Pierce) solution. Signals were recorded as pixel intensity, and exposure times were adjusted such that there were no saturated pixels.
Antibody Procedure
Blots were blocked in 5% milk in TBS + 0.1% Tween (1hr, room temp). Primary antibodies were incubated at 4°C overnight, and were as follows: mouse polyclonal anti-PSD-95 (1:2000, Affinity BioReagents), mouse anti-pERK (1:1000, Cell Signaling), rabbit anti-GAPDH (1:4000, Santa Cruz), mouse anti- β-actin (1:10,000, Sigma). Secondary antibodies were HRP-linked polyclonal anti-mouse and anti-rabbit (1:1000, Cell Signaling), incubated for 2 hours at room temperature in 5% milk. All incubation steps and washes were completed on a Rocker Platform (Bellco), positioned perpendicular to the pivot axis such that each lane was evenly exposed to all reagents. Between antibodies, blots were reblocked, but no chemical stripping was used. Each blot was stained using submersion in Amido Black (0.03% Napthol Blue Black in 3% acetic acid, Sigma.) for 3 minutes and allowed to air-dry overnight on plastic wrap before scanning at 300dpi using a HP Scanjet 7400C. For the primary antibody GAPDH (Santa Cruz), concentrations ranging from 1:2000 to 1:10,000 of primary antibody and different incubation times (15min, 1 hour, overnight) were tested, but did not improve linearity. A second antibody to GAPDH (Sigma, G9545) was also tested using concentrations of 1:1000 and 1:10,000, with blots either stained solely with this antibody or after preliminary staining with PSD-95.
Densitometry
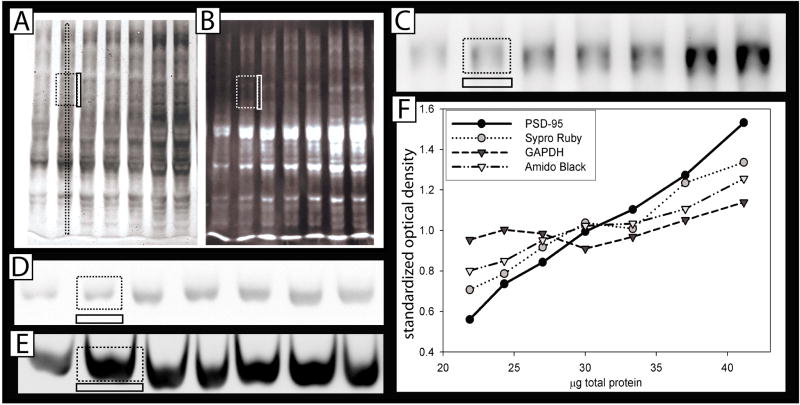
Images were analyzed using AlphaEaseFC software (Alpha Innotech). For relative quantification, the integrated optical density value (defined as Σ(each pixel value -background)) was determined for equal sized boxes (for each antibody) drawn around bands, with background values taken below each band of interest to account for non-specific antibody staining in the lane. For total protein stains, different box sizes were tested, either around most of the lane, a small portion of the lane, or a thin strip through the center of the lane running from top to bottom. Background values were taken between lanes to remove background due to non-specific staining from neighboring lanes (see figure 1). However, in both cases background values did not significantly alter the data, and high values should be considered a warning sign. A preliminary test showed that the placement and size of the boxes shown in figure 1 (either a small rectangle, or a thin strip) is optimal for the total protein stains. A small rectangle allows multiple single proteins to be quantified, and can exclude the protein of interest, while a thin strip allows a majority of proteins to be included, while minimizing errors due to lane bending. The correlation coefficient data reported (figure 2B) are for small rectangles.
Figure 1. Quantification of relative abundance using Western Blotting.
Serial dilutions (21.9, 24.3, 27, 30, 33, 37 and 41μg of protein) derived from mouse cortex were run on SDS polyacrylamide gels. Large dotted rectangles represent quantified area (both a long strip or a smaller rectangle worked sufficiently to quantify total protein). Smaller, solid adjacent rectangles represent subtracted background, either between the lanes (for total stains) or below the bands (for single proteins). A: Amido black total protein stain, B: SYPRO® Ruby total protein stain, C: Representative blots stained with anti-PSD-95 D: anti-GAPDH (Santa Cruz.) and E: anti-GAPDH (sigma). F: Optical density at each protein concentration averaged over 10 blots, showing the different “relative abundance” slopes determined using each potential loading control. (β-actin and pERK were omitted for clarity, and because they were represented on <10 blots.) These data were examined statistically in figure 2B.
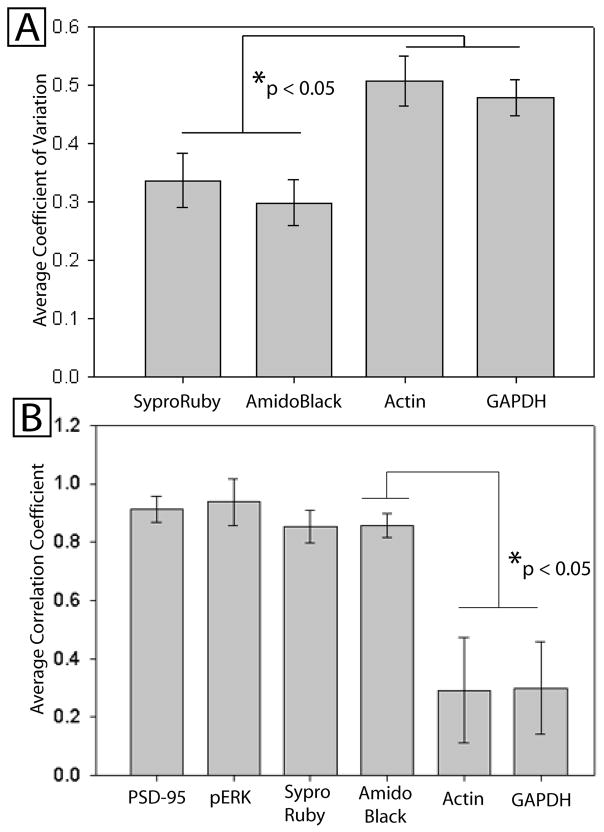
Figure 2.
Serial dilutions between 21.9 and 41μg of protein homogenate were loaded in duplicate on each blot and stained both for the protein of interest (PSD-95) as well as each potential loading control. Relative optical density of the stain was used as a measure of abundance. A: The Average Coefficient of Variation (COV) of the “relative abundance” derived from the measure of PSD-95 normalized to each loading control. The COV is the standard deviation divided by the average normalized optical density, and should be low when the loading control accurately reflects loaded differences. There was an overall difference in COV based on loading control (F=5.6, p<0.01). B: The average correlation (over all blots) between the known amount of loaded protein and measured optical density of the loading control. There was an overall difference in correlation coefficients by loading control (F=10.4, p<0.001). Post hoc findings corrected for multiple comparisons are shown for both graphs.
For calculations of coefficients of variation, blots were standardized so that data from replicate blots could be combined. When all experimental samples can be represented on a single gel, each band can be standardized (divided by) the mean of bands of that gel in order to average between technical replicates. (When several gels are needed to represent all groups, a standard protein or standard dilution series serves this purpose instead. Similarly, if the experimental groups vary enough that multiple digital exposures are necessary to avoid saturation, relative concentration should be extrapolated from a dilution series.)
Results
Western analysis was completed on serially diluted samples of brain homogenate (between 22 and 41μg total protein), typical of experiments used to detect differences in brain protein levels. Two total protein stains, SYPRO® Ruby and aqueous Amido black, were tested along with PSD-95 (always first), followed by one or more of the following: pERK, GAPDH, and β-actin (Figure 1A-D). A second antibody to GAPDH was also tested alone (no other antibodies) (Figure 1E). Differences in the chemiluminescence emission were detected digitally. However, we initially compared digital imaging to film exposure for quantifying the optical density of β-actin and GAPDH staining and found that film was more likely to become saturated (unpublished observations). Small rectangles were drawn around each band, as shown in figure 1A–E, and used to quantify the integrated density value. Identical sized boxes (same number of pixels) were necessarily used for each antibody throughout the experiment, as integrated density is highly dependent on pixel number, and average density does not reliably reduce variance if box sizes differ. Figure 1F shows the average optical density values for each concentration used, averaged over all 10 blots. To test differences statistically between loading control performance, the following measures were made:
First, we used the coefficient of variation (COV) to determine how well the commonly used practice of dividing the protein of interest by the loading control (the POI/LC ratio) is able to normalize for “loading errors” due to human error, incomplete transfer, or position effect on the blot. In this case, “loading errors” were intentional, known differences created using a serial dilution. The COV is calculated using the standard deviation of all the normalized amounts (PSD-95/LC), divided by the average of these numbers. The COV is used instead of standard deviation alone because it is resistant to effects due to the magnitude of the optical density, which necessarily differs between loading controls. Lower coefficients of variation signify a better ability of the loading control to normalize loading errors. The average coefficient of variation for PSD-95 when divided by each loading control, respectively, was 0.34 for SYPRO® Ruby, 0.30 for Amido Black, 0.47 for GAPDH (Santa Cruz) and 0.50 for β-actin (figure 2A). There was an overall difference in the coefficient of variation due to loading control (F=5.6, p<0.01). Post hoc analysis showed that Amido Black had significantly lower COV compared with either GAPDH or β-actin (p<0.05), but was not significantly different from SYPRO® Ruby (p>0.05).
Secondly, relative optical density measures were taken from each blot (14 lanes per blot, two averaged lanes per concentration) and the correlation coefficient (r) between loaded amount and optical density was computed for each blot (Figure 2B). PSD-95 (n=10 blots) had an average r of 0.914, SYPRO® Ruby (n=11): 0.848, GAPDH (SC) (n=10): 0.253, Amido Black (n=11): 0.865, β-actin (n=6): 0.268, and pERK (n=2): 0.938. Using ANOVA, there was an overall difference in correlation coefficients due to loading control (F=10.4, p<0.001). Post hoc analysis (Tukey’s HSD correction) showed that SYPRO® Ruby and Amido Black had significantly higher correlation coefficients than GAPDH(SC) or β-actin (p<0.01), but were not significantly different from each other (p>0.05). GAPDH (Sigma) was tested separately (no other antibodies used, n=2 gels, or after measuring PSD-95, n=2) and showed a negative correlation coefficient (−0.38), despite the fact that amido black on these gels correlated well with concentration (either quantified with small boxes (r= 0.92), or using long thin boxes (r=0.94), (different box sizes shown in Figure 1)). Prior staining with PSD-95 did not alter measurement of GAPDH.
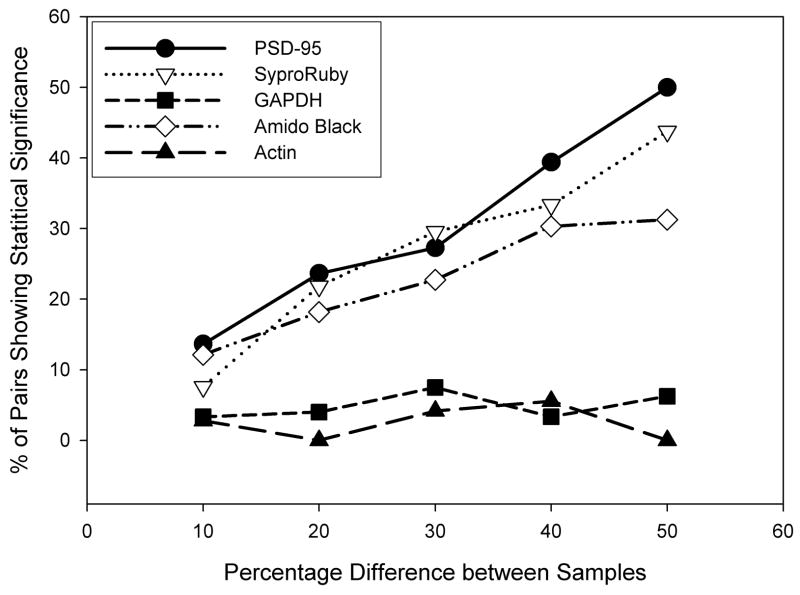
Finally, we were able to calculate the approximate detectable difference given proteins loaded in these ranges. “Detect” was defined as finding a positive, statistically significant difference using a 1-tailed t-test (p<0.05) to compare a pair of different protein dilutions on one gel, loaded in duplicate (see figure 3). A known 50% difference was experimentally “detectable” between replicate pairs of bands 50% of the time for PSD-95 and ~44 and 31% of the time for SYPRO® Ruby and AmidoBlack, respectively. The difference was detectable by anti-GAPDH (SC) only 6%, or β-actin, 0% of the time, suggesting detection was at levels equivalent to chance for these proteins loaded at these concentrations. Using a less strict definition of detectability (eg: a positive difference between the means of each pair) suggested a much higher “detectability”, but revealed the same relative trend regarding each loading control.
Figure 3. Total protein stains are better than high-abundance loading controls at detecting known differences in protein level.
The x-axis indicates the known concentration difference (ranging from 10–50%) between individual lanes containing serial dilutions, in order to test for the ability to determine these differences experimentally. The y-axis depicts the percentage of possible pairs that, within a blot, were able to demonstrate a statistical difference (in the correct direction) between concentrations run in duplicate (p < 0.05).
Discussion
Our results show that total protein stains are superior for detecting differences in protein amounts loaded within the range typically used for quantification of low-abundance proteins of interest, such as pERK and PSD-95. However, the coefficients of variation, even for the total protein stains, were still quite high, and several explanations for this effect are possible. First, we noticed that there is a significant edge-effect which varies between stain and antibody types. Although this effect was not always present, often the lanes nearest to the edge of the gel would stain more intensely than the interior lanes. This effect was especially pronounced for GAPDH, but also was noticeable with SYPRO® Ruby and Amido Black staining. For this reason, most of the samples analyzed were positioned in random order on the gels. However, this effect still contributes to higher variation, a major reason why multiple gel repetitions are suggested. Second, because each stain and antibody has a different correlation with loaded protein concentration, dividing the POI by the LC may be able to reduce, but not remove, variance that is attributable to differences in the amount loaded into each lane. Figure 1E shows the average relationship between antibody staining and loaded amount, and reveals that PSD-95 and the total protein stains show a linear relationship to the loaded amount on average, although the protein stains show a shallower slope. Dividing by one of the total protein stains should reduce some of the variance due to concentration, whereas dividing by GAPDH or β-actin should only introduce more variance as both are nearly uncorrelated with total concentration. However, for typical experiments, variation between the loaded amounts will be drastically lower compared with the serial dilutions used here. Thus, it is expected that in a typical experiment the coefficient of variation for repeated samples normalized with each loading control would be significantly lower. Given our findings and observations, our protocol for semi-quantitative blotting is as follows:
Test for Differences in Loading
Samples that have been equalized by first using a BCA test, or equivalent, are loaded in pseudo-randomized order for at least one of the replicate blots (a publication replicate can be run in order). Total protein stain lanes are compared for unequal loading. A cutoff (eg: >20% deviation from the mean) is established, and samples from lanes where the deviation is high are discarded or rerun, as this type of deviation is probably due to improper transfer. A Student’s t-test or ANOVA is used to determine if there is a statistically significant difference between the loading controls for the experimental conditions being compared. If a difference is found and enough sample remains, the dilutions of the sample should be retested using the BCA (or equivalent) test for total protein, and rediluted.
Normalization
As discussed above, normalization (dividing by the LC) will not completely remove differences due to unequal loading and thus should be relied on cautiously, especially in cases where there appears to be a difference between groups of interest. Instead, it is more important that lanes and gels be replicated, and some measure be used to test statistically for overall differences in loading. However, it is important to point out that our data do not specifically suggest that normalization will aberrantly alter data, especially if the samples are within a tight concentration range. Therefore, if normalization is desired or required (e.g., if there is a difference between experimental groups but samples cannot be rerun), our data suggest that total protein stains are better suited to correct for differences in loading than single-protein high-abundance loading controls such as GAPDH, and can reduce at least some of the variation due to loading differences. Alternatively, our results suggest that one or, preferably, multiple single protein loading controls (such as PSD-95) that are of similar abundance to the protein of interest could be used successfully, with the caveat that the investigator must demonstrate either that such proteins do not vary under the conditions tested, or that the specific hypothesis being tested warrants the use of such a control. Proteins of the same molecular weight should not be used as controls (eg: ERK as a control for pERK, on the same blot) as this can lead to a dramatic reduction in the signal of the second antibody (unpublished observations). Similarly, we have observed “ghosting,” when the center of the band ceases glowing at very high concentrations, and “visible bands” where the nitrocellulose appears burned. These three phenomena may explain why overloaded concentrations are less quantitative. Although these phenomena are not visible at the (relatively) lower concentrations used in this experiment, the irreversible reactions creating them may be occurring at rates sufficient to alter the chemiluminescence. Another possibility is that less of the binding site on the protein is available because of the high density of the excess protein in the gel lane.
In our experience, differences due to position on the gel and improper mixing of samples can be more significant than potential loading effects (as long as a BCA test is run first). Therefore it is most important that samples be positioned randomly on the gel, and gels run at least in duplicate (we prefer triplicate). Directly before loading each sample, tubes should be carefully mixed by flicking, as even a few minutes of settling can lead to loading errors. Additionally, researchers should run a dilution titration for each antibody and protein stain employed to establish the range of linearity in their own samples of interest, as well as to determine the size and placement of the box used to quantify optical density. Furthermore, the order of stains and antibodies should be tested to ensure that prior steps do not affect subsequent binding. Although we found that interference between antibodies was not the cause of the non-linearity of the anti-GAPDH (Sigma) staining, it is possible that interference between antibodies and protein stains in this study had some effect on the coefficients of variance. As this is likely to differ based on the antibodies and homogenates being used, it is suggested that researchers test for this possibility within their own systems.
Although we cannot rule out the possibility that certain high-abundance proteins will function adequately under certain conditions (especially low protein loading), the vast majority of protein controls that are displayed in manuscript figures show dark, overexposed bands that, in our experience, are always well outside the range where differences can accurately be measured. Findings from such studies are not necessarily incorrect, as this type of normalization, in our experience, is more likely to add random noise to the data than specifically bias the findings. However, studies using such Westerns to claim a lack of difference between groups may be grossly statistically underpowered; a more careful use and understanding of western blotting technique should reduce the rate of false negatives in the field.
Acknowledgments
This work was supported by MH35321, HD007333 and the Spastic Paralysis Research Foundation of the Illinois-Eastern Iowa District of Kiwanis International. We would like to thank Shawn Kohler for his help on the analysis and Der-I Kao, Ning Weng, Soong Ho Kim, Xiaofeng Huo, and Claudia Lutz for discussions leading to this paper.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Alexander M, Curtis G, Avruch J, Goodman HM. Insulin regulation of protein biosynthesis in differentiated 3T3 adipocytes. Regulation of glyceraldehyde-3-phosphate dehydrogenase. J Biol Chem. 1985;260:11978–85. [PubMed] [Google Scholar]
- Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis. 2006;21:217–27. doi: 10.1016/j.nbd.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Dittmer A, Dittmer J. Beta-actin is not a reliable loading control in Western blot analysis. Electrophoresis. 2006;27:2844–5. doi: 10.1002/elps.200500785. [DOI] [PubMed] [Google Scholar]
- Huggett J, Dheda K, Bustin S, Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes and immunity. 2005;6:279–84. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- Kitchener P, Di Blasi F, Borrelli E, Piazza PV. Differences between brain structures in nuclear translocation and DNA binding of the glucocorticoid receptor during stress and the circadian cycle. Eur J Neurosci. 2004;19:1837–46. doi: 10.1111/j.1460-9568.2004.03267.x. [DOI] [PubMed] [Google Scholar]
- Liu NK, Xu XM. beta-tubulin is a more suitable internal control than beta-actin in western blot analysis of spinal cord tissues after traumatic injury. Journal of neurotrauma. 2006;23:1794–801. doi: 10.1089/neu.2006.23.1794. [DOI] [PubMed] [Google Scholar]
- Moskowitz PF, Oblinger MM. Transcriptional and post-transcriptional mechanisms regulating neurofilament and tubulin gene expression during normal development of the rat brain. Brain Res Mol Brain Res. 1995;30:211–22. doi: 10.1016/0169-328x(95)00006-e. [DOI] [PubMed] [Google Scholar]
- Nahlik KW, Mleczko AK, Gawlik MK, Rokita HB. Modulation of GAPDH expression and cellular localization after vaccinia virus infection of human adherent monocytes. Acta biochimica Polonica. 2003;50:667–76. [PubMed] [Google Scholar]
- Vasudevan KM, Gurumurthy S, Rangnekar VM. Suppression of PTEN expression by NF-kappa B prevents apoptosis. Molecular and cellular biology. 2004;24:1007–21. doi: 10.1128/MCB.24.3.1007-1021.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner B, Natarajan A, Grunaug S, Kroismayr R, Wagner EF, Sibilia M. Neuronal survival depends on EGFR signaling in cortical but not midbrain astrocytes. Embo J. 2006;25:752–62. doi: 10.1038/sj.emboj.7600988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yperman J, De Visscher G, Holvoet P, Flameng W. Beta-actin cannot be used as a control for gene expression in ovine interstitial cells derived from heart valves. The Journal of heart valve disease. 2004;13:848–53. [PubMed] [Google Scholar]