Abstract
Purpose
To determine whether CD4+CD25+ T-regulatory cells (Tregs) from the eyes of rats with recurrent (r) experimental autoimmune uveitis (EAU) were less efficient in suppressing intraocular inflammation than those from rats with monophasic (m) disease (m-EAU).
Methods
m-EAU and r-EAU were induced in Lewis rats by immunization with R16 or by adoptive transfer of R16-specific T cells, respectively. Ocular CD4+CD25+ Tregs were separated from CD4+CD25− T-effector cells, and the inhibitory functions of Tregs were determined. Aqueous humor (AqH) from m-EAU and r-EAU was collected and studied for its ability to enhance ocular Treg function.
Results
The authors found that the number of ocular CD4+CD25+ (Tregs) increased in the eye during resolution of the first acute attack of intraocular inflammation in m-EAU and r-EAU. However, the suppressor function of these cells was weaker in r-EAU. The suppressor function of ocular Tregs in r-EAU was enhanced by incubation with AqH from animals recovering from m-EAU. Moreover, the weaker suppressor function of ocular Tregs in r-EAU correlated with low or undetectable levels of IL-10 in the AqH and was reversed by the addition of IL-10 to the AqH. Finally, the transfer of ocular Tregs from animals with m-EAU converted r-EAU to a monophasic disease.
Conclusions
This study demonstrated that although a number of mechanisms may contribute to the recurrence of intraocular inflammation, dysregulation and malfunction of Tregs in the eye are important factors in disease recurrence.
Three patterns of autoimmune (idiopathic) uveitis are seen in humans—monophasic, recurrent, and chronic. Chronic and recurrent uveitis are difficult to treat and they account for approximately 10% of legal blindness in the Western world.1 To understand the mechanisms that result in the natural resolution of monophasic uveitis and, thus, to identify optimal therapeutic strategies for recurrent or chronic uveitis, we previously generated experimental animal models of autoimmune uveitis with characteristics of the human disease.2–4 The major pathogenic events in experimental autoimmune uveitis (EAU) are initiated by the activation of T cells reactive with retinal antigens, such as interphotoreceptor retinoid-binding protein (IRBP).5,6 Activated T cells have an increased ability to enter the target organ7 and to produce cytokines and chemokines to recruit other inflammatory cells that result in retinal tissue damage. Immunization with the immunodominant 1177–1191 peptide (R16) of IRBP results in monophasic disease in the Lewis rat; intraocular inflammation appears on day 8, lasts for 2 weeks, and spontaneously resolves. In contrast, the adoptive transfer of R16-specific T cells in Lewis rats results in recurrent disease that starts on day 4, with repeated recurrences for the next 2 to 3 months. These two models provide us with useful tools for studying the regulation of pathogenic effector T cells in vivo.
A number of mechanisms are likely to contribute to recovery from autoimmune disease, including uveitis.8–10 Recent studies have demonstrated that among CD4 T cells, a subset expressing CD25 has strong suppressor activity.11,12 These naturally arising CD4+CD25+ regulatory T cells (Tregs) are powerful inhibitors of T-cell activation in vivo and in vitro, acting partially through IL-10 or transforming growth factor β (TGF-β)13,14; they also contribute to the maintenance of immunologic self-tolerance and negative control of various immune responses. Their depletion or alteration of function leads to the development of autoimmune disease in otherwise healthy animals.15
The eye is the prototypic immunoprivileged organ. The ability of the ocular microenvironment, especially the aqueous humor (AqH),16 to suppress immune effector responses and inflammation is believed to be important to the existence of ocular immune privilege.16–18 Normal AqH displays many immunosuppressive and anti–inflammatory properties in vitro. With regard to adaptive immunity, AqH suppresses T-cell activation after ligation of the T-cell receptor (TCR) by antigen, inhibiting the proliferation of T-effector cells and the secretion of IFN-γ.17 It also alters the functional program of TCR ligand–activated, primed T cells, converting the cells to TGF-β–producing regulatory cells.19 Numerous immunomodulatory factors, such as TGF-β2,20,21 α-melanocyte–stimulating hormone,22 vasoactive intestinal peptide,23 calcitonin gene-related peptide,24 macrophage migration inhibitory factor,25 and IL-10,26 account for the immunosuppressive properties of AqH. This study provides evidence that Treg cells mediate spontaneous recovery from an active disease process in the target organ.
Materials and Methods
Animals and Reagents
Pathogen-free female Lewis rats (5–6 weeks old) were purchased from Harlan Sprague–Dawley (Indianapolis, IN) and were housed and maintained at the animal facilities of the University of Louisville (Louisville, KY).
All animal studies conformed to the ARVO Statement on the Use of Animals in Ophthalmic and Vision Research. Institutional approval was obtained, and institutional guidelines regarding animal experimentation were followed. Bovine IRBP peptide R16 (residues 1177–1191, ADGSSWEGVGVVPDV) was synthesized by Sigma-Aldrich (St. Louis, MO).
Animal Model of EAU
The induction of uveitis in Lewis rats by immunization with R16 has been described previously.2,3 Briefly, the rats were immunized subcutaneously with 200 μL of an emulsion containing 50 μg R16 and 500 μg Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI) in incomplete Freund adjuvant (Sigma, St. Louis, MO), distributed over six spots on the tail base and flank.
For induction of uveitis by adoptive transfer of R16-specific T cells,3 naive Lewis rats were injected with in vitro–restimulated R16-specific T cells prepared from rats with uveitis and were examined daily by slit lamp biomicroscopy for clinical signs of uveitis.
Intensity of uveitis was scored blind on an arbitrary scale of 0 to 4,2,3 with 0 representing no disease, 1 representing engorged blood vessels in the iris and an abnormal pupil configuration, 2 representing a hazy anterior chamber, 3 representing a moderately opaque anterior chamber with the pupil still visible, and 4 representing an opaque anterior chamber, obscured pupil, and, frequently, proptosis.
Inflammation in the eye was confirmed by histopathologic examination. Many inflammatory cells, together with a disorganized retinal architecture, retinal detachment, and photoreceptor cell damage, were seen in the anterior and posterior chambers.
Aqueous Humor Collection
Aqueous humor was collected from both eyes by an anterior chamber puncture (20 μL/rat) using a 30-gauge needle under a surgical microscope and was stored immediately in a −80°C freezer until use. The concentration of IL-10 in the AqH was measured by ELISA (rat IL-10 ELISA kit; R&D Systems, Minneapolis, MN). To modulate Treg function in vitro, the AqH was used after the addition of monoclonal antibodies (mAbs) of anti–IL-10 (1 μg/mL; R&D Systems), TGF-β (25 μg/mL; R&D Systems), or an isotype control or after the addition of recombinant rat IL-10 (25 ng/mL; R&D Systems).
Preparation of R16-Specific T Cells
R16-specific T cells were isolated from R16-immunized Lewis rats using previously described methods for generating antigen-specific T cells.2,3 T cells were isolated from lymph node cells or spleen cells 10 days postimmunization (p.i.) by passage through a nylon wool column. The cells (1 × 107) were stimulated for 2 days with 20 μg/mL R16 in 2 mL medium in a six-well plate (Costar, Cambridge, MA) in the presence of 1 × 107 irradiated syngeneic spleen cells as antigen-presenting cells (APCs). Then activated lymphoblasts were isolated by gradient centrifugation (Lymphoprep; Robbins Scientific, Mountain View, CA) and cultured in RPMI 1640 medium (Mediatech, Herndon, VA) supplemented with 15% IL-2–containing medium (supernatant from Con A-stimulated rat spleen cells).
Isolation of the CD4+CD25+ T-Cell Subset
Monoclonal antibodies OX6 (mouse anti–rat major histocompatibility complex [MHC class II]), OX8 (mouse anti–rat CD8), OX12 (mouse anti–rat kappa light chain), NKR-P1a (CD161a), and OX39 (mouse anti–rat CD25), used for the purification of T-cell subpopulations and for flow cytometry, were purchased from BD Biosciences (La Jolla, CA). Rat CD4+ T cells were purified from spleen or eye cells by the removal of B cells, CD8 T cells, class II-positive cells, and NK cells using a cocktail of OX-12, OX8, OX6, and NKR-P1a and a MACS magnetic column. Briefly, spleen or eye cells were shaken for 30 minutes in ice with the mAb cocktail, then for 15 minutes at 4°C with anti–mouse IgG microbeads. The cells were then separated into bound and nonbound cells on a separator column (auto-MACS; Miltenyi Biotec Inc., Auburn, CA) that was washed with 15 mL medium according to the manufacturer’s protocol, and the flow-through fraction containing CD4+ enriched cells was collected. CD4+CD25+ T cells were enriched by binding to anti–PE magnetic beads after labeling with PE-conjugated OX-39 mAb; the CD4+CD25+-depleted cells were designated as CD4+CD25− T cells. The purity of the isolated cell fraction was determined by flow cytometric analysis using FITC-conjugated anti–CD4 antibodies and PE-conjugated antibodies directed against the TCR or CD25.2 Purity of CD4+CD25+ cells was routinely above 98% for spleen cells and above 85% for ocular cells.
Immunofluorescence Flow Cytometry
Aliquots of 2 × 105 cells were double stained with combinations of FITC- or PE-conjugated mAbs against rat αβTCR (R73), CD4, CD25, IL-10, or Foxp3 (all from BD Biosciences). For intracellular staining, the cells were incubated for 30 minutes at 4°C with anti–CD4 or isotype control antibodies, fixed overnight with 1 mL fixation buffer (Fix and Perm Cell Permeabilization Kit; eBioscience, San Diego, CA), washed, and incubated for 30 minutes 4°C with anti–rat-Foxp3 or with anti–rat IL-10 antibodies. Data collection and analysis were performed on a flow cytometer (FACSCalibur; BD Biosciences) using flow cytometry software (CellQuest; BD Biosciences).
Proliferation Assay
R16-specific T cells (4 × 105 cells/well) were cultured at 37°C for 48 hours in 96-well microtiter plates with or without R16 in the presence of 1 × 105 APCs in a total volume of 200 μL. [3H]-Thymidine was added to each culture for the last 8 hours, and incorporation was assessed using a microplate scintillation counter (Packard Instruments, Meriden, CT). For the in vitro suppression assay, CD4+CD25+ T cells were used as responder cells because they gave a stronger response than total unfractionated T cells. Responder T cells (4 × 105 cells/well) were prepared on day 9 from rats immunized with R16 and were stimulated for 48 hours with R16 (10 μg/mL) and APCs in the presence or absence of various numbers of CD4+CD25+ Treg cells, then processed as above. To avoid a direct effect of AqH on the effector T cells, Tregs pretreated with AgH were collected and washed before coculture with R16-specific effector T cells. The proliferative response was expressed as mean ± SD cpm of triplicate determinations.
Examination of Pathologic Findings
Inflammation of the eye was confirmed by histopathology. Whole eyes were collected, immersed for 1 hour in 4% phosphate-buffered glutaraldehyde, and transferred to 10% phosphate-buffered formaldehyde until processed. The fixed and dehydrated tissue was embedded in methacrylate, and 5-μm sections were cut through the pupillary-optic nerve plane and stained with hematoxylin and eosin. Presence or absence of disease was evaluated blind by examining six sections cut at different levels for each eye. Severity of EAU was scored on a scale of 0 (no disease) to 4 (maximum disease) in half-point increments, as described previously.4
Isolation of Cells from Inflamed Eyes
After perfusion of the anesthetized rat with PBS on the indicated day after immunization or injection of T cells, the eyes were collected, and a cell suspension was prepared by digestion for 10 minutes at 37°C with collagenase (1 mg/mL) and DNase (100 μg/mL) in RPMI 1640, followed by gradient centrifugation on 25% Percoll and subsequent Ficoll separation. Then the cells were washed and resuspended in staining buffer (PBS containing 3% FCS and 0.1% sodium azide) for antibody staining.27,28 To identify regulatory T cells in the eye, mouse anti–rat mAbs against TCR (for T cells), CD4, CD25, or Foxp3 were used for staining, and the cells were analyzed by flow cytometry.
Results
Accumulation of CD4+CD25+ Cells within the Eye Correlates with Resolution of the First Acute Attack of the Disease in Both m-EAU and r-EAU
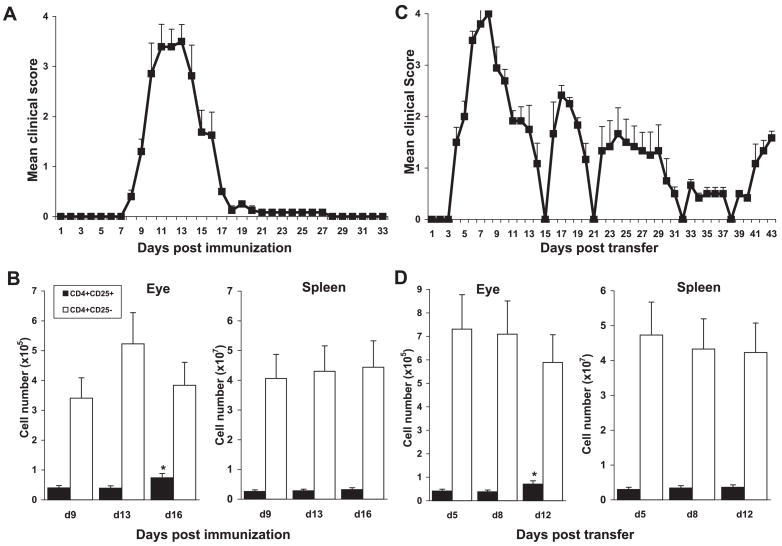
After immunization or in response to tissue inflammation, antigen-specific Tregs have been shown to expand or accumulate in draining lymph nodes29–33 and at the site of inflammation.34 We found that during m-EAU induced by immunization with R16 (Fig. 1A), there was a marked increase in the CD4+ cells expressing CD25+ in the eye as intraocular inflammation evolved from the peak of disease (day 13) to recovery (day 16; Fig. 1B, left). In contrast, the proportion of CD4+CD25+ cells in the spleen remained fairly constant throughout the disease course (Fig. 1B, right). Similar results were also seen in rats with r-EAU induced by the transfer of R16-specific T cells (Fig. 1C). CD4+CD25+ cells increased in the eye during resolution of the first acute attack of the disease (days 8–12) but did not change in the spleen (Fig. 1D).
Figure 1.
Proportions of CD4+ cells expressing CD25 in the eye, but not the spleen, increased during recovery in m-EAU and r-EAU. m-EAU (A) or r-EAU (C) was induced and disease severity was determined (n = 10 rats). The mean score at each time point is the average clinical score of 10 independent scores. Eyes and spleens of m-EAU (B) or r-EAU (D) rats were taken at the times indicated (4 rats/day), and the numbers of CD4+CD25+ and CD4+CD25+ cells in the eye and spleen were assessed by FACS staining. Data are expressed as mean ± SD of four individual rats per time point, representative of three separate experiments. Statistical analyses were performed using one-way ANOVA with Tukey post hoc analysis. CD4+CD25+ cell number in the recovery phase was significantly different from that at the onset or peak of disease (*P < 0.01).
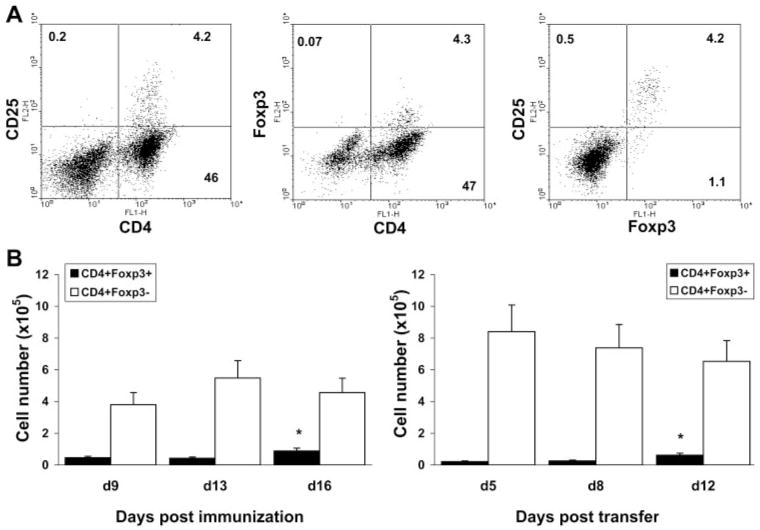
Because CD25 expression is not a definitive marker for Tregs, we also assessed the expression of the transcription factor FoxP3, which determines differentiation of the regulatory phenotype, and distinguished Tregs from activated T cells35,36 in relation to CD25 expression by ocular CD4+ cells. Most CD4+CD25+ cells in the eye were found to express FoxP3 (Fig. 2A), at all times analyzed (Fig. 2B), in m-EAU and r-EAU, confirming that ocular CD25+ cells are regulatory T cells and not activated T-effector cells.
Figure 2.
Ocular CD4+CD25+ cells expressed FoxP3. Ocular cells from m-EAU and r-EAU rats were analyzed by flow cytometry for expression of FoxP3 and CD25. (A) Representative plots from ocular infiltrating cells of r-EAU rats stained with mAbs for CD4/CD25, CD4/Foxp3, and CD25/Foxp3. (B) Numbers of CD4+FoxP3+ cells in eyes over time in m-EAU (left) and r-EAU (right) rats. Data were obtained from three experiments and analyzed by one-way ANOVA and Tukey post hoc analysis. The CD4+Foxp3+ cell number in the recovery phase was significantly different from that at the onset or peak of disease (*P < 0.01).
CD4+CD25+ Tregs Derived from the Eye Are More Potent in m-EAU Than in r-EAU
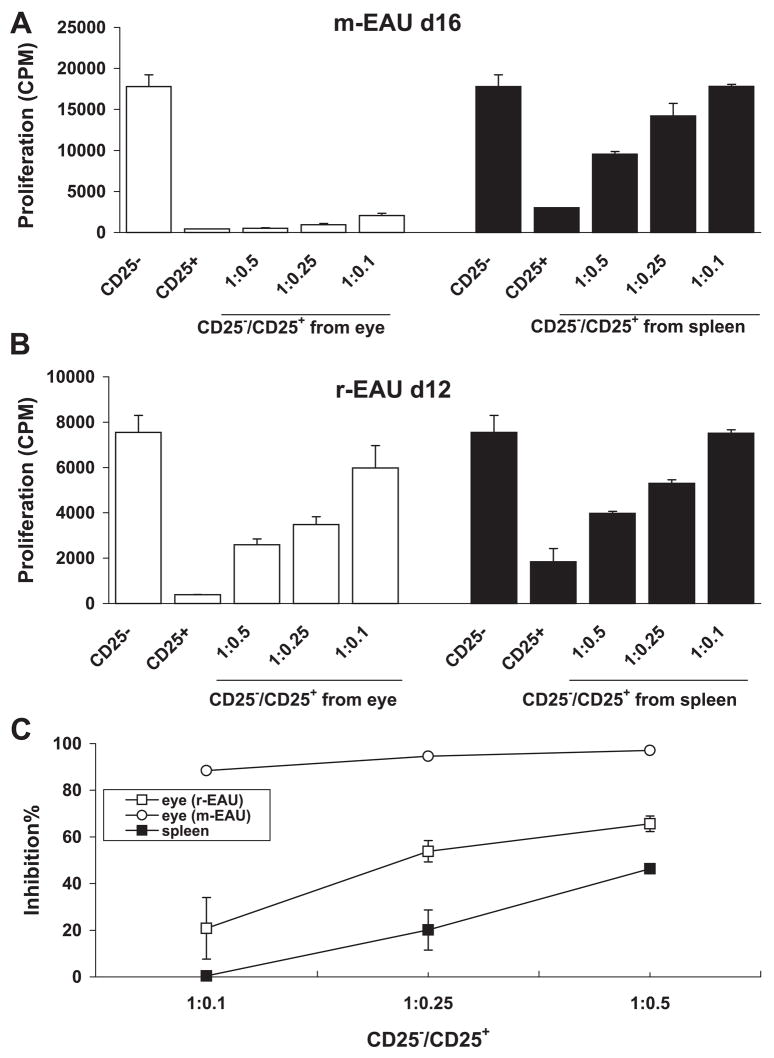
To determine whether the inhibitory function of intraocular CD4+CD25+ T cells on infiltrated effector T cells accounted for monophasic or recurrent EAU, we compared the ability of ocular Tregs from animals recovering from m-EAU or r-EAU to suppress the proliferation of R16 effector T cells in vitro. The CD4+CD25+ Treg cells were derived from the eyes and spleens of rats with m-EAU (day 16 p.i.) and r-EAU (day 12 after transfer); CD4+CD25− T effector cells were derived from the spleens of m-EAU rats (9 days p.i.). The CD4+CD25− T effector cells proliferated in response to specific antigen, whereas CD25+ Tregs did not. Furthermore, the eye-derived CD4+CD25+ Treg cells from the m-EAU rats completely abolished the proliferation of responder CD4+CD25− T cells at a Treg/T-responder ratio as low as 1:10 (Fig. 3A), whereas the same eye-derived Treg cells from r-EAU rats (Fig. 3B) or the spleen of m-EAU rats (Fig. 3A) suppressed the proliferation of responder CD4+CD25− T cells at a Treg/T-responder ratio of only 1:4. Interestingly, there was a hierarchical potency of suppression by CD4+CD25+ cells from different sources, with the m-EAU eye > r-EAU eye > m-EAU spleen (Fig. 3C). These results are consistent with the idea that CD4+CD25+ Tregs in the eye are functionally activated and antigen specific.
Figure 3.
CD4+CD25+ Tregs from the eye of recovering m-EAU rats inhibited CD4+CD25− effector T cells. (A) CD4+CD25− responder T cells (4 × 105 cells/well) from the spleens of m-EAU rats (9 days p.i.) were mixed at the indicated ratios with CD4+CD25+ T cells from the eyes or spleens of recovering m-EAU rats (16 days p.i.) and stimulated with R16 (10 μg/mL) presented by irradiated syngeneic spleen APCs (1 × 105/well). T-cell proliferation was measured at 48 hours by [3H]-thymidine uptake and expressed as cpm of triplicate wells. (B) As in (A), but using CD4+CD25+ T cells from the eyes or spleens of recovering r-EAU rats (12 days p.i.). (C) Percentage inhibition of the proliferation of R16-specific CD4+CD25− effector T cells by CD4+CD25+ Tregs derived from the eyes of recovering m-EAU or r-EAU rats or the spleens of recovering m-EAU rats calculated from (A) and (B). Results shown are representative of those from three independent experiments.
Recurrent Uveitis Is Converted to m-EAU by the Transfer of Ocular CD4+CD25+ Tregs from m-EAU
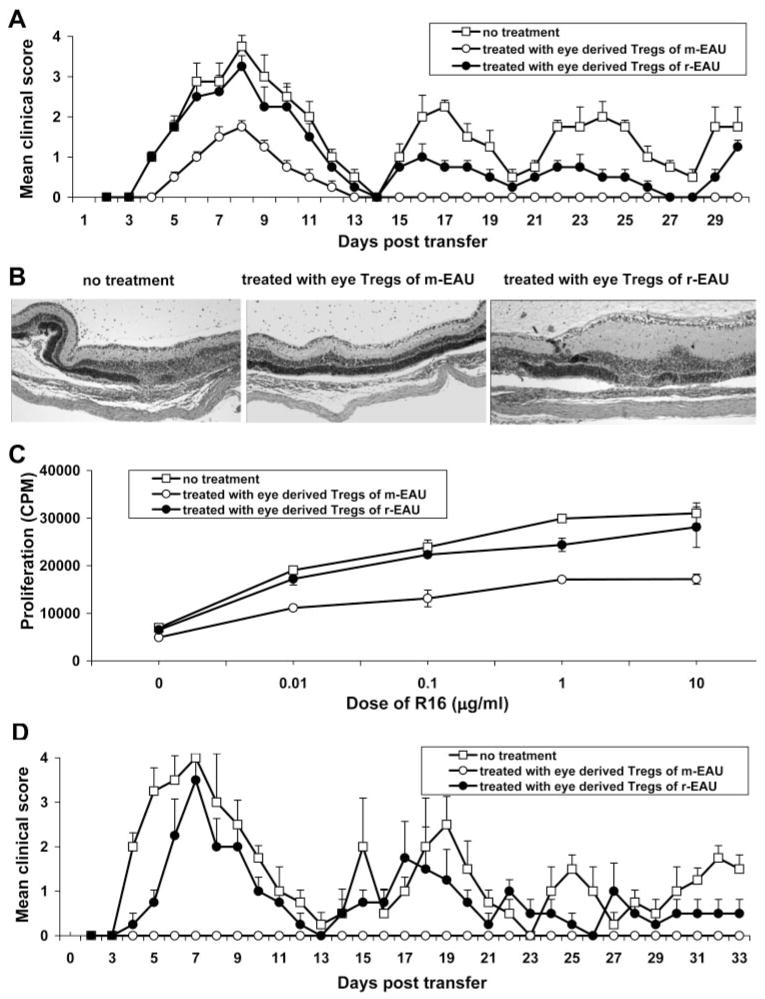
We demonstrated that the population of ocular CD4+CD25+ Tregs in m-EAU was inhibitory at a higher dilution of cells than those from r-EAU in vitro. We sought to extend these observations in vivo by the induction of r-EAU, as previously described. These rats were then injected intraperitoneally on day 0 with 3 × 105 ocular Treg cells prepared from m-EAU (day 16 p.i.) or r-EAU (day 12 after transfer) rats. Tregs from m-EAU rats significantly reduced disease severity and converted the disease pattern in recipients to monophasic, whereas the same number of ocular Tregs from r-EAU rats had a less suppressive effect (Fig. 4A). These results suggested that r-EAU is at least partially attributed to the low inhibitory efficiency of ocular CD4+CD25+ Tregs generated in the r-EAU model. Histologic examination on day 8 showed that although infiltration of inflammatory cells was seen in the anterior segment in all three groups (data not shown), only limited retinal damage was seen in the eyes of rats treated with Tregs from m-EAU rats, in contrast to the other two groups (Fig. 4B). Furthermore, R16-specific splenic T cells on day 8 from the rats treated with ocular CD4+CD25+ m-EAU Tregs responded poorly to in vitro stimulation with R16 (Fig. 4C). Interestingly, spleen cells recovered on day 30 from rats treated with ocular Tregs from r-EAU or PBS (control) rats induced recurrent disease, whereas spleen cells from rats treated with ocular Tregs from m-EAU animals did not (Fig. 4D). These results supported the conclusion that ocular Tregs from m-EAU rats inhibited R16-specific effector T cells in vivo, as well as in vitro.
Figure 4.
CD4+CD25+ Tregs from the eyes of m-EAU rats converted recurrent uveitis to monophasic uveitis. r-EAU was induced as previously described, and recipients were then injected intraperitoneally on day 0 with 3 × 105 ocular Treg cells prepared from m-EAU or r-EAU in the recovery phase. (A, B) Severity of uveitis was scored over 30 days (A) or determined by histologic examination on day 8 after transfer (B). (C) Splenic T cells collected from the three groups of rats were tested on day 8 after transfer for proliferation to R16. (D) Splenic T cells from these same three groups on day 30 after transfer were restimulated in vitro with R16 and irradiated APCs for 2 days and transferred into naive Lewis rats. Disease induction was observed for 30 days by clinical examination. Data in A, C, and D are the mean clinical score ± SD for three rats per group and are representative of two independent experiments.
IL-10 Produced by CD4 Cells Is Increased in m-EAU Compared with r-EAU
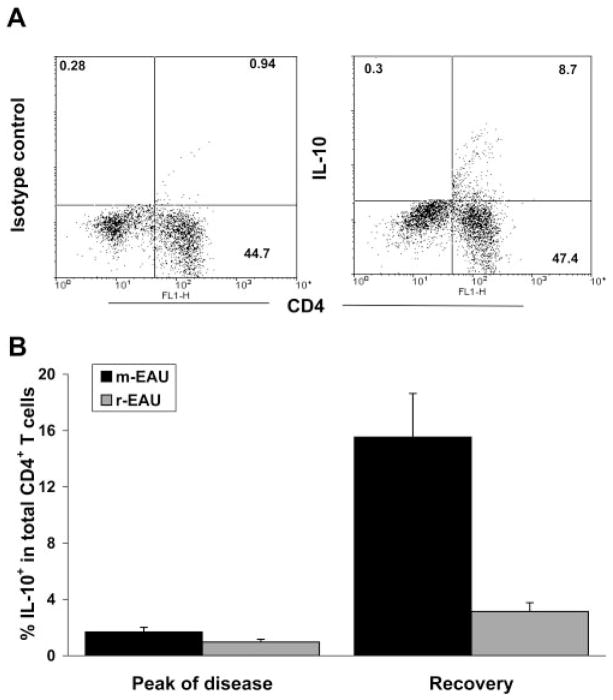
The inhibitory function of Tregs occurs through IL-10, which is likely to be important during recovery from EAU.37 Thus, the production of IL-10 by lymphoid cells in the eye of m-EAU and r-EAU was compared to determine whether the amount of IL-10 produced contributed to the function of Treg. CD4+ cells were found to be major producers of IL-10 (Fig. 5A). Although the proportion of CD4+ cells in the eye producing IL-10 was small, there was an overall increase in the proportion of IL-10+ CD4+ cells over time (Fig. 5B) correlating with recovery in m-EAU, whereas the proportion of IL-10+ CD4+ cells in r-EAU did not significantly increase over time.
Figure 5.
Proportions of CD4+ IL-10+ cells were higher in the eyes of m-EAU than r-EAU rats. (A) Representative plots from ocular infiltrating cells of m-EAU rats stained with mAbs for CD4/isotype control (left) and CD4/IL-10 (right). Cells were harvested on day 16 and stimulated in vitro with PMA/ionomycin. (B) Ocular infiltrated cells were harvested from rats with m-EAU and r-EAU at the peak and recovery phases of disease (4 rats/day), stimulated with PMA/ionomycin, and analyzed for the expression of CD4 and intracellular IL-10. The percentage of IL-10+CD4+ T cells was calculated and is representative of two experiments.
AqH in m-EAU Enhanced the Inhibitory Function of CD4+CD25+ Tregs in r-EAU
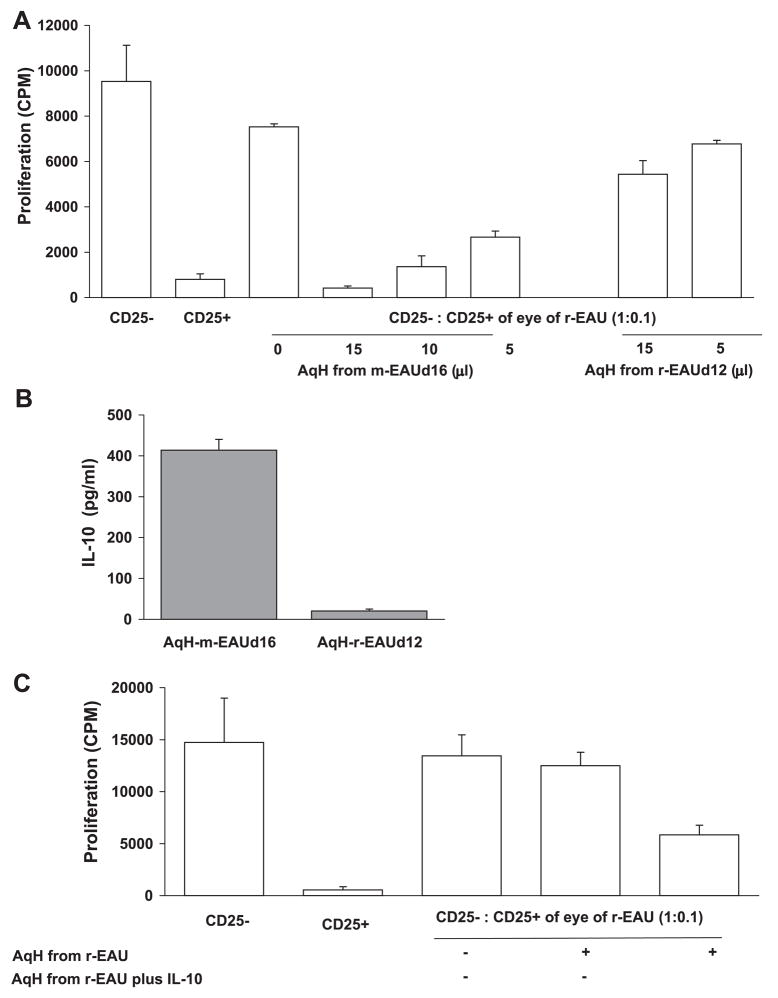
We then hypothesized that molecules present in the AqH in m-EAU contributed to the potent inhibitory effect of ocular Tregs. We incubated CD4+CD25+ Tregs from the eyes of r-EAU rats (12 days p.i.) with AqH from the eyes of either m-EAU (16 days p.i.) or r-EAU rats (12 days p.i.). As shown in Figure 6A, AqH from m-EAU rats, but not r-EAU rats, enhanced the inhibitory function of ocular Tregs from r-EAU rats in a dose-dependent manner.
Figure 6.
Inhibitory activity of CD4+CD25+ Tregs from the eyes of recovering r-EAU rats was enhanced by AqH from m-EAU rats. (A) CD4+CD25− R16-specific effector T cells prepared from the spleens of m-EAU rats (9 days p.i.) were stimulated with R16 (10 μg/mL) and APCs in the presence of CD4+CD25+ Tregs (CD25−:CD25+, 1:0.1) from the eyes of recovering r-EAU rats, with or without overnight pretreatment with the indicated volume of AqH from recovering m-EAU or r-EAU rats. Before coculture with R16-specific effector T cells, Tregs treated with AqH were collected and washed with media to avoid a direct effect of AqH on the effector T cells. T-cell proliferation was measured at 48 hours by [3H]-thymidine uptake and expressed as cpm of triplicate wells for each condition. (B) IL-10 was measured in the AqH of recovering m-EAU and r-EAU rats by ELISA. (C) As in (A) but using Tregs from recovering r-EAU rats pretreated with AqH from recovering r-EAU rats to which IL-10 (25 ng/mL) was added. Results shown are representative of three independent experiments.
IL-10 has been observed in the aqueous humor of human eyes with uveitis.26,38,39 To determine whether IL-10 is present in higher concentrations in the AqH of m-EAU rather than r-EAU and whether IL-10 influences the function of Tregs, we used ELISA to study IL-10 levels in the AqH. The AqH of recovering m-EAU rats contained high levels of IL-10, but little or no IL-10 was detected in the AqH of r-EAU rats (Fig. 6B). When IL-10 was added to the AqH of recovering r-EAU rats, the suppressive effect of CD4+CD25+ cells was significantly enhanced (Fig. 6C).
Discussion
Understanding the mechanisms that mediate the natural resolution of monophasic uveitis may facilitate the design of optimal therapeutic strategies for recurrent uveitis. We found that the accumulation of CD4+CD25+ T regulatory cells in the eye correlated with the recovery phase of m-EAU. Moreover, transfer of small numbers of ocular CD4+CD25+ Treg cells from recovering m-EAU rats prevented recurrences in r-EAU recipients. Although there is extensive evidence for the important role of CD4+CD25+ Tregs in preventing spontaneous or induced autoimmune diseases, including EAU, it was unknown whether these cells mediated natural remission once inflammation was established. Our results demonstrated that CD4+CD25+ Tregs made a significant contribution to controlling an ongoing inflammatory response in the target organ. Dysregulation and malfunction of CD4+CD25+ Tregs in the eye may be an important factor in disease recurrence, persistence, and progression, though a number of other mechanisms may also contribute to the recurrence of inflammation.
The adoptive transfer of CD4+CD25+ Tregs into mice before immunization with IRBP is known to prevent the development of EAU.40,41 In contrast, CD25-depleted wild-type and knockout mice immunized with IRBP show enhanced antigen-specific immunologic responses and severity of intraocular inflammation.42 Depletion of CD25+ cells before the induction of EAU may result in an increased expansion of pathogenic effector T cells. Additionally, the rate of recovery from EAU was markedly slow, suggesting that CD4+CD25+ cells may play an active role in mediating recovery from intraocular inflammation. Indeed, we observed that the adoptive transfer of ocular CD25+ Tregs from the recovery phase of m-EAU prevented the development of r-EAU (Fig. 4), confirming that CD25+ Tregs played a central role in controlling ongoing intraocular inflammation and in the prevention of recurrent autoimmune uveitis.
The number of CD4+CD25+ Tregs in the eye in EAU was significantly increased during the recovery phase (Figs. 1, 2). A relatively small number of these highly potent cells may be sufficient to begin downregulation of local intraocular inflammation, and the increased proportion seen in the later phase of the disease may reflect their continued recruitment/expansion in the eye. Although Tregs were initially described in in vitro studies as hypoproliferative, it is now clear that these cells can be expanded in vivo by antigen,30–33,43 particularly in the presence of inflammatory signals such as up-regulated costimulatory molecules on activated APCs and dendritic cells.31 Tregs that have recently been activated or expanded in vitro are more potent than resting Tregs.44–46 We demonstrated that CD4+CD25+ T cells in the eye are more potent as suppressors in vitro (Fig. 3) than those in the spleen. Moreover, very low numbers of CD4+CD25+ T cells from the eye were able to transfer protection to naive recipients (Fig. 4), whereas the same number of CD4+CD25+ T cells from the spleen had no effect (Ke Y, Shao H, unpublished observation, 2007).
The different protocols used to induce m-EAU and r-EAU may result in different Treg subsets and Tregs with strong and weak antigen specificity. We have observed that CD4+CD25+ Tregs from naive rats also have suppressive activity in vitro (data not shown). In addition, the regulatory T cells isolated from IRBP-induced EAU were more inhibitory for uveitogenic T cells than for MOG-induced encephalitogenic T cells.47,48 Thus, we believe that naive and inducible Tregs exist in m-EAU and r-EAU. Unfortunately, because of technical limitations, the differences between these Treg populations cannot be discerned at this time.
We observed that IL-10 was important for the regulation of EAU by Tregs. CD4+ cells were the major source of IL-10 in the eye (Fig. 5A), and IL-10 production is known to be crucial for the resolution of intraocular inflammation.37 Although the frequency of ocular CD4+CD25+ and CD4+ FoxP3 T cells was similar in m-EAU and r-EAU (Figs. 1, 2), there were many fewer CD4+IL-10+ cells in r-EAU than in m-EAU (Fig. 5B). The presence of more IL-10–producing T cells in the eyes of m-EAU than r-EAU rats strongly suggests that IL-10 is central to the resolution of intraocular inflammation. Treg cells may also produce TGF-β; the production and action of these two cytokines are interrelated and are likely to involve positive feedback loops in which IL-10 enhances the expression of TGF-β and vice versa. IL-10 may act locally at the site of inflammation, whereas TGF-β seems to have a more systemic effect on the immune response. Because no anti–rat TGF-β antibody is available to detect this cytokine by flow cytometry, we focused only on IL-10 in this study.
Furthermore, the observed inability of ocular Tregs from r-EAU to protect against subsequent episodes of intraocular inflammation could not be explained by a subpopulation of CD4+CD25+ effector T cells because most CD4+CD25+ cells in the eye were found to express FoxP3 (Fig. 2A).
The strong inhibitory ability of ocular Tregs was dependent on the AqH within the eye. Although a similar percentage of ocular Tregs was seen during recovery from the first acute episode of m-EAU and r-EAU, the inhibitory function of these cells on effector R16-specific T cells was different. Those from m-EAU rats were more potent inhibitors (Fig. 6), presumably because of a difference in the amount of regulatory factors such as IL-10, TGF-β, and α-melanocyte stimulating hormone49 in the AqH. Interestingly, the weaker suppressive activity of CD4+CD25+ Tregs from r-EAU rats was enhanced by incubation with AqH from m-EAU rats. It is known that many immunoregulatory factors in AqH protect the eye from inflammation. AqH not only suppresses the activation of Th1 cells, it promotes the induction of CD4+CD25+ Treg cells.50,51 Our studies provide additional evidence that factors in the AqH contribute to the functional potency of CD4+CD25+ Treg cells, thus leading to the different clinical presentations of autoimmune uveitis. Better understanding of the mechanism(s) by which Treg cells are influenced by the local microenvironment may identify novel methods of immune regulation.
Despite the weak inhibitory activity of ocular Tregs during recovery from the first acute episode of r-EAU, remission still occurred. It seems that the weak inhibition of ocular Tregs temporarily reduced the pathogenic effect of effector T cells, but, given that uveitis recurred, they did not induce anergy or elimination of effector T cells. In contrast, the anergy or elimination of effector T cells was seen in rats treated with ocular Tregs of m-EAU animals because few (day 8 after transfer; Fig. 4C) or no (day 30 after transfer; data not shown) R16-specific responder T cells were seen in the in vitro proliferative response assay. Indeed, no uveitis was induced by T cells derived from the rats treated with ocular Tregs from m-EAU animals because of the anergy or elimination of R16 effector T cells (Fig. 4D). Therefore, CD4+CD25+ T regulatory cells within the eye played an active and central role in mediating recovery from the first episode of acute EAU and in preventing the development of recurrent or chronic disease.
In conclusion, our data showed that CD4+CD25+ Treg cells derived from the eye during recovery from m-EAU have strong suppressor activity and are involved in the remission of intraocular inflammation. A similar effect of CD4+CD25+ T cells has been shown in other disease models, such as colitis,34,52 early-onset diabetes,44 and experimental autoimmune encephalitis.53 Understanding the physiological mechanisms (e.g., self-antigen, costimulatory molecules on APCs, cytokines) that drive the expansion and recruitment of Tregs in EAU may provide an effective therapy for autoimmune conditions, particularly in diseases with a relapsing-remitting course such as autoimmune uveitis.
Acknowledgments
Supported in part by National Institutes of Health Grants EY12974 and EY14599, Vision Research Infrastructure Development Grant R24 EY015636, a Research to Prevent Blindness Career Development Award (HS), and the Commonwealth of Kentucky Research Challenge Trust Fund (HJK).
Footnotes
Disclosure: Y. Ke, None; G. Jiang, None; D. Sun, None; H.J. Kaplan, None; H. Shao, None
References
- 1.Ganley JP. Current Ocular Therapy. Philadelphia: WB Saunders; 1980. p. 485. [Google Scholar]
- 2.Shao H, Shi H, Kaplan HJ, Sun D. Chronic recurrent autoimmune uveitis with progressive photoreceptor damage induced in rats by transfer of IRBP-specific T cells. J Neuroimmunol. 2005;163:102–109. doi: 10.1016/j.jneuroim.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 3.Shao H, Lei S, Sun SL, Kaplan HJ, Sun D. Conversion of monophasic to recurrent autoimmune disease by autoreactive T cell subsets. J Immunol. 2003;171:5624–5630. doi: 10.4049/jimmunol.171.10.5624. [DOI] [PubMed] [Google Scholar]
- 4.Shao H, Liao T, Ke Y, Shi H, Kaplan HJ, Sun D. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 5.Caspi RR. Immune mechanisms in uveitis. Springer Semin Immunopathol. 1999;21:113–124. doi: 10.1007/BF00810244. [DOI] [PubMed] [Google Scholar]
- 6.Caspi RR. Immunogenetic aspects of clinical and experimental uveitis. Reg Immunol. 1992;4:321–330. [PubMed] [Google Scholar]
- 7.Dullforce PA, Seitz GW, Garman KL, et al. Antigen-specific accumulation of naive, memory and effector CD4 T cells during anterior uveitis monitored by intravital microscopy. Cell Immunol. 2006;239:49–60. doi: 10.1016/j.cellimm.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 8.MacPhee IA, Antoni FA, Mason DW. Spontaneous recovery of rats from experimental allergic encephalomyelitis is dependent on regulation of the immune system by endogenous adrenal corticosteroids. J Exp Med. 1989;169:431–445. doi: 10.1084/jem.169.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien NC, Charlton B, Cowden WB, Willenborg DO. Nitric oxide plays a critical role in the recovery of Lewis rats from experimental autoimmune encephalomyelitis and the maintenance of resistance to reinduction. J Immunol. 1999;163:6841–6847. [PubMed] [Google Scholar]
- 10.Lenz DC, Swanborg RH. Suppressor cells in demyelinating disease: a new paradigm for the new millennium. J Neuroimmunol. 1999;100:53–57. doi: 10.1016/s0165-5728(99)00208-8. [DOI] [PubMed] [Google Scholar]
- 11.Sakaguchi S, Ono M, Setoguchi R, et al. Foxp3+ CD25+ CD4+ natural regulatory T cells in dominant self-tolerance and autoimmune disease. Immunol Rev. 2006;212:8–27. doi: 10.1111/j.0105-2896.2006.00427.x. [DOI] [PubMed] [Google Scholar]
- 12.Shevach EM, Piccirillo CA, Thornton AM, McHugh RS. Control of T cell activation by CD4+CD25+ suppressor T cells. Novartis Found Symp. 2003;252:24–36. [PubMed] [Google Scholar]
- 13.Asseman C, Mauze S, Leach MW, Coffman RL, Powrie F. An essential role for interleukin 10 in the function of regulatory T cells that inhibit intestinal inflammation. J Exp Med. 1999;190:995–1004. doi: 10.1084/jem.190.7.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roncarolo MG, Bacchetta R, Bordignon C, Narula S, Levings MK. Type 1 T regulatory cells. Immunol Rev. 2001;182:68–79. doi: 10.1034/j.1600-065x.2001.1820105.x. [DOI] [PubMed] [Google Scholar]
- 15.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25): breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 16.Cousins SW, Trattler WB, Streilein JW. Immune privilege and suppression of immunogenic inflammation in the anterior chamber of the eye. Curr Eye Res. 1991;10:287–297. doi: 10.3109/02713689108996334. [DOI] [PubMed] [Google Scholar]
- 17.Kaiser CJ, Ksander BR, Streilein JW. Inhibition of lymphocyte proliferation by aqueous humor. Reg Immunol. 1989;2:42–49. [PubMed] [Google Scholar]
- 18.Ohta K, Wiggert B, Yamagami S, Taylor AW, Streilein JW. Analysis of immunomodulatory activities of aqueous humor from eyes of mice with experimental autoimmune uveitis. J Immunol. 2000;164:1185–1192. doi: 10.4049/jimmunol.164.3.1185. [DOI] [PubMed] [Google Scholar]
- 19.Taylor AW, Alard P, Yee DG, Streilein JW. Aqueous humor induces transforming growth factor-beta (TGF-beta)-producing regulatory T-cells. Curr Eye Res. 1997;16:900–908. doi: 10.1076/ceyr.16.9.900.5043. [DOI] [PubMed] [Google Scholar]
- 20.Granstein RD, Staszewski R, Knisely TL, et al. Aqueous humor contains transforming growth factor-beta and a small (less than 3500 daltons) inhibitor of thymocyte proliferation [published erratum appears in J Immunol 1991 May 15;146:3687] J Immunol. 1990;144:3021–3027. [PubMed] [Google Scholar]
- 21.Cousins SW, McCabe MM, Danielpour D, Streilein JW. Identification of transforming growth factor-beta as an immunosuppressive factor in aqueous humor. Invest Ophthalmol Vis Sci. 1991;32:2201–2211. [PubMed] [Google Scholar]
- 22.Taylor AW, Streilein JW, Cousins SW. Identification of alpha-melanocyte stimulating hormone as a potential immunosuppressive factor in aqueous humor. Curr Eye Res. 1992;11:1199–1206. doi: 10.3109/02713689208999545. [DOI] [PubMed] [Google Scholar]
- 23.Taylor AW, Streilein JW, Cousins SW. Immunoreactive vasoactive intestinal peptide contributes to the immunosuppressive activity of normal aqueous humor. J Immunol. 1994;153:1080–1086. [PubMed] [Google Scholar]
- 24.Taylor AW, Yee DG, Streilein JW. Suppression of nitric oxide generated by inflammatory macrophages by calcitonin gene-related peptide in aqueous humor. Invest Ophthalmol Vis Sci. 1998;39:1372–1378. [PubMed] [Google Scholar]
- 25.Apte RS, Sinha D, Mayhew E, Wistow GJ, Niederkorn JY. Cutting edge: role of macrophage migration inhibitory factor in inhibiting NK cell activity and preserving immune privilege. J Immunol. 1998;160:5693–5696. [PubMed] [Google Scholar]
- 26.Ongkosuwito JV, Feron EJ, van Doornik CE, et al. Analysis of immunoregulatory cytokines in ocular fluid samples from patients with uveitis. Invest Ophthalmol Vis Sci. 1998;39:2659–2665. [PubMed] [Google Scholar]
- 27.Liao T, Ke Y, Shao WH, et al. Blockade of the interaction of leukotriene b4 with its receptor prevents development of autoimmune uveitis. Invest Ophthalmol Vis Sci. 2006;47:1543–1549. doi: 10.1167/iovs.05-1238. [DOI] [PubMed] [Google Scholar]
- 28.Shao H, Lei S, Sun SL, Xiang J, Kaplan HJ, Sun D. CpG-containing oligodeoxynucleotide 1826 converts the weak uveitogenic rat interphotoreceptor retinoid-binding protein peptide 1181–1191 into a strong uveitogen. J Immunol. 2003;171:4780–4785. doi: 10.4049/jimmunol.171.9.4780. [DOI] [PubMed] [Google Scholar]
- 29.Green EA, Choi Y, Flavell RA. Pancreatic lymph node-derived CD4(+)CD25(+) Treg cells: highly potent regulators of diabetes that require TRANCE-RANK signals. Immunity. 2002;16:183–191. doi: 10.1016/s1074-7613(02)00279-0. [DOI] [PubMed] [Google Scholar]
- 30.Walker LS, Chodos A, Eggena M, Dooms H, Abbas AK. Antigen-dependent proliferation of CD4+ CD25+ regulatory T cells in vivo. J Exp Med. 2003;198:249–258. doi: 10.1084/jem.20030315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yamazaki S, Iyoda T, Tarbell K, et al. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–247. doi: 10.1084/jem.20030422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klein L, Khazaie K, von Boehmer H. In vivo dynamics of antigen-specific regulatory T cells not predicted from behavior in vitro. Proc Natl Acad Sci U S A. 2003;100:8886–8891. doi: 10.1073/pnas.1533365100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fisson S, Rasse-Jeze G, Litvinova E, et al. Continuous activation of autoreactive CD4+ CD25+ regulatory T cells in the steady state. J Exp Med. 2003;198:737–746. doi: 10.1084/jem.20030686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mottet C, Uhlig HH, Powrie F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J Immunol. 2003;170:3939–3943. doi: 10.4049/jimmunol.170.8.3939. [DOI] [PubMed] [Google Scholar]
- 35.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nat Immunol. 2003;4:330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 36.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299:1057–1061. [PubMed] [Google Scholar]
- 37.Rizzo LV, Xu H, Chan CC, Wiggert B, Caspi RR. IL-10 has a protective role in experimental autoimmune uveoretinitis. Int Immunol. 1998;10:807–814. doi: 10.1093/intimm/10.6.807. [DOI] [PubMed] [Google Scholar]
- 38.Sijssens KM, Rijkers GT, Rothova A, Stilma JS, Schellekens PA, de Boer JH. Cytokines, chemokines and soluble adhesion molecules in aqueous humor of children with uveitis. Exp Eye Res. 2007;85:443–449. doi: 10.1016/j.exer.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 39.Takase H, Futagami Y, Yoshida T, et al. Cytokine profile in aqueous humor and sera of patients with infectious or noninfectious uveitis. Invest Ophthalmol Vis Sci. 2006;47:1557–1561. doi: 10.1167/iovs.05-0836. [DOI] [PubMed] [Google Scholar]
- 40.Keino H, Takeuchi M, Usui Y, et al. Supplementation of CD4+CD25+ regulatory T cells suppresses experimental autoimmune uveoretinitis. Br J Ophthalmol. 2007;91:105–110. doi: 10.1136/bjo.2006.099192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Siepmann K, Biester S, Plskova J, Muckersie E, Duncan L, Forrester JV. CD4(+)CD25 (+) T regulatory cells induced by LPS-activated bone marrow dendritic cells suppress experimental autoimmune uveoretinitis in vivo. Graefes Arch Clin Exp Ophthalmol. 2007;245:221–229. doi: 10.1007/s00417-006-0356-9. [DOI] [PubMed] [Google Scholar]
- 42.Grajewski RS, Silver PB, Agarwal RK, et al. Endogenous IRBP can be dispensable for generation of natural CD4+CD25+ regulatory T cells that protect from IRBP-induced retinal autoimmunity. J Exp Med. 2006;203:851–856. doi: 10.1084/jem.20050429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gavin MA, Clarke SR, Negrou E, Gallegos A, Rudensky A. Homeostasis and anergy of CD4+CD25+ suppressor T cells in vivo. Nat Immunol. 2002;3:33–41. doi: 10.1038/ni743. [DOI] [PubMed] [Google Scholar]
- 44.Tang Q, Henriksen KJ, Bi M, et al. In vitro-expanded antigen-specific regulatory T cells suppress autoimmune diabetes. J Exp Med. 2004;199:1455–1465. doi: 10.1084/jem.20040139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thornton AM, Shevach EM. Suppressor effector function of CD4+CD25+ immunoregulatory T cells is antigen nonspecific. J Immunol. 2000;164:183–190. doi: 10.4049/jimmunol.164.1.183. [DOI] [PubMed] [Google Scholar]
- 46.Tarbell KV, Yamazaki S, Steinman RM. The interactions of dendritic cells with antigen-specific, regulatory T cells that suppress autoimmunity. Semin Immunol. 2006;18:93–102. doi: 10.1016/j.smim.2006.01.009. [DOI] [PubMed] [Google Scholar]
- 47.Han G, Shao H, Peng Y, et al. Suppressor role of rat CD8(+)CD45RC(low) T cells in experimental autoimmune uveitis (EAU) J Neuroimmunol. 2007;183:81–88. doi: 10.1016/j.jneuroim.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Peng Y, Shao H, Ke Y, et al. Minimally activated CD8 autoreactive T cells specific for IRBP express a high level of Foxp3 and are functionally suppressive. Invest Ophthalmol Vis Sci. 2007;48:2178–2184. doi: 10.1167/iovs.06-1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nishida T, Taylor AW. Specific aqueous humor factors induce activation of regulatory T cells. Invest Ophthalmol Vis Sci. 1999;40:2268–2274. [PubMed] [Google Scholar]
- 50.Taylor AW. Ocular immunosuppressive microenvironment. Chem Immunol Allergy. 2007;92:71–85. doi: 10.1159/000099255. [DOI] [PubMed] [Google Scholar]
- 51.Taylor AW. Modulation of regulatory T cell immunity by the neuropeptide alpha-melanocyte stimulating hormone. Cell Mol Biol (Noisy-le-grand) 2003;49:143–149. [PubMed] [Google Scholar]
- 52.Liu H, Hu B, Xu D, Liew FY. CD4+CD25+ regulatory T cells cure murine colitis: the role of IL-10, TGF-β, and CTLA4. J Immunol. 2003;171:5012–5017. doi: 10.4049/jimmunol.171.10.5012. [DOI] [PubMed] [Google Scholar]
- 53.McGeachy MJ, Stephens LA, Anderton SM. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J Immunol. 2005;175:3025–3032. doi: 10.4049/jimmunol.175.5.3025. [DOI] [PubMed] [Google Scholar]