Abstract
Aims
Contractile dysfunction associated with myocardial ischaemia is a significant cause of morbidity and mortality in the elderly. Strategies to protect the aged heart from ischaemia-mediated pump failure are needed. We hypothesized that troponin I-mediated augmentation of myofilament calcium sensitivity would protect cardiac function in aged mice.
Methods and results
To address this, we investigated transgenic (Tg) mice expressing a histidine-substituted form of adult cardiac troponin I (cTnI A164H), which increases myofilament calcium sensitivity in a pH-dependent manner. Serial echocardiography revealed that Tg hearts showed significantly improved systolic function at 4 months, which was sustained for 2 years based on ejection fraction and velocity of circumferential fibre shortening. Age-related diastolic dysfunction was also attenuated in Tg mice as assessed by Doppler measurements of the mitral valve inflow and lateral annulus Doppler tissue imaging. During acute hypoxia, cardiac contractility significantly improved in aged Tg mice made evident by increased stroke volume, end systolic pressure, and +dP/dt compared with non-transgenic mice.
Conclusion
This study shows that increasing myofilament function by means of a pH-responsive histidine button engineered into cTnI results in enhanced baseline heart function in Tg mice over their lifetime, and during acute hypoxia improves survival in aged mice by maintaining cardiac contractility.
Keywords: Troponin I, Ageing, Cardiac function, Hypoxia
1. Introduction
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality worldwide, accounting for 16.7 million deaths globally each year.1–3 Advancing age is a major risk factor for acquiring CVD.4–6 Over one-third of all patients with CVD are 65 years of age or older. Despite significant advances in medical therapy and technology, ischaemic heart disease in the elderly is associated with high mortality.1,7 Myocardial ischaemia results from inadequate regional blood flow and leads to reduced contractility, often leading to heart failure.
Myocardial ischaemia causes a drop in myocardial intracellular pH which desensitizes the myofilament to cytosolic Ca2+ fluxes, compromising myocyte contractility.8 The pH-mediated changes in thin filament proteins, particularly the interaction between troponin C and troponin I, are primarily responsible for this reduction in myofilament calcium responsiveness and resulting decrement in contractile function.9 Troponin I (TnI), the inhibitory subunit of the ternary troponin complex, is the Ca2+-sensitive molecular switch of the myofilament.10 During diastole, the C-terminus of TnI interacts with actin which allows tropomyosin to sterically inhibit actomyosin ATPase activity. During systole, a conformation change in the troponin complex occurs wherein the C-terminal switch domain of TnI binds to the hydrophobic patch of the Ca2+ saturated N-terminal lobe of TnC.11 This releases the inhibition on actomyosin ATPase resulting in cross-bridge cycling and subsequent sarcomeric contraction.
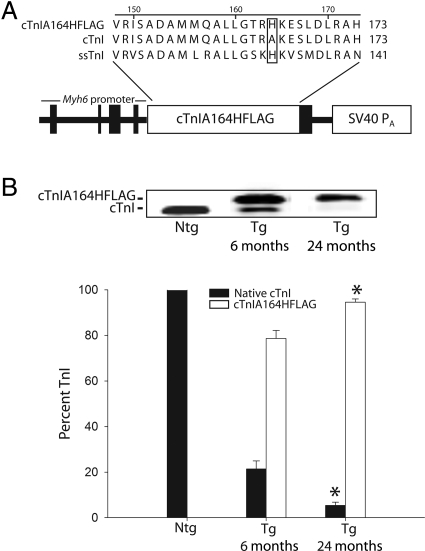
Carboxy-terminal charge differences between the neonatal and adult isoforms of TnI, slow skeletal TnI and cardiac TnI, respectively, are responsible for alterations in pH sensitivity of the myofilament.12,13 Recent studies indicate that a critical histidine residue underlies TnI regulation of myofilament pH sensitivity (Figure 1A).12–15 When cardiac TnI is modified to contain a histidine at codon 164, it behaves as a titratable molecular switch regulating myofilament tension development in response to biochemical changes in the myocyte in vitro and in the whole heart in vivo.15 Importantly, this histidine substitution increases myofilament sensitivity to activating calcium while retaining important PKA phosphorylation sites on the N-terminus of cardiac troponin I (cTnI). PKA-mediated phosphorylation of TnI is a critical mediator of the β-adrenergic response in the adult heart.16
Figure 1.
Age-dependent stoichiometric replacement in cardiac troponin I A164H transgenic mice. (A) Sequence alignment showing location of the single histidine substitution originally studied in the slow skeletal isoform of troponin I and subsequently introduced into cardiac troponin I (cTnI A164HFLAG). Expression cassette of cDNA-encoding cardiac troponin I A164H with an SV40 polyadenylation (pA) signal was driven by the 5.5 kb Myh6 promoter. A C-terminal flag epitope was used to aid detection of cardiac troponin I A164H. (B) Immunoblot and bar graph showing expression of native cardiac troponin I (filled square) in non-transgenic mice as well as stoichiometric incorporation of cardiac troponin I A164HFLAG (open square) relative to endogenous cardiac troponin I (filled square) in transgenic mice at 6 months (n = 12) and 2 years (n = 6). Values are expressed as mean ± SEM. *P < 0.05 for 6 month vs. 2 year replacement.
Numerous studies have shown that increasing myofilament calcium sensitivity through mutations in the troponin complex results in varying forms of cardiomyopathy in the context of normal physiological growth and function (e.g. cTnI R193H, cTnT R92Q).17,18 In contrast, we have shown that cTnI A164H has no baseline pathology and significantly protects cardiac function in response to a variety of pathophysiological challenges including acute and chronic ischaemia.15 Adding to the evidence supporting the therapeutic role of histidine-modified troponin I, this paper is the first to show that improvement in inotropy through modification of a myofilament protein attenuates cardiovascular dysfunction in the aged heart in vivo.
2. Methods
2.1. Mouse model
Generation and analysis of transgenic (Tg) mice expressing a histidine-modified cTnI A164H with FLAG epitope was previously described (Figure 1A).15 The University of Michigan is accredited by the American Association of Accreditation of Laboratory Animal Health Care, and the animal care use programme conforms to the standards of the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Pub. No. 85-23). (see Supplemental Methods online).
2.2. Conductance micromanometry
Measurements of in vivo cardiovascular haemodynamics were obtained using conductance micromanometry as previously performed by this laboratory15 (see Supplemental Methods online).
2.3. Echocardiography
Two-dimensional, M-mode, Doppler and tissue Doppler echocardiographic images were recorded using the Visual Sonics Vevo 770 high-resolution in vivo micro-imaging system (see Supplemental Methods online).
2.4. Adult mouse myocyte isolation and analysis
Adult mouse cardiac myocytes were isolated from 3- to 6-month-old mice and analysed for SR load as described previously19 (see Supplemental Methods online).
2.5. Immunoblot detection
The stoichiometric ratio of cTnI A164HFLAG to native cTnI was accomplished by detection with the pan-TnI antibody MAB 1691 (Chemicon; 1:1000). Calcium-handling proteins were detected using the following antibodies: phospholamban (Clone A1, Upstate), SERCA2a (MA3-919, ABR), NCX (11-13, Swant), and calsequestrin (PA1-913, ABR). Phosphorylation status was performed by Western blot using antibodies directed against the serine 16 phosphorylation site on phospholamban (07-052, Upstate) and the serine 23/24 tandem phosphorylation sites on cardiac troponin I (4004, Cell Signaling). Indirect immunodetection was carried out using a fluorescently labelled secondary antibody (Rockland, IRDye 680 conjugated affinity purified; 1:5000). Western blot analysis was accomplished using the infrared imaging system, Odyssey (Li-Cor, Inc.) and images analysed using Odyssey software v. 1.2. For protein detection, samples from young and aged non-transgenic (Ntg) and Tg mice were run on the same gel. After protein transfer, the gel was stained with Coomassie for use in quantification to account for any loading differences.
2.6. Statistics
All results are expressed as mean ± SEM. All two-group comparisons were assessed by two-tailed t-test. All multi-group comparisons were assessed using two-way analysis of variance (ANOVA) with Tukey post hoc test. Survival after the in vivo acute hypoxic challenge was assessed by the Fisher's exact test.
3. Results
3.1. cTnIA164H transgene expression increases in aged murine hearts
Consistent with recent findings,15 6-month-old mice (line 892) showed high levels of stoichiometric replacement of endogenous cTnI with cTnI A164H (78.6 ± 3.5%) (Figure 1B). Ageing of generation F2–F3 Tg mice to 2 years revealed a significant increase in cTnI A164H replacement relative to native cTnI (94.6 ± 3.1%; P < 0.05, Tg 6 vs. 24 month expression) as determined by Western blotting (Figure 1B).
3.2. cTnI A164H improves global cardiac function during ageing
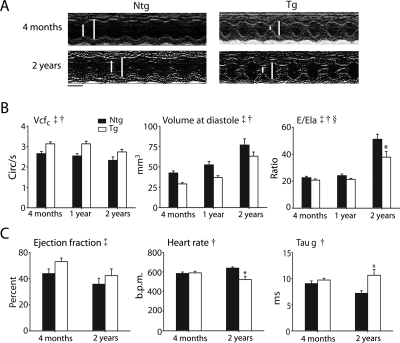
Systolic and diastolic cardiac function at 4 months, 1, and 2 years of age was assessed by serial echocardiography. Comparison of Ntg and Tg mice revealed significant differences in contractility and chamber geometry at all ages (Figure 2A and B). Compared with Ntg mice, Tg mice had significantly higher contractility as measured by the velocity of circumferential fibre shortening (Vcfc) (Figure 2B). Ejection fraction correlated with the Vcfc during this time course further validating the increased inotropy of Tg mice (Table 1). Tg mice also had significantly smaller LV diastolic volume (Vol d) compared with Ntg mice at all ages (Figure 2B). Although the ageing process had similar effects on both cohorts resulting in an age-dependent deterioration of cardiac function and geometry, cTnI A164H Tg mice had sustained improvements in contractility and reduced LV cavitary dilation compared with Ntg mice. These conclusions were supported by ANOVA main effects for genotype and age with measurements of contractility (Vcfc), and cardiac geometry (Vol d) (P < 0.05).
Figure 2.
Age-dependent changes in baseline cardiac function by echocardiography and Millar catheterization. (A) Representative M-mode echocardiographic images along the parasternal short axis view showing changes in contractility of non-transgenic and transgenic mice between 4 months and 2 years (scale bar = 0.1 s). White bars delineate systole and diastole. (B) Summarized mean data showing age-related differences in cardiac function as assessed by echocardiography including velocity of circumferential fibre shortening corrected for heart rate (Vcfc), volume at diastole (Vol d), and the ratio of the Mitral E wave to lateral annular E wave (E/Ela) showing changes in cardiac function in non-transgenic (filled square) and transgenic (open square) mice at 4 months (non-transgenic, n = 26; transgenic, n = 32), 1 year (non-transgenic, n = 12; transgenic, n = 13), and 2 years (non-transgenic, n = 13; transgenic, n = 15). Values are expressed as mean ± SEM. Two-way ANOVA main effects: age (double dagger) and genotype (single dagger): Vcfc, Vol d, E/Ela, P < 0.05. ANOVA interaction effects between genotype and age (section symbol): E/Ela, *P < 0.05 for non-transgenic vs. transgenic at 2 years of age. (C) Summarized conductance micromanometry mean data showing age-related differences in haemodynamic function including the ejection fraction (EF), time constant for relaxation (tau g), and heart rate of non-transgenic (filled square) and transgenic (open square) mice at 4 months (n = 12–14) and 2 years (n = 3–4) of age. Values are expressed as mean ± SEM. *P < 0.05 for non-transgenic vs. transgenic at 24 months. ANOVA main effects: genotype (single dagger): tau g, HR, P < 0.05; age (double dagger): EF, P < 0.05. Ntg, nontransgenic; Tg, transgenic.
Table 1.
Baseline cardiac function of aged mice
| Parameter | Ntg | Tg |
|---|---|---|
| Conductance micromanometry | ||
| Cardiac output (µL/min) | 11 440.7 ± 1542.2 | 12 471.4 ± 2984.3 |
| Stroke volume (µL) | 17.8 ± 2.2 | 23.1 ± 4.5 |
| Stroke work (mmHg × µL) | 1228.7 ± 360.5 | 1737.0 ± 478.3 |
| End systolic pressure (mmHg) | 97.4 ± 20.7 | 97.1 ± 15.8 |
| dP/dt min (mmHg/s) | −9858.8 ± 2756.6 | −7456.5 ± 1789.6 |
| Tau w (ms) | 5.75 ± 0.2687 | 7.95 ± 0.6695 |
| Echocardiography | ||
| Ela (m/s) | 23.2 ± 2.4 | 31.7 ± 3.4 |
| Ala (m/s) | 22.5 ± 1.6 | 25.3 ± 2.3 |
| MV E (m/s) | 1050.4 ± 51.8 | 1143.2 ± 72.9 |
| MV A (cm/s) | 771.4 ± 42.0 | 846.5 ± 40.5 |
| EF (%) | 64.0 ± 3.9 | 74.4 ± 2.3* |
For conductance micromanometry measurements n = 3–4/group. For echocardiography measurements n = 13–15/group. All values are from animals aged to 2 years. Echocardiography parameters: Doppler tissue imaging of the early (Ela) and late (Ala) tissue velocities of the lateral annulus; conventional Doppler imaging of the mitral valve early (MV E) and late (MV A) filling velocities; ejection fraction (EF). All values are expressed as mean ± SEM.
Ntg, non-transgenic; Tg, transgenic.
*P < 0.05 for Ntg vs. Tg.
Diastolic function was also assessed by conventional Doppler of the mitral valve early (Ea) and late (Aa) waves as well as Doppler tissue imaging (DTI) of the early (Ela) and late (Ala) tissue velocities of the lateral annulus. Although no differences in diastolic function were observed at 4 months, age-related decrements in diastolic function were observed in Ntg mice that were significantly attenuated in Tg mice at 2 years. The lateral annular E wave (Ela), as measured by DTI, was not statistically different between groups (Table 1). The ratio of the mitral valve E wave to the lateral annular E wave (E/Ela) was significantly lower in Tg mice compared with Ntg mice at 2 years of age (Ntg vs. Tg: 51.0 ± 3.8 vs. 37.8 ± 4.1; P < 0.05) (Figure 2B). Taken together, the progression of diastolic dysfunction that accompanies the ageing process, as observed in Ntg mice, was reduced by cTnI A164H resulting in an attenuation of diastolic dysfunction in aged Tg mice. These conclusions were supported by ANOVA main effects for genotype and age (P < 0.05) as well as an interaction effect (P < 0.05) for E/Ela.
Cardiac pressure–volume analysis was assessed by implantation of a Millar catheter into the left ventricle for acquisition of real-time conductance micromanometry measurements. Continuous monitoring of cardiac function revealed significant differences in age-dependent baseline haemodynamics between Ntg and Tg mice. Measurements of systolic function, such as the positive derivative of pressure development (data not shown) and ejection fraction, were comparable between groups (Figure 2C). In terms of diastolic function, conductance micromanometry measurements were inconclusive based on the rate constant for isovolumic relaxation (Tau) which showed either an increase [Tau Glanz (Figure 2C)] or no change [Tau Weiss (Table 1)] during the ageing process. A significant decrease in heart rate was also observed in 2-year-old Tg mice compared with their Ntg littermates (Figure 2C).
Analysis of heart-weight-to-body-weight ratio revealed significant age-dependent changes (Table 2). At 4 months of age Tg mice had significantly lower body weights and heart weights compared with Ntg mice (4 month HW/BW ratio: 4.6 ± 0.1 vs. 4.0 ± 0.1; Ntg vs. Tg; P < 0.05). However, at 2 years the HW/BW ratio was similar between Tg and Ntg mice (2 year HW/BW ratio: 5.6 ± 0.2 vs. 5.9 ± 0.4; Ntg vs. Tg).
Table 2.
Heart and body weight analysis
| Ntg | Tg | |
|---|---|---|
| Four months | ||
| Heart weight (mg) | 126 ± 3.5 | 99.7 ± 3.7* |
| Body weight (g) | 27.1 ± 0.8 | 25.1 ± 1.2 |
| HW/BW ratio | 4.6 ± 0.1 | 4.0 ± 0.1* |
| Two years | ||
| Heart weight (mg) | 168 ± 14.6 | 167 ± 13.2 |
| Body weight (g) | 31.3 ± 2.2 | 31.0 ± 0.5 |
| HW/BW ratio | 5.6 ± 0.2 | 5.9 ± 0.4 |
All values are expressed as mean ± SEM.
*P < 0.05.
3.3. cTnI A164H aged mice retain cardiac haemodynamics during hypoxia
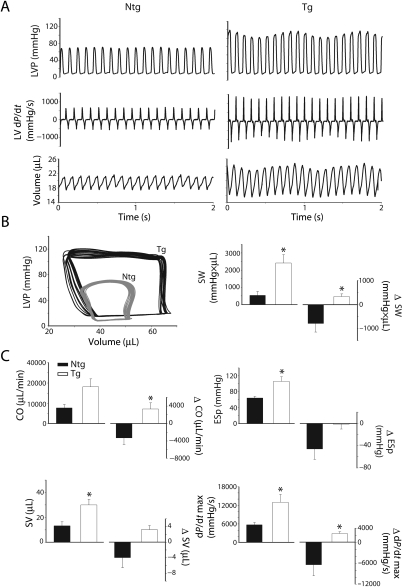
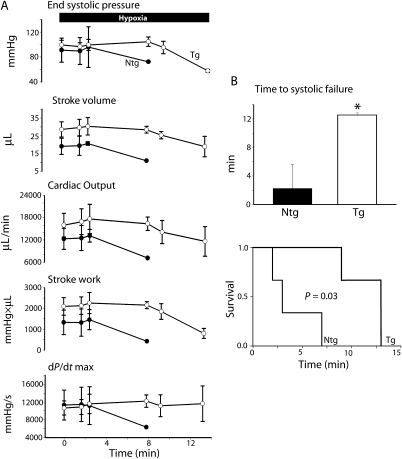
Previous studies have shown that young cTnI A164H Tg mice sustain cardiac function during an acute hypoxic challenge and consequently survive significantly longer than Ntg mice.15 To assess the physiological and therapeutic role of cTnI A164H in the aged murine heart, mice were exposed to conditions of controlled hypoxia (12% O2) during Millar catheterization and assessed for global cardiac function and survival (Figures 3 and 4). After 4 min of hypoxia, raw traces of LV pressure (LVP), the derivatives of LV pressure development (dP/dt) and volume, together with their corresponding pressure–volume loops, revealed that aged Ntg mice have significantly compromised cardiac haemodynamics compared with aged Tg mice (Figure 3A and B). Mean haemodynamic data taken at 4 min into the acute hypoxic challenge and the delta change from baseline (Table 1) to hypoxia showed improved systolic and diastolic performance of Tg mice compared with Ntg mice (Figure 3C). Specifically, compared with their Ntg littermates, LV performance was maintained during hypoxia in Tg mice as represented by higher stroke work (SW), end systolic pressure (ESP), and the positive derivative of pressure development (dP/dt max) (Figure 3C). Compared with baseline values, aged Tg mice showed improved contractility during hypoxia compared with a significant decrease in contractile function in aged Ntg mice based on measurements of stroke work (Δ SW), and the positive derivative of pressure development (Δ dP/dt max) (Figure 3C). The negative derivative of pressure development (dP/dt min) was also maintained during an acute hypoxic challenge in Tg mice (data not shown). Finally, compared to Ntg littermates, Tg mice were able to sustain global cardiac function during hypoxia as shown by a higher stroke volume (SV) and cardiac output (Δ CO), (Figure 3C). These data show improved cardiac function in response to hypoxia in Tg mice compared with their Ntg littermates (Figure 4A). Consistent with improved haemodynamic function, aged Tg mice survived significantly longer during an acute hypoxic challenge than Ntg mice (11.6 ± 1.3 vs. 3.7 ± 1.9 min, P < 0.05) (Figure 4B).
Figure 3.
In vivo haemodynamic differences between aged non-transgenic and transgenic mice during an acute hypoxic challenge. (A) Two second raw data sweeps derived by conductance micromanometry of left ventricular pressure (LVP), left ventricular pressure derivatives (dP/dt), and left ventricular volume of 2-year-old non-transgenic and transgenic mice during an acute hypoxic challenge (12% O2). (B) Representative pressure–volume loops in vivo of non-transgenic (grey) and transgenic (black) mice during hypoxia. (C) Mean data showing haemodynamic function as well as the delta change (Δ, right side axes) from baseline values for cardiac output (CO), stroke volume (SV), stroke work (SW), end systolic pressure (ESp), and positive pressure derivatives (dP/dt max) derived by Millar catheterization of 2-year-old non-transgenic (filled square; n = 3) and transgenic (open square; n = 3) mice. *P < 0.05. Values are expressed as mean ± SEM. Ntg, nontransgenic; Tg, transgenic.
Figure 4.
In vivo haemodynamic function and survival of aged mice during an acute hypoxic challenge. (A) Averaged left ventricular haemodynamic function including end systolic pressure (ESp), stroke volume (SV), cardiac output (CO), stroke work (SW), and the positive derivative of pressure development (dP/dt max) during the time course of an acute hypoxic challenge for non-transgenic (filled circle; n = 3) and transgenic (open circle; n = 3) aged mice. (B) Summarized mean survival data and survival curve showing time to systolic heart failure for 2-year-old non-transgenic (n = 3) and transgenic (n = 3) mice. Values are expressed as mean ± SEM. *P < 0.05.
3.4. Calcium cycling is different between Ntg and Tg mice
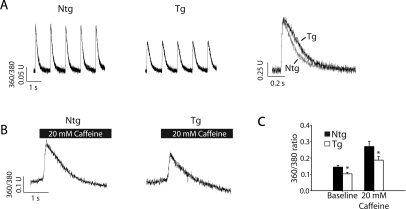
Functional analysis of calcium handling in acutely isolated cardiac myocytes showed that under conditions of 1 Hz pacing, young Tg mice had a reduced calcium transient amplitude compared with young Ntg myocytes (Figure 5A and C), consistent with previous reports.15 Furthermore, experiments were performed to measure SR calcium load by means of acute addition of 20 mM caffeine (Figure 5B and C). Similar to baseline calcium transients, these data show that SR calcium load was lower in Tg vs. Ntg mice (Figure 5A–C). This supports the hypothesis that increased myofilament calcium sensitivity reduces calcium requirements in the cell and may contribute to the protection of Tg hearts during ageing as well as cardiomyopathic challenges such as ischaemia/reperfusion injury.15
Figure 5.
Analysis of calcium homoeostasis in young mice. (A) Raw traces of calcium transients from FURA 2AM loaded isolated myocytes during pacing with 1 Hz (left and middle) as well as raw calcium transient traces normalized to the peak amplitude (right). (B) Raw calcium transients after acute addition of 20 mM caffeine to release sarcoplasmic reticulum calcium (horizontal black bars). (C) Summarized data from calcium-handling experiments (n = 20–24 myocytes per group). Values are expressed as mean ± SEM. *P < 0.05 for non-transgenic vs. transgenic. Ntg, nontransgenic; Tg, transgenic.
3.5. Calcium-handling protein expression and phosphorylation levels
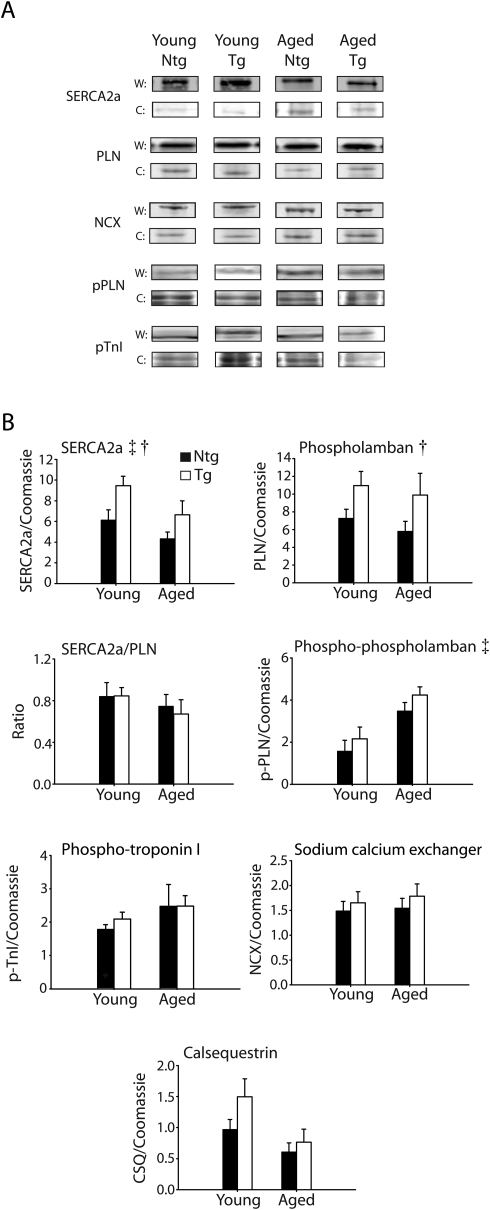
Western blot analysis was performed to assess changes in calcium-handling proteins between young (2–4 months) and aged (24 months) Ntg and Tg mice (Figure 6A and B). Alterations in the expression of proteins specifically involved in the regulation of calcium homoeostasis have been established as one of the factors that contribute to the development of age-related cardiac dysfunction.20–26 These data show that calsequestrin (CSQ), the sodium calcium exchanger (NCX), and phospholamban (PLN) were not altered during ageing by two-way ANOVA. In contrast, SERCA2a was reduced in both Ntg and Tg aged mice based on a significant ANOVA main effect for age (P < 0.05), a finding that is consistent with previous studies of the ageing heart.26,27 Furthermore, these data show that the SERCA2a/PLN ratio was not different between Ntg and Tg mice during the ageing process (Figure 6B). Although the SERCA2a levels are elevated in Tg hearts by Western blot (based on an ANOVA main effect for genotype), in vitro calcium-handling measurements indicate a reduced level of SR calcium load in Tg hearts (Figure 5).
Figure 6.
Changes in protein expression and phosphorylation in non-transgenic and transgenic mice. (A) Western blots of calcium-handling proteins including the sarco-endoplasmic reticulum ATPase (SERCA2a), phospholamban (PLN), the sodium calcium exchanger (NCX), serine 16 phosphorylation of phospholamban (pPLN), and tandem serine 23/24 phosphorylation of troponin I (pTnI) for young and aged non-transgenic and transgenic mice. Coomassie was used to normalize for protein loading (W, Western; C, Coomassie). (B) Mean summary data for differences in the expression of calcium-handling proteins including CSQ, NCX, PLN, SERCA2a, the SERCA2a/PLN ratio, pPLN, and pTnI. Two-way ANOVA main effects (P < 0.05): age (double dagger), SERCA2a, and pPLN; and genotype (single dagger), PLN, SERCA2a. Ntg, nontransgenic; Tg, transgenic.
In addition, young and aged Ntg and Tg hearts were assessed for changes in protein phosphorylation (Figure 6A and B). These data showing serine 16 phosphorylation of phospholamban indicate that aged mice have a higher baseline level of phosphorylated PLN compared with young mice based on a significant ANOVA main effect for age. cTnI serine 23/24 phosphorylation showed no statistical significance between groups.
4. Discussion
This study focused on the physiological impact of histidine-modified cTnI A164H in aged mice. These data provide new evidence that molecular manipulation of myofilament calcium sensitivity can preserve heart function during ageing. Specifically, this study showed that age-related decrements in baseline cardiac systolic function were significantly attenuated in Tg mice compared with Ntg littermates. Echocardiography studies also demonstrated that age-dependent diastolic dysfunction (elevated E/Ela) was attenuated in Tg animals. Importantly, when exposed to an acute hypoxic challenge in vivo, aged Tg mice maintained cardiac performance that significantly extended their survival compared with Ntg mice. These data support the hypothesis that myofilament-based enhanced function by single histidine-modified cTnI provides a mechanism for attenuating age-related decrements in cardiac function.
Increasing calcium sensitivity of the myofilament consequent to alterations in the biochemistry of cTnI has been well studied.10,12,13,15,16,28 A recent report of the crystal structure of cTnI places Ala164 at the critical switch domain that is key in regulating myofilament calcium activation.11 Codon 164 is situated at the interface between the amphiphilic switch region, H3, and the C-terminal actin-binding domain, H4. These regions are defined as the regulatory segment. In the calcium-saturated state, the entire regulatory segment of cTnI (residues 137–210) undergoes a conformational change concomitant with binding of the H3 domain to the conserved N-terminal hydrophobic patch of TnC. In the slow skeletal isoform of TnI (ssTnI), which is the foetal/neonatal isoform in mammals, a unique biochemical characteristic of the switch region was found to confer pH-dependent increases in calcium sensitivity to the myofilament.29,30 In initial studies, mutagenesis experiments identified a critical histidine at position 132 as responsible for this pH-dependent functional outcome.12,14,15 The physiological effects of this mutation in the heart directly reflect the unique biochemical properties of histidine’s imidazole moiety which, unique among all the amino acids, ionizes within the physiological range (pKR = 6.0). Numerous studies have shown that under conditions of acidosis, ssTnI protects contractility of myocytes in vitro and the whole heart in vivo.12,14,29–32 In light of these findings, we sought to introduce this pH sensitivity into the switch domain of cTnI. An engineered histidine modification in codon 164 of cTnI (A164H) takes advantage of this critical functional property of ssTnI transposed into the context of cTnI. This modification takes place without altering important physiological functions of cTnI in regulating global cardiac inotropic and lusitropic responses to β-adrenergic signalling.15 Cardiac TnI A164H provides a titratable molecular switch mechanism regulating myofilament tension development in response to biochemical changes in the adult myocyte. One of the central hypotheses of this study was that increasing myofilament calcium sensitivity by means of cTnI A164H is a powerful molecular therapy for attenuating morbidity and mortality associated with ischaemia/hypoxia-induced contractile failure in aged mice.
Declining cardiac function contributes to waning health in geriatric populations.5 In many cases these age-related changes are secondary to coronary artery disease.6 Of particular salience to this study is the propensity for chronic ischaemia associated with vascular stenosis to contribute to poor cardiovascular health of the elderly.4 A portion of this study was based on an experimental protocol to impose a controlled state of hypoxia in aged mice through low inhaled oxygen. In the clinical setting, hypoxia is usually secondary to cardio-pulmonary pathologies frequently seen in geriatric populations. Physiologically these conditions are usually due to hypoventilation, vascular shunting, ventilation/perfusion mismatch, and interstitial diffusion defects. Clinical diseases of the lungs such as COPD, pneumonia, interstitial lung disease, or pulmonary embolic disease are common conditions associated with hypoxia which may lead to secondary ischaemic cardiac injury. The aged heart is particularly at risk in these settings because of intrinsic muscle disease and underlying coronary artery disease with areas of marginally perfused myocardium. These clinically relevant aetiologies, known risk factors for CVD, provide contextual relevance for the key findings of this study. These important results include the finding that aged cTnI A164H Tg mice have enhanced cardiac function and extended survival capacity during an acute hypoxic challenge compared with Ntg mice.
Irrespective of the aetiology, systolic and diastolic dysfunction are strong predictors of heart failure in geriatric populations.33 In this study we show that cTnI A164H Tg mice have improved contractility during the ageing process compared with Ntg mice. The equalization of the HW/BW ratio at 2 years of age neutralizes any heart size-dependent variables that influence contractility measurements between groups at this time point. This lends credence to the conclusion that Tg mice, which retained the same relative increase in contractility over Ntg mice during the ageing process, actually underwent an age-dependent and heart size-independent increase in absolute contractility. This could be explained in part by the increase in stoichiometric incorporation of cTnI A164H in old vs. young Tg mice and could provide mechanistic basis for the observed increase in contractility at 2 years of age. Additionally, compared with Tg mice, increased LV cavitary dilation in aged Ntg mice may also, at least in part, contribute to the divergence of contractile efficiency at the 2-year time point. A shift towards higher sarcomere lengths, which occurs with dilated cardiomyopathies and may be a component of age-related dysfunction, could reduce the efficiency of contractility based on the Frank–Starling principle.
In addition to systolic dysfunction, decrements in diastolic function are also characteristic of the ageing process. LV dilation together with stiffening of the aged myocardium prevents sufficient relaxation of the heart during the filling phase of the cardiac cycle. Thus, another key finding of this study is that cTnI A164H protects diastolic function during the ageing process. These data indicate that Ntg mice show evidence of diastolic dysfunction based on a decrease in the velocity of the lateral annulus during the early filling phase (Ela) of the LV as well as an increase in the mitral valve E wave flow velocity to lateral annular E wave ratio (E/Ela). This latter parameter (E/Ela) shows the ratio of the inflow velocity to the tissue velocity providing insight into the elastic properties of the ventricle. In essence, the E wave velocity controls for cardiac output, heart rate, and filling so that the ratio (E/Ela) correlates with left atrial pressure. Here, the E/Ela shows evidence of the transgene altering the typical progression of diastolic dysfunction based on a significant interaction effect (P < 0.05). These data indicate that cTnI A164H Tg mice are able to retain improved diastolic function during the ageing process compared with Ntg mice which experience predictable age-related diastolic dysfunction.
The relationship between cTnI A164H and diastolic performance, however, is complex as indicated by differences in echocardiographic and micromanometry data at baseline. Conflicting data based on the measures of isovolumic relaxation (Tau) provide inconclusive evidence regarding baseline diastolic function in Tg mice, which is consistent with previous findings.15 However, the mechanical component of relaxation during the late (filling) phase of diastole, as measured non-invasively by DTI indicates that Ntg mice have compromised viscoelastic properties of the ventricle resulting in a decline in diastolic function during the ageing process which is significantly attenuated in Tg mice.
Taking this whole organ functional analysis into consideration, it has been established that, at the sub-cellular level, changes in the expression of calcium-handling proteins contribute to the progression of pump dysfunction during ageing.34 Our study supports these findings specifically with regard to the observation that SERCA2a levels are diminished at 2 years of age in Ntg and Tg mice. Reduced expression of SERCA2a has been implicated directly in the progression of age-induced cardiomyopathy26 and provides a sub-cellular basis, at least in part, for the whole organ diastolic dysfunction observed in this study during ageing. The lack of any genotypic differences in CSQ, NCX and the SERCA2a to PLN stoichiometry during ageing suggests an alternative mechanism for improvement of cardiac function in the Tg mice during the ageing process. We propose that this mechanism is predominantly the result of increasing inotropy by molecular manipulation of myofilament performance by means of cTnI A164H.
The results of this study show that Tg hearts have reduced SR calcium loading. Previous reports have found that aged hearts are more susceptible to calcium overload than young hearts.3,35,36 This suggests that a reduced SR Ca2+ load may benefit cTnI A164H hearts in ageing, similar to our recent report in the context of myocardial injury such as ischaemia/reperfusion.15 We hypothesize that the lower SR Ca2+ load and Ca2+ transient are made possible by myofilament activation enhancement by cTnIA164H. We propose that there is an interplay between the myofilament enhancement and SR functionality that could account for higher SERCA2a levels in Tg hearts.
The cardiac functional readout of these findings at the sub-cellular level is seen in the whole organ serial echocardiographic analysis of Ntg and Tg mice during the 2-year ageing process. The decline in cardiac contractility (e.g. EF) increase in LV chamber geometry and development of diastolic dysfunction (e.g. increased E/Ela) particularly evident at the 2-year time point are likely the consequence, at least in part, of the changes observed in calcium-handling proteins.
In conclusion, ischaemia-related cardiac dysfunction in aged populations remains a significant cause of morbidity and mortality. Therapies for ischaemic heart disease and heart failure could be directed specifically towards improving myofilament function. The consequent improvement in contractility that results from increased myofilament performance may appear to counter the logic of commonly prescribed therapeutics, which call for the use of beta blockers that decrease contractility acutely, allowing for reduced oxygen consumption and energy expenditure. Although there is proven value in the diverse effects of beta blockers, we have shown that targeted alteration of the myofilaments to increase contractile performance is an effective mechanism for treatment of ischaemic heart disease and heart failure in small mammals.15 This is in concurrence with Mann and Bristow’s view that augmentation of myofilament responsiveness to calcium would improve the force-generating capacity of the sarcomere and thus redress global cardiac dysfunction.37 The present study, together with previous work,15 strengthens the hypothesis that altering the functionality of troponin I is an effective means of specifically augmenting myofilament function and consequently enhancing cardiac contractility. Adding to the growing evidence for the therapeutic role of histidine-modified TnI in the heart, this study provides new evidence that specific replacement of native cTnI with cTnI A164H in the adult heart protects cardiac function during ageing. We propose that the progression of pathologic and age-related diminutions in cardiac function may be improved by myofilament- based molecular therapeutics for increasing cardiac performance.
Supplementary material
Supplementary material is available at Cardiovascular Research online.
Funding
The authors acknowledge support from National Institutes of Health (RO1 HL 059301 to J.M.) and American Heart Association (0715632Z to N.P.).
Supplementary Material
Acknowledgements
We thank Jaime Predmore for technical expertise in mouse myocyte isolation with which SR calcium load experiments were performed. We appreciate Dr Mark Russell for his assistance with interpretation of echocardiography data and Dr Margaret Westfall for helpful comments. We also thank Dr Samuel Palpant for his medical advice regarding cardio-pulmonary diseases in geriatric populations.
Conflict of interest: none declared.
References
- 1.Michaud CM, Murray CJL, Bloom BR. Burden of disease–implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 2.Thom T, Haase N, Rosamond W, Howard VJ, Rumsfeld J, Manolio T, et al. Heart disease and stroke statistics–2006 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2006;113:e85–e151. doi: 10.1161/CIRCULATIONAHA.105.171600. [DOI] [PubMed] [Google Scholar]
- 3.Jahangir A, Sagar S, Terzic A. Aging and cardioprotection. J Appl Physiol. 2007;103:2120–2128. doi: 10.1152/japplphysiol.00647.2007. [DOI] [PubMed] [Google Scholar]
- 4.Lakatta EG. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part III: cellular and molecular clues to heart and arterial aging. Circulation. 2003;107:490–497. doi: 10.1161/01.cir.0000048894.99865.02. [DOI] [PubMed] [Google Scholar]
- 5.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part II: the aging heart in health: links to heart disease. Circulation. 2003;107:346–354. doi: 10.1161/01.cir.0000048893.62841.f7. [DOI] [PubMed] [Google Scholar]
- 6.Lakatta EG, Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises. Part I: aging arteries: a ‘set up’ for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- 7.Deedwania PC. The key to unraveling the mystery of mortality in heart failure – an integrated approach. Circulation. 2003;107:1719–1721. doi: 10.1161/01.CIR.0000014688.12415.C0. [DOI] [PubMed] [Google Scholar]
- 8.Parsons B, Szczesna D, Zhao J, Van Slooten G, Kerrick WG, Putkey JA, et al. The effect of pH on the Ca2+ affinity of the Ca2+ regulatory sites of skeletal and cardiac troponin C in skinned muscle fibres. J Muscle Res Cell Motil. 1997;18:599–609. doi: 10.1023/a:1018623604365. [DOI] [PubMed] [Google Scholar]
- 9.Ball KL, Johnson MD, Solaro RJ. Isoform specific interactions of troponin I and troponin C determine pH sensitivity of myofibrillar Ca2+ activation. Biochemistry. 1994;33:8464–8471. doi: 10.1021/bi00194a010. [DOI] [PubMed] [Google Scholar]
- 10.Farah CS, Reinach FC. The troponin complex and regulation of muscle contraction. FASEB J. 1995;9:755–767. doi: 10.1096/fasebj.9.9.7601340. [DOI] [PubMed] [Google Scholar]
- 11.Takeda S, Yamashita A, Maeda K, Maeda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003;424:35–41. doi: 10.1038/nature01780. [DOI] [PubMed] [Google Scholar]
- 12.Westfall MV, Borton AR, Albayya FP, Metzger JM. Specific charge differences in troponin I isoforms influence myofilament Ca2+ sensitivity of tension in adult cardiac myocytes. Biophys J. 2001;80:356A–356A. [Google Scholar]
- 13.Dargis R, Pearlstone JR, Barrette-Ng I, Edwards H, Smillie LB. Single mutation (A162H) in human cardiac troponin I corrects acid pH sensitivity of Ca2+-regulated actomyosin S1 ATPase. J Biol Chem. 2002;277:34662–34665. doi: 10.1074/jbc.C200419200. [DOI] [PubMed] [Google Scholar]
- 14.Westfall MV, Metzger JM. Single amino acid substitutions define isoform-specific effects of troponin I on myofilament Ca2+ and pH sensitivity. J Mol Cell Cardiol. 2007;43:107–118. doi: 10.1016/j.yjmcc.2007.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day SM, Westfall MV, Fomicheva EV, Hoyer K, Yasuda S, La Cross NC, et al. Histidine button engineered into cardiac troponin I protects the ischemic and failing heart. Nat Med. 2006;12:181–189. doi: 10.1038/nm1346. [DOI] [PubMed] [Google Scholar]
- 16.Metzger JM, Westfall MV. Covalent and noncovalent modification of thin filament action: the essential role of troponin in cardiac muscle regulation. Circ Res. 2004;94:146–158. doi: 10.1161/01.RES.0000110083.17024.60. [DOI] [PubMed] [Google Scholar]
- 17.Davis J, Wen H, Edwards T, Metzger JM. Thin filament disinhibition by restrictive cardiomyopathy mutant R193H troponin I induces Ca2+-independent mechanical tone and acute myocyte remodeling. Circ Res. 2007;100:1494–1502. doi: 10.1161/01.RES.0000268412.34364.50. [DOI] [PubMed] [Google Scholar]
- 18.Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104:469–481. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coutu P, Bennett CN, Favre EG, Day SM, Metzger JM. Parvalbumin corrects slowed relaxation in adult cardiac myocytes expressing hypertrophic cardiomyopathy-linked alpha-tropomyosin mutations. Circ Res. 2004;94:1235–1241. doi: 10.1161/01.RES.0000126923.46786.FD. [DOI] [PubMed] [Google Scholar]
- 20.Hasenfuss G, Pieske B. Calcium cycling in congestive heart failure. J Mol Cell Cardiol. 2002;34:951–969. doi: 10.1006/jmcc.2002.2037. [DOI] [PubMed] [Google Scholar]
- 21.Koretsune Y, Marban E. Cell calcium in the patho-physiology of ventricular-fibrillation and in the pathogenesis of postarrhythmic contractile dysfunction. Circulation. 1989;80:369–379. doi: 10.1161/01.cir.80.2.369. [DOI] [PubMed] [Google Scholar]
- 22.Lakatta EG, Sollott SJ. Perspectives on mammalian cardiovascular aging: humans to molecules. Comp Biochem Physiol A Mol Integr Physiol. 2002;132:699–721. doi: 10.1016/s1095-6433(02)00124-1. [DOI] [PubMed] [Google Scholar]
- 23.Lee JA, Allen DG. Mechanisms of acute ischemic contractile failure of the heart. Role of intracellular calcium. J Clin Invest. 1991;88:361–367. doi: 10.1172/JCI115311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wehrens XHT, Lehnart SE, Marks AR. Intracellular calcium release and cardiac disease. Annu Rev Physiol. 2005;67:69–98. doi: 10.1146/annurev.physiol.67.040403.114521. [DOI] [PubMed] [Google Scholar]
- 25.Kranias EG, Bers DM. Calcium and cardiomyopathies. Subcell Biochem. 2007;45:523–537. doi: 10.1007/978-1-4020-6191-2_20. [DOI] [PubMed] [Google Scholar]
- 26.Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res Cardiol. 1997;92(Suppl. 1):87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- 27.del Monte F, Hajjar RJ, Harding SE. Overwhelming evidence of the beneficial effects of SERCA gene transfer in heart failure. Circ Res. 2001;88:E66–E67. doi: 10.1161/hh1101.092004. [DOI] [PubMed] [Google Scholar]
- 28.Solaro RJ, el Saleh SC, Kentish JC. Ca2+, pH and the regulation of cardiac myofilament force and ATPase activity. Mol Cell Biochem. 1989;89:163–167. doi: 10.1007/BF00220770. [DOI] [PubMed] [Google Scholar]
- 29.Westfall MV, Metzger JM. Troponin I isoforms and chimeras: tuning the molecular switch of cardiac contraction. News Physiol Sci. 2001;16:278–281. doi: 10.1152/physiologyonline.2001.16.6.278. [DOI] [PubMed] [Google Scholar]
- 30.Westfall MV, Albayya FP, Turner II, Metzger JM. Chimera analysis of troponin I domains that influence Ca2+-activated myofilament tension in adult cardiac myocytes. Circ Res. 2000;86:470–477. doi: 10.1161/01.res.86.4.470. [DOI] [PubMed] [Google Scholar]
- 31.Westfall MV, Rust EM, Metzger JM. Slow skeletal troponin I gene transfer, expression, and myofilament incorporation enhances adult cardiac myocyte contractile function. Proc Natl Acad Sci USA. 1997;94:5444–5449. doi: 10.1073/pnas.94.10.5444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Urboniene D, Dias FAL, Pena JR, Walker LA, Solaro RJ, Wolska BM. Expression of slow skeletal troponin I in adult mouse heart helps to maintain the left ventricular systolic function during respiratory hypercapnia. Circ Res. 2005;97:70–77. doi: 10.1161/01.RES.0000173849.68636.1e. [DOI] [PubMed] [Google Scholar]
- 33.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 34.Hunter WC. Role of myofilaments and calcium handling in left ventricular relaxation. Cardiol Clin. 2000;18:443–457. doi: 10.1016/s0733-8651(05)70155-7. [DOI] [PubMed] [Google Scholar]
- 35.Ataka K, Chen D, Levitsky S, Jimenez E, Feinberg H. Effect of aging on intracellular Ca2+, pHi, and contractility during ischemia and reperfusion. Circulation. 1992;86(Suppl. 5):II371–II376. [PubMed] [Google Scholar]
- 36.Frolkis VV, Frolkis RA, Mkhitarian LS, Shevchuk VG, Fraifeld VE, Vakulenko LG, et al. Contractile function and Ca2+ transport system of myocardium in ageing. Gerontology. 1988;34:64–74. doi: 10.1159/000212932. [DOI] [PubMed] [Google Scholar]
- 37.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111:2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.