Abstract
Background and purpose:
The beneficial effect of 5-HT6 receptor antagonism in cognition remains controversial. This study has been undertaken to reassess the cognition enhancing properties of acute vs subchronic treatment with the selective 5-HT6 receptor antagonist SB-271046 in unimpaired rats, as well as against scopolamine (cholinergic-) or MK-801 (glutamatergic-mediated) deficits.
Experimental approach:
The Morris water maze was used, measuring behaviour acquisition and retention, and swim speed. Other behavioural measures included yawning and motor activity. SB-271046 was given acutely before each trial or subchronically for 7 days before the trials. The AChE inhibitor galanthamine was also used alone or in combination with SB-271046.
Key results:
Subchronic treatment with SB-271046 improved acquisition in the Morris water maze, while the acute treatment only improved retention. Neither acute nor subchronic SB-271046 treatment reversed scopolamine-induced learning deficits. MK-801 induced learning impairment associated with a behavioural syndrome, reversed by acute, but not subchronic, SB-271046 treatment. Interestingly, combined treatment with galanthamine and SB-271046 reversed the scopolamine- or MK-801-induced learning impairments. Subchronic treatment with SB-271046 did not modify motor activity or the increased number of yawns, a cholinergic-mediated behaviour, induced by single administration of SB-271046.
Conclusions and implications:
These data suggest a potential therapeutic role of 5-HT6 receptor antagonists such as SB-271046, alone or in combination with galanthamine, in the treatment of cognitive dysfunction, such as those seen in Alzheimer's disease and schizophrenia.
Keywords: Morris water maze, scopolamine, MK-801, galanthamine, motor activity
Introduction
The 5-HT6 receptor has become an increasingly promising target for improving cognition (Woolley et al., 2004; Mitchell and Neumaier 2005; Mitchell et al., 2006; Meneses et al., 2007). The 5-HT6 receptor antagonist Ro 04-6790 improved retention of the Morris water maze task (Woolley et al., 2001) and reversed a scopolamine-induced deficit in an autoshaping task (Meneses, 2001) and in a rodent test of recognition memory (Lieben et al., 2005). Two other structurally different 5-HT6 receptor antagonists, SB-399885 and SB-271046, increased novel object recognition in adult rats treated either acutely or subchronically (Hirst et al., 2003a, 2003b; King et al., 2004) and improved water maze retention (Rogers and Haga, 2001), although failing to alter acquisition of spatial learning. In contrast, Russell and Dias (2002) and Lindner et al. (2003) failed to detect any effects of Ro 04-6790 or SB-271046 upon acquisition of an autoshaping task, scopolamine-induced deficits in contextual fear-conditioning or retention of a water maze task.
There is convincing evidence that the purported 5-HT6 receptor's influence on memory is mediated at least partially by increased cholinergic neurotransmission (Bentley et al., 1999; Shirazi-Southall et al., 2002; Riemer et al., 2003; Marcos et al., 2006). However, Ro 04-6790 also reversed impairment in learning consolidation produced by the NMDA receptor antagonist, MK-801 (Meneses, 2001), suggesting that 5-HT6 receptor modulation of glutamate (Dawson et al., 2000, 2001) may also contribute to the effects of 5-HT6 receptor antagonists on cognition. Chronic treatment with glutamatergic receptor antagonists resulted in a decrease in striatal mRNA levels of 5-HT6 receptors (Healy and Meador-Woodruff, 1999). Moreover, atypical antipsychotics with high affinities for 5-HT6 receptors, such as clozapine, enhance glutamate levels in the frontal cortex (Daly and Moghaddam, 1993), and this neurochemical effect has been proposed to contribute, at least in part, to the efficacy of these drugs in improving negative symptoms and cognitive dysfunction of schizophrenia (Tollefson, 1996; Meltzer and McGurk, 1999; Roth et al., 2004).
Some of the discrepancies found on the purported effects of 5-HT6 receptor antagonists on learning and memory may be due to differences in the administration protocol (acute vs chronic administration) or methodological differences (Perez-Garcia and Meneses, 2005). Therefore, our first aim was to reassess the cognition enhancing properties of acute vs subchronic treatment with SB-271046 in unimpaired animals and against a scopolamine (cholinergic-mediated) or MK-801 (glutamatergic-mediated) deficit in the Morris water maze. In addition, and on the basis of a preliminary report demonstrating that the combined treatment with SB-271046 with an AChE inhibitor produced an additive increase in passive avoidance learning (Callahan et al., 2004), the second aim of this work was to study the effects of the combined treatment of SB-271046 with galanthamine, an AChE inhibitor with neuroprotective effects (reviewed by Geerts, 2005), in unimpaired animals as well as against a scopolamine or MK-801 deficit in the Morris water maze. The present data suggest a procognitive effect of the 5-HT6 receptor antagonist SB-271046 in unimpaired rats in the Morris water maze. In addition, SB-271046, alone or in combination with galanthamine, was able to reverse not only scopolamine-induced cognitive impairments, but also MK-801-induced cognitive/behavioural dysfunctions.
Methods
Animal care
All the procedures and experiments performed were in strict compliance with the ‘Principles of laboratory animal care' and the recommendations of the EU (DOCE L 358/1/18/2/1986) for the care and use of laboratory animals. Male Wistar rats (n=224), weighing 230–250 g were used. Animals were kept at constant room temperature (21±1 °C) and relative humidity (55±5%) with a 12-h light/dark cycle (dark from 2000 hours) and free access to food and water.
Experimental design
SB-271046 (provided by Glaxo SmithKline, Harlow, UK) was administered orally (p.o.) in 1% methylcellulose solution. Scopolamine (Sigma, St Louis, MO, USA) 0.4 mg kg−1 (Diez-Ariza et al., 2003), MK-801 (Tocris, Bristol, UK) 0.2 mg kg−1 (Filliat and Blanchet, 1995) and galanthamine (Tocris) 2.5 and 5 mg kg−1 were dissolved in saline. In a pilot set of experiments, the subchronic administration of saline (n=10) showed no effect on latency, path length and swim speed compared with naive controls (n=10). Because of the large number of animals needed, each experiment was performed in two blocks, half of the animals from each group being tested in each block. No statistical difference between these two blocks was found in controls, and therefore, data from each were combined before statistical analysis. For acute studies, rats were treated with SB-271046 (10 mg kg−1) every day except on the habituation day, 4 h before starting the Morris water maze procedure, as the maximal whole brain SB-271046 concentration is not reached until at least 3 h after oral administration (Routledge et al., 2000). All other drugs were administered i.p., 30 min (scopolamine and MK-801) or 45 min (galanthamine) before the first trial on each day. For subchronic studies, rats were treated twice a day for 7 days with SB-271046 (10 mg kg−1) or vehicle, and the Morris water maze experiment took place subsequently as described above. Yawning and motor activity were assayed twice, on first and seventh day of the treatment.
Yawning
After treating for 30 min with SB-271046, rats were placed in individual transparent chambers. Numbers of yawns were counted for 60 min as previously reported (Sleight et al., 1998).
Motor activity measurement
Horizontal locomotor activity was measured for 30 min in an open field, which consisted of a square arena (43 × 43 cm, 51 cm height) made of black wood, using a video tracking system (Ethovision 3.0, Noldus Information Technology B.V., The Netherlands), in a softly illuminated room. Tracking system was set to determine the position of the animal five times per second. Total path length (cm) was analysed.
Morris water maze
Equipment
The water maze consisted of a black circular pool (145 cm diameter × 55 cm high) constructed from black polyethylene and filled with water at 22 °C. The pool was virtually divided into four equal quadrants identified as north-east, north-west, south-east and south-west. A black invisible platform, 10 cm diameter, was located in a constant position in the middle of the south-west quadrant. The water level was 40, 2 cm above the platform, which was completely concealed. The training room offered black geometric paintings, to serve as visual extramaze cues for the animals. The information was analysed using a video tracking system (Ethovision 3.0).
Water maze procedure
The protocol was adapted from previous reports (Rogers and Hagan, 2001; Diez-Ariza et al., 2003). On the first day of the experimental procedure, animals performed a single trial of 60 s, without the platform present, to get used to the pool. In the acquisition phase, rats performed six training trials per day (120 s each) for two consecutive days (days 2 and 3) with the escape platform in a fixed position. Trials were started by placing the animals into the pool, close to and facing the wall at starting points designed as west, north, east, south, west and north. Rats were allowed to swim freely until they found the submerged platform or until 120 s elapsed. If the rat found the platform, it was allowed to remain there for 15 s and then returned to its home cage. If it was unable to find the platform within 120 s, it was then placed on the platform for 15 s and a maximum score of 120 s was assigned. Results were expressed as latency to find the hidden platform. The results on distances swum are not shown as, in all cases, they were parallel with latencies. For illustrative purposes, data were averaged across trials for each test day and presented in this format. On the fourth and seventh day (retention phase) a single transfer test was performed, in which the platform was removed from the pool. Rats were allowed to swim for 60 s in search of the platform. Time spent by the animals in the south-west quadrant, where the platform used to be located, was recorded. Swim speed (cm s−1) was calculated by dividing the swim path length (cm) by the latency (s). If the rat demonstrated a persistent preference during the trial to navigate in the pool quadrant where the escape platform had previously been placed, this was taken as an index of acquisition of the spatial task (Gage et al., 1984).
AChE activity
The degree of AChE inhibition by galanthamine was measured in homogenates from the frontal cortex and hippocampus according to the colorimetric method described by Wang et al. (1999).
Statistical analysis
Normality was checked by Shapiro–Wilk's test (P>0.05) before any other statistical analysis. In the acquisition phase of the Morris water maze, overall treatment effects were examined by a two-way repeated measures ANOVA. One-way ANOVA was used to test differences between treatment groups within trials, and differences between trials within groups were analysed using a repeated measures ANOVA. All analysis was followed by a Tukey test as post hoc testing. Time spent in the south-west quadrant in the retention phase was analysed with a one-way ANOVA followed by a Tukey-b test.
Yawning and motor activity measured in the first and seventh day were also analysed by a two-way repeated measures ANOVA with post hoc testing using Tukey-b test.
Results
Effects of acute vs subchronic treatment with SB-271046 on the Morris water maze
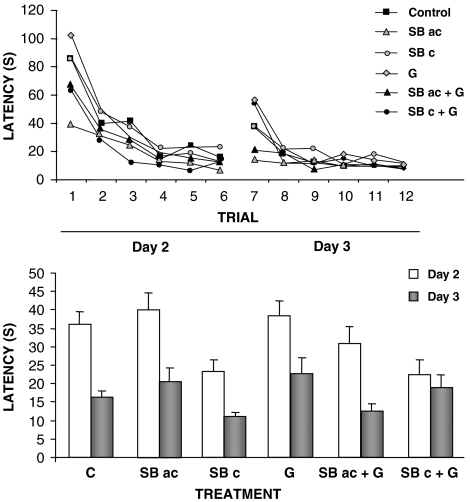
In the acquisition phase of the Morris water maze, statistical analysis (repeated measures two-way ANOVA) to compare the effects of acute vs subchronic treatment with SB-271046 did not show interaction between treatment × trial in latency or swim speed. As shown in Figure 1, time spent to reach the platform position for all groups (control, acute and subchronic treatment with SB-271046) improved significantly over trials on the acquisition phase (repeated measures, two-way ANOVA, F11,538=19.225, P<0.001). Comparing treatments, overall analysis of the latency to find the platform showed that animals with subchronic SB-271046 treatment (F2,49=3.150, P<0.05) reached the platform in a significantly shorter time.
Figure 1.
Effects of acute (10 mg kg−1) or subchronic treatment with SB-271046 (10 mg kg−1, twice daily, 7 days), alone or in combination with galanthamine (2.5 mg kg−1) on latency to find the platform in the acquisition phase of the Morris water maze (upper panel) and mean latency on days 2 and 3 of the Morris water maze (lower panel). Owing to the large amount of data, error bars have been omitted in the upper panel. C, control; SBac, acute SB-271046; SBc, subchronic SB-271046; G, galanthamine. Data are mean±s.e.mean of latency to find the platform (s) of 12 rats on each treatment.
In the first retention test (day 4), acute treatment with SB-271046 improved significantly (F2,46=3.808, P<0.05) the ability to remember the position of the platform compared with the other two experimental groups. No differences between groups were found on the second retention test (day 7). All these results are shown in Table 1.
Table 1.
Effect of the different treatments on Morris water maze retention
| Treatment | n |
Time spent in SW quadrant |
|
|---|---|---|---|
| Day 4 | Day 7 | ||
| Control | 10 | 18±1 | 15±1 |
| SB acute | 8 | 24±3* | 16±2 |
| SB subchronic | 12 | 15±1 | 12±1 |
| Gal | 8 | 18±3 | 12±1 |
| SB ac.+Gal | 9 | 17±1 | 13±2 |
| SB sbcr.+Gal | 9 | 14±2 | 13±2 |
| Control | 10 | 19±1 | 17±1 |
| Scop | 9 | 13±1* | 14±1* |
| SB ac.+Scop | 9 | 15±1 | 14±1 |
| SB sbcr.+Scop | 9 | 13±1 | 15±1 |
| Gal+Scop | 9 | 14±1 | 15±2 |
| SB ac.+Gal+Scop | 9 | 16±1 | 16±1 |
| SB sbcr.+Gal+Scop | 9 | 16±1 | 13±1 |
| Control | 10 | 21±1 | 17±1 |
| MK801 | 9 | 19±2 | 14±2 |
| SB ac.+MK801 | 9 | 17±2 | 17±1 |
| SB sbcr.+MK801 | 9 | 16±3 | 11±1 |
| Gal+MK801 | 9 | 14±2 | 15±3 |
| SB ac.+Gal+MK801 | 9 | 15±1 | 16±2 |
| SB sbcr.+Gal+MK801 | 9 | 8±2* | 13±1 |
Abbreviations: ac, acute; Gal, galanthamine; SB, SB-27104; Scop, scopolamine; sbcr, subchronic; SW, south-west.
Data are means±s.e.mean of time (in s) spent in the quadrant (SW) where the platform used to be located during the acquisition phase, 4 and 7 days after the beginning of the Morris water maze procedure. *P<0.05 vs control.
Choice of the dose of galanthamine
In a pilot study, no differences were found in time to reach the platform between the two most commonly used doses of galanthamine (2.5 and 5 mg kg−1). In addition, the degree of AChE inhibition was similar with using galanthamine, 2.5 mg kg−1 (% control: 51.5±3.2 in the frontal cortex, 31.2±3.7 in the hippocampus) or 5 mg kg−1 (% control: 42.3±6.5 in the frontal cortex, 23.2±3.3 in the hippocampus). Therefore, the lowest dose of 2.5 mg kg−1 was chosen.
Effects of a combined treatment of SB-271046 with galanthamine
There were no statistical difference in escape latency between the control group and galanthamine alone. The combined treatment of acute or subchronic treatment with SB-271046+galanthamine decreased latency over trials (F11,692=33.871, P<0.001), although did not show any significant differences in the overall average escape latency with respect to control animals (Figure 1).
Scopolamine-induced learning impairments
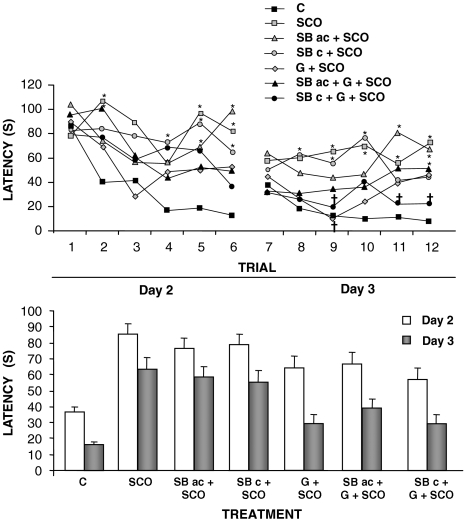
The ability of acute or subchronic treatment with SB-271046, alone or in combination with galanthamine, to reverse the amnesic effects of scopolamine, 0.4 mg kg−1, in the Morris water maze was first studied. In the acquisition phase of the Morris water maze, repeated measures two-way ANOVA showed a significant interaction between treatment × trial in latency to find the platform (F121,1197=1.440, P<0.01, Figure 2). Time spent to reach the platform position improved significantly over trials on the acquisition phase in the control (factorial ANOVA with replicates, F11,252=17.291, P<0.001) and combined galanthamine+subchronic treatment with SB-271046 (F11,77=2.866, P<0.001) groups. As expected, scopolamine-treated animals showed a significant impairment in learning as compared with the control group and no learning over trials was found. Neither galanthamine nor acute/subchronic SB-271046 treatment alone was able to reverse scopolamine effects. Combined treatment with galanthamine+subchronic treatment with SB-271046 partially reversed the scopolamine-induced learning impairment (Figure 2; one-way ANOVA, F11,110=3.371, P<0.001; F11,110=4.634, P<0.001 and F11,110=6.125, P<0.001 for trials 9, 11 and 12, respectively).
Figure 2.
Effects of acute (10 mg kg−1) or subchronic treatment with SB-271046 (10 mg kg−1, twice daily, 7 days), alone or in combination with galanthamine (2.5 mg kg−1) on learning impairments induced by scopolamine (0.4 mg kg−1) (upper panel) and mean latency on days 2 and 3 of the Morris water maze (lower panel). C, control; SCO, scopolamine; SBac, acute SB-271046; SBc, subchronic SB-271046; G, galanthamine. Data represented are mean±s.e.mean of latency to find the platform (s) of 12 rats on treatment. Statistical analysis of data on upper panel, repeated measures followed by analysis of simple effects (see text for details). *P<0.05 vs control; †P<0.05 vs scopolamine.
In the retention phase (Table 1), none of the treatments was able to counteract the amnesic effects of scopolamine in either of the two testing days (4 and 7).
Regarding swim speed, the acute treatment of SB-271046 and scopolamine produced a significant increase on the swim speed with respect to controls in the acquisition phase (42.1±1.7 vs 25.6±1.1 cm s−1, respectively, repeated measures, two-way ANOVA F11,109=3.593, P=0.000). No other effects were found.
MK-801-induced learning impairments
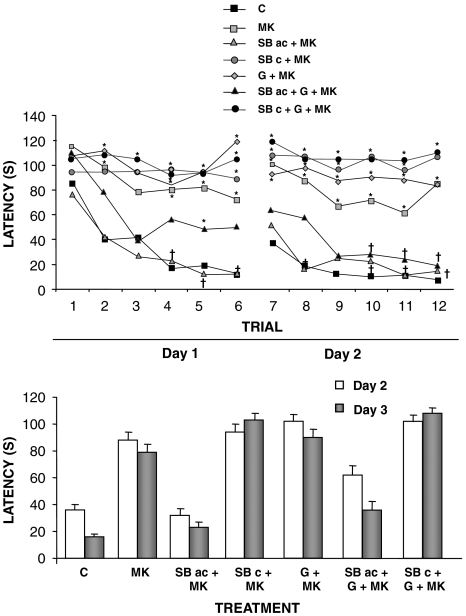
Rats given MK-801, 0.2 mg kg−1, showed a significant learning impairment, together with a behavioural syndrome consisting of thigmotaxic swimming, inability to stay on the platform when guided to it, locomotor hyperactivity and several motor responses such as wet-dog shakes and body rolling (as measured by gross visual examination). The acute administration of SB-271046, alone or combined with galanthamine, normalized the behaviour.
When studying the effects of acute or subchronic treatment with SB-271046, alone or in combination with galanthamine, to reverse the amnesic effects of MK-801 in the acquisition phase of the Morris water maze, there was a significant interaction between treatment × trial in latency to find the platform (repeated measures two-way ANOVA, F121,1192=1.768, P<0.001, Figure 3). Latency of rats treated with MK-801 was significantly higher than controls (repeated measures, two-way ANOVA, F11,108=15.54, P<0.001). Simple effect contrast showed that administration of acute SB-271046 reversed significantly the MK-801-induced impairments on trials 4, 5, 6, 8 and 11 (one-way ANOVA, F11,108=8.500, P<0.001; F11,106=11.306, P<0.001; F11,107=20.722, P<0.001; F11,109=18.713, P<0.001; F11,109=14.470, P<0.001 and respectively). On the last trials, administration of both acute SB-271046 or combined treatment of acute SB-271046+galanthamine reversed significantly the MK-801-induced impairments (F11,109=21.879 P<0.001; F11,109=31.822, P<0.001 for trial 10 and 12, respectively).
Figure 3.
Effects of acute (10 mg kg−1) or subchronic treatment with SB-271046 (10 mg kg−1, twice daily, 7 days), alone or in combination with galanthamine (2.5 mg kg−1) on learning impairments induced by MK-801 (0.2 mg kg−1) (upper panel) and mean latency on days 2 and 3 of the Morris water maze (lower panel). C, control; MK, MK-801; SBac, acute SB-271046; SBc, subchronic SB-271046; G, galanthamine. Data represented are mean±s.e.mean of latency to find the platform (s) of 12 rats on treatment. Statistical analysis of data on upper panel, repeated measures followed by analysis of simple effects (see text for details). *P<0.05 vs control; †P<0.05 vs MK-801.
The administration of MK-801 did not affect retention in the Morris water maze, and time searching for the platform was similar to that in the control animals (Table 1).
Swim speed was significantly affected in all rats that received MK-801 treatment (22.9 and 31.9 cm s−1 for control and MK-801, respectively, (repeated measures, two-way ANOVA, F121,1192=1.960, P<0.001), although acute administration of SB-271046, alone or in combination with galanthamine, normalized swim speed to values similar to controls.
Behavioural effects of a subchronic treatment with SB-271046
Cholinergic-mediated behaviours
A two-way repeated measures ANOVA revealed that the significant increases in the number of yawns induced by SB-271046 (F1,17.3=7.014, P<0.01, n=8–12) were similar at both days 1 and 7 after the beginning of treatment (Table 2).
Table 2.
Behavioural effects of a subchronic treatment with SB-271046
|
Day 1 |
Day 7 |
|||
|---|---|---|---|---|
| Control | SB-271046 | Control | SB-271046 | |
| Yawning | 1.7±0.8 | 4.4±1.2* | 1.2±0.5 | 4.4±1.3* |
| Motor activity | 6979±574 | 6606±321 | 6094±458 | 5875±312 |
Effects were measured at days 1 and 7 after the beginning of treatment with SB-271046. Motor activity is expressed as total path length (in cm). Yawning represents number of yawns per hour. Values are means±s.e.mean. *Significant difference (P<0.05) vs control at each day.
Motor activity
Overall analysis (two-way ANOVA) showed that motor activity (expressed as total path length) was not modified by treatment with SB-271046 at either day 1 or 7 after the beginning of the treatment (Table 2).
Discussion
Trying to elucidate the purported role of 5-HT6 receptors in cognition (see Introduction for discrepancies in literature), we reassessed the cognition enhancing properties of acute vs subchronic treatment with SB-271046, a selective 5-HT6 receptor antagonist with a pKi of 8.9 at the human 5-HT6 receptor, with greater than 200-fold selectivity over more than 51 other receptors, ion channels and enzymes (Routledge et al., 2000). The dose of 10 mg kg−1 of SB-271046 was chosen as several studies on cognition have reported consistent effects with SB-271046 at this optimal dose (Routledge et al., 2000; Rogers and Hagan, 2001; Dawson et al., 2000, 2001; Foley et al., 2004). Chemicals producing procognitive effects often produce bell-shaped dose-response curves. Here, the 5-HT6 receptor antagonist has been used only at this single dose (10 mg kg−1), sometimes in the presence of various other compounds, that is, AChE inhibitor, muscarinic antagonist and NMDA channel blocker (see below). Consequently, different treatment regimens might have the same effects on the animal behaviour, but with shifted dose-response curves. Using a standard spatial maze protocol (adapted from Morris, 1984) previously used by our group (Diez-Ariza et al., 2003), acute administration of SB-271046 did not produce any effect on acquisition phase of the Morris water maze, as reported (Rogers and Hagan, 2001), but significantly improved memory on the first day of the retention phase. The surprising loss of the beneficial effects of SB-271046 when administered subchronically is noteworthy. These results do not seem to be related to alterations in motor behaviour after the subchronic treatment.
There is convincing evidence that the influence of 5-HT6 receptors on memory is mediated at least partially by increased ACh neurotransmission. Direct evidence of the modulation of cholinergic activity by 5-HT6 receptors has been provided, as blockade of 5-HT6 receptors by SB-271046 has been shown to enhance ACh release (Shirazi-Southall et al., 2002; Riemer et al., 2003; Marcos et al., 2006). In fact, one of the first behavioural phenomena associated with 5-HT6 receptor inhibition, stretching and yawning behaviour, is attenuated by the cholinergic antagonist atropine (Bentley et al., 1999). In our hands, this cholinergic-mediated behaviour was induced to a similar extent by acute and subchronic dosing of SB-271046, indicating that the enhancement of cholinergic transmission by 5-HT6 receptor antagonism endures even after repeat dosing of the antagonist.
Effects of SB-271046, alone or in combination with galanthamine, on cholinergic-mediated deficits
As the cholinergic deficit hypothesis has become central to the study of Alzheimer's disease, including the notion that activation of muscarinic receptors is required during memory formation, cholinergic muscarinic antagonists, mainly scopolamine, have been used for modelling mechanisms of dysfunctional memory and for developing new drugs acting on dementia. SB-271046 had previously showed ability to restore this scopolamine-induced learning impairment in the passive avoidance task (Foley et al., 2004). However, we were unable to reproduce these effects in the Morris Water Maze, either after acute or subchronic treatment.
Interestingly, in support of a preliminary report demonstrating that combined treatment with SB-271046 with an AChE inhibitor produced an additive increase in passive avoidance (Callahan et al., 2004), we found that combined treatment of subchronic SB-271046 with galanthamine, significantly reversed the scopolamine-induced amnesic effects. Such benefits gained from the combined administration of a 5-HT6 receptor antagonist and a cholinesterase inhibitor may not be surprising, because the increased ACh release induced by the former and the reduced ACh degradation induced by the latter is expected to result in higher levels of extracellular ACh, thus resulting in enhanced cognitive performance. In addition, accumulating evidence suggest that galanthamine is an allosteric modulator of α-7 nicotinic receptors, which may also be implicated in the effects observed. It could be argued that the lack of reversal of the effects of scopolamine in cognition by galanthamine alone in our study could be due to an inadequate cholinesterase inhibition. However, we showed a >50% cholinesterase inhibition, which is comparable to the levels achieved in Alzheimer's disease patients, treated with AChE inhibitors (Barnes et al., 2000).
Effects of SB-271046 on glutamatergic-mediated deficits
NMDA blockade by MK-801 has been used to imitate cognitive impairments associated with schizophrenia or Alzheimer's disease. However, it is also of note that motor disturbances associated with MK-801 administration (Åhlander et al., 1999) may be responsible for the purported learning impairment induced by this NMDA receptor antagonist. In fact, in our hands, there were no differences between the control and the MK-801 groups on retention, so rats have presumably learnt the spatial location of the platform. Interestingly, the acute administration of SB-271046 that reverses the MK-801 motor syndrome was also able to reverse the learning impairment induced by MK-801. The fact that acute SB-271046 treatment reverses MK-801 induced impact on latency only during acquisition might be seen as further evidence for an impact on side effects rather than cognition. Previous studies have indicated that MK801 administration increases 5-HT transmission (Whitton et al., 1992; Loscher et al., 1993; Qing-Shan et al., 1997; Callado et al., 2000) that may be responsible for motor abnormalities (Åhlander et al., 1999). It could be speculated that SB-271046, blocking 5-HT6 receptors, would counteract increased 5-HT transmission.
On the other hand, and assuming that MK-801 really induced a learning deficit, a subchronic treatment with SB-271046 failed to improve learning in the model of MK-801-induced impairments. These results further emphasize the hypothesis that 7-day treatment with SB-271046 may be activating unknown mechanisms that counteract the otherwise beneficial effects of this molecule.
Conclusions
Taken together, all these data suggest a potential therapeutic role of 5-HT6 receptor antagonists in the treatment of cognitive dysfunction, particularly those associated with cholinergic hypofunction. However, it is also worth mentioning that SB-271046, alone or in combination with galanthamine, was also able to reverse MK-801-induced cognitive/behavioural dysfunctions. The relatively higher affinity for 5-HT6 receptors might explain some of the beneficial effects of clozapine upon cognitive impairments in schizophrenia (Meltzer and McGurk, 1999; Roth et al., 2004). It is worth mentioning that some clinical papers have reported that adjunct galanthamine may improve negative and cognitive symptoms in schizophrenic patients (Ferreri et al., 2006; Schubert et al., 2006). Therefore, the beneficial effects of co-administering SB-271046 and galanthamine indicate that a combined 5-HT6 receptor antagonist and cholinesterase inhibitor strategy could be a novel approach for the treatment of cognitive disorders such as those seen in Alzheimer's disease or schizophrenia.
Acknowledgments
SB-271046 was generously provided by Glaxo SmithKline, Harlow, UK. B Marcos and FJ Gil-Bea have a scholarship from Gobierno de Navarra (Spain).
Conflict of interest
Beatriz Marcos, Francisco J Gil-Bea and María J Ramirez have no conflicts of interest. Tsu T Chuang is an employee of Glaxo SmithKline.
References
- Åhlander M, Misane I, Schött PA, Ogren SO. A behavioral analysis of the spatial learning deficit induced by the NMDA receptor antagonist MK801 (dizolcipine) in the rat. Neuropsychopharmacol. 1999;21:414–426. doi: 10.1016/S0893-133X(98)00116-X. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Meltzer J, Houston F, Orr G, McGann K, Wenk GL. Chronic treatment of old rats with donepezil or galantamine: effects on memory, hippocampal plasticity and nicotinic receptors. Neuroscience. 2000;99:17–23. doi: 10.1016/s0306-4522(00)00180-9. [DOI] [PubMed] [Google Scholar]
- Bentley JC, Bourson A, Boess FG, Fone KCF, Marsden CA, Petit N, et al. Investigation of stretching behaviour induced by the selective 5-HT6 receptor antagonist, Ro 04-6790, in rats. Br J Pharmacol. 1999;126:1537–1542. doi: 10.1038/sj.bjp.0702445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callado LF, Hopwood SE, Hancock PJ, Stamford JA. Effects of dizolcipine (MK801) on noradrenaline, serotonin and dopamine release and uptake. Neuroreport. 2000;17:173–176. doi: 10.1097/00001756-200001170-00034. [DOI] [PubMed] [Google Scholar]
- Callahan PM, Ilch CP, Rowe WB, Tehim A. Characterization of the selective 5-HT6 receptor antagonist SB-271046. Soc Neurosci Meet. 2004;776:19. [Google Scholar]
- Daly DA, Moghaddam B. Actions of clozapine and haloperidol on the extracellular levels of excitatory amino acids in the prefrontal cortex and striatum of conscious rats. Neurosci Lett. 1993;152:61–64. doi: 10.1016/0304-3940(93)90483-2. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Li P, Nguyen HQ. The 5-HT6 receptor antagonist SB-271046 selectively enhances excitatory neurotransmission in the rat frontal cortex and hippocampus. Neuropsychopharmacol. 2001;25:662–668. doi: 10.1016/S0893-133X(01)00265-2. [DOI] [PubMed] [Google Scholar]
- Dawson LA, Nguyen HQ, Li P. In vivo effects of the 5-HT6 antagonist SB-271046 on striatal and frontal cortex extracellular concentrations of noradrenaline, dopamine, 5-HT, glutamate and aspartate. Br J Pharmacol. 2000;130:23–26. doi: 10.1038/sj.bjp.0703288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez-Ariza M, Redondo C, Garcia-Alloza M, Lasheras B, Del Rio J, Ramirez MJ. Flumazenil and tacrine increase the effectiveness of ondansetron on scopolamine-induced impairment of spatial learning in rats. Psychopharmacol. 2003;169:35–41. doi: 10.1007/s00213-003-1467-1. [DOI] [PubMed] [Google Scholar]
- Ferreri F, Agbokou C, Gauthier S. Cognitive dysfunctions in schizophrenia: potential benefits of cholinesterase inhibitor adjunctive therapy. J Psychi Neurosci. 2006;31:369–376. [PMC free article] [PubMed] [Google Scholar]
- Filliat P, Blanchet G. Effects of TCP on spatial memory: comparison with MK-801. Pharmacol Biochem Behav. 1995;51:429–434. doi: 10.1016/0091-3057(95)00002-e. [DOI] [PubMed] [Google Scholar]
- Foley AG, Murphy KJ, Hirst WD, Gallagher HC, Hagan JJ, Upton N, et al. The 5-HT6 receptor antagonist SB-271046 reverses scopolamine-disrupted consolidation of a passive avoidance task and ameliorates spatial task deficits in aged rats. Neuropsychopharmacol. 2004;29:93–100. doi: 10.1038/sj.npp.1300332. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Bjorklund A. Spatial learning and motor deficits in aged rats. Neurobiol Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Geerts H. Indicators of neuroprotection with galantamine. Brain Res Bull. 2005;64:519–524. doi: 10.1016/j.brainresbull.2004.11.002. [DOI] [PubMed] [Google Scholar]
- Healy DJ, Meador-Woodruff JH. Glutamate receptor modulation of 5-HT6 and 5-HT7 mRNA expression in rat brain. Neuropsychopharmacol. 1999;21:341–351. doi: 10.1016/S0893-133X(99)00043-3. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Abrahamsen B, Blaney FE, Calver AR, Aloj L, Price GW, et al. Differences in the central nervous system distribution and pharmacology of the mouse 5-hydroxytryptamine-6 receptor compared with rat and human receptors investigated by radioligand binding, site-directed mutagenesis, and molecular modeling. Mol Pharmacol. 2003a;64:1295–1308. doi: 10.1124/mol.64.6.1295. [DOI] [PubMed] [Google Scholar]
- Hirst WD, Moss SF, Bromidge SM, Riley G, Stean TO, Rogers SC. Characterization of SB-399885, a potent and selective 5-HT6 receptor antagonist. Soc Neurosci Meet. 2003b;576:7. [Google Scholar]
- King MV, Sleight AJ, Woolley ML, Topham IA, Marsden CA, Fone KC. 5-HT6 receptor antagonists reverse delay-dependent deficits in novel object discrimination by enhancing consolidation—an effect sensitive to NMDA receptor antagonism. Neuropharmacol. 2004;47:195–204. doi: 10.1016/j.neuropharm.2004.03.012. [DOI] [PubMed] [Google Scholar]
- Lieben CK, Blokland A, Sik A, Sung E, van Nieuwenhuizen P, Schreiber R. The selective 5-HT6 receptor antagonist Ro4368554 restores memory performance in cholinergic and serotonergic models of memory deficiency in the rat. Neuropsychopharmacol. 2005;30:2169–2179. doi: 10.1038/sj.npp.1300777. [DOI] [PubMed] [Google Scholar]
- Lindner MD, Hodges DB, Jr, Hogan JB, Orie AF, Corsa JA, Barten DM, et al. An assessment of the effects of serotonin 6 (5-HT6) receptor antagonists in rodent models of learning. J Pharmacol Exp Ther. 2003;307:682–691. doi: 10.1124/jpet.103.056002. [DOI] [PubMed] [Google Scholar]
- Loscher W, Annies R, Honack D. Comparison of competitive and uncompetitive NMDA receptor antagonists with regard to monoaminergic neuronal activity and behavioral effects in rats. Eur J Pharmacol. 1993;242:263–274. doi: 10.1016/0014-2999(93)90250-l. [DOI] [PubMed] [Google Scholar]
- Marcos B, Gil-Bea FJ, Hirst W, García-Alloza M, Ramírez MJ. Lack of localization of 5-HT6 receptors on cholinergic neurons: implication of multiple neurotransmitter systems in 5-HT6 receptor-mediated acetylcholine release. Eur J Neurosci. 2006;24:1299–1306. doi: 10.1111/j.1460-9568.2006.05003.x. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizo Bull. 1999;25:233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- Meneses A. Effects of the 5-HT6 receptor antagonist Ro 04-6790 on learning consolidation. Behav Brain Res. 2001;118:107–110. doi: 10.1016/s0166-4328(00)00316-8. [DOI] [PubMed] [Google Scholar]
- Meneses A, Manuel-Apolinar L, Castillo C, Castillo E. Memory consolidation and amnesia modify 5-HT6 receptors expression in rat brain: an autoradiographic study. Behav Brain Res. 2007;178:53–61. doi: 10.1016/j.bbr.2006.11.048. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Hoplight BJ, Lear SP, Neumaier JF. BGC20-761, a novel tryptamine analog, enhances memory consolidation and reverses scopolamine-induced memory deficit in social and visuospatial memory tasks through a 5-HT6 receptor-mediated mechanism. Neuropharmacol. 2006;50:412–420. doi: 10.1016/j.neuropharm.2005.10.002. [DOI] [PubMed] [Google Scholar]
- Mitchell ES, Neumaier JF. 5-HT6 receptors: a novel target for cognitive enhancement. Pharmacol Ther. 2005;108:320–333. doi: 10.1016/j.pharmthera.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Morris R. Developments of a water maze procedure for studing spatial learning in the rat. J Neurosci Meth. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Perez-Garcia G, Meneses A. Oral administration of the 5-HT6 receptor antagonists SB-357134 and SB-399885 improves memory formation in an autoshaping learning task. Pharmacol Biochem Behav. 2005;81:673–682. doi: 10.1016/j.pbb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Qing-Shan Y, Maarten EAR, Jobe PC, Dailey JW. Dizolcipine (MK801) increases not only dopamine but also serotonin and norepinephrine transmissions in the nucleus accumbens as measured by microdialysis in freely moving rats. Brain Res. 1997;765:149–158. doi: 10.1016/s0006-8993(97)00568-4. [DOI] [PubMed] [Google Scholar]
- Riemer C, Borroni E, Levet-Trafit B, Martin JR, Poli S, Porter RH, et al. Influence of the 5-HT6 receptor on acetylcholine release in the cortex: pharmacological characterization of 4-(2-bromo-6-pyrrolidin-1-ylpyridine-4-sulfonyl)phenylamine, a potent and selective 5-HT6 receptor antagonist. J Med Chem. 2003;46:1273–1276. doi: 10.1021/jm021085c. [DOI] [PubMed] [Google Scholar]
- Rogers DC, Hagan JJ. 5-HT6 receptor antagonists enhance retention of a water maze task in the rat. Psychopharmacol. 2001;158:114–119. doi: 10.1007/s002130100840. [DOI] [PubMed] [Google Scholar]
- Roth BL, Roth BL, Hanizavareh SM, Blum AE. Serotonin receptors represent highly favorable molecular targets for cognitive enhancement in schizophrenia and other disorders. Psychopharmacol. 2004;174:17–24. doi: 10.1007/s00213-003-1683-8. [DOI] [PubMed] [Google Scholar]
- Routledge C, Bromidge SM, Moss SF, Price G, Hirst W, Newman H, et al. Characterization of SB-271046: a potent, selective and orally active 5-HT6 receptor antagonist. Br J Pharmacol. 2000;130:1606–1612. doi: 10.1038/sj.bjp.0703457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell MG, Dias R. Memories are made of this (perhaps): a review of serotonin 5-HT(6) receptor ligands and their biological functions. Curr Top Med Chem. 2002;2:643–654. doi: 10.2174/1568026023393877. [DOI] [PubMed] [Google Scholar]
- Schubert MH, Young KA, Hicks PB. Galantamine improves cognition in schizophrenic patients stabilized on risperidone. Biol Psychi. 2006;60:530–533. doi: 10.1016/j.biopsych.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Shirazi-Southall S, Rodriguez DE, Nomikos GG. Effects of typical and atypical antipsychotics and receptor selective compounds on acetylcholine efflux on the hippocampus of the rat. Neuropsychopharmacol. 2002;26:583–594. doi: 10.1016/S0893-133X(01)00400-6. [DOI] [PubMed] [Google Scholar]
- Sleight AJ, Boess FG, Bös M, Levet-Trafit B, Riemer C, Bourson A. Characterization of Ro 04-6790 and Ro 63-0563: potent and selective antagonists at human and rat 5-HT6 receptors. Br J Pharmacol. 1998;125:556–562. doi: 10.1038/sj.bjp.0701851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson GD. Cognitive function in schizophrenic patients. J Clin Psychi. 1996;11:31–39. [PubMed] [Google Scholar]
- Wang H, Carlier PR, Ho WL, Wu DC, Lee NT, Li CPL, et al. Effects of bis(7)-tacrine, a novel anti-Alzheimer's agent, on rat brain AChE. Neuroreport. 1999;10:789–793. doi: 10.1097/00001756-199903170-00023. [DOI] [PubMed] [Google Scholar]
- Whitton PS, Biggs CS, Pearce BR, Fowler LJ. MK801 increases extracellular 5-hydroxytryptamine in rat hippocampus and striatum in vivo. J Neurochem. 1992;58:1573–1575. doi: 10.1111/j.1471-4159.1992.tb11381.x. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Bentley JC, Sleight AJ, Marsden CA, Fone KC. A role for 5-ht6 receptors in retention of spatial learning in the Morris water maze. Neuropharmacol. 2001;41:210–219. doi: 10.1016/s0028-3908(01)00056-9. [DOI] [PubMed] [Google Scholar]
- Woolley ML, Marsden CA, Fone KC. 5-ht6 receptors. Curr Drug Targets. 2004;3:59–79. doi: 10.2174/1568007043482561. [DOI] [PubMed] [Google Scholar]