Abstract
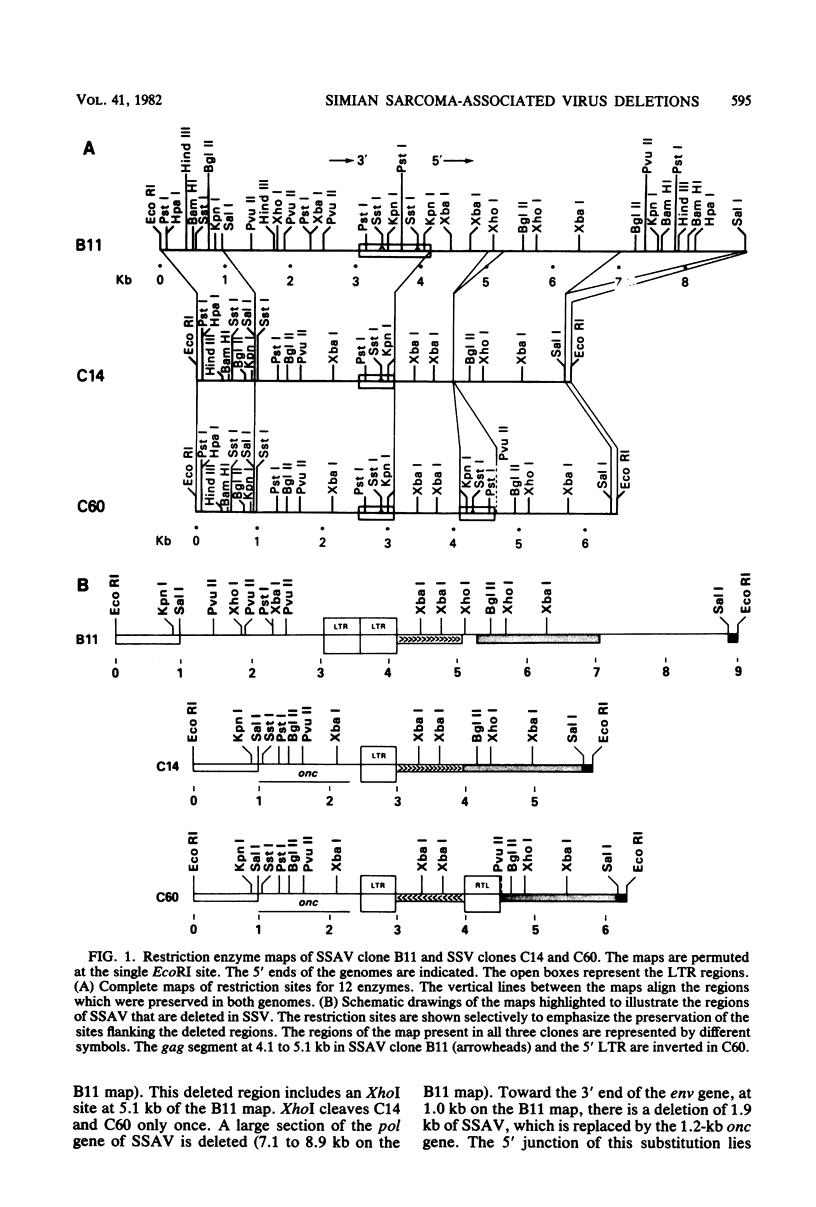
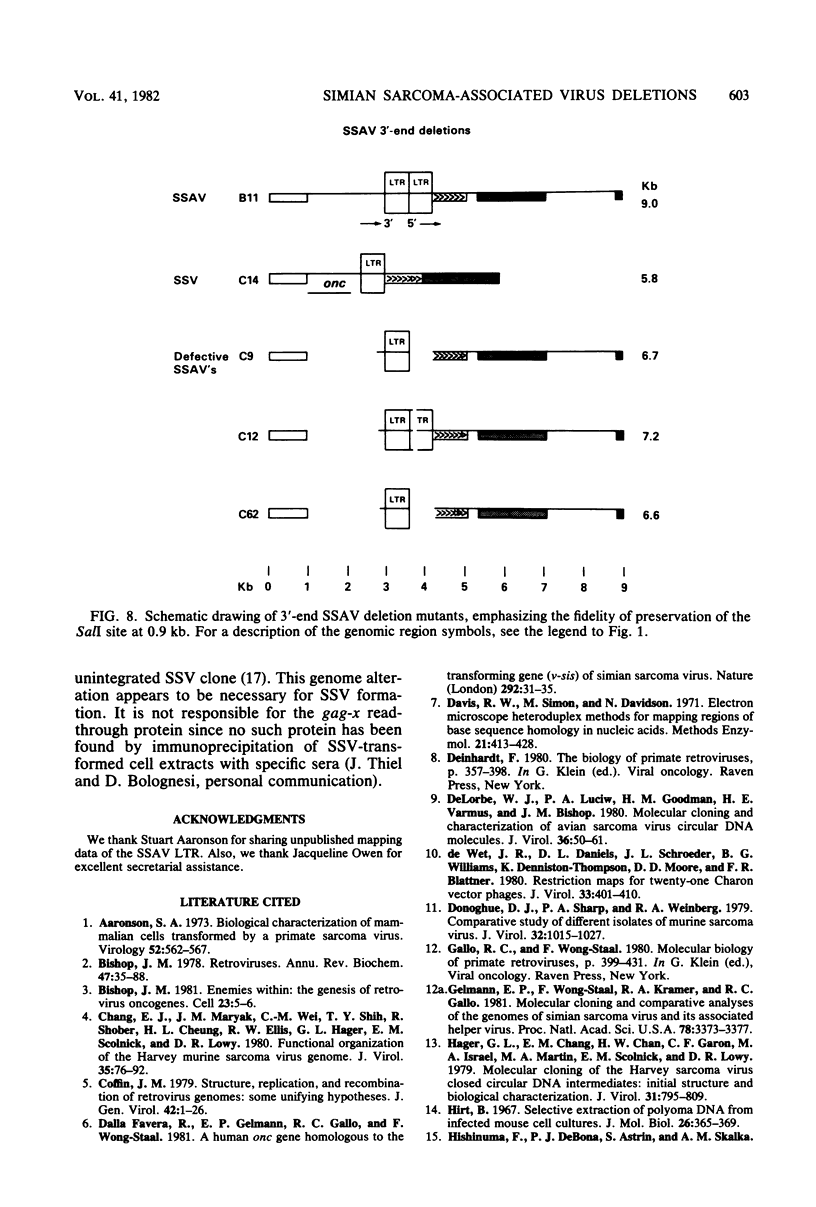
Extrachromosomal DNA was isolated from tissue culture cells that were acutely infected with simian sarcoma virus (SSV) and its associated helper (simian sarcoma-associated virus [SSAV]). Two sizes of closed circular viral genomic DNA intermediates were isolated, cleaved at the single EcoRI site, and ligated to the Charon 21A phage lambda vector. Cloned molecules of the larger size all represented the full-length (9.0-kilobase [kb]) SSAV molecule. A heterogeneous group of clones was derived from the smaller DNA circles. These included the SSV genome and SSAV deletion mutants. When two SSV clones were compared with the helper, they contained the following three characteristic deletions: (i) a 250-base pair deletion in the gag gene about 1.0 kb from the 5' end of the genome; (ii) a 1.85-kb deletion in the pol gene; and (iii) a 1.9-kb deletion at the 3' end, which included part of the env gene. This latter deletion was the site of the onc gene substitution. Six other clones of the smaller molecules represented the following variants of the SSAV genome: (i) two clones of the entire genome containing only one long terminal repeat unit; (ii) one clone with the 1.85-kb deletion of the pol gene observed in SSSV; and (iii) three clones having a deletion of the 3' end of the SSAV genome. In each of the latter clones, the 5' border of the deletion was indistinguishable from the 5' border of the onc substitution in SSV. The fidelity of genetic deletions observed suggested that certain regions of the SSAV genome were deleted at a high frequency. In certain cases, these deletions may have been accompanied by a substitution of cellular sequences to generate SSV.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaronson S. A. Biologic characterization of mammalian cells transformed by a primate sarcoma virus. Virology. 1973 Apr;52(2):562–567. doi: 10.1016/0042-6822(73)90351-6. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Enemies within: the genesis of retrovirus oncogenes. Cell. 1981 Jan;23(1):5–6. doi: 10.1016/0092-8674(81)90263-4. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Chang E. H., Maryak J. M., Wei C. M., Shih T. Y., Shober R., Cheung H. L., Ellis R. W., Hager G. L., Scolnick E. M., Lowy D. R. Functional organization of the Harvey murine sarcoma virus genome. J Virol. 1980 Jul;35(1):76–92. doi: 10.1128/jvi.35.1.76-92.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffin J. M. Structure, replication, and recombination of retrovirus genomes: some unifying hypotheses. J Gen Virol. 1979 Jan;42(1):1–26. doi: 10.1099/0022-1317-42-1-1. [DOI] [PubMed] [Google Scholar]
- Dalla-Favera R., Gelmann E. P., Gallo R. C., Wong-Staal F. A human onc gene homologous to the transforming gene (v-sis) of simian sarcoma virus. Nature. 1981 Jul 2;292(5818):31–35. doi: 10.1038/292031a0. [DOI] [PubMed] [Google Scholar]
- DeLorbe W. J., Luciw P. A., Goodman H. M., Varmus H. E., Bishop J. M. Molecular cloning and characterization of avian sarcoma virus circular DNA molecules. J Virol. 1980 Oct;36(1):50–61. doi: 10.1128/jvi.36.1.50-61.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue D. J., Sharp P. A., Weinberg R. A. Comparative study of different isolates of murine sarcoma virus. J Virol. 1979 Dec;32(3):1015–1027. doi: 10.1128/jvi.32.3.1015-1027.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
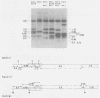
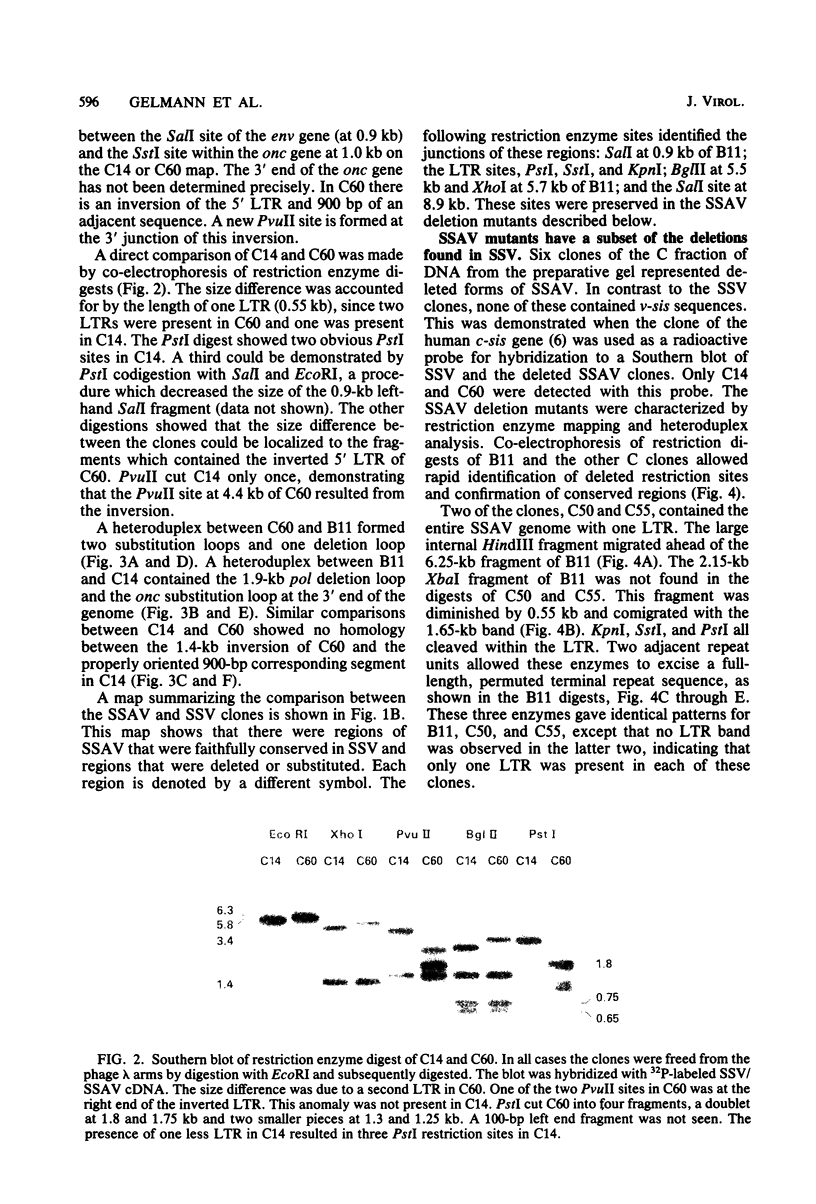
- Gelmann E. P., Wong-Staal F., Kramer R. A., Gallo R. C. Molecular cloning and comparative analyses of the genomes of simian sarcoma virus and its associated helper virus. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3373–3377. doi: 10.1073/pnas.78.6.3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hager G. L., Chang E. H., Chan H. W., Garon C. F., Israel M. A., Martin M. A., Scolnick E. M., Lowy D. R. Molecular cloning of the Harvey sarcoma virus closed circular DNA intermediates: initial structural and biological characterization. J Virol. 1979 Sep;31(3):795–809. doi: 10.1128/jvi.31.3.795-809.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Aaronson S. A. Molecular cloning of integrated simian sarcoma virus: genome organization of infectious DNA clones. Proc Natl Acad Sci U S A. 1981 May;78(5):2918–2922. doi: 10.1073/pnas.78.5.2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scolnick E. M. Transformation by rat-derived oncogenic retroviruses. Microbiol Rev. 1981 Mar;45(1):1–8. doi: 10.1128/mr.45.1.1-8.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr C. J., Fedele L. A., Oskarsson M., Maizel J., Vande Woude G. Molecular cloning of Snyder-Theilen feline leukemia and sarcoma viruses: comparative studies of feline sarcoma virus with its natural helper virus and with Moloney murine sarcoma virus. J Virol. 1980 Apr;34(1):200–212. doi: 10.1128/jvi.34.1.200-212.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Tsuchida N., Uesugi S. Structure and functions of the Kirsten murine sarcoma virus genome: molecular cloning of biologically active Kirsten murine sarcoma virus DNA. J Virol. 1981 May;38(2):720–727. doi: 10.1128/jvi.38.2.720-727.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Beveren C., Galleshaw J. A., Jonas V., Berns A. J., Doolittle R. F., Donoghue D. J., Verma I. M. Nucleotide sequence and formation of the transforming gene of a mouse sarcoma virus. Nature. 1981 Jan 22;289(5795):258–262. doi: 10.1038/289258a0. [DOI] [PubMed] [Google Scholar]
- Vennström B., Fanshier L., Moscovici C., Bishop J. M. Molecular cloning of the avian erythroblastosis virus genome and recovery of oncogenic virus by transfection of chicken cells. J Virol. 1980 Nov;36(2):575–585. doi: 10.1128/jvi.36.2.575-585.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma I. M., Lai M. H., Bosselman R. A., McKennett M. A., Fan H., Berns A. Molecular cloning of unintegrated Moloney mouse sarcoma virus DNA in bacteriophage lambda. Proc Natl Acad Sci U S A. 1980 Apr;77(4):1773–1777. doi: 10.1073/pnas.77.4.1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong-Staal F., Dalla-Favera R., Gelmann E. P., Manzari V., Szala S., Josephs S. F., Gallo R. C. The v-sis transforming gene of simian sarcoma virus is a new onc gene of primate origin. Nature. 1981 Nov 19;294(5838):273–275. doi: 10.1038/294273a0. [DOI] [PubMed] [Google Scholar]
- Yamamoto T., Tyagi J. S., Fagan J. B., Jay G., deCrombrugghe B., Pastan I. Molecular mechanism for the capture and excision of the transforming gene of avian sarcoma virus as suggested by analysis of recombinant clones. J Virol. 1980 Aug;35(2):436–443. doi: 10.1128/jvi.35.2.436-443.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wet J. R., Daniels D. L., Schroeder J. L., Williams B. G., Denniston-Thompson K., Moore D. D., Blattner F. R. Restriction maps for twenty-one Charon vector phages. J Virol. 1980 Jan;33(1):401–410. doi: 10.1128/jvi.33.1.401-410.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]