Abstract
Background and purpose:
The regulation of vascular soluble guanylyl cyclase (sGC) expression by nitric oxide (NO) is still under discussion. In vitro, NO has been shown to downregulate the expression of sGC but it is unclear if this mechanism is operative in vivo and occurs during nitrate treatment.
Experimental approach:
We investigated whether high dose isosorbide mononitrate (ISMN) or pentaerythrityl tetranitrate (PETN) treatment changes vascular sGC expression and activity in vivo. New Zealand White rabbits received a standard diet, 2 or 200 mg ISMN kg−1 d−1 for 16 weeks, and C57BL/6 mice received a standard diet, 6, 60 or 300 mg PETN kg−1 d−1 for four weeks. Absorption was checked by measuring the plasma levels of the drug/metabolite.
Key results:
Western blots of rabbit aortic rings showed similar protein levels of sGC α1- (P=0.2790) and β1-subunits (P=0.6900) in all groups. Likewise, ANOVA showed that there was no difference in the expression of sGC in lungs of PETN-treated mice (P=0.0961 for α1 and P=0.3709 for β1). The activities of isolated sGC in response to SNAP (1 μM–1 mM) were identical in aortae of ISMN-treated rabbits (P=0.0775) and lungs of PETN-treated mice (P=0.6348). The aortic relaxation response to SNAP slightly decreased at high ISMN but not at high PETN.
Conclusions and implications:
These data refute the hypothesis that therapeutic treatment with long acting NO donors has a significant impact on the regulation of vascular sGC expression and activity in vivo.
Keywords: organic nitrates, nitric oxide, soluble guanylyl cyclase, endothelium, nitric oxide synthase, chronic treatment
Introduction
Organic nitrates are commonly used drugs for prevention and acute treatment of coronary artery disease symptoms. They are activated to nitric oxide (NO), which is involved in physiological processes such as smooth muscle relaxation, neurotransmission, platelet aggregation, host defence mechanisms and apoptosis and has antioxidative effects (Moncada and Higgs, 1993). The effects of NO greatly contribute to the physiological vascular functions and probably protect the vascular wall from vasotoxic compounds such as reactive oxygen species (Gewaltig and Kojda, 2002). In the vasculature, the majority of the effects of NO are mediated by the activation of soluble guanylyl cyclase (sGC), generation of cyclic guanosine monophosphate (cGMP), activation of protein kinase G (PKG) and phosphorylation of various cellular proteins regulating calcium haemostasis (Ignarro et al., 1999). The sGC enzyme is composed of two subunits, α and β, and a prosthetic haem group. The vast majority of vascular sGC is formed by the subunits α1 and β1 (Buechler et al., 1991; Russwurm and Koesling, 2004).
A disturbance of the NO–cGMP pathway induced by changes of the expression of sGC has been previously observed. The expression of vascular sGC is downregulated in spontaneously hypertensive rats (Bauersachs et al., 1998; Ruetten et al., 1999), in smooth muscle cells of Fischer 344 rats (Chen et al., 2000) and in aged Wistar Kyoto rats (Kloss et al., 2000; Friebe and Koesling, 2003). Other studies have shown a downregulation of sGC mRNA and protein expression by endotoxin, cAMP, cytokines and oestradiol (Shimouchi et al., 1993; Papapetropoulos et al., 1996; Kojda et al., 1998a; Ruetten et al., 1999; Krumenacker et al., 2001; Takata et al., 2001; Friebe and Koesling, 2003). In contrast, hypercholesterolaemia increases the expression of rabbit aortic vascular sGC, particularly in atherosclerotic plaques (Laber et al., 2002), and a qualitatively similar but smaller effect has been observed in experimental chronic myocardial infarction and in nitrate tolerance (Bauersachs et al., 1998; Mulsch et al., 2001). In late-stage atherosclerosis, this may be different, in particular in the neointima (Melichar et al., 2004).
The mechanisms underlying these changes of sGC expression are currently unknown. Studies in cultured smooth muscle cells have suggested a crucial role of vascular NO generation, which may downregulate sGC expression in a negative feedback manner (Filippov et al., 1997; Weber et al., 2001). Likewise, transfection of HEK 293 cells with an endothelial nitric oxide synthase (eNOS)-containing plasmid and incubation of rat aortic rings with the NO-donor N-[4[1-(3-aminopropyl)-2-hydroxy-2-nitrosohydrazino]butyl]-1,3-propanediamine (SPER/NO) reduced mRNA expression of the β1 subunit (Schmidt et al., 2001; Weber et al., 2001).
To investigate whether alterations of sGC protein expression and activity may be involved in oral therapy with long-acting NO donors such as isosorbide mononitrate (ISMN) and pentaerythrityl tetranitrate (PETN), we sought to determine the effect of nitrate treatment on vascular smooth muscle sGC in vivo. To accomplish this, we measured the vascular sGC protein expression and activity as well as NO effects in aortic segments after treatment with different doses of ISMN and in mice treated with different doses of PETN.
Methods
Test systems used
A total of 30 New Zealand white rabbits (10–12 weeks old) with a mean body weight of 2105±47 g were housed individually as previously described (Kojda et al., 1995). The rabbits were randomly assigned to three groups of 10 animals and were fed a standard diet (ISMN-0) and a diet supplemented with ISMN to achieve a daily dose of ISMN of 2 mg kg−1 (ISMN-2) or 200 mg kg−1 (ISMN-200) for 16 weeks. The dose of ISMN was given in two identical portions in the morning at 0800 hours and in the early afternoon at 1500 hours. Body weight was determined weekly and the animals were supervised by a veterinerian. Previous studies have shown that 200 mg kg−1 per day of ISMN induces a nitrate tolerance as indicated selectively by reduced vasodilator activity of ISMN, both in normal and in hypercholesterolaemic rabbits (Muller et al., 2003, 2004).
C57BL/6 mice (male, 5-month old) were divided into four groups and were then randomly allocated to receive placebo (n=8) or PETN treatment (n=12 per group) for 4 weeks. The groups were treated with placebo (PETN-0) or 6 (PETN-6), 60 (PETN-60) or 300 mg kg−1 per day (PETN-300) of PETN, according to an average body weight of 25 g and an assumed daily amount of 5 g of consumed food. According to the manufacturer's recommendations, PETN was given continuously.
Permission for the animal studies was provided by the regional government of Germany (AZ 23.05-230-3-77/99, AZ 23.05-230-3-52/99, AZ 50.05-230-3-65/99, AZ 50.05-230-3-94/00 AZ 50.05-230-18/06). The experiments were performed according to the guidelines for the use of experimental animals, as given by the German ‘Tierschutzgesetz' and the ‘Guide for the Care and Use of Laboratory Animals' of the US National Institutes of Health.
Measurements of plasma levels of drug/metabolite
Plasma concentrations of ISMN, pentaerythrityl dinitrate and mononitrate were determined by ACC GmbH (Leidensbach, Germany) with gas chromatography/mass spectrometry (GC/MS, HP6890, Hewlett-Packard, Germany) after liquid–liquid extraction with ethyl acetate as previously described (Muller et al., 2004).
Measurement of blood pressure
Systolic blood pressure and heart rate were measured in awake male C57BL/6 (n=4 of each PETN treatment group) at 3–4 months of age using an automated tailcuff system as previously described (Kojda et al., 1999). On day 7, the PETN diet was started and blood pressure measurement was continued for a maximum of 7 days.
Vasorelaxation studies, sGC activity and western blots
Preparation of rabbit thoracic ring segments, equilibration and relaxation to the NO-donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP) as well as preparation of cytosols from rabbit aorta and mouse lung were performed as previously described (Kojda et al., 1995; Laber et al., 2002). Specific activity of sGC was measured as described by Kojda et al. (1998a) and Schultz and Böhme (1984). Western blots for sGC α1 and β1 subunits were performed in rabbit aorta and mouse lung using specific antibodies. In addition, blots for vasodilator-stimulated phosphoprotein (VASP) were performed in lung and heart homogenates of PETN-treated mice. Please refer to Supplementary information for detailed protocols.
Data analyses and statistical procedures
All data were analysed by a standard computer programme (GraphPad Prism PC software, version 3.03) and are expressed as mean±s.e.m. of n individual samples. Statistical comparisons between groups were performed by Newman–Keuls multiple comparisons test following analysis of variance (ANOVA) for pD2 values and protein expression or two-way ANOVA for concentration–response curves. P<0.05 was considered statistically significant.
Drugs, chemical reagents and other materials
[α-32P]-GTP was obtained from PerkinElmer (Rodgau, Germany). Antibodies against sGC α1 subunit (catalogue no. G4280) and actin (no. A2066) were from Sigma (Munich, Germany); against sGC β1 subunit (no. 160897) from Cayman (Biozol, Eching, Germany); against rabbit immunoglobulin G (IgG) (no. 401315) from Calbiochem (Darmstadt, Germany); against phosphorylated and total VASP (no. 804–240 and no. 210–880) from Alexis Biochemicals (Lörrach, Germany) and against mouse IgG (no. 170–6516) from Bio-Rad (Munich, Germany). Polyvinylidene fluoride membranes were from Millipore (Schwalbach, Germany). SNAP was synthesized in our laboratory as previously described (Kojda et al., 1996). ISMN was provided by Schwarz Pharma (Monheim, Germany); PETN by Actavis (Langenfeld, Germany). All other chemicals were obtained from Merck (Darmstadt, Germany) or from Sigma in analytical grade. The stock solutions of acetylcholine (10 mM) and phenylephrine (10 mM) and ISMN (100 mM) were prepared in distilled water. Solutions of SNAP (200 mM) were prepared in dimethylsulphoxide. All stock solutions were prepared daily, diluted with Krebs buffer as required, kept on ice and protected from daylight until use. The blood pressure measurement system was from Visitech Systems (Apex, NC, USA).
The molecular target nomenclature in this paper conforms with the BJP's Guide to Receptors and Channels (Alexander et al., 2008).
Results
ISMN-treated rabbits
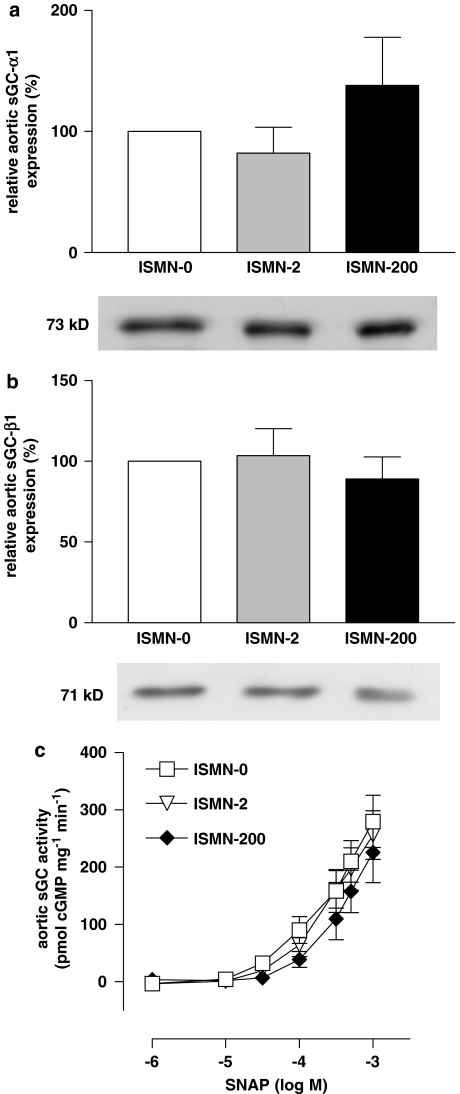
Treatment with ISMN resulted in plasma ISMN concentrations of 6.2±1.9 ng mL−1 (n=10) in ISMN-2 and 1.77±0.469 μg mL−1 (n=9) in ISMN-200. Densitometric analyses of the α1 subunit revealed that its expression in the ISMN-2 and ISMN-200 groups (each n=10) was not different from that in the ISMN-0 group (Figure 1a). Expression of β1 in the ISMN-2 and ISMN-200 groups also showed no difference from that in the ISMN-0 group (Figure 1b). In addition, we measured sGC protein expression using actin protein as a standard. These data showed protein levels of 77.4±21.0% (n=10) in ISMN-2 and 97.5±18.9% (n=8) in ISMN-200 for α1 (P=0.1598, ANOVA) and 105.1±17.9% (ISMN-2) or 136.7±26.9% (ISMN-200) for β1 (P=0.3072, each n=10, ANOVA). Thus, the method of standardization had no influence on the main result.
Figure 1.
Effect of isosorbide mononitrate (ISMN) treatment on soluble guanylyl cyclase (sGC) in rabbits. Protein expression of (a) sGC α1 subunit (P=0.2790) and (b) sGC β1 subunit in aortae of ISMN-treated rabbits measured by western blot densitometry. Neither the low-dose (ISMN-2) nor the high-dose (ISMN-200, each n=10, P=0.6900, analysis of variance (ANOVA)) group showed a significant change in protein expression. (c) Activity of sGC protein measured in pmol cGMP per mg protein per min in ISMN-treated rabbits. There was no significant difference among all three groups (n=6–7, P=0.0775, ANOVA). Results shown represent mean±s.e.m.
Measurement of sGC activity in response to increasing concentrations of SNAP (1μM–1 mM) in aortic cytosols showed comparable pD2 values (half-maximal effective concentrations in −logM) in ISMN-2 (3.41±0.24, n=6), ISMN-200 (3.05±0.39, n=7) and ISMN-0 (3.39±0.26, n=6, P=0.6524, ANOVA). Likewise, the dose–response curves showed no significant differences between all three groups, as determined by two-way ANOVA (Figure 1c).
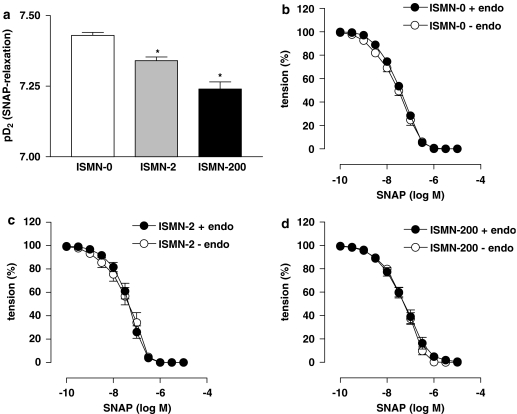
The dose–response curves for SNAP-induced vasorelaxation in rabbit aortic rings were slightly shifted to the right in the ISMN-2 and ISMN-200 groups. However, the shift was very small and the maximal response to SNAP was not changed. The corresponding pD2 values were significantly less in the treated groups compared to the untreated group (Figure 2a). Measurements after removal of the endothelium did not significantly change this SNAP response pattern or pD2 values for ISMN-0 (Figure 2b), ISMN-2 (Figure 2c) and ISMN-200 (Figure 2d). Again, significant differences occurred between treatment groups and ISMN-0 (P=0.0206, ANOVA) but not between ISMN-2 and ISMN-200.
Figure 2.
Vasorelaxation experiments with rabbit aorta. (a) The nitric oxide (NO)-sensitivity of aortic rings progressively declined with increasing isosorbide mononitrate (ISMN) dosage (n=9–10, P=0.0161, analysis of variance (ANOVA)). (b–d) Removal of the endothelium (–endo) in all three groups did not change the responses to S-nitroso-N-acetyl-D,L-penicillamine (SNAP) in any of the groups (P=0.1999 for ISMN-0, P=0.3706 for ISMN-2 and P=1.000 for ISMN-200, n=9–10, t-tests). All values represent mean±s.e.m.
PETN-treated mice
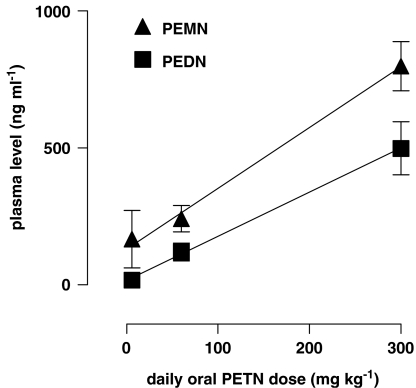
The plasma concentrations of the PETN dinitrate (PEDN) and mononitrate (PEMN) metabolites, which were detectable by GC/MS, increased with the daily oral dose of PETN. The concentrations of both metabolites increased in a directly proportional way (Figure 3).
Figure 3.
Plasma levels of the pentaerythrityl tetranitrate (PETN) metabolites PETN dinitrate (PEDN) and mononitrate (PEMN) detected in PETN-treated mice. The concentrations of both metabolites increased significantly with increased PETN dose in a directly proportional manner; r2 is 0.5606 for PEDN (significant deviation from 0, P=0.0013) and 0.7374 for PEMN (P<0.0001, n=7). All values represent mean±s.e.m.
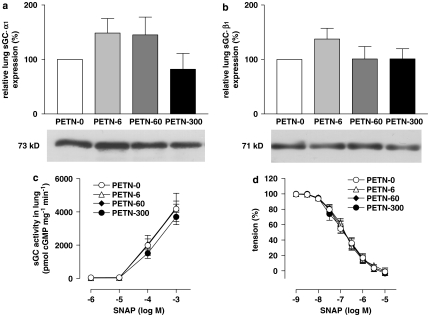
Western blot analyses of the sGC α1 subunit standardized by total protein revealed that its expression in the lungs of PETN-treated mice did not differ significantly from that in the untreated (PETN-0) group (Figure 4a). Likewise, expression of the β1 subunit showed no significant difference between the groups (Figure 4b). Additional western blots for β1 in aortic tissues showed an aortic expression of 109.8±25.8% in PETN-6 and 144.9±25.3% in PETN-60 group (n=5, P=0.3135, ANOVA).
Figure 4.
Effects of pentaerythrityl tetranitrate (PETN) treatment on soluble guanylyl cyclase (sGC) in mice. Protein expression of (a) sGC α1 subunit (P=0.0961, n=8) and (b) sGC β1 subunit (P=0.3709, n=8, analysis of variance (ANOVA)) in lungs of PETN-treated mice measured by western blot densitometry. None of the groups treated with different doses of PETN showed a significant change in protein expression. (c) Activity of sGC protein measured in pmol cGMP per mg protein per min in PETN-treated mice. There was no significant difference among all four groups (P=0.6348, n=7, two-way ANOVA). (d) NO-dependent vasorelaxation in aortic rings of PETN-treated mice induced by the NO-donor S-nitroso-N-acetyl-D,L-penicillamine (SNAP). Again, there was no significant difference between the groups (P=0.8865, two-way ANOVA). All values represent mean±s.e.m.
Phosphorylation of VASP at serine 239 relating to total VASP showed no difference induced by PETN treatment, either. In lung tissue, phosphorylation was 103±47.4% (PETN-6, n=6) and 121±34.0% (PETN-60, n=5, P=0.8996, ANOVA) compared to PETN-0 (n=6), in hearts 112.3±32.0% (PETN-6, n=7) and 99.7±42.8% (PETN-60, n=7, P=0.9460, ANOVA).
The maximal activities of sGC in response to 1 mM SNAP in lung cytosols (in pmol cGMP per mg protein per min) did not differ significantly between the groups (Figure 4c). Likewise, the pD2 values (in −logM) for SNAP in PETN-6 (3.84±0.07), PETN-60 (3.89±0.15) and PETN-300 (3.73±0.14) were not significantly different from PETN-0 (3.86±0.31, each n=7, P=0.9412, ANOVA), as well as the dose–response curves (P=0.6348, two-way ANOVA).
The NO-dependent vasorelaxation was determined in organ bath experiments using the NO-donor SNAP in concentrations from 1 nM up to 10 mM. The dose–response patterns did not differ between the groups (Figure 4d), and the half-maximal concentrations of SNAP in this experiment (−logM) were similar in all four groups (PETN-0: 6.85±0.08, PETN-6: 6.82±0.06, PETN-60: 6.81±0.07 and PETN-300: 6.84±0.09, P=0.7217, ANOVA).
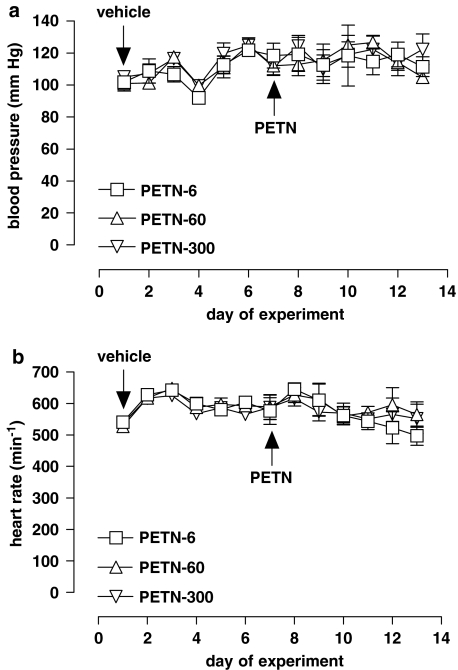
PETN had no effect on systolic blood pressure, either on the first day (acute effect) or on days 5–7 of treatment (chronic effect) in all three groups as compared to pretreatment values (Figure 5a). Measurements of heart rate in the three PETN-treated groups also showed no significant differences between the groups and from the pretreatment values (Figure 5b).
Figure 5.
Effects of pentaerythrityl tetranitrate (PETN) treatment on blood pressure and heart rate. (a) Blood pressure measurements before and during PETN treatment of C57BL/6 mice. On day 7, vehicle food was replaced by diets enriched with 6, 60 or 300 mg kg−1 per day of PETN. None of these doses showed a significant acute or chronic effect on the blood pressure compared to pretreatment measurements (P=0.5314, n=4, two-way analysis of variance (ANOVA)). (b) Heart rate of PETN-treated mice before and during treatment. The different PETN doses had no significant effect on the heart rate compared to pretreatment measurements (P=0.4416, n=4, two-way ANOVA). All values represent mean±s.e.m.
Discussion and conclusions
The aim of this study was to determine the influence of pharmacological nitrate treatment on the expression and function of vascular sGC in vivo. Our main finding is that none of the conditions changed the protein expression of sGC α1 and β1 subunits and sGC activity. In addition, the functional efficacy of the vascular NO–cGMP pathway was maintained in all experimental models used. These data suggest that oral therapy with long-acting nitrates does not impair the vascular NO–cGMP pathway.
Studies in cultured rat aortic smooth muscle cells have provided evidence for an NO-dependent downregulation of sGC protein expression, suggesting a negative feedback loop where NO acts as a signalling molecule regulating sGC expression (Filippov et al., 1997). In another study, rat pulmonary artery smooth muscle cells responded to treatment with lipopolysaccharide, which is known to induce the expression of inducible NOS, with a downregulation of sGC mRNA levels (Scott and Nakayama, 1998). In eNOS-transfected cells showing a strong western blot signal for eNOS, we found an approximately fourfold downregulation of sGC mRNA expression and a reduction of sGC activity (Schmidt et al., 2001). Likewise, incubation of rat aortic rings with the NO-donor SPER/NO at NO generation levels of approximately 2 μmol L−1 min−1 resulted in both, a reduction of activity and of protein expression (Weber et al., 2001). Therefore, it appears that our in vivo findings contradict the results obtained in cultured cells (Filippov et al., 1997; Scott and Nakayama, 1998) and isolated rat aortic rings (Weber et al., 2001).
In contrast, our data are consistent with reports that failed to show the existence of an NO-dependent feedback loop controlling sGC expression in vivo. Mice overexpressing eNOS in the vasculature show a resistance to endothelium-dependent and NO-induced vasodilatation but not a decrease of sGC expression (Yamashita et al., 2000). Instead, the authors describe a 50% reduction of basal unstimulated sGC activity and a 20% reduction of PKG expression. However, other groups have found that changes of PKG activity do not occur in eNOS knockout mice and that there is no change of sGC protein expression in this animal model (Hussain et al., 1999; Brandes et al., 2000). Inhibition of NOS by chronic treatment of mice with the NOS inhibitor L-NAME has been shown to have no effect on vascular sGC expression but potentiate the aortic cGMP response to the NO-donor sodium nitroprusside (Mullershausen et al., 2003).
There is some debate as to whether NO is indeed the pharmacologically active principle of organic nitrates (Kleschyov et al., 2003), although spin trap-based NO analyses in rabbits have demonstrated vascular NO formation from glyceryl trinitrate in both venous and arterial vessels (Mülsch et al., 1995). Detailed investigations identified a novel reductase activity of mitochondrial aldehyde dehydogenase as the most important pathway for glyceryl trinitrate bioactivation, whereas nitrite but no NO was detectable as an intermediate of this reaction (Chen et al., 2002). It has been shown that PETN and its trinitrate metabolite PETriN is effectively metabolized by ALDH-2 (Wenzel et al., 2007), whereas the PEDN and PEMN metabolites as well as ISMN are probably bioactivated by other pathways, for example, in a cytochrome P 450-dependent manner to generate NO or at least an sGC stimulating intermediate compound (McGuire et al., 1998; Minamiyama et al., 1999). These data suggest that vascular generation of such a compound or NO from PEDN, PEMN and ISMN is a prerequisite for the therapeutic efficacy of PETN and ISMN.
The apparent contradiction between the in vitro and in vivo results (Francois and Kojda, 2004) might be caused by various mechanisms, such as the difference between bioactive concentrations of vascular NO, the time of exposure and possibly associated compensatory changes of the NO–cGMP pathway. In this study, increasing doses of the NO-donor ISMN in rabbits causing a 280-fold increase of maximal ISMN plasma concentrations in ISMN-200 compared to ISMN-2 and a moderate nitrate tolerance (Muller et al., 2003) had no effect on sGC activity and protein expression, whereas the NO sensitivity of aortic rings progressively declined with increasing ISMN dosage. Although, the overall effect on SNAP-induced vasodilatation was very small. These data suggest that the bioactive concentration of vascular NO even in the ISMN-200 group did not reach a concentration threshold necessary to induce inhibition of sGC expression.
To confirm these results and to avoid species- or treatment-specific artefacts, we repeated our experiments using a different species (mice) and various doses of a different nitrate (PETN). Although plasma concentrations of PEDN and PEMN were detectable in a dose-dependent manner, PETN treatment did not have an effect on sGC activity, sGC expression, NO-dependent vasorelaxation and blood pressure. However, our VASP measurements in the lung and in the heart showed no difference as well, although the plasma concentrations of PEDN and PEMN at 60 mg kg−1 per day of PETN largely exceed any therapeutic plasma concentration. So far, there is no other report available showing blood pressure reduction in mice receiving long-term treatment with PETN and just one paper describes a blood pressure drop 3 h after oral bolus application of 20 mg kg−1 of ISMN, a dose exceeding the human dose by approximately 80-fold (Momi et al., 2007), whereas another newly developed nitrate had no effect. Of the several possible explanations for the lack of blood pressure reduction in response to PETN, physiological adaptation to any initial arterial vasodilator effect appears to be the most likely. Furthermore, PETN has been shown to dilate venous vessels predominantly (Mullenheim et al., 2001). The supine position of the animals may blunt the blood pressure response as well.
Nevertheless, our data suggest that the bioactive concentration of vascular NO even in the PETN-300 and ISMN-200 groups did not reach a concentration threshold necessary to induce a negative feedback signalling on sGC expression that has been described to occur in vitro. One explanation for this phenomenon might be that vascular metabolism of organic nitrates to NO is decreased by NO itself (Kojda et al., 1998b). Using 14C-glyceryl trinitrate, we have previously provided data to support this hypothesis.
In another study, chronic glyceryl trinitrate treatment induced severe nitrate tolerance and elicited a small upregulation of sGC that could be related to a negative feedback signalling between NO and sGC expression (Mulsch et al., 2001), but the results of this study and those previously obtained in vivo (Laber et al., 2002) and in vitro (Weber et al., 2001) rather suggest that this effect was mediated by the increase of vascular superoxide production associated with nitrate tolerance. Taken together, these results suggest that treatment with NO donors does not increase vascular bioavailable NO enough to induce a negative feedback signalling on sGC expression.
Previous in vitro observations in cultured smooth muscle cells expressing either sGC or PKG showed that transfection of PKG-deficient smooth muscle cells with the catalytic domain of PKG-I reduced protein expression of the β1 subunit, whereas transfection of sGC-deficient smooth muscle cells with both α1 and β1 subunits reduced protein expression of PKG after a 48 h treatment with the NO-donor 2,2′-(hydroxynitrosohydrazono)-bis-ethanimine (DETA/NO) (Browner et al., 2004). The effect of NO on the expression of sGC appears to involve a post-transcriptional regulation (Filippov et al., 1997), which might be caused by decreased binding of the sGC-mRNA-stabilizing family protein HuR (Kloss et al., 2003). In view of our in vivo results we suggest such inhibition by NO of sGC expression occurs at concentrations of NO exceeding normal in vivo concentrations and which are not even achievable with extremely high nitrate doses.
Supplementary Material
Acknowledgments
We thank Dr Dirk Stalleicken (Actavis Deutschland GmbH and Co. KG, Langenfeld, Germany) for providing PETN and Dr Gudrun Walosek (Schwarz Pharma AG, Monheim, Germany) for providing ISMN.
This study was supported by the Forschungskommission of the Heinrich-Heine-Universität Düsseldorf (projects 9772 109 and 9772 272 to GK and project 9772 345 to TS).
Abbreviations
- eNOS
endothelial nitric oxide synthase
- ISMN
isosorbide mononitrate
- NO
nitric oxide
- PETN
pentaerythrityl tetranitrate
- sGC
soluble guanylyl cyclase
- SNAP
S-nitroso-N-acetyl-D,L-penicillamine
- VASP
vasodilator-stimulated phosphoprotein
Conflict of interest
GK received an unrestricted small educational grant from Actavis GmbH, Germany.
Supplementary Information accompanies the paper on British Journal of Pharmacology website (http://www.nature.com/bjp)
References
- Alexander SP, Mathie A, Peters JA.Guide to Receptors and Channels (GRAC) Br J Pharmacol 2008153Suppl 2S1–S209.3rd edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauersachs J, Bouloumié A, Mülsch A, Wiener G, Fleming I, Busse R. Vasodilator dysfunction in aged spontaneously hypertensive rats: changes in NO sythase III and soluble guanylyl cyclase expression, and in superoxide anion production. Cardiovasc Res. 1998;37:772–779. doi: 10.1016/s0008-6363(97)00250-2. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Kim DY, Schmitz-Winnenthal FH, Amidi M, Gödecke A, Mülsch A, et al. Increased nitrovasodilator sensitivity in endothelial nitric oxide synthase knockout mice—role of soluble guanylyl cyclase. Hypertension. 2000;35:231–236. doi: 10.1161/01.hyp.35.1.231. [DOI] [PubMed] [Google Scholar]
- Browner NC, Dey NB, Bloch KD, Lincoln TM. Regulation of cGMP-dependent protein kinase expression by soluble guanylyl cyclase in vascular smooth muscle cells. J Biol Chem. 2004;279:46631–46636. doi: 10.1074/jbc.M408518200. [DOI] [PubMed] [Google Scholar]
- Buechler WA, Nakane M, Murad F. Expression of soluble guanylate cyclase activity requires both enzyme subunits. Biochem Biophys Res Commun. 1991;174:351–357. doi: 10.1016/0006-291x(91)90527-e. [DOI] [PubMed] [Google Scholar]
- Chen LH, Daum G, Fischer JW, Hawkins S, Bochaton-Piallat ML, Gabbiani G, et al. Loss of expression of the β subunit of soluble guanylyl cyclase prevents nitric oxide-mediated inhibition of DNA synthesis in smooth muscle cells of old rats. Circ Res. 2000;86:520–525. doi: 10.1161/01.res.86.5.520. [DOI] [PubMed] [Google Scholar]
- Chen Z, Zhang J, Stamler JS. Identification of the enzymatic mechanism of nitroglycerin bioactivation. Proc Natl Acad Sci USA. 2002;99:8306–8311. doi: 10.1073/pnas.122225199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filippov G, Bloch DB, Bloch KD. Nitric oxide decreases stability of mRNAs encoding soluble guanylate cyclase subunits in rat pulmonary artery smooth muscle cells. J Clin Invest. 1997;100:942–948. doi: 10.1172/JCI119610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francois M, Kojda G. Effect of hypercholesterolemia and of oxidative stress on the nitric oxide–cGMP pathway. Neurochem Int. 2004;45:955–961. doi: 10.1016/j.neuint.2004.03.026. [DOI] [PubMed] [Google Scholar]
- Friebe A, Koesling D. Regulation of nitric oxide-sensitive guanylyl cyclase. Circ Res. 2003;93:96–105. doi: 10.1161/01.RES.0000082524.34487.31. [DOI] [PubMed] [Google Scholar]
- Gewaltig MT, Kojda G. Vasoprotection by nitric oxide: mechanisms and therapeutic potential. Cardiovasc Res. 2002;55:250–260. doi: 10.1016/s0008-6363(02)00327-9. [DOI] [PubMed] [Google Scholar]
- Hussain MB, Hobbs AJ, MacAllister RJ. Autoregulation of nitric oxide-soluble guanylate cyclase-cyclic GMP signalling in mouse thoracic aorta. Br J Pharmacol. 1999;128:1082–1088. doi: 10.1038/sj.bjp.0702874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ignarro LJ, Cirino G, Casini A, Napoli C. Nitric oxide as a signaling molecule in the vascular system: an overview. J Cardiovasc Pharmacol. 1999;34:879–886. doi: 10.1097/00005344-199912000-00016. [DOI] [PubMed] [Google Scholar]
- Kleschyov AL, Oelze M, Daiber A, Huang Y, Mollnau H, Schulz E, et al. Does nitric oxide mediate the vasodilator activity of nitroglycerin. Circ Res. 2003;93:e104–e112. doi: 10.1161/01.RES.0000100067.62876.50. [DOI] [PubMed] [Google Scholar]
- Kloss S, Bouloumie A, Mulsch A. Aging and chronic hypertension decrease expression of rat aortic soluble guanylyl cyclase. Hypertension. 2000;35:43–47. [PubMed] [Google Scholar]
- Kloss S, Furneaux H, Mulsch A. Post-transcriptional regulation of soluble guanylyl cyclase expression in rat aorta. J Biol Chem. 2003;278:2377–2383. doi: 10.1074/jbc.M206453200. [DOI] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Hacker A, Noack E. Alterations of the vascular and the myocardial guanylate cyclase/cGMP-system induced by long-term hypertension in rats. Pharm Acta Helv. 1998a;73:27–35. doi: 10.1016/s0031-6865(97)00044-7. [DOI] [PubMed] [Google Scholar]
- Kojda G, Kottenberg K, Nix P, Schlüter KD, Piper HM, Noack E. Low increase in cGMP induced by organic nitrates and nitrovasodilators improves contractile response of rat ventricular myocytes. Circ Res. 1996;78:91–101. doi: 10.1161/01.res.78.1.91. [DOI] [PubMed] [Google Scholar]
- Kojda G, Laursen JB, Ramasamy S, Kent JD, Kurz S, Burchfield J, et al. Protein expression, vascular reactivity and soluble guanylate cyclase activity in mice lacking the endothelial nitric oxide synthase: contributions of NOS isoforms to blood pressure and heart rate control. Cardiovasc Res. 1999;42:206–213. doi: 10.1016/s0008-6363(98)00315-0. [DOI] [PubMed] [Google Scholar]
- Kojda G, Patzner M, Hacker A, Noack E. Nitric oxide inhibits vascular bioactivation of glyceryl trinitrate. A novel mechanism to explain preferential venodilation of organic nitrates. Mol Pharmacol. 1998b;53:547–554. doi: 10.1124/mol.53.3.547. [DOI] [PubMed] [Google Scholar]
- Kojda G, Stein D, Kottenberg E, Schnaith EM, Noack E. In vivo effects of pentaerythrityl-tetranitrate and isosorbide-5-mononitrate on the development of atherosclerosis and endothelial dysfunction in cholesterol-fed rabbits. J Cardiovasc Pharmacol. 1995;25:763–773. doi: 10.1097/00005344-199505000-00012. [DOI] [PubMed] [Google Scholar]
- Krumenacker JS, Hyder SM, Murad F. Estradiol rapidly inhibits soluble guanylyl cyclase expression in rat uterus. Proc Natl Acad Sci USA. 2001;98:717–722. doi: 10.1073/pnas.98.2.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laber U, Kober T, Schmitz V, Schrammel A, Meyer W, Mayer B, et al. Effect of hypercholesterolemia on expression and function of vascular soluble guanylyl cyclase. Circulation. 2002;105:855–860. doi: 10.1161/hc0702.103975. [DOI] [PubMed] [Google Scholar]
- McGuire JJ, Anderson DJ, McDonald BJ, Narayanasami R, Bennett BM. Inhibition of NADPH-cytochrome P450 reductase and glyceryl trinitrate biotransformation by diphenyleneiodonium sulfate. Biochem Pharmacol. 1998;56:881–893. doi: 10.1016/s0006-2952(98)00216-0. [DOI] [PubMed] [Google Scholar]
- Melichar VO, Behr-Roussel D, Zabel U, Uttenthal LO, Rodrigo J, Rupin A, et al. Reduced cGMP signaling associated with neointimal proliferation and vascular dysfunction in late-stage atherosclerosis. Proc Natl Acad Sci USA. 2004;101:16671–16676. doi: 10.1073/pnas.0405509101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamiyama Y, Takemura S, Akiyama T, Imaoka S, Inoue M, Funae Y, et al. Isoforms of cytochrome P450 on organic nitrate-derived nitric oxide release in human heart vessels. FEBS Lett. 1999;452:165–169. doi: 10.1016/s0014-5793(99)00612-2. [DOI] [PubMed] [Google Scholar]
- Momi S, Impagnatiello F, Guzzetta M, Caracchini R, Guglielmini G, Olivieri R, et al. NCX 6560, a nitric oxide-releasing derivative of atorvastatin, inhibits cholesterol biosynthesis and shows anti-inflammatory and anti-thrombotic properties. Eur J Pharmacol. 2007;570:115–124. doi: 10.1016/j.ejphar.2007.05.014. [DOI] [PubMed] [Google Scholar]
- Moncada S, Higgs A. Mechanisms of disease: the L-arginine-nitric oxide pathway. N Engl J Med. 1993;329:2002–2012. doi: 10.1056/NEJM199312303292706. [DOI] [PubMed] [Google Scholar]
- Mullenheim J, Muller S, Laber U, Thamer V, Meyer W, Bassenge E, et al. The effect of high-dose pentaerythritol tetranitrate on the development of nitrate tolerance in rabbits. Naunyn Schmiedebergs Arch Pharmacol. 2001;364:269–275. doi: 10.1007/s002100100464. [DOI] [PubMed] [Google Scholar]
- Muller S, Konig I, Meyer W, Kojda G. Inhibition of vascular oxidative stress in hypercholesterolemia by eccentric isosorbide mononitrate. J Am Coll Cardiol. 2004;44:624–631. doi: 10.1016/j.jacc.2004.02.062. [DOI] [PubMed] [Google Scholar]
- Muller S, Laber U, Mullenheim J, Meyer W, Kojda G. Preserved endothelial function after long-term eccentric isosorbide mononitrate despite moderate nitrate tolerance. J Am Coll Cardiol. 2003;41:1994–2000. doi: 10.1016/s0735-1097(03)00392-9. [DOI] [PubMed] [Google Scholar]
- Mullershausen F, Russwurm M, Koesling D, Friebe A. The enhanced NO-induced cGMP response induced by long-term L-NAME treatment is not due to enhanced expression of NO-sensitive guanylyl cyclase. Vascul Pharmacol. 2003;40:161–165. doi: 10.1016/s1537-1891(03)00049-1. [DOI] [PubMed] [Google Scholar]
- Mülsch A, Mordvintcev P, Bassenge E, Jung F, Clement B, Busse R. In vivo spin trapping of glyceryl trinitrate-derived nitric oxide in rabbit blood vessels and organs. Circulation. 1995;92:1876–1882. doi: 10.1161/01.cir.92.7.1876. [DOI] [PubMed] [Google Scholar]
- Mulsch A, Oelze M, Kloss S, Mollnau H, Topfer A, Smolenski A, et al. Effects of in vivo nitroglycerin treatment on activity and expression of the guanylyl cyclase and cGMP-dependent protein kinase and their downstream target vasodilator-stimulated phosphoprotein in aorta. Circulation. 2001;103:2188–2194. doi: 10.1161/01.cir.103.17.2188. [DOI] [PubMed] [Google Scholar]
- Papapetropoulos A, Abou-Mohamed G, Marczin N, Murad F, Caldwell RW, Catravas JD. Downregulation of nitrovasodilator-induced cyclic GMP accumulation in cells exposed to endotoxin or interleukin-1β. Br J Pharmacol. 1996;118:1359–1366. doi: 10.1111/j.1476-5381.1996.tb15545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruetten H, Zabel U, Linz W, Schmidt HHHW. Downregulation of soluble guanylyl cyclase in young and aging spontaneously hypertensive rats. Circ Res. 1999;85:534–541. doi: 10.1161/01.res.85.6.534. [DOI] [PubMed] [Google Scholar]
- Russwurm M, Koesling D. NO activation of guanylyl cyclase. EMBO J. 2004;23:4443–4450. doi: 10.1038/sj.emboj.7600422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Andrew P, Schrammel A, Groschner K, Schmitz V, Kojda G, et al. Comparison of neuronal and endothelial isoforms of nitric oxide synthase in stably transfected HEK 293 cells. Am J Physiol Heart Circ Physiol. 2001;281:H2053–H2061. doi: 10.1152/ajpheart.2001.281.5.H2053. [DOI] [PubMed] [Google Scholar]
- Schultz G, Böhme E.Guanylate Cyclase Methods of Enzymatic Analysis 1984Verlag Chemie: Weinheim, FRG; 379–389.In: Bergmeyer HU (ed). [Google Scholar]
- Scott WS, Nakayama DK. Escherichia coli lipopolysaccharide downregulates soluble guanylate cyclase in pulmonary artery smooth muscle. J Surg Res. 1998;80:309–314. doi: 10.1006/jsre.1998.5442. [DOI] [PubMed] [Google Scholar]
- Shimouchi A, Janssens SP, Bloch DB, Zapol WM, Bloch KD. cAMP regulates soluble guanylate cyclase β1-subunit gene expression in RFL-6 rat fetal lung fibroblasts. Am J Physiol Lung Cell Mol Physiol. 1993;265:L456–L461. doi: 10.1152/ajplung.1993.265.5.L456. [DOI] [PubMed] [Google Scholar]
- Takata M, Filippov G, Liu H, Ichinose F, Janssens S, Bloch DB, et al. Cytokines decrease sGC in pulmonary artery smooth muscle cells via NO-dependent and NO-independent mechanisms. Am J Physiol Lung Cell Mol Physiol. 2001;280:L272–L278. doi: 10.1152/ajplung.2001.280.2.L272. [DOI] [PubMed] [Google Scholar]
- Weber M, Lauer N, Mulsch A, Kojda G. The effect of peroxynitrite on the catalytic activity of soluble guanylyl cyclase. Free Radic Biol Med. 2001;31:1360–1367. doi: 10.1016/s0891-5849(01)00706-7. [DOI] [PubMed] [Google Scholar]
- Wenzel P, Hink U, Oelze M, Seeling A, Isse T, Bruns K, et al. Number of nitrate groups determines reactivity and potency of organic nitrates: a proof of concept study in ALDH-2−/− mice. Br J Pharmacol. 2007;150:526–533. doi: 10.1038/sj.bjp.0707116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T, Kawashima S, Ohashi Y, Ozaki M, Rikitake Y, Inoue N, et al. Mechanisms of reduced nitric oxide/cGMP-mediated vasorelaxation in transgenic mice overexpressing endothelial nitric oxide synthase. Hypertension. 2000;36:97–102. doi: 10.1161/01.hyp.36.1.97. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.