Abstract
Background and purpose:
Micturition is controlled by central 5-HT-containing pathways. 5-HT2 receptors have been implicated in this system especially in control of the urethra, which is a drug target for treating urinary incontinence. This study investigates the role of each of the three subtypes of this receptor with emphasis on sphincter regulation.
Experimental approach:
Recordings of urethral and bladder pressure, external urethral sphincter (EUS) EMG, as well as the micturition reflex induced by bladder distension along with blood pressure and heart rate were made in anaesthetized rats. The effects of agonists and antagonists for 5-HT2 receptor subtypes were studied on these variables.
Key results:
The 5-HT2C agonists Ro 60-0175, WAY 161503 and mCPP, i.v., activated the EUS, increased urethral pressure and inhibited the micturition reflex. The effects of Ro 60-0175 on the EUS were blocked by the 5-HT2C antagonist SB 242084 and the 5-HT2A antagonists, ketanserin and MDL 100907. SB 242084 also blocked the inhibitory action on the reflex, while the 5-HT2B antagonist RS 127445 only blocked the increase in urethral pressure. The 5-HT2A receptor agonist DOI given i.v. or i.t. but not i.c.v. activated the EUS.
Conclusions and implications:
5-HT2A/2C receptors located in the sacral spinal cord activate the EUS, while central 5-HT2C receptors inhibit the micturition reflex and 5-HT2B receptors, probably at the level of the urethra, increase urethral smooth muscle tone. Furthermore, 5-HT2B and 5-HT2C receptors do not seem to play an important role in the physiological regulation of micturition.
Keywords: external urethral sphincter, bladder, micturition, blood pressure, MAP, mean arterial pressure, 5-HT2A receptors, 5-HT2B receptors, 5-HT2C receptors, Ro 60-0175, mCPP, DOI
Introduction
Central 5-HT-containing pathways are now known to play an important role in the control of micturition (see Ramage, 2006). The major receptor subtypes that have been identified to play a role in this pathway are 5-HT1A receptors (Lecci et al., 1992; Testa et al., 1999; Conley et al., 2001) at both the supra-spinal and spinal levels (Kakizaki et al., 2001; Secker et al., 2003; Yoshiyama et al., 2003) and 5-HT7 receptors, that act only at a supra-spinal level (Read et al., 2003). Interestingly, the data from these experiments would imply that 5-HT-containing neurones play an excitatory role in the control of micturition. This contradicts the established view that 5-HT pathways are inhibitory. To deal with this contradiction a 5-HT2C receptor pathway has been proposed, which excites inhibitory interneurones controlling the parasympathetic preganglionic neurones to the bladder (see De Groat, 2002). In this scenario, activation of 5-HT2C receptors would cause inhibition of the micturition reflex as reported by Steers and De Groat (1989) using the archetypical 5-HT2C receptor agonist mCPP, although mCPP has poor selectivity between the 5-HT2 receptor subtypes (see Barnes and Sharp, 1999; Alexander et al., 2007). Further, in the paper by Steers and De Groat (1989) it was also suggested that 5-HT2C receptors activate ‘somatic muscle contraction' that is, the external urethral sphincter (EUS). In this respect, in the cat, 5-HT2 receptors have been implicated in the control of the EUS (Danuser and Thor, 1996). Thus, the present experiments were carried out to investigate the role of 5-HT2 receptor subtypes in the control of the urethra and the regulation of micturition in anaesthetized female rats. Preliminary accounts of some of these data have been previously published (Mbaki et al., 2005, 2006).
Methods
The experiments were carried out under the Animals (scientific procedures) Act, 1986. After the completion of experiments, animals were killed by an overdose of sodium pentobarbital i.v.
General preparation
Experiments were performed on 110 anaesthetized female Sprague–Dawley rats (200–300 g). Anaesthesia was induced and maintained during initial surgery with isoflurane (4 in 100% oxygen, which was reduced to 3 in 100% oxygen). The left jugular vein was cannulated for anaesthetic and drug administration. The trachea was cannulated to maintain a patent airway. Isoflurane administration was then stopped and anaesthesia was maintained for the duration of the experiment with intravenous administration of urethane (1.2 g kg−1, i.v.). Supplementary doses of urethane (0.1 g kg−1, i.v.) were given as required. The right common carotid artery was cannulated with a heparinized cannula (20 U mL−1 heparin in 0.9% w v−1 saline) for the measurement of arterial blood pressure and sampling arterial blood gases for analysis. Blood pressure was measured using a pressure transducer (Gould Statham P23XL), and the heart rate (HR) derived electronically on-line from the blood pressure signal using AcqKnowledge version 3.7.3 software (Biopac Systems Inc., USA). The depth of anaesthesia was assessed by the stability of blood pressure and heart rate, and by an absence of hind limb withdrawal in response to paw pinch. Animals were allowed to spontaneously breathe oxygen-enriched air supplied by the use of a positive pressure pump (Harvard Rodent Ventilator 683). Body temperature was monitored by placing the rectal temperature probe underneath the animal and was maintained at 37–38 °C using a homeothermic blanket system (Harvard). Blood samples were taken from the carotid arterial cannula and blood gases and pH were monitored with a Corning pH/blood gas analyser (Model 238). Blood gases were maintained between 90–130 mm Hg PO2, 40–50 mm Hg PCO2 and pH 7.3–7.4. Adjustments of the supplemented oxygen levels were made necessary to maintain blood gas and pH balance. The animals were infused (6 mL kg−1 h−1, i.v.) with a solution comprising 10 mL plasma substitute (gelofusine), 10 mL distilled water, 0.04 g glucose and 0.168 g sodium bicarbonate, to prevent the development of non-respiratory acidosis and to maintain blood volume. The rats were placed in a stereotaxic frame and the head tilted approximately 10–15° to allow the animal to lie in a supine position to prevent the weight of the animal affecting the bladder and urethral pressure recordings.
Measurement of bladder and urethral pressures
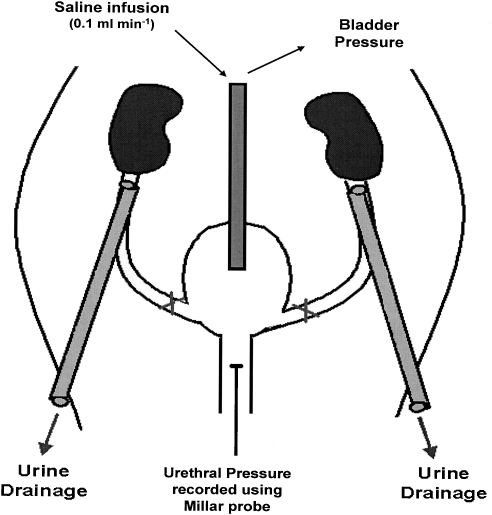
The ureters were exposed by retroperitoneal incisions and the proximal end of each ureter leading from the kidney was cannulated to prevent the bladder filling with urine during the experiments (Figure 1). The distal ends of the ureters were tied to ensure no backflow and hence no leakage of saline from the bladder. The urinary bladder was exposed by a midline laparotomy. A small incision was made in the bladder dome and a single-cuffed cannula (0.86 mm internal diameter and 1.52 mm external diameter) was inserted into the bladder dome. The cannula was secured with a suture around the top of the bladder dome and this was followed by closure of the abdominal incision. The cuffed cannula was connected through a T-piece to a pressure transducer (Gould Statham P23XL) to record bladder pressure, and a syringe pump for the infusion of saline (0.9% w v−1) at a rate of 0.1 mL min−1, to evoke the micturition reflex. Backflow through this cannula allowed the bladder to be emptied of residual fluid after micturition had occurred. For measurement of urethral pressure a 1.4 F nylon catheter with a side-mounted microtip transducer located 1 mm from the catheter tip (SPR-671, Millar Instruments Inc., Houston, TX, USA) was inserted into the urethra from the urethral orifice into the mid region of the urethra where high frequency pressure fluctuations were observed during urethral relaxation when the micturition reflex was tested.
Figure 1.
Schematic representation of the experimental method used. The ureters were tied and cut at the level of the bladder and cannulated at the level of the kidneys. A single-cuffed cannula connected through a T-piece to a pressure transducer and a syringe pump was inserted into the bladder dome. Electrodes used to record EUS–EMG activity were inserted on either side of the urethral orifice. The Millar probe was inserted into the urethra from the urethral orifice. Adapted from Wibberley et al. (2002).
External urethral sphincter—EMG recordings
Two fine copper wire electrodes (0.2 mm diameter) were used to measure the EMG of the external urethral sphincter. The tip of an electrode was positioned in the bevel of a needle (25 G) and the needle was inserted percutaneously approximately 0.5 mm lateral and 0.5 mm caudal to the urethral orifice. The needle and wire were advanced approximately 5 mm through the skin and the needle slowly withdrawn leaving the wire inserted in the external urethral sphincter. The second electrode was inserted in the same position on the opposite side of the urethral orifice. The electrodes were connected to a Neurolog head stage (NL100; Digitimer, Welwyn Garden City, UK) and the signal was amplified (NL104) and filtered (500 Hz; NL125, and displayed on an oscilloscope (Tektronix, 2205).
Cannulation of the lateral cerebral ventricle (i.c.v.) and placement of cannula for i.t. injections
A stainless steel guide cannula (22 G) was implanted into the right lateral cerebral ventricle. The coordinates used from bregma were 3.5 mm ventral, 1.5 mm lateral and 1 mm caudal. Drugs and vehicle solutions (5 μL) were administered through an i.c.v. injection cannula (28 G) attached by a length of polythene tubing to a 25 μL syringe (Hamilton). For i.t. administration (Yaksh and Rudy, 1976) the animal was placed on its front and a midline incision was made to expose the vertebral column. Using the last rib as a guide (T13 of the vertebral column), a guide cannula (26 G, 9.5 mm in length; Plastics One Inc.) was inserted at the level between the L1–L2 vertebrae (L6–S1 region of the spinal cord). A tail flick or hind limb reflex established the placement of the guide cannula in the spinal cord. The guide cannula was secured in place using the tissue adhesive Vetbond. The animal was thereafter turned on to its right side to prevent the animal's weight affecting bladder recordings. Owing to the positioning of the animal, the Millar probe was not inserted into the urethral orifice and thus urethral pressure was not recorded for the i.t. experiments. Drugs and vehicle (10 μi) were administered through an internal cannula (33 G) attached through a length of polythene tubing to a 50 μL syringe (Hamilton). The location of the cannula placement for both injection sites was subsequently confirmed by the administration of 5 μL of 2% Pontamine sky blue dye.
Experimental protocols
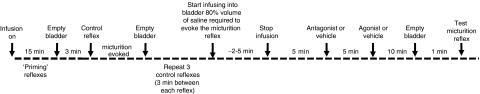
Animals were left for 1 h to stabilize after completion of surgery. A diagram of the main experimental protocol used is shown in Figure 2. The bladder was infused with saline until threshold was reached and the micturition reflex was evoked. Saline was continuously infused for 15 min to ‘prime' the system and cause a series of infusion-induced micturition reflexes. After ‘priming the system', infusion was stopped and the bladder emptied. Three minutes later, saline was infused into the bladder until micturition was evoked, the infusion was discontinued, the bladder was emptied and the residual volume was collected and measured. This was repeated until three consecutive reflex-evoked bladder contractions of similar amplitude were obtained to ensure reproducibility of the response. After a period of 3 min, the bladder was filled slowly with 80% volume of saline required to evoke the micturition reflex and all variables were recorded for 5 min. The test drug or vehicle (saline or 100% DMSO) was administered i.v. and all variables were recorded for a further 5 min before administering the 5-HT2 receptor agonist or vehicle. After 10 min the bladder was emptied and infusion was commenced once more to evoke the micturition reflex (a single micturition) in the presence of test drugs or vehicle. In some experiments, the test drug caused a continuous rise in bladder pressure with no obvious contraction observed. In these cases, after a certain period of time, a few drops of saline leaked from the urethral opening and once this was observed, the infusion was switched off. These responses were taken to indicate that the micturition response had been abolished. In the experiments where drug/vehicle was administered i.c.v. or i.t. the protocol was the same as i.v. but drug agonist/vehicle was given 5 min after the bladder was filled with 80% volume of saline required to evoke the micturition reflex. The variables were then measured for 10 min and then the bladder was emptied and infusion was commenced once more to evoke the micturition reflex (a single micturition).
Figure 2.
Diagram to illustrate the main experimental protocol for the i.v. studies used in the present experiments.
Data capture and analysis
Arterial blood and bladder pressure were continuously displayed on a chart recorder (Grass Instruments) and captured (2000 samples per second) by a MP 100 WSW interface (Biopac Systems Inc., USA) to allow data to be acquired and analysed offline using Acknowledge version 3.7.3 software (Biopac Systems Inc., USA). Heart rate (HR) was derived electronically online from the blood pressure signal using the Biopac system. The amplified raw EMG signal was captured (2000 samples per second) and the input integrated offline using Acknowledge version 3.7.3 software (Biopac Systems Inc., USA). For EUS–EMG activity, the noise levels were verified at the end of the experiment by slowly administering the neuromuscular blocker decamethonium bromide (3 mg kg−1, i.v.) to abolish activity. This was done under artificial respiration and the animal was then killed by an overdose of pentobarbital. Then background noise was determined and subtracted from the measured values. Urethral pressure was captured (500 samples per second) and displayed using the Acknowledge version 3.7.3 software and analysed offline.
Analysis of results
Saline infusion into the bladder evoked large-amplitude bladder contractions with corresponding urethral relaxations and bursts of the EUS–EMG, each of which represents a micturition reflex (Maggi et al., 1986). The following variables were measured; the volume threshold (mL)—defined as the volume of saline required to elicit voiding, the pressure threshold (mm Hg)—the bladder pressure recorded at the start of a micturition contraction, and the residual volume—expressed as a percentage of the total volume infused. The total integrated EUS–EMG signal using the root mean square of the raw EUS–EMG was used for analysis as it produced waveforms that were more easily analysable than the raw EMG signal. Baseline values of the area under the curve of the rectified EUS–EMG signal were analysed over a 1 min period before administration of test drug or vehicle and for 5 min after drug/vehicle administration. The percentage change of the total integrated EUS–EMG signal following deduction of background noise was calculated thereafter. Similarly, the mean baseline variables for urethral pressure, blood pressure and heart rate were also measured over a 1 min period before addition of test drug/vehicle with post-treatment effects measured for 5 min thereafter.
Statistical analysis
Changes in all the above variables were measured after drug administration and all values are expressed as mean±s.e. mean. Drug evoked changes on the micturition reflex were compared with control reflexes (calculated mean of the variables measured for the three control micturition reflexes) using Student's unpaired t-test. Changes in mean integrated EUS–EMG signal, urethral pressure, blood pressure and heart rate caused by the test drugs were analysed using the General Linear Model (SPSS (PC+)) with ‘drug/vehicle group' as the between subjects factor and ‘time' as a within subjects factor in the split-plot analysis. Group differences in the incremental response (with respect to the baseline at T0) were analysed across the cluster of four consecutive time points (2–5 min): that is after the phasic response to drug treatment had stabilized. The data were first tested for homogeneity of sample variance and log 10 transformed, where appropriate, and the Greenhouse—Geisser ‘ɛ‘ correction was applied to compensate for any violation of sphericity of the variance—covariance matrix. A significant effect on the main factor(s), or relevant interactions between them, was the criterion for progressing to post hoc comparisons between specific treatment groups and their respective vehicle control (DMSO or saline) or another treatment group. Group differences (at plateau) were evaluated across the same cluster of data points as before. Statistical significance was set at P⩽0.05.
Drugs and solutions
Drugs and chemicals were obtained from the following sources: WAY 161503 (8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazinol[1,2,-α]quinoxaline-5(6H)-one hydrochloride), Ro 60-0175 ((αS)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate), BW 723C86 (α-methyl-5-(2-thienylmethoxy)-1 H-indole-3-ethanamine), ketanserin and mianserin from Tocris Cookson Ltd, Avonmouth, Bristol, UK; urethane, decamethonium bromide, mCPP (1-(3-Chlorophenyl) piperazine hydrochloride), DOI, (2,5-dimethoxy-4-idophenyl)-2-aminopropane hydrochloride), SB 242084 (6-Chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride) and dimethyl sulphoxide (DMSO) from Sigma Aldrich Chemicals, Poole, Dorset, UK; isoflurane from Abbot Labs, Queenborough, Kent, UK; gelofusine from Braun Medical Ltd, Aylesbury, Bucks, UK; sodium chloride, glucose, sodium bicarbonate from Merck/BDH, Poole, Dorset, UK; heparin from CP Pharmaceuticals Ltd, Wrexham, UK. RS 127445 (2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine) was synthesized by Pfizer Global Research and Development, Sandwich, Kent, UK. BW501C67 (α-anilino-N-2-m-chlorophenoxypropylacetamide) was a gift from Wellcome Research Laboratories, Beckenham, Kent, UK. MDL 100907 (R-(+)-a-(2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol) a gift from Marion Merrell Dow Inc., Cincinnati, OH, USA; sodium pentobarbital (Pentoject) from Animalcare Ltd, York, UK. mCPP, WAY161503, Ro 60-0175, DOI BW723C86 and BW 501C67 were dissolved in 0.9% w v−1 saline. Mianserin, ketanserin, MDL 100907, SB 242084 and RS 127445 were dissolved in 100% DMSO. All i.v. bolus doses were administered in a 0.1 mL volume followed by a flush of 0.1 mL saline. All drugs/vehicles given i.c.v. were administered in a 5 μL volume over a 20 s period whereas for the i.t. route drugs/vehicles were administered in a 10 μL volume over the same time period. All drugs were given as their salts, except MDL 100907.
Results
Controls
Administration of 5-HT2 receptor antagonist vehicle (100% DMSO; 0.1 mL; i.v.; n=5) followed 5 min later by the 5-HT2 receptor agonist vehicle (0.9% w v−1 saline; i.v.; n=5) evoked no significant changes in baseline EUS–EMG signal, urethral pressure and bladder distension caused by infusion of saline at a rate of 0.1 mL min−1 (micturition reflex). Baseline MAP and HR were also unaffected. Additionally, for mCPP experiments the following control was performed; saline followed 5 min later by saline (n=5; 0.1 mL), and again no effects were observed on any of the variables. Furthermore, saline i.c.v. (n=5; 5 μL) and i.t (n=3; 10 μL) had no effect on any of the recorded variables. Table 1 shows the absolute values for all recorded baseline variables and the control reflex. The control reflex values are the mean of three control micturition reflexes.
Table 1.
Baseline values for external urethral sphincter (EUS)-EMG signal, urethral pressure, control reflex evoked changes caused by bladder distension in bladder pressure, volume and residual volume and baseline mean arterial pressure (MAP) and heart rate (HR) for each experimental group in urethane-anaesthetized female rats
| Experimental group | n | EUS-EMG signal (μV) | Urethral pressure (mm Hg) |
Bladder |
MAP (mm Hg) | HR (b.p.m.) | ||
|---|---|---|---|---|---|---|---|---|
| Volume thresholds | Pressure (mm Hg) | Residual Vol (mL) | ||||||
| Saline i.v.+saline i.v. | 5 | 0.26±0.02 | 22±2 | 0.27±0.01 | 9±0.5 | 0.19±0.01 | 125±3 | 329±12 |
| DMSO i.v.+saline i.v. | 5 | 0.26±0.04 | 22±2 | 0.42±0.06 | 9±1 | 0.20±0.06 | 125±3 | 329±12 |
| WAY 161503 300 μg kg−1 i.v. | 5 | 0.26±0.04 | 22±2 | 0.33±0.09 | 8±0.5 | 0.12±0.05 | 119±8 | 336±9 |
| Ro 60-0175 300 μg kg−1 i.v. | 5 | 0.28±0.03 | 22±2 | 0.30±0.06 | 10±1 | 0.15±0.05 | 116±4 | 346±10 |
| mCPP 300 μg kg−1 i.v. | 5 | 0.42±0.10 | 19±2 | 0.29±0.01 | 10±1 | 0.13±0.01 | 140±4 | 363±11 |
| DOI 30 μg kg−1 i.v. | 3 | 0.40±0.06 | 24±2 | 0.38±0.03 | 10±1 | 0.15±0.05 | 121±5 | 369±12 |
| DOI 50 μg kg−1 i.v. | 3 | 0.37±0.07 | 21±1 | 0.31±0.09 | 11±3 | 0.20±0.08 | 111±10 | 377±12 |
| DOI 100 μg kg−1 i.v. | 5 | 0.56±0.12 | 20±2 | 0.31±0.06 | 12±1 | 0.12±0.05 | 121±8 | 337±30 |
| BW723C86 300 μg kg−1 i.v. | 5 | 0.34±0.02 | 23±3 | 0.40±0.06 | 9±1 | 0.10±0.03 | 116±4 | 349±9 |
| SB 242084 30 μg kg−1 i.v.+Ro 60-0175 300 μg kg−1 i.v. | 5 | 0.24±0.11 | 15±2 | 0.30±0.03 | 8±1 | 0.11±0.02 | 107±6 | 396±29 |
| Ketanserin 30 μg kg−1 i.v.+Ro 60-0175 300 μg kg−1 i.v. | 5 | 0.22±0.01 | 20±0.1 | 0.22±0.02 | 8±1 | 0.11±0.01 | 95±0.3 | 390±15 |
| MDL 100907 30 μg kg−1 i.v.+Ro 60-0175 300 μg kg−1 i.v. | 5 | 0.22±0.02 | 15±0.1 | 0.34±0.11 | 8±1 | 0.24±0.10 | 102±2 | 369±18 |
| Ketanserin 30 μg kg−1 i.v.+DOI 30 μg kg−1 i.v. | 5 | 0.34±0.06 | 19±1 | 0.37±0.06 | 8±0.5 | 0.13±0.04 | 137±9 | 335±10 |
| MDL 100907 30 μg kg−1 i.v.+DOI 30 μg kg−1 i.v. | 3 | 0.40±0.06 | 25±0.5 | 0.32±0.10 | 10±1 | 0.20±0.10 | 112±4 | 360±1 |
| BW 100 μg kg−1 i.v.+mCPP 300 μg kg−1 i.v. | 5 | 0.74±0.31 | 19±2 | 0.39±0.05 | 9±0.5 | 0.17±0.02 | 114±5 | 340±13 |
| BW 100 μg kg−1 i.v.+DOI 100 μg kg−1 i.v. | 4 | 0.35±0.06 | 18±2 | 0.27±0.06 | 11±1 | 0.11±0.04 | 108±4 | 319±23 |
| BW 1 mg kg−1 i.v.+DOI 1 mg kg−1 i.v. | 3 | 0.44±0.08 | 24±2 | 0.44±0.15 | 9±1 | 0.32±0.12 | 124±6 | 361±9 |
| Ketanserin 100 μg kg−1 i.v. | 5 | 0.36±0.07 | 20±2 | 0.27±0.02 | 11±1 | 0.13±0.01 | 110±5 | 369±6 |
| MDL 100907 30 μg kg−1 i.v. | 5 | 0.36±0.06 | 24±1 | 0.25±0.03 | 8±1 | 0.10±0.02 | 115±5 | 356±14 |
| BW 1 mg kg−1 i.v. | 5 | 0.46±0.11 | 23±2 | 0.40±0.06 | 8±1 | 0.18±0.05 | 122±3 | 352±10 |
| Saline i.c.v. 5 μL | 5 | 0.32±0.06 | 19±2 | 0.27±0.01 | 9±1 | 0.19±0.01 | 136±5 | 324±31 |
| mCPP 300 μg kg−1 i.c.v. | 3 | 0.50±0.12 | 24±4 | 0.43±0.04 | 8±1 | 0.24±0.11 | 130±2 | 311±6 |
| DOI 100 μg kg−1 i.c.v. | 5 | 0.54±0.12 | 21±2 | 0.33±0.05 | 8±1 | 0.17±0.04 | 116±2 | 358±14 |
| Saline i.t. 10 μL | 3 | 0.30±0.01 | — | 0.22±0.01 | 12±1 | 0.10±0.02 | 108±2 | 337±10 |
| DOI 100 μg kg−1 i.t. | 3 | 0.34±0.07 | — | 0.25±0.01 | 13±1 | 0.11±0.02 | 103±9 | 342±21 |
NB. The peripheral 5-HT receptor antagonist BW 501C67 has been abbreviated in this table to BW.
5-HT2 receptor agonists (Ro 60-0175, WAY 161503, mCPP, DOI and BW723C86)
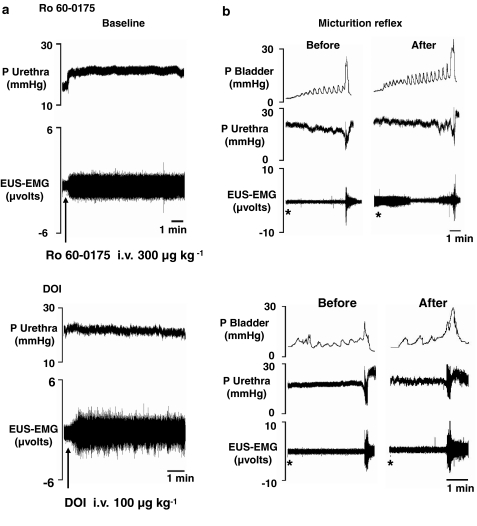
Traces showing the effects of Ro 60-0175 and DOI on baseline urethral pressure and EUS–EMG signal, and distension-induced micturition reflex are shown in Figure 3.
Figure 3.
Traces showing the effects of Ro 60-0175 (300 μg kg−1, i.v.) and DOI (100 μg kg−1, i.v.) on (a) baseline urethral pressure and EUS–EMG activity and (b) on bladder-distension (micturition) induced changes in bladder and urethral pressures and EUS–EMG activity. *Denotes onset of saline infusion into the bladder.
Urethral variables
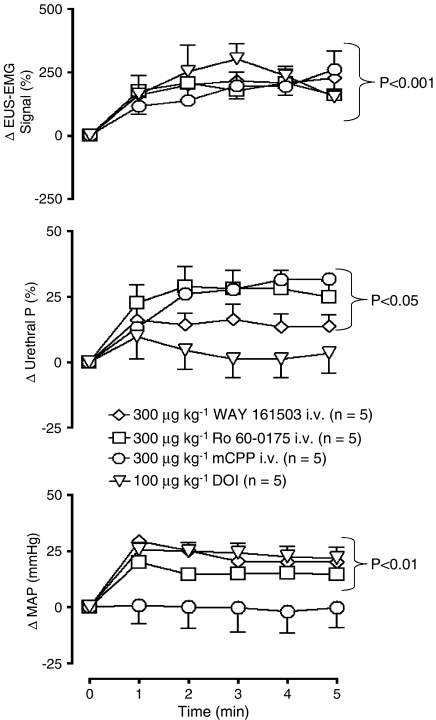
Administration i.v. of 300 μg kg−1 (n=5) of Ro 60-0175, WAY 161503 and mCPP evoked EUS–EMG activity and significantly (P<0.05) increased the EUS–EMG signal by 154±1, 179±17 and 166±17%, respectively. This was associated with a significant increase in urethral pressure reaching a maximum of 23±2, 13±2 and 24±3%, respectively (see Figure 4). For Ro 60-0175 and WAY 161503, but not mCPP this evoked EUS–EMG activity was observed to be ongoing for up to 10 min. When the bladder was emptied to begin testing the micturition reflex, the EUS–EMG firing usually stopped. However, in some experiments, EUS–EMG activity was observed to be continuous while testing the micturition reflex (see Figure 3b, Ro 60-0175 data). For mCPP evoked EUS–EMG activity, the duration was only between 5–8 min. The onset of appearance of EUS–EMG activity for all three agonists was 28±11, 36±13 and 20±5 s, respectively. DOI (30, 50 and 100 μg kg−1, n=3/5) increased baseline EUS–EMG signal activity in a similar manner to the other agonists causing significant increases in the EUS–EMG signal of 139±27, 224±36 and 198±15%, respectively. However, none of the three doses of DOI had any significant effect on urethral pressure (see Figure 4). The onset of appearance of EUS–EMG activity for DOI for these doses was 48±9, 46±6 and 17±3 s, respectively. Again this evoked EUS–EMG activity was observed to be ongoing up to 10 min. When the bladder was emptied to test the micturition reflex, EUS–EMG firing usually stopped, but again in some experiments EUS–EMG was observed to be continuous while testing the micturition reflex in the presence of all three doses of DOI. BW723C86 (300 μg kg−1) failed to have any significant effect on the EUS–EMG signal and urethral pressure although, between 4 and 5 min after administration, there was a tendency for urethral pressure to increase.
Figure 4.
Urethane anaesthetized female rats: a comparison of the effects of 5-HT2 receptor agonists WAY 161503, Ro 60-0175, mCPP and DOI on changes (Δ) in external urethral sphincter (EUS) EMG signal (%), urethral pressure (urethral P; %) and mean arterial blood pressure (MAP; mm Hg). Each point represents the mean value and vertical bars show the s.e.mean. Changes caused by WAY 161503, Ro 60-0175 and DOI were compared with DMSO+saline control, whereas for mCPP the control was saline+saline control (these control data have not been illustrated for the sake of clarity, see Figures 7a and 8a for DMSO+saline control data) using a split-plot analysis.
Micturition
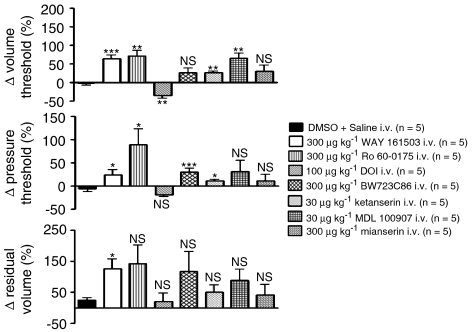
Ro 60-0175 and WAY 161503 attenuated the micturition reflex causing significant increases in volume threshold of 71±15 and 55±13% and pressure threshold of 90±33 and 25±11%, whereas BW723C86 only caused a significant increase in the pressure threshold of 30±9% (see Figure 5). WAY 161503 also caused a significant increase in residual volume of 121±35%. Both drugs had no significant effects on the evoked urethral relaxation or EUS–EMG. This was found to be the case with all the other experiments carried out throughout this study (except i.v. mCPP; see the sentence below), thus the reflex-evoked urethral relaxation and mean area of integrated EUS–EMG burst in the presence of these compounds were not analysed any further. mCPP completely blocked the micturition reflex causing a continuous rise in bladder pressure resulting after 5 min in the appearance of a few drops of saline leaking from the urethral orifice (data not illustrated). However, DOI had an excitatory action on the reflex both at 30 and 100 μg kg−1, causing a significant decrease in volume threshold of 27±5 and 35±7% (see Figure 5).
Figure 5.
Urethane anaesthetized female rats: a comparison of the effects of the 5-HT2 receptor agonists WAY 161503, Ro 60-0175, DOI and BW723C86 and the antagonists ketanserin, MDL 100907 and mianserin on percentage (%) changes (Δ) in volume threshold, pressure threshold and residual volume. Each bar represents mean value and vertical bars show the s.e.mean. Changes caused by WAY 161503, Ro 60-0175, DOI, BW723C86, ketanserin, MDL 100907 and mianserin were compared with vehicle (DMSO+saline) control using Student's unpaired t-test. *P<0.05, **P<0.01, ***P<0.001, NS, non-significant. NB mCPP was not included as part of the reflex data as the reflex was completely abolished hence it was not possible to measure any of the bladder variables.
Blood pressure
Ro 60-0175, WAY 161503, DOI (only at the highest dose of 100 μg kg−1) and BW723C67 significantly increased MAP by 14±1, 21±1, 21±1 and 10±5 mm Hg, respectively. WAY 161503 and DOI failed to affect HR but Ro 60-0175 and BW501C67 caused a decrease of 13±2 and 12±3 b.p.m. mCPP (Figure 4) failed to affect MAP but caused a bradycardia of 21±3 b.p.m.
Effects of Ro 60-0175 in the presence of 5-HT2 receptor antagonists (SB 242084, RS 127445, ketanserin and MDL 100907)
SB 242084, a 5-HT2C receptor antagonist
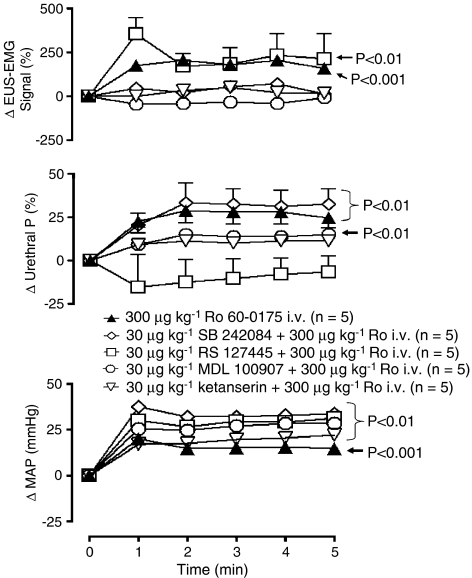
SB 242084 (30 μg kg−1, i.v.; n=5) blocked the effects of Ro 60-0175 (n=5) on baseline EUS–EMG signal but not the evoked increase in urethral pressure (27±3%; Figure 6). On the micturition reflex, the inhibitory action on the volume threshold was reversed to an excitatory action. Ro 60-0175 now caused a significant decrease in volume threshold of 53±16% whereas the effect on pressure threshold was just blocked (data not illustrated).
Figure 6.
Urethane anaesthetized female rats: a comparison of the effects of Ro 60-0175 alone and Ro 60-0175 in the presence of SB 242084, RS 127445, MDL 100907 or ketanserin on changes (Δ) in external urethral sphincter (EUS) EMG signal (%), urethral pressure (urethral P; %) and mean arterial blood pressure (MAP; mm Hg). Each point represents the mean value and vertical bars show the s.e.mean. Changes in baseline variables were compared with vehicle controls using a split-plot analysis. Vehicle control has not been illustrated for the sake of clarity (see Figures 7a and 8a for DMSO+saline control data). It should be noted that changes caused between antagonist pretreatments were found not to be significant.
The pressor response evoked by Ro 60-0175 was significantly potentiated (Figure 6), but the bradycardia was unaffected. SB 242084 on its own had no effect on baseline variables.
RS 127445 a 5-HT2B receptor antagonist
In the presence of RS 127445 (300 μg kg−1, i.v.; n=5), Ro 60-0175 evoked EUS–EMG activity was unaffected (218±30%) but the increase in urethral pressure was now blocked, with Ro 60-0175 now evoking a non-significant decrease of 11±4% (see Figure 6). On the micturition reflex, RS 127445 failed to have a significant effect on the Ro 60-0175 evoked decrease in both volume and pressure threshold. However due to a reduction in the variability, Ro 60-0175 now evoked a significant increase in residual volume of 64±11% (data not illustrated).
RS 127445 failed to block the pressor (25±2 mm Hg; Figure 6) and bradycardia (19±6 b.p.m.) responses evoked by Ro 60-0175. RS 127445 alone had no effect on baseline variables.
Ketanserin and MDL 100907 (5-HT2A receptor antagonists)
In the presence of either ketanserin or MDL 100907 (30 μg kg−1, i.v.; n=5), Ro 60-0175 (n=5) no longer evoked EUS–EMG activity and the increase in urethral pressure was reduced to 9±1 and 11±2% respectively (Figure 6). In fact the increase in urethral pressure in the presence of MDL 100907 was no longer significant compared with control. On the micturition reflex, both ketanserin and MDL 100907 failed to block the inhibitory actions of Ro 60-0175 on volume (61±5 and 63±6%) and pressure threshold (50±3 and 44±6%). The tendency of Ro 60-0175 to increase residual volume was now non-existent (data not illustrated).
Both ketanserin and MDL 100907 failed to block the pressor effect (16±2 and 24±1 mm Hg respectively) of Ro 60-0175 (Figure 6), and the bradycardia was larger although not significantly, 34±2 and 22±3 b.p.m. respectively. Ketanserin alone had no effect on baseline variables; however, MDL 100907 caused a rise in BP (14±1 mm Hg) associated with no effect on HR.
Effects of DOI in the presence of the 5-HT2A receptor antagonists (ketanserin and MDL 100907)
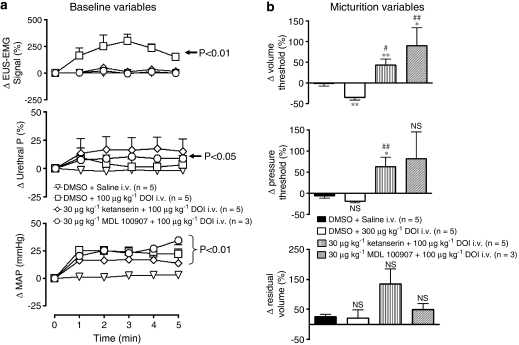
In the presence of ketanserin (30 μg kg−1, i.v.; n=5) or MDL 100907 (n=3), DOI (100 μg kg−1) also failed to activate EUS–EMG (Figure 7a). Interestingly, DOI now tended to increase urethral pressure and, in the case of MDL 100907, this was significant compared with control. On the micturition reflex, DOI effects were reversed, evoking an increase of 43±15 and 90±43%, respectively in the volume threshold (Figure 7b). Furthermore in the presence of ketanserin, pressure threshold was also increased by 63±22%, and there was a non-significant tendency for residual volume to increase.
Figure 7.
Urethane anaesthetized female rats: Comparison of the effects of vehicle (DMSO+saline) with DOI in the presence of DMSO, ketanserin or MDL 100907 (30 μg kg−1) on changes (Δ) in (a) baseline variables, external urethral sphincter (EUS) EMG signal (%), urethral pressure (urethral P; %) and mean arterial blood pressure (MAP; mm Hg) and (b) on percentage (%) changes (Δ) in volume threshold, pressure threshold and residual volume evoked by infusion of saline into the bladder (micturition reflex). Changes in baseline variables were compared with vehicle controls using a split-plot analysis. Changes in micturition were compared with vehicle control using Student's unpaired t-test. In (b) *compared with vehicle, #compared with DMSO+DOI. *,#P<0.05, **,##P<0.01, ***P<0.001, NS, non-significant. Each point or bar represents mean value and vertical bars show the s.e.mean.
Ketanserin and MDL 100907 failed to interfere with the pressor effect (14±1 and 24±3 mm Hg respectively) evoked by DOI (Figure 7a). HR again was unaffected.
Effects of 5-HT2 receptor antagonist alone (mianserin, ketanserin, MDL 100907 & BW 501C67)
Mianserin (300 μg kg−1, n=5), ketanserin (100 μg kg−1, n=5), MDL 100907 (30 μg kg−1, n=5) and BW 501C67 (1 mg kg−1, n=5) given i.v. had no effect on EUS–EMG and urethral pressure. Mianserin (Figure 5) and BW 501C67 (Figure 8a) also failed to affect the micturition reflex. However, ketanserin and MDL 100907 had an inhibitory effect on the micturition reflex (Figure 5). Ketanserin caused a significant increase in volume threshold (27±3%) and pressure threshold (11±4%; Figure 7), but had no effect on residu+al volume. MDL 100907, however, only significantly increased volume threshold (65±15%).
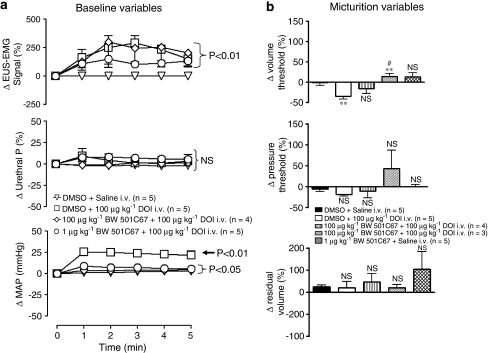
Figure 8.
Urethane anaesthetized female rats: Comparison of the effects of vehicle (DMSO+saline) with DOI in the presence of DMSO or BW 501C67 (100 μg kg−1 and 1 mg kg−1) on changes (Δ) in (a) baseline variables, external urethral sphincter (EUS) EMG signal (%), urethral pressure (urethral P, %) and mean arterial blood pressure (MAP; mm Hg) and (b) on percentage (%) changes (Δ) in volume threshold, pressure threshold and residual volume evoked by infusion of saline into the bladder (micturition reflex). Changes in baseline variables were compared with vehicle controls using a split-plot analysis. Changes in micturition were compared with vehicle control using Student's unpaired t-test. *,#P<0.05, **,##P<0.01, NS, non-significant. Each point or bar represents mean value and vertical bars show the s.e.mean.
Ketanserin and BW 501C67 had no effect on MAP and HR. Mianserin tended to cause an increase in MAP whereas MDL 100907 significantly increased MAP by 14±1 mm Hg, with no significant effects observed on HR.
Effect of the peripherally acting 5-HT receptor antagonist BW 501C67 on the actions of mCPP and DOI
In the presence of BW 501C67 (100 μg kg−1, n=4/5) both mCPP (300 μg kg−1, i.v.) and DOI (100 μg kg−1, i.v.) still increased EUS–EMG signal by 269±44 and 211±29% (Figure 8a), respectively. Even in the presence of the high dose of BW 501C67 (1 mg kg−1, n=3), DOI still increased the EUS–EMG signal by 133±12% (Figure 8a). BW 501C67 also failed to block the increase in urethral pressure (20±2%) evoked by mCPP whereas DOI in the presence of BW 501C67 still failed to affect urethral pressure (Figure 8a). The evoked increases in EUS–EMG activity were again observed to be ongoing for up to 10 min until the bladder was emptied to test the micturition reflex. In the presence of BW 501C67 (100 μg kg−1), mCPP still blocked the micturition reflex, whereas the DOI-evoked decrease in volume threshold was no longer significant (Figure 8b). However, the high dose of BW 501C67 (1 mg kg−1) reversed this effect of DOI on volume threshold (23±7%; Figure 8b).
Both doses of BW 501C67 significantly attenuated the rise in blood pressure evoked by DOI (Figure 8a). Again HR was unaffected by DOI whereas mCPP still had no effect on BP and HR.
Effects of mCPP and DOI given i.c.v.
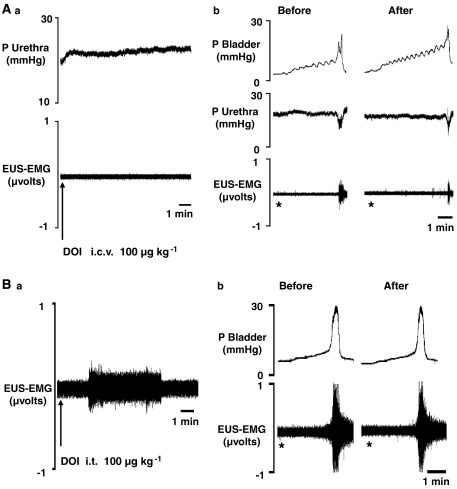
Neither mCPP (300 μg kg−1; 5 μL, n=3) nor DOI (100 μg kg−1; 5 μL, n=5) had a significant effect on baseline EUS–EMG signal, urethral pressure, MAP and HR when compared with saline control. Traces showing the effect of DOI given i.c.v. are shown in Figure 9. On the micturition reflex mCPP significantly increased volume threshold by 50±11% whereas DOI evoked a significant increase in pressure threshold of 34±9%. mCPP had no effect on MAP whereas DOI significantly increased MAP by 13±2 mm Hg. HR was unaffected.
Figure 9.
Urethane anaesthetized female rats: Traces showing the effects of (A) i.c.v. DOI (100 μg kg−1) on (a) baseline urethral pressure and EUS–EMG activity and (b) on distention caused by infusion of saline into the bladder (the micturition reflex) in bladder and urethral pressure and raw urethral striated muscle. (B) Traces showing the effects of i.t. DOI (100 μg kg−1) on (a) baseline EUS–EMG activity and (b) on distention caused by infusion of saline into the bladder (the micturition reflex) in bladder and EUS–EMG activity. *Denotes onset of saline infusion into the bladder.
Effect of DOI given intrathecally (i.t.)
DOI (100 μg kg−1; 10 μL, n=3) significantly increased baseline EUS–EMG signal by 24±5% (Figure 9). Unlike the i.v. route, the duration of EUS–EMG firing was shorter, lasting for approximately 3 min. On the micturition reflex, i.t. DOI had no significant effect on any of the variables.
Discussion
Micturition variables
The present data demonstrate that with the exception of BW723C86, all the 5-HT2 receptor agonists tested, mCPP, WAY 161503, Ro 60-0175 and DOI, when given i.v. caused activation of the external urethral sphincter (EUS) in urethane-anaesthetized rats with a primed (that is 80% full) urinary bladder. In addition, with the exceptions of DOI and BW723C86, all drugs increased urethral pressure and inhibited the micturition reflex. In this latter respect DOI had an excitatory effect, whereas BW723C86 had no effect on the micturition reflex. The agonists mCPP (see Bonhaus et al., 1997) WAY 161503 (Cryan and Lucki, 2000) and Ro-60-0175 (Martin et al., 1998) are considered to be selective for 5-HT2C receptors. If taken alone these agonist data would suggest that activation of 5-HT2C receptors causes external urethral sphincter activation, urethral contraction and the inhibition of micturition. It should be noted that an increase in urethral pressure could involve both urethral smooth muscle and striated muscle. Furthermore, the present data confirm the observations by Steers and De Groat (1989) that mCPP activates the external urethral sphincter. However, the suggestion by these authors that the inhibition of isovolumetric contractions by mCPP is caused by activation of the EUS would seem to be unfounded. The present data indicate that this is a separate action as, although mCPP completely inhibited the micturition reflex, the other agonists activated the EUS in a similar fashion but only attenuated the micturition reflex.
To determine that these effects were mediated by 5-HT2C receptors, the effects of Ro 60-0175 were examined in the presence of the highly selective 5-HT2C receptor antagonist SB 242084 (Kennett et al., 1997). In the presence of SB 242084, Ro 60-0175 no longer activated the EUS and inhibited the micturition reflex. However, Ro 60-0175 still caused a similar increase in urethral pressure. This latter action was completely blocked by pretreatment with the selective 5-HT2B receptor antagonist RS 127445 (Bonhaus et al., 1999), without affecting the evoked EUS–EMG activity and inhibition of the micturition reflex by Ro 60-0175. This again indicates that increases in both urethral pressure and EUS activity are not linked. These combined data indicate that activation of 5-HT2C receptors mediates EUS–EMG activity and the inhibition of the micturition reflex, whereas activation of 5-HT2B receptors causes an increase in urethral pressure. It is therefore surprising that the 5-HT2B receptor agonist BW723C86 (Kennett et al., 1996) did not affect urethral pressure, although there was a tendency for it to increase this variable. This might be related to a lack of expected selectivity in vivo. Indeed, in vitro data give conflicting information on the selectivity of BW723C86 for 5-HT2B receptors; it is either 100-fold greater for 5-HT2A/C receptors (Porter et al., 1999) or not (Knight et al., 2004). However the ability of DOI, which shows some selectivity for 5-HT2A receptors (Porter et al., 1999; Knight et al., 2004) to activate the EUS, indicates that 5-HT2A receptors are also involved in this action. Furthermore, DOI also has an excitatory action on the micturition reflex indicating that 5-HT2A receptors are also involved in the reflex, although having the reverse action. This was confirmed by the observations that DOI in the presence of the selective 5-HT2A receptor antagonists, ketanserin (see Bonhaus et al., 1997) or MDL 100907 (Kehne et al., 1996) failed to evoke EUS–EMG activity and reversed the excitatory action of DOI on the micturition reflex to inhibition, a 5-HT2C receptor-mediated action. This suggests that DOI, at the dose used in the present study, also has an additional action on 5-HT2C receptors. Thus the 5-HT2C receptor-mediated effects of DOI are usually overridden by its 5-HT2A receptor agonist action on the micturition reflex. In this respect, DOI might be expected to also have a residual action on the external urethral sphincter if both receptor subtypes are responsible for this action; however, no effect was observed. Overall the data indicate that 5-HT2A receptors are also involved in the activation of the EUS.
To determine if any 5-HT2A receptor-mediated component was involved in the action of Ro 60-0175 in activating the EUS, the effects of Ro 60-0175 were investigated in the presence of either ketanserin or MDL 100907. Both antagonists surprisingly completely blocked the ability of Ro 60-0175 to activate the EUS but as expected, failed to interfere with the inhibitory action of Ro 60-0175 on the micturition reflex. Interestingly, although ketanserin did not interfere with the ability of Ro 60-0175 to evoke an increase in urethral pressure, MDL 100907 did prevent Ro 60-0175 evoking a significant increase in urethral pressure when compared with vehicle control. DOI also in the presence of MDL 1000907 now showed small but significant increase in urethral pressure. This former observation implies that, even at the low dose of MDL 100907 used in the present study, there might be some weak 5-HT2B receptor blocking actions. However if so, then the ability of DOI to increase urethral pressure in the presence of MDL 100907 is somewhat surprising unless again this reflects the different potency of these two drugs at 5-HT2B receptors at the particular doses chosen. Overall the data indicate that both 5-HT2A and 5-HT2C receptors are involved in mediating the ability of these agonists to activate the EUS. The relationship between these two 5-HT receptor subtypes in this action remains to be determined, although on the micturition reflex they have opposing actions. However, the data with the selective SB 242084 could simply indicate that at the dose used this antagonist may also be blocking, rather surprisingly, 5-HT2A receptors as well.
The present data, examining the effects of ketanserin and MDL 100907 alone on the micturition reflex indicate that 5-HT2A receptors may play a physiological role in micturition as these antagonists caused an increase in the volume threshold. However, these antagonists had no effect on the reflex-evoked EUS–EMG activity indicating that these receptors are not involved in this part of the micturition pathway. In this respect, ketanserin has previously been reported to also inhibit bladder isovolumetric contractions (Testa et al., 2001). However, the involvement of 5-HT2A receptors in the reflex control of micturition may be considered a minor one as a similar dose to that of ketanserin of a 5-HT7 receptor antagonist completely blocked the reflex (Read et al., 2003). Further, Testa et al. (2001) and Mbaki (2007) found that the selective 5-HT2C receptor antagonist SB 242084 (Kennett et al., 1997) and the so-called classical 5-HT2C receptor antagonist mesulergine, which again according to binding data shows poor selectivity for this receptor (see Bonhaus et al., 1997 and Knight et al., 2004) also failed to affect the micturition reflex (Testa et al., 1999, 2001). Although the present data support the view that 5-HT2C receptors have an inhibitory action on the micturition reflex (see Introduction), the published data do not support a physiological role for this receptor subtype. Overall this supports the view that, at least in the rat, 5-HT pathways have an excitatory role in the control of micturition not an inhibitory one.
Blood pressure
5-HT2 receptors are also known to play a role in central and peripheral regulation of blood pressure. Activation of central 5-HT2A receptors causes sympathoexcitation and vasopressin release (see Ramage, 2001) and, peripherally, vasoconstriction (see Kaumann and Levy, 2006) all leading to a rise in blood pressure. The role of 5-HT2C receptors in central cardiovascular regulation is very poorly understood, although there is some evidence to suggest that they may cause sympathoexcitation and thus a rise in blood pressure (Knowles and Ramage, 2000). In the peripheral cardiovascular system, 5-HT2C receptors have not been identified (see Kaumann and Levy, 2006). Consistent with this is the fact that DOI, the so called selective 5-HT2A receptor agonist in the present experiments, caused an increase in blood pressure although the selective 5-HT2C receptor agonist Ro 60-0175 also caused a rise. However, the classical 5-HT2C receptor agonist mCPP had no effect on blood pressure. Interestingly, the selective 5-HT2B receptor BW723C86 caused a rise in blood pressure as reported by Centurión et al. (2004). Whether this is because of a lack of selectivity that is an action on 5-HT2A receptors (in section on Micturition variables top of page left column) or because of an action on 5-HT2B receptors remains to be determined. In this respect, BW723C86 given centrally caused a fall in blood pressure, which was believed to be mediated by 5-HT2B receptors (Knowles and Ramage, 2000). The pressor effect of Ro 60-0175 was not blocked by the 5-HT2C receptor antagonist SB 242084 but in fact was observed to be potentiated, probably because of blockade of the bradycardia preventing this counteracting effect of reduction in cardiac output on the evoked pressor response. Further, the 5-HT2B receptor antagonist also failed to block the pressor action of Ro 60-0175. These observations are consistent with the pressor action being mediated by activation of 5-HT2A receptors. However, both the 5-HT2A receptor antagonist ketanserin, and MDL 100907 failed to block the pressor response of Ro 60-0175 and DOI, although the peripheral acting 5-HT2 receptor antagonist BW 501C67 did block the pressor response. There is little data on the affinity of BW 501C67 for 5-HT2 receptor subtypes; however, Anderson et al. (1992) did report that BW 501C67 had a pKD of 9.5 at 5-HT2A and a pKD of 8.5 at 5-HT2C receptors, although nothing was reported on BW 501C67's affinity for the 5-HT2B receptor. The BW 501C67 data suggest that the pressor response of these agonists is peripherally mediated by activation of the 5-HT2A receptors on vascular smooth muscle. The failure of ketanserin and MDL 100907 to block the pressor response is still somewhat surprising but could be because of the lower doses of these antagonists used. However, the antagonists were capable of blocking the 5-HT2A receptor-mediated activation of the EUS and this may just reflect the fact that the concentration of Ro 60-0175 and DOI is much lower at the site of action that is sacral spinal cord, than at the smooth muscle. Thus the overall data suggest that the pressor action of the agonists in the present study is mainly because of a peripheral action on 5-HT2A receptors on vascular smooth muscle. In this respect, the observation that the 5-HT2A receptor-mediated excitatory action of DOI on the micturition reflex is blocked by these 5-HT2A receptor antagonists as well as by the peripheral acting antagonist BW 501C67 supports the view that this excitatory action of DOI is because of an increase in the smooth muscle tone of the bladder.
The selective 5-HT2A receptor antagonist MDL 100907 caused a significant increase in blood pressure and in this respect mianserin also tended to increase blood pressure although in all cases heart rate was unaffected; however, ketanserin had no effect. This is somewhat surprising as blockade of 5-HT2 receptors would be expected to cause a fall in blood pressure and this is the first report of a 5-HT2 receptor antagonist causing an increase in blood pressure (see Ramage, 2001). The failure to see this trend with ketanserin may be related to its ability to block α1-adrenoceptors (Ramage, 1985). A possible explanation for this increase in blood pressure could be related to blockade of 5-HT2B receptors, which could be under tonic activation as MDL 100907 did interfere with the ability of Ro 60-0175 to increase urethral pressure, a putative 5-HT2B receptor-mediated action. However, the selective 5-HT2B receptor antagonist RS 127445 given before the agonist challenge in the present experiments did not affect resting blood pressure, so the mechanism by which MDL 100907 increases blood pressure may be unrelated to such a mechanism. Thus, 5-HT2 receptor subtypes do not seem to be physiologically involved in the regulation of blood pressure.
Site of action
Data with the peripherally acting 5-HT2 receptor antagonist BW 501C67 (Mawson and Whittington, 1970; Fuller et al., 1986) indicate that the ability of mCPP and DOI to activate the EUS is because of a central action. Interestingly, the low dose of BW 501C67 had a tendency to cause a potentiation of the mCPP evoked EUS–EMG activity in the first minute. This tendency to cause a potentiation was not observed for DOI. DOI was also tested against a very high dose of BW 501C67, which also failed to block the evoked EUS–EMG activity. Further, the increase in urethral pressure caused by mCPP and DOI was unaffected by BW 501C67 as was the inhibitory action of mCPP on the micturition reflex. This indicates that 5-HT2C receptors located centrally cause inhibition of the micturition reflex and is consistent with the view that 5-HT2C receptors have not been found outside the CNS. As mentioned earlier nothing has been reported on BW 501C67's affinity for 5-HT2B receptors. Thus it is not possible to conclude whether the failure of BW 501C67 to interfere with the evoked increase in urethral pressure is because of this effect, being a centrally and/or peripherally-mediated action. On the other hand, i.c.v. administration of mCPP failed to cause an increase in urethral pressure or evoke EUS–EMG activity. Furthermore, it has been reported that the urethra predominantly expresses 5-HT2B receptors and the relative expression ratios are 300-fold that of the brain and 6-fold that of the bladder (see Mbaki, 2007). Furthermore, both mCPP and DOI (i.c.v.) had an inhibitory effect on the bladder and, surprisingly, mCPP failed to completely block the micturition reflex as it did i.v. suggesting additional central site/s of action for 5-HT2C receptors in the control of the micturition reflex. This could be at a sacral spinal level along with the site involved in evoking EUS–EMG activity. In this respect, 5-HT2C receptors have been localized in the parasympathetic nucleus of the sacral spinal cord (Bancila et al., 1999). However, DOI given intrathecally at the level of the sacral spinal cord failed to affect the micturition reflex but did evoke EUS–EMG activity. The most obvious site at the sacral spinal level for this effect on the EUS is Onuf's nucleus whose neurones innervate the EUS. This nucleus also innervates the anal sphincter and contains a high density of 5-HT2A and 5-ht5A receptors (Doly et al., 2004a, 2004b) as well 5-HT2C receptors (Bancila et al., 1999). As the bladder is required to be 80% full before this effect of 5-HT2A/2C receptor agonists can be observed this would imply that a level of afferent input from the bladder to these motoneurones is required with the possibility of their location on the afferent terminals. However, the motoneurones in most cases have the 5-HT2A receptor located postsynaptically (Doly et al., 2004b) and this has been recently confirmed for Onuf's nucleus, which also receives 5-HT innervation (Xu et al., 2007). Further, the present study again does not indicate that these 5-HT2A/2C receptors are physiologically involved in the control of the EUS nor do the present data indicate a physiological role for 5-HT2C receptors in the micturition reflex. Recently, in the guinea-pig, EUS excitation has been demonstrated to be because of activation of 5-HT2C rather than 5-HT2A receptors (Conlon et al., 2005). Interestingly, the function of the external urethral sphincter in this species is similar to that of man but not the rat (see McMurray et al., 2006). In the guinea-pig as in man it is part of the ‘guarding reflex', that is, activity gradually increases as the bladder fills to prevent incontinence, whereas in the rat the external sphincter has pulsatile activity during micturition. This pulsatile activity in the rat is meant to aid voiding by acting as a pump. So, in the rat the external sphincter can be considered to aid in micturition, whereas in the guinea-pig it can be considered to be part of the mechanism where urine is retained that is inhibitory to micturition, which seems to be the general role of 5-HT2C receptors in micturition.
In conclusion, the data indicate that activation of 5-HT2A/2C receptors at the level of sacral spinal cord is responsible for excitation of the EUS and further support the view that activation of central 5-HT2C receptors causes inhibition of the micturition reflex. Further, 5-HT2B receptor activation increases urethral pressure presumably by increasing tone in the urethral smooth muscle. Overall these data indicate that 5-HT2C and 5-HT2B receptors are not physiologically involved in micturition, although there is some indication that central 5-HT2A may have a minor excitatory role. In addition, the data indicate that assumptions about the selectivity of these agonists and antagonists for the various 5-HT2 receptor subtypes has to be treated with caution, especially when studying a system in which activation of all three receptor subtypes can have effects.
Acknowledgments
YM was supported by a BBSRC collaborative studentship with Pfizer UK. We are also grateful for the technical assistance of Mr S Wilkinson. We also thank Simon Westbrook and Gordon McMurray at Pfizer UK for their support.
Abbreviations
- BP
blood pressure
- BW501C67
α-anilino-N-2-m-chlorophenoxypropylacetamide
- BW723C86
α-methyl-5-(2-thienylmethoxy)-1 H-indole-3-ethanamine
- DMSO
Dimethyl sulphoxide
- DOI
2,5-dimethoxy-4-idophenyl)-2-aminopropane hydrochloride
- EMG
electromyogram
- EUS
external urethral sphincter
- HR
heart rate
- i.c.v.
intracerebroventricular
- i.t.
intrathecal
- mCPP 1-(3-chlorophenyl) piperazine hydrochloride; MDL 100907
R-(+)-a- (2,3-dimethoxyphenyl)-1-[2-(4-fluorophenylethyl)]-4-piperidine methanol
- Ro 60-0175
(αS)-6-chloro-5-fluoro-α-methyl-1H-indole-1-ethanamine fumarate
- RS 127445
2-amino-4-(4-fluoronaphth-1-yl)-6-isopropylpyrimidine
- SB 242084
6-chloro-2,3-dihydro-5-methyl-N-[6-[(2-methyl-3-pyridinyl)oxy]-3-pyridinyl]-1H-indole-1-carboxyamide dihydrochloride
- WAY 161503
8,9-dichloro-2,3,4,4a-tetrahydro-1H-pyrazinol[1,2,-α]quinoxaline-5(6H)-one hydrochloride
Conflict of interest
The authors state no conflict of interest.
References
- Anderson IK, Martin GR, Ramage AG. Evidence that i.c.v. administration of 5-HT causes sympathoexcitation through activation of 5-HT1A receptors and vasopressin release through activation of 5-HT2/1C receptors in anaesthetized rats. Br J Pharmacol. 1992;107:1020–1028. doi: 10.1111/j.1476-5381.1992.tb13401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 2nd edition (2007 revision) Br J Pharmacol. 2007;150 Suppl. 1:S6–S8. doi: 10.1038/sj.bjp.0707199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancila M, Vergé D, Rampin O, Backstrom JR, Sanders-Bush E, McKenna KE, et al. 5-Hydroxytryptamine2C receptors on spinal neurons controlling penile erection in the rat. Neuroscience. 1999;92:1523–1537. doi: 10.1016/s0306-4522(99)00082-2. [DOI] [PubMed] [Google Scholar]
- Barnes NM, Sharp T. A review of central 5-HT receptors and their function. Neuropharmacol. 1999;38:1083–1152. doi: 10.1016/s0028-3908(99)00010-6. [DOI] [PubMed] [Google Scholar]
- Bonhaus DW, Flippin LA, Greenhouse RJ, Jaime S, Rocha C, Dawson M, et al. RS-127445: a selective, high affinity, orally bioavailable 5-HT2B receptor antagonist. Br J Pharmacol. 1999;127:1075–1082. doi: 10.1038/sj.bjp.0702632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonhaus DW, Weinhardt KK, Taylor M, DeSouza A, McNeeley PM, Szczepanski K, et al. RS-102221: a novel high affinity and selective, 5-HT2C receptor antagonist. Neuropharmacol. 1997;36:621–629. doi: 10.1016/s0028-3908(97)00049-x. [DOI] [PubMed] [Google Scholar]
- Centurión D, Glusa E, Sánchez-López A, Valdivia LF, Saxena PR, Villalón CM. 5-HT7, but not 5-HT2B, receptors mediate hypotension in vagosympathectomized rats. Eur J Pharmacol. 2004;502:239–242. doi: 10.1016/j.ejphar.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Conlon K, Miner W, Christy C, McCleary S, Brinkman H, Rees H, et al. Identification of 5-HT2C- mediated mechanisms involved in urethral sphincter reflexes 20052005Society for Neuroscience: Washington, DC; Program No. 48.14. Abstract Viewer/Itinerary PlannerOnline [Google Scholar]
- Conley RK, Williams TJ, Ford AP, Ramage AG. The role of α1-adrenoceptors and 5-HT1A receptors in the control of the micturition reflex in male anaesthetized rats. Br J Pharmacol. 2001;133:61–72. doi: 10.1038/sj.bjp.0704043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cryan JF, Lucki I. Antidepressant-like behavioral effects mediated by 5-Hydroxytryptamine2C receptors. J Pharmacol Exp Ther. 2000;295:1120–1126. [PubMed] [Google Scholar]
- Danuser H, Thor KB. Spinal 5-HT2 receptor-mediated facilitation of pudendal nerve reflexes in the anaesthetized cat. Br J Pharmacol. 1996;118:150–154. doi: 10.1111/j.1476-5381.1996.tb15378.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Groat WC. Influence of central serotonergic mechanisms on lower urinary tract function. Urology. 2002;59 5 Suppl 1:30–36. doi: 10.1016/s0090-4295(01)01636-3. [DOI] [PubMed] [Google Scholar]
- Doly S, Fischer J, Brisorgueil MJ, Vergé D, Conrath M. 5-HT5A receptor localization in the rat spinal cord suggests a role in nociception and control of pelvic floor musculature. J Comp Neurol. 2004a;476:316–329. doi: 10.1002/cne.20214. [DOI] [PubMed] [Google Scholar]
- Doly S, Madeira A, Fischer J, Brisorgueil MJ, Daval G, Bernard R, et al. The 5-HT2A receptor is widely distributed in the rat spinal cord and mainly localized at the plasma membrane of postsynaptic neurons. J Comp Neurol. 2004b;472:496–511. doi: 10.1002/cne.20082. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Kurz KD, Mason NR, Cohen ML. Antagonism of a peripheral vascular but not an apparently central serotonergic response by xylamidine and BW501C67. Eur J Pharmacol. 1986;125:71–77. doi: 10.1016/0014-2999(86)90084-1. [DOI] [PubMed] [Google Scholar]
- Kakizaki H, Yoshiyama M, Koyanagi T, De Groat WC. Effects of WAY100635, a selective 5-HT1A-receptor antagonist on the micturition-reflex pathway in the rat. Am J Physiol Regul Integr Comp Physiol. 2001;280:R1407–R1413. doi: 10.1152/ajpregu.2001.280.5.R1407. [DOI] [PubMed] [Google Scholar]
- Kaumann AJ, Levy FO. 5-Hydroxytryptamine receptors in the human cardiovascular system. Pharmacol & Ther. 2006;111:674–706. doi: 10.1016/j.pharmthera.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Kehne JH, Ketteler HJ, McCloskey TC, Sullivan CK, Dudley MW, Schmidt CJ. Effects of the selective 5-HT2A receptor antagonist MDL 100, 907 on MDMA-induced locomotor stimulation in rats. Neuropsychopharmacol. 1996;15:116–124. doi: 10.1016/0893-133X(95)00160-F. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Bright F, Trail B, Baxter GS, Blackburn TP. Effects of the 5-HT2B receptor agonist, BW 723C86, on three rat models of anxiety. Br J Pharmacol. 1996;117:1443–1448. doi: 10.1111/j.1476-5381.1996.tb15304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Trail B, Riley G, Holland V, et al. SB 242084, a selective and brain penetrant 5-HT2C receptor antagonist. Neuropharmacol. 1997;36:609–620. doi: 10.1016/s0028-3908(97)00038-5. [DOI] [PubMed] [Google Scholar]
- Knight AR, Misra A, Quirk K, Benwell K, Revell D, Kennett G, et al. Pharmacological characterisation of the agonist radioligand binding site of 5-HT2A, 5-HT2B and 5-HT2C receptors. Naunyn Schmiedebergs Arch Pharmacol. 2004;370:114–1123. doi: 10.1007/s00210-004-0951-4. [DOI] [PubMed] [Google Scholar]
- Knowles ID, Ramage AG. Evidence that activation of central 5-HT2B receptors causes renal sympathoexcitation in anaesthetized rats. Br J Pharmacol. 2000;129:177–183. doi: 10.1038/sj.bjp.0703011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecci A, Giuliani S, Santicioli P, Maggi CA. Involvement of 5-hydroxytryptamine1A receptors in the modulation of micturition reflexes in the anesthetized rat. J Pharmacol Exp Ther. 1992;262:181–189. [PubMed] [Google Scholar]
- Maggi CA, Santicioli P, Meli A. The nonstop transvesical cystometrogram in urethane-anesthetized rats: a simple procedure for quantitative studies on the various phases of urinary bladder voiding cycle. J Pharmacol Meth. 1986;15:157–167. doi: 10.1016/0160-5402(86)90064-1. [DOI] [PubMed] [Google Scholar]
- Martin JR, Bos M, Moreau J-L, Mutel V, Wichmann J, Andrews JS, et al. 5-HT2C receptor agonists: Pharmacological characteristics and therapeutic potential. J Pharmacol Exp Ther. 1998;286:913–924. [PubMed] [Google Scholar]
- Mawson C, Whittington H. Evaluation of the peripheral and central antagonistic activities against 5-hydroxytryptamine of some new agents. Br J Pharmacol. 1970;39:223P–224P. [PMC free article] [PubMed] [Google Scholar]
- Mbaki Y.The role of 5-HT2 receptor subtypes in the control of micturition in urethane anaesthetized female rats 2007. PhD Thesis: University of London
- Mbaki Y, Gardiner J, McMurray G, Ramage AG. The effect of 5-HT2C agonists Ro 60-0175 in the control of the urethra and micturition in anaesthetized female rats. Proc Physiol Soc. 2006;3:PC108. [Google Scholar]
- Mbaki Y, Westbrook S, Ramage AG. The role of 5-HT2 receptors in the control of the urethra and micturition reflex in anaesthetized rats. Proceedings of the BPS. 2005;65P [Google Scholar]
- McMurray G, Casey JH, Naylor AM. Animal models in urological disease and sexual dysfunction. Br J Pharmacol. 2006;147 Suppl 2:S62–S79. doi: 10.1038/sj.bjp.0706630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter RH, Benwell KR, Lamb H, Malcolm CS, Allen NH, Revell DF, et al. Functional characterization of agonists at recombinant human 5-HT2A, 5-HT2B and 5-HT2C receptors in CHO-K1 cells. Br J Pharmacol. 1999;128:13–20. doi: 10.1038/sj.bjp.0702751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramage AG. The effects of ketanserin, methysergide and LY 53857 on sympathetic nerve activity. Eur J Pharmacol. 1985;113:295–303. doi: 10.1016/0014-2999(85)90076-7. [DOI] [PubMed] [Google Scholar]
- Ramage AG. Central cardiovascular regulation and 5-hydroxytryptamine receptors. Brain Res Bull. 2001;56:425–439. doi: 10.1016/s0361-9230(01)00612-8. [DOI] [PubMed] [Google Scholar]
- Ramage AG. The role of central 5-hydroxytryptamine (5-HT, serotonin) receptors in the control of micturition. Br J Pharmacol. 2006;147 Suppl 2:S120–S131. doi: 10.1038/sj.bjp.0706504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read KE, Sanger GJ, Ramage AG. Evidence for the involvement of central 5-HT7 receptors in the micturition reflex in anaesthetized female rats. Br J Pharmacol. 2003;140:53–60. doi: 10.1038/sj.bjp.0705399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Secker AG, Westbrook SL, Ramage AG.Evidence that central 5-HT1A receptors control micturition at both a supraspinal and sacral spinal sites in female urethane anaesthetized rats Br J Pharmacol 200314020Por [Google Scholar]
- Steers WD, De Groat WC. Effects of m-chlorophenylpiperazine on penile and bladder function in rats. Am J Physiol. 1989;257:R1441–R1449. doi: 10.1152/ajpregu.1989.257.6.R1441. [DOI] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Angelico P, Velasco C, Poggesi E, Cilia A, et al. Effect of different 5-hydroxytryptamine receptor subtype antagonists on the micturition reflex in rats. Br J Urol Int. 2001;87:256–264. doi: 10.1046/j.1464-410x.2001.02038.x. [DOI] [PubMed] [Google Scholar]
- Testa R, Guarneri L, Pogessie E, Angelico P, Ibba M, Cilia A, et al. Effect of several 5-hydroxytryptamine1A receptor ligands on the micturition reflex in rats: comparison with WAY 100635. J Pharmacol Exp Ther. 1999;290:1258–1269. [PubMed] [Google Scholar]
- Wibberley A, Nunn PA, Naylor AM, Ramage AG. An investigation of the effects of zaprinast, a PDE inhibitor, on the nitrergic control of the urethra in anaesthetized female rats. Br J Pharmacol. 2002;136:399–414. doi: 10.1038/sj.bjp.0704735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C, Giuliano F, Sun XQ, Brisorgueil MJ, Leclerc P, Vergé D, et al. Serotonin 5-HT2A and 5-HT5A receptors are expressed by different motoneuron opulations in rat Onuf's nucleus. J Comp Neurol. 2007;502:620–634. doi: 10.1002/cne.21344. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Rudy TA. Chronic catheterization of the spinal subarachnoid space. Physiol Behav. 1976;17:1031–1036. doi: 10.1016/0031-9384(76)90029-9. [DOI] [PubMed] [Google Scholar]
- Yoshiyama M, Kakizaki H, De Groat WC. Suppression of the micturition reflex in urethane-anaesthetized rats by intracerebroventricular injection of WAY 100635, a 5-HT1A receptor antagonist. Brain Res. 2003;980:281–287. doi: 10.1016/s0006-8993(03)02996-2. [DOI] [PubMed] [Google Scholar]