Abstract
Background and purpose:
The recent development of the UT ligand palosuran (1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulphate salt) has led to the proposition that urotensin-II (U-II) plays a significant pathological role in acute and chronic renal injury in the rat.
Experimental approach:
In the present study, the pharmacological properties of palosuran were investigated further using a series of radioligand binding and functional bioassays.
Key results:
Palosuran functioned as a ‘primate-selective' UT ligand in recombinant cell membranes (monkey and human UT Ki values of 4±1 and 5±1 nM), lacking appreciable affinity at other mammalian UT isoforms (rodent and feline Ki values >1 μM). Paradoxically, however, palosuran lost significant (10- to 54-fold) affinity for native and recombinant human UT when radioligand binding was performed in intact cells (Ki values of 50±3 and 276±67 nM). In accordance, palosuran also exhibited diminished activity in hUT (human urotensin-II receptor)-CHO (Chinese hamster ovary) cells (IC50 323±67 nM) and isolated arteries (Kb>10 μM in rat aorta; Kb>8.5 μM in cat arteries; Kb>1.6 μM in monkey arteries; Kb 2.2±0.6 μM in hUT transgenic mouse aorta). Relative to recombinant binding Ki values, palosuran was subjected to a 392- to 690-fold reduction in functional activity in monkey isolated arteries. Such phenomena were peculiar to palosuran and were not apparent with an alternative chemotype, SB-657510 (2-bromo-N-[4-chloro-3-((R)-1-methyl-pyrrolidin-3-yloxy)-phenyl]-4,5-dimethoxybenzenesulphonamide HCl).
Conclusions and implications:
Collectively, such findings suggest that caution should be taken when interpreting data generated using palosuran. The loss of UT affinity/activity observed in intact cells and tissues cf. membranes offers a potential explanation for the disappointing clinical efficacy reported with palosuran in diabetic nephropathy patients. As such, the (patho)physiological significance of U-II in diabetic renal dysfunction remains uncertain.
Keywords: urotensin-II, UT, GPR14, SB-657510, palosuran, ACT-058362, G-protein-coupled receptor, diabetic nephropathy, antagonist
Introduction
Human urotensin-II (hU-II), cognate ligand for the ‘orphan' G-protein-coupled receptor UT (Ames et al., 1999; Douglas and Ohlstein, 2000; NC-IUPHAR database: http://www.iuphar-db.org/index.jsp), is purported to play a significant role in the aetiology of several cardiorenal and metabolic disease states, including heart failure (Douglas et al., 2002; Richards et al., 2002), atherosclerosis (Bousette et al., 2004; Maguire et al., 2004), hypertension (Cheung et al., 2004) and diabetes (Totsune et al., 2003, 2004; Wenyi et al., 2003). Such a proposition has led to the rapid development in recent years of several distinct UT receptor antagonists (Dhanak et al., 2003; Douglas et al., 2004a).
Palosuran (ACT-058362; 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulphate salt), one such high-affinity human UT ligand provides cardiorenal and metabolic protection in rat models of both ischaemic (Clozel et al., 2004) and diabetic (Clozel et al., 2006) nephropathy. Additional in vitro studies demonstrate that this moiety also potentiates glucose-induced insulin secretion in rat isolated, perfused pancreas (Egido et al., 2005; Marco et al., 2005) and, most significantly, reduces microalbuminuria in diabetic nephropathy patients (Sidharta et al., 2006). Recently, however, it was found that palosuran failed to provide substantial efficacy in diabetic nephropathy patients based on collective data compiled from three separate proof-of-concept clinical studies (http://www.actelion.com/uninet/www/www_main_p.nsf/Content/me+23+May+2005; Clozel et al., 2006). As such, the precise pathophysiological significance of U-II in the diabetic kidney remains unclear. The underlying reason for the ‘insufficient' clinical efficacy reported is, at present, unknown but could reflect the (a) possibility that U-II is not a clinically significant pathological mediator in diabetic nephropathy, (b) use of inadequate dosage regimens (resulting in insufficient exposure to active drug) and/or (c) fact that palosuran is an ineffective antagonist of U-II function. Such potential explanations clearly have contrasting implications for the continued development of UT antagonists in this indication.
Despite the disappointing clinical data described above, palosuran has the potential to become a ‘benchmark' UT antagonist, a standard pharmacological tool used extensively within the field. However, upon close inspection, it is apparent that conflicting efficacy data exist within the literature for this moiety. Whereas palosuran (1–10 μM) purportedly regulates angiogenesis and adrenocortical function in the rat (Albertin et al., 2006; Spinazzi et al., 2006), the antagonist concentrations employed in such studies (1–10 μM) are inactive in intact cells expressing rat recombinant UT isoform (IC50 values >10 μM in radioligand binding and [Ca2+]i-mobilization assays; Clozel et al., 2004). More alarmingly, such actions are not replicated by another UT antagonist, SB-710411 (Albertin et al., 2006; Spinazzi et al., 2006).
In view of these disparities, in the present study the pharmacological characteristics of palosuran were assessed in detail using a broad spectrum of radioligand binding (rodent, feline and primate recombinant UT whole cell and membrane-binding formats) and functional U-II assays. These properties were compared to those of a chemically distinct, sulphonamide UT antagonist, SB-657510 (2-bromo-N-[4-chloro-3-((R)-1-methyl-pyrrolidin-3-yloxy)-phenyl]-4,5-dimethoxybenzenesulphonamide HCl) (Douglas et al., 2005).
In accord with the results of Clozel et al. (2004), palosuran behaved as a high-affinity, ‘primate-selective' UT ligand in recombinant membrane preparations. Subsequently, however, palosuran was found to be subject to a profound loss in affinity/activity when profiled in intact cells or isolated arteries. Such phenomena were specific for palosuran and were not evident with the alternative chemotype, SB-657510. It is, therefore, concluded that palosuran is not an optimal preclinical pharmacological tool for use in non-primate studies. Furthermore, such findings (loss of affinity/activity in intact cells and tissues) perhaps offer insight into the poor clinical efficacy attained in diabetic nephropathy subjects. On the basis of the present observations, a significant role for U-II in the aetiology of diabetic nephropathy cannot be dismissed.
Methods
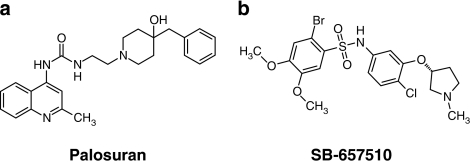
The pharmacological properties of palosuran, a quinolinyl-urea, were assessed in both radioligand (membrane cf. whole cell)-binding studies and in vitro functional bioassays (isolated arterial contraction) and were compared directly to those of a ‘reference' (chemically distinct sulphonamide) UT antagonist, SB-657510 (Figure 1; Douglas et al., 2005). Pharmacological properties were profiled across multiple mammalian species, including mouse, rat, cat, monkey and human.
Figure 1.
Structures of the UT receptor antagonists (a) palosuran (1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulphate salt; Aissaoui et al., 2003) and (b) SB-657510 (2-bromo-N-[4-chloro-3-((R)-1-methyl-pyrrolidin-3-yloxy)-phenyl]-4,5-dimethoxybenzenesulphonamide hydrochloride; Douglas et al., 2005).
All studies were performed in accredited facilities in accordance with institutional guidelines.
Radioligand binding at recombinant hUT in cell membrane preparations
Cell membranes were prepared from human embryonic kidney (HEK293) cells stably transfected with mouse or monkey UT or human osteosarcoma (U2OS) cells transiently expressing rat, cat or human UT (Ames et al., 1999; Behm et al., 2006). [125I]hU-II competition binding assays (scintillation proximity assay, SPA) were performed using 400 pM [125I(Tyr9)]hU-II in the presence or absence of 1 pM–1 μM cold hU-II, SB-657510 or palosuran in assay buffer (20 mM Tris-HCl (pH 7.4), 5 mM MgCl2 and 0.05% bovine serum albumin) containing cell membranes pre-coupled to wheat germ agglutinin coated-SPA (WGA-SPA) beads. Nonspecific binding was defined using 1 μM unlabelled hU-II. Assay plates were sealed, shaken gently (45 min, 20 °C) and left overnight before scintillation counting (TopCount; Perkin Elmer, Shelton, CT, USA).
Radioligand binding at recombinant and native hUT in whole cell preparations
Whole cell [125I]hU-II competition binding assays were performed using transient recombinant hUT (human urotensin-II receptor)-U2OS cells (Behm et al., 2006). Assays were performed with 125 pM [125I(Tyr9)]hU-II in the presence or absence of 1 pM–1 μM unlabelled hU-II, SB-657510 or palosuran in Dulbecco's phosphate-buffered saline (DPBS; 0.9 mM CaCl2, 0.5 mM MgCl2) and 0.1% bovine serum albumin. Nonspecific binding was defined using 1 μM cold hU-II. Assay plates were incubated at 37 °C for 30 min. Following three washes with DPBS, the cells were lysed with 2 N NaOH and the resulting lysate was counted using a gamma counter (Wallac Wizard, Perkin Elmer).
Radioligand binding at native hUT in intact SJRH30 cells
Binding of [125I]hU-II to native hUT receptors was studied according to Douglas et al. (2004b) using a whole cell-binding assay format in the human rhabdomyosarcoma cell line, SJRH30. Briefly, SJRH30 cells were washed once in DPBS buffer (with 10 mM MgCl2, 0.7 mM CaCl2, 1.4 mM glucose, 0.2% bovine serum albumin) immediately prior to exposure (37 °C for 1 or 4 h) to 180 pM [125I]hU-II and competing ligands. Following three washes with DPBS, the cells were lysed with 2 N NaOH and the resulting lysate was counted using a gamma counter (Wallac Wizard).
Intracellular [Ca2+]-mobilization assay
Intracellular [Ca2+]-mobilization experiments were performed at 22 °C in hUT recombinant Chinese hamster ovary (CHO) cells using a fluorometric imaging plate reader (Cerep, Paris, France). Antagonists (palosuran, SB-657510; 0.1 nM–10 μM) were added 10 min prior to the addition of 10 nM hU-II and IC50 curves were generated by nonlinear regression analysis.
Assessment of contractility in mammalian isolated arteries
Endothelium-denuded arterial rings (approximately 3 mm in length) were cleaned of adherent tissues and suspended in Krebs solution of the following composition (mM): NaCl, 112.0; KCl, 4.7; KH2PO4, 1.2; MgSO4, 1.2; CaCl2, 2.5; NaHCO3, 25.0; dextrose, 11.0. Indomethacin (0.01 mM) was included in all experiments unless stated otherwise. Isometric force responses (measured using MLT0201/D transducers) were recorded digitally (Chart 5.0 software; ADInstruments, Colorado Springs, CO, USA) under optimal resting tension (1.0, 2.0, 2.0 and 0.5 g in rat, cat, monkey and transgenic mouse vessels, respectively; Douglas et al., 2000; Behm et al., 2004). Following 1 h equilibration, vessels were treated with 60 mM KCl and 1 μM phenylephrine (subsequent responses were normalized to KCl). Functional endothelial loss was confirmed using 10 μM carbachol. Paired arteries were pretreated (30 min) with vehicle (0.1% dimethylsulphoxide), palosuran or SB-657510 following which cumulative concentration–response curves to hU-II were constructed (tissues were used to generate only one concentration–response curve).
Rat isolated aortae
Following induction of anaesthesia (5% isoflurane in O2), male Sprague–Dawley or Wistar rats (400 g; Charles River, Raleigh, NC, USA) were killed by exsanguination and proximal thoracic aortae were isolated (Douglas et al., 2000). Care was taken to isolate consistently a ∼15–20 mm length of proximal descending aorta and isolated rings were randomly assigned to organ baths (to minimize any ‘reactivity bias' resulting from differential receptor distribution/spare receptor reserve or drug treatment; Itoh et al., 1988; Douglas et al., 2000; Maguire et al., 2000). The influence of cyclooxygenase inhibition was also investigated where Wistar rat studies were performed in the presence and absence of 10 μM indomethacin.
Cat isolated aortae and femoral arteries
Femoral arteries and thoracic aortae were isolated from adult male cats (4–5 kg; Liberty Research Inc., Waverly, NY, USA) following sodium pentobarbitone overdose (100 mg kg−1, i.v. Fatal-Plus) as previously described (Behm et al., 2004).
Cynomolgus monkey isolated renal and mesenteric arteries
Renal and superior mesenteric arteries were isolated from male cynomolgus monkeys (4–7 kg; Primate Products, Miami, FL, USA; Covance, Alice, TX, USA: Charles River, Andover, MA, USA; Mannheimer, Homestead, FL, USA) following sodium pentobarbitone overdose (100 mg kg−1, i.v. Fatal-Plus; Douglas et al., 2000).
hUT transgenic mouse isolated thoracic aorta
Male hUT transgenic mice (25–30 g) were anaesthetized (5% isoflurane: O2) and thoracic aortae were isolated following exsanguination (Douglas et al., 2000). Transgenic mice were originally generated using an overexpression cassette consisting of the promoter of the SM22α gene (22 kDa smooth muscle-specific protein; MscI restriction fragment containing 2736 bp of 5′-flanking region, the 65 bp first exon and 1188 bp of the first intron; Moessler et al., 1996) driving expression of the hUT cDNA linked to a LacZ (gene encoding β-galactosidase) reporter gene through an internal ribosome entry site element. The SM22α promoter drives expression in vascular smooth muscle cells (Li et al., 1996; Moessler et al., 1996). The gene construct was microinjected into isogenic C57B1/6 single-celled mouse oocytes. Positive founder mice were identified by PCR (LacZ reporter gene primers: 5 primer: 5-GACCAGCCCTTCCCGGCTGTGCCG-3; 3 primer: 5-GCCGACCACGGGTTGCCGTTTTCA-3; 30 cycles of 94 °C (45 s), 65 °C (45 s) and 72 °C (45 s) giving a product size of 300 bp) and numbers were expanded to produce experimental animals.
Reagents and materials
SB-657510 (Douglas et al., 2005; Figure 1) and palosuran (Figure 1) were synthesized at GlaxoSmithKline. Palosuran structure was confirmed through one-dimensional [1H]NMR, two-dimensional NMR and liquid chromatography with mass spectroscopic detection. Fatal-Plus was supplied by Vortech Pharmaceuticals (Dearborn, MI, USA). Carbachol, indomethacin, phenylephrine and bovine serum albumin were from Sigma (St Louis, MO, USA). All other reagents were of analytical grade. WGA-SPA beads were from Amersham (Arlington Heights, IL, USA); DPBS from Gibco (Grand Island, NY, USA). SJRH30, American Type Culture Collection number CRL-2061 was from ATCC (Manassas, VA, USA). The MLT0201/D transducers were from Letica (Barcelona, Spain) and the ADInstruments Chart 5.0 software from ADInstruments.
Data analysis
Binding curves were analysed by nonlinear regression (GraphPad Prism) and concentration–contraction curves were fitted to a logistic equation (Douglas et al., 2005). Antagonist affinity (Kb) was determined using the Schild equation (Jenkinson et al., 1998). All values are mean±s.e.mean (n represents the number of independent triplicate experiments or the number of animals from which vessels were isolated). Statistical comparisons were made using a paired, two-tailed t-test or ANOVA (Dunnett's post-test analysis). Differences were considered significant when P⩽0.05.
Results
The pharmacological properties of palosuran were assessed in both radioligand (membrane cf. whole cell) binding and in vitro functional bioassay ([Ca2+]i mobilization, isolated arterial contraction) studies. Palosuran functioned as a high-affinity, ‘primate-selective' UT ligand in membrane-binding assays but lost almost two orders of magnitude in affinity when profiled in a whole cell radioligand-binding format. Accordingly, palosuran lacked appreciable (that is, commensurate with primate membrane Ki values) activity in functional, intact tissue bioassays.
Radioligand-binding studies in cell membrane preparations demonstrate that palosuran exhibits primate UT selectivity
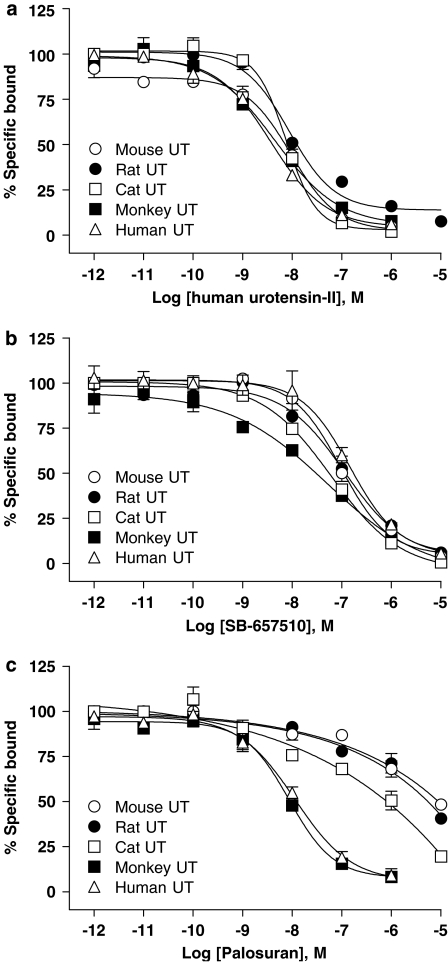
hU-II was a high-affinity UT ligand across species when assessed in a recombinant UT cell membrane-binding assay (Ki values of 2–8 nM at rat, cat and primate UT, Ki 28 nM at mouse UT; Table 1 and Figure 2a). Whereas the sulphonamide SB-657510 also functioned as a high-affinity, pan-species hUT ligand (Ki values of 17–65 nM, differing by <3-fold in mouse, rat, cat and monkey UT membranes cf. human UT Ki; Table 1 and Figure 2b), palosuran exhibited primate UT selectivity (>200-fold selectivity cf. non-primate receptors; Table 1 and Figure 2c) under identical conditions, that is, identical high-affinity binding was evident in primate and non-human primate UT cell membranes (Ki values of 4–5 nM) but no affinity was evident (Ki values >1 μM) at rat, mouse or cat UT.
Table 1.
Palosuran is a high-affinity, ‘primate-specific' UT receptor ligand in recombinant cell membranes: radioligand-binding affinity constants (Ki values, [125I]hU-II competition binding) for human urotensin-II (hU-II), palosuran (ACT-058362) and SB-657510 in mammalian recombinant UT receptor membranes
|
hU-II |
SB-657510 |
Palosuran (ACT-058362) |
||||
|---|---|---|---|---|---|---|
| Ki (nM) | Affinity relative to hUTa | Ki (nM) | Affinity relative to hUTa | Ki (nM) | Affinity relative to hUTa | |
| Human (n=7) | 2±1 | — | 61±8 | — | 5±1 | — |
| Monkey (n=3) | 5±2 | 2.5 | 17±5 | 0.3 | 4±1 | 0.8 |
| Cat (n=3) | 8±1 | 4.0 | 30±7 | 0.5 | >1000 | >200.0 |
| Rat (n=3) | 5±1 | 2.5 | 65±7 | 1.1 | >1000 | >200.0 |
| Mouse (n=3) | 28±5 | 14.0 | 56±5 | 0.9 | >1000 | >200.0 |
Abbreviation: hUT, human urotensin-II receptor.
Relative affinities for hU-II, SB-657510 and palosuran in non-human UT isoforms are expressed relative to their respective human UT Ki values. All values are expressed as mean±s.e.mean.
Figure 2.
[125I]hU-II (human urotensin-II) radioligand competition binding at recombinant mouse, rat, cat, monkey and human UT in membrane preparations. In contrast to cold hU-II and SB-657510, which exhibited similar affinities for rodent, feline and primate UT (a, b), palosuran (c) exhibited ‘primate UT selectivity'.
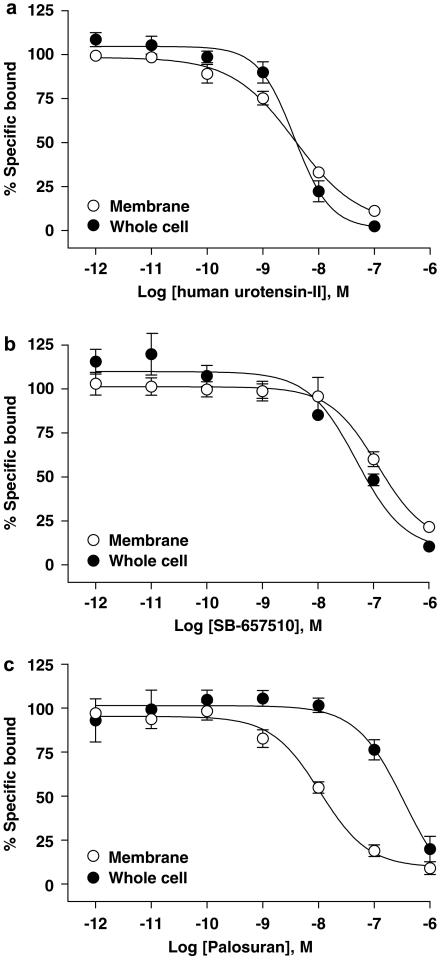
Palosuran exhibits a selective loss in human recombinant UT affinity in an intact cell radioligand-binding assay
hU-II was a high-affinity human UT ligand irrespective of whether cell membrane- (Ki 2 nM) or whole cell (Ki 3 nM; Table 2 and Figure 3a)-binding formats were employed (1.5-fold difference). Similarly, SB-657510 Ki values were identical (0.8-fold difference) in cell membrane and whole cell-binding formats (Ki values of 61 and 46 nM, respectively; Table 2 and Figure 3b). In contrast, however, palosuran experienced a significant 54-fold loss in hUT affinity when profiled in a whole cell-binding format (0.3 μM Ki in intact cells cf. 5 nM Ki in hUT cell membranes; Table 2 and Figure 3c).
Table 2.
Palosuran lacks high affinity for recombinant hUT receptor expressed in intact cells: radioligand-binding affinity constants (Ki values, [125I]hU-II competition) for human urotensin-II (hU-II), palosuran and SB-657510 at the recombinant hUT receptor in membrane and whole cell preparations
| Membrane binding Ki (nM; n=7) | Whole cell binding Ki (nM; n=4) | Relative affinitya | |
|---|---|---|---|
| Urotensin-II | 1.9±0.5 | 2.9±0.5 | 1.5 |
| SB-657510 | 61.4±7.5 | 46.2±3.0 | 0.8 |
| Palosuran | 5.1±0.7 | 276.0±66.9*** | 54.1 |
Abbreviation: hUT, human urotensin-II receptor.
Relative affinity is expressed as the whole cell binding human UT Ki relative to the corresponding value obtained in hUT membrane preparations. All values are expressed as mean±s.e.mean. Membrane data are from Table 1 and are included for ease of comparison. Statistical comparisons were made using an unpaired, two-tailed t-test where ***P<0.001 (versus Ki obtained for the corresponding ligand in membrane preparation).
Figure 3.
[125I]hU-II (human urotensin-II) competed for binding at recombinant hUT (human urotensin-II receptor) in membrane and intact cell preparations with (a) hU-II, (b) SB-657510 and (c) palosuran. In contrast to both hU-II and SB-657510 where hUT-binding affinities were similar on membranes and intact cells, the inhibitory binding affinity of palosuran for hUT was 54-fold less potent in intact cells as compared to membrane preparations.
Palosuran lacks high affinity for native hUT receptor endogenously expressed in the rhabdomyosarcoma cell line SJRH30
hU-II and SB-657510 competed with [125I]hU-II for binding at native hUT receptor in intact SJRH30 cells with affinities similar to those determined at human recombinant UT cell membranes (Table 3). In contrast, however, palosuran exhibited a ⩾10-fold reduction in affinity in intact SJRH30 cells as compared to human recombinant UT cell membranes. Ki values for all three ligands were not significantly altered by length of incubation (1–4 h; Table 3).
Table 3.
Palosuran lacks high affinity for native hUT receptor endogenously expressed in the rhabdomyosarcoma cell line SJRH30: radioligand-binding affinity constants (Ki values, [125I]hU-II competition) for human urotensin-II (hU-II), palosuran and SB-657510 at the native hUT receptor in whole cell preparations following 1 and 4 h incubation periods
| Ki (nM) |
Relative affinitya |
||||
|---|---|---|---|---|---|
| 1 h incubation | 4 h incubation | Ratio | 1 h incubation | 4 h incubation | |
| Urotensin-II | 0.2±0.0 | 0.3±0.1 | 1.5 | 0.1 | 0.2 |
| SB-657510 | 56.9±9.7 | 213.0±71.2 | 3.7 | 0.9 | 3.5 |
| Palosuran | 49.8±3.3 | 192.5±57.2 | 3.9 | 9.8 | 37.7 |
Abbreviation: hUT, human urotensin-II receptor.
Relative affinity is expressed as the native human UT Ki relative to the corresponding value obtained in recombinant hUT membrane preparations (see Table 1). All values are expressed as mean±s.e.m. Statistical comparisons were made using an unpaired, two-tailed t-test and no values were determined from 1 h (n=3).
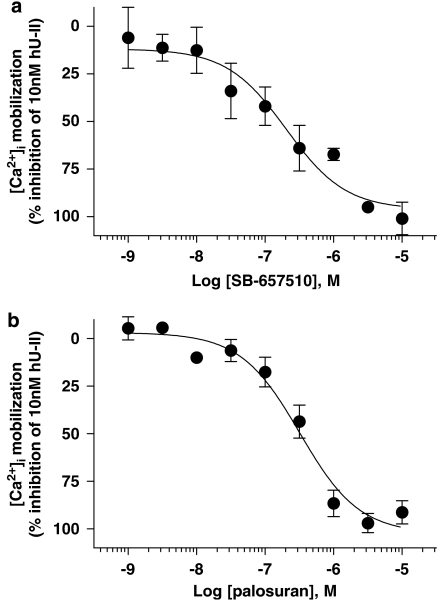
Palosuran inhibits hU-II-induced [Ca2+]i mobilization in recombinant hUT-CHO cells with a diminished functional activity (IC50) as compared to its binding affinity (Ki)
Pretreatment with SB-657510 inhibited [Ca2+]i mobilization elicited by 10 nM hU-II with an IC50 of 180±46 nM (Figure 4a), a value consistent with membrane-binding data (61 nM Ki at human UT membranes; Table 1). In contrast to SB-657510, however, palosuran was significantly (65-fold) less active at inhibiting [Ca2+]i mobilization (IC50 323±67 nM; Figure 4b) as compared to its membrane-binding affinity (5 nM Ki at human UT membranes; Table 1).
Figure 4.
Inhibition of 10 nM hU-II (human urotensin-II)-induced [Ca2+]i mobilization in recombinant hUT (human urotensin-II receptor)-CHO (Chinese hamster ovary) cells by (a) SB-657510 and (b) palosuran. SB-657510 inhibited [Ca2+]i mobilization with an IC50 of 180±46 nM, a value consistent with membrane-binding affinity (Ki 61 nM at human UT membranes; Table 1). In contrast, palosuran was significantly (65-fold) less active at inhibiting [Ca2+]i mobilization (IC50 323±67 nM) as compared to its membrane-binding affinity (Ki 5 nM at human UT membranes; Table 1).
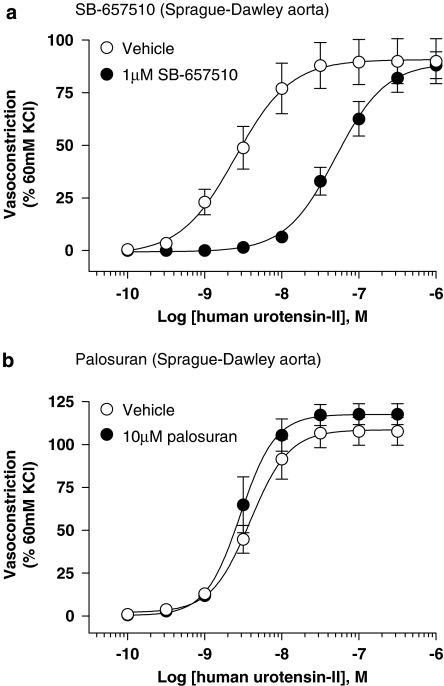
Palosuran is devoid of significant antagonist activity in rat intact, isolated aortae
SB-657510 (1 μM) functioned as an active UT antagonist in Sprague–Dawley and Wistar rat isolated aortae causing significant (P<0.01–0.001), ∼20-fold rightward shifts in the hU-II concentration–response curve (Tables 4 and 6, and Figures 5 and 6). Antagonism was competitive with no alteration in Emax. In accord with the rat UT membrane Ki (65 nM; Table 1), SB-657510 Kb ranged from 49 to 79 nM in the rat aorta in both strains. In contrast, Tables 5 and 6 and Figures 5 and 6 demonstrate that palosuran was inactive in both Sprague–Dawley and Wistar rat isolated aortae (Kb values >10 μM), commensurate with rat UT binding Ki>1 μM (Table 1). As such, this quinolinyl-urea was >100-fold less active than SB-657510 in these two rat UT functional bioassays.
Table 4.
SB-657510 is a competitive, ‘pan-species' active UT antagonist in mammalian isolated arteries
| Emax (% KCl) |
EC50 (nM) |
SB-657510 Kb (nM) | Kb/Ki | |||
|---|---|---|---|---|---|---|
| Vehicle-treated control | SB-657510-treated | Vehicle-treated control | SB-657510-treated | |||
| Sprague–Dawley rat | ||||||
| Thoracic aorta (n=8) | 90±11 | 89±6 | 3.1±0.6 | 59.5±10.3*** | 62±10 | 1.0 |
| Cat | ||||||
| Femoral artery (n=4) | 206±36 | 239±43 | 0.9±0.2 | 138.3±53.8* | 106±47 | 3.5 |
| Thoracic aorta (n=5) | 126±10 | 140±10 | 1.5±0.3 | 50.1±9.9** | 824±446 | 27.5 |
| Monkey | ||||||
| Renal artery (n=6) | 130±10 | 143±22 | 0.7±0.1 | 146.5±38.4* | 71±18 | 4.2 |
| Mesenteric artery (n=5) | 155±24 | 135±19 | 2.1±0.8 | 189.3±59.9* | 158±71 | 9.3 |
| Human UT transgenic mouse | ||||||
| Thoracic aorta (n=5) | 68±5 | 74±10 | 1.7±0.3 | 153.7±22.9** | 117±23 | 1.9 |
All values are expressed as mean±s.e.mean. Tissues were pretreated for 30 min with either 1 μM (rat aorta) or 10 μM SB-657510 prior to generating cumulative concentration–response curves to hU-II (human urotensin-II). Kb/Ki is a ratio comparing functional activity of SB-657510 in an isolated arterial preparation (Kb above) with the corresponding affinity (Ki, see Table 1) in the UT recombinant binding assay in the same species. Statistical comparisons were made using a two-tailed t-test where *P<0.05, **P<0.01 and ***P<0.001 versus vehicle.
Table 6.
SB-657510 is a competitive UT antagonist, whereas palosuran lacks inhibitory activity in the Wistar rat isolated aorta
| Emax (% KCl) |
EC50 (nM) |
Kb (nM) |
||||||
|---|---|---|---|---|---|---|---|---|
| Vehicle-treated control | SB-657510- treated | Palosuran- treated | Vehicle-treated control | SB-657510- treated | Palosuran- treated | SB-657510- treated | Palosuran- treated | |
| No indomethacin | 117±9 | 130±5 | 111±8 | 2.4±0.7 | 48.3±11.4** | 4.9±1.4 | 49±10 | >10 000 |
| Indomethacin 10 μM | 104±9 | 108±6 | 94±12 | 2.2±0.5 | 41.1±12.6** | 3.5±0.8 | 79±14 | >10 000 |
All values are expressed as mean±s.e.mean. Tissues were pretreated for 30 min with vehicle, 1 μM SB-657510 or 10 μM palosuran prior to generating cumulative concentration–response curves to hU-II (human urotensin-II). Statistical comparisons of Emax and EC50 values were made using a paired, two-tailed t-test where **P<0.01 versus vehicle. The presence of indomethacin did not alter the contractile responses to hU-II (Emax, EC50) nor the inhibitory effects of the antagonists (Kb; paired, two-tailed t-test; P>0.05).
Figure 5.
Inhibition of hU-II (human urotensin-II)-induced contraction of Sprague–Dawley rat isolated aorta by (a) 1 μM SB-657510 and (b) 10 μM palosuran. Whereas SB-657510 inhibited hU-II contraction with a Kb of 62 nM (Table 4), palosuran failed to elicit an inhibitory effect (Kb>10 000 nM; Table 5).
Figure 6.
Inhibition of hU-II (human urotensin-II)-induced contraction of Wistar rat isolated aorta by 1 μM SB-657510 in the (a) absence and (c) presence of 10 μM indomethacin and 10 μM palosuran in the (b) absence and (d) presence of 10 μM indomethacin. Whereas SB-657510 inhibited hU-II contraction with Kb values of 49–79 nM, palosuran failed to elicit an inhibitory effect (Kb>10 000 nM; Table 6).
Table 5.
Palosuran exhibits poor activity as a UT antagonist in mammalian isolated arteries
| Emax (% KCl) |
EC50 (nM) |
Palosuran Kb (nM) | Kb/Ki | |||
|---|---|---|---|---|---|---|
| Vehicle-treated control | Palosuran-treated | Vehicle-treated control | Palosuran-treated | |||
| Rat | ||||||
| Thoracic aorta (n=5) | 109±8 | 118±6 | 4.0±0.6 | 3.3±0.6 | >10 000 | >10.0 |
| Cat | ||||||
| Femoral artery (n=3) | 235±25 | 202±16 | 0.5±0.1 | 1.1±0.2 | 8541±684 | 8.5 |
| Thoracic aorta (n=3) | 160±6 | 153±15 | 2.9±0.6 | 3.6±1.1 | >10 000 | >10.0 |
| Monkey | ||||||
| Renal artery (n=4) | 127±33 | 124±27 | 1.2±0.4 | 14.0±5.0 | 1568±773 | 392.0 |
| Mesenteric artery (n=3) | 127±42 | 143±47 | 1.2±0.2 | 7.7±3.0 | 2760±984 | 690.0 |
| Human UT transgenic mouse | ||||||
| Thoracic aorta (n=4) | 69±4 | 66±7 | 2.6±0.6 | 14.8±0.7*** | 2,214±637 | 442.8 |
All values are expressed as mean±s.e.mean. Tissues were pretreated for 30 min with 10 μM palosuran prior to generating cumulative concentration–response curves to hU-II (human urotensin-II). Kb/Ki is a ratio comparing functional activity of palosuran in an isolated arterial preparation (Kb above) with the corresponding affinity (Ki, see Table 1) in the UT recombinant binding assay in the same species. Statistical comparisons of Emax and EC50 values were made using a paired, two-tailed t-test where ***P<0.001 versus vehicle.
Palosuran was soluble under such conditions (5.0±0.1 μM calculated organ bath concentration at 30 min following incubation).
The presence of indomethacin (10 μM) did not alter the contractile responses to hU-II or the inhibitory effects of the antagonists (Table 6 and Figure 6).
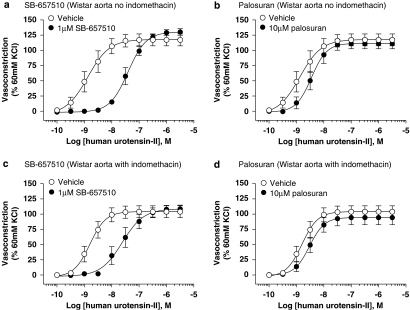
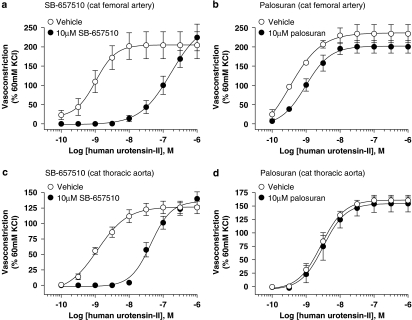
Palosuran lacks appreciable activity as a hU-II antagonist in cat intact, isolated aortae and femoral arteries
SB-657510 (10 μM) antagonized the contractile actions of hU-II in both cat isolated femoral arteries and aortae, causing significant 154- (P<0.05) and 33-fold (P<0.01) rightward shifts in the agonist concentration–response curves, respectively (Kb values of 106 and 824 nM; Table 4 and Figure 7). Antagonism was competitive in nature (concentration–response curves were shifted in a parallel manner with no alteration in Emax).
Figure 7.
Inhibition of hU-II (human urotensin-II)-induced contraction by 10 μM SB-657510 and 10 μM palosuran in cat isolated femoral artery (a, b) and thoracic aorta (c, d). Whereas SB-657510 inhibited hU-II contraction of cat isolated femoral artery and thoracic aorta with Kb values of 106 and 824 nM (Table 4), palosuran was a weak UT receptor functional antagonist in cat isolated arteries (Kb values of 8541 and >10 000 nM, respectively; Table 5).
In contrast, palosuran was inactive in cat isolated aortae (Kb>10 μM; Table 5 and Figure 7). This was in accord with the cat membrane binding (Ki>1 μM; Table 1). Although palosuran exhibited surmountable inhibitory activity in the cat isolated femoral artery, this was extremely weak (Kb ∼9 μM; Table 5 and Figure 7).
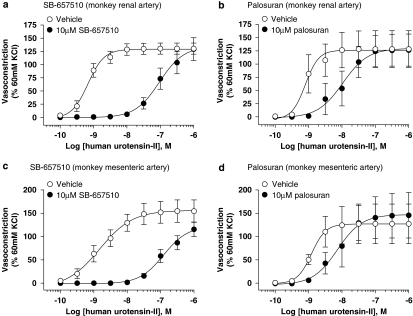
The high-affinity primate UT ligand palosuran is a weak hU-II antagonist in monkey intact isolated renal and mesenteric arteries
SB-657510 (10 μM) functioned as a UT antagonist in both monkey isolated renal and mesenteric arteries causing significant (P<0.05) 209- and 90-fold rightward shifts in the hU-II concentration–response curves, respectively (Table 4 and Figure 8). Antagonism was competitive (concentration–response curves were shifted in a parallel, manner with no alteration in Emax). Kb values were 71 and 158 nM in renal and mesenteric vessels, respectively.
Figure 8.
Inhibition of hU-II (human urotensin-II)-induced contraction by 10 μM SB-657510 and 10 μM palosuran in monkey isolated renal artery (a, b) and superior mesenteric artery (c, d). SB-657510 inhibited hU-II contraction of monkey isolated arteries (Kb values of 71 and 158 nM; Table 4) with activities similar to binding affinities at the monkey UT receptor (Ki 17 nM; Table 1). In contrast, palosuran was a weak functional antagonist; in monkey isolated artery Kb values (1568 and 2760 nM; Table 5) were 392- to 690-fold lower than monkey UT Ki values (4 nM; Table 1).
In contrast to the membrane-binding data (4 nM Ki at monkey UT membranes; Table 1), palosuran (10 μM) was significantly (392- to 690-fold) less active in monkey isolated renal and mesenteric arteries with Kb values of 1.6 and 2.8 μM, respectively (indeed, the modest 6- to 11-fold rightward shifts in the hU-II EC50 s were not statistically significant; Table 5, and Figures 8b and d).
Antagonism in the human UT transgenic mouse isolated thoracic aorta
As predicted from the human recombinant UT membrane/intact cell-binding studies (Ki values of 61 and 46 nM, respectively; Tables 1 and 2), SB-657510 was an active (Kb 117 nM) UT antagonist in mouse isolated aortae expressing the human UT transgene (Table 4 and Figure 9). SB-657510 (10 μM) caused a significant (P<0.01), parallel, 90-fold rightward shift in the hU-II concentration–response curve with no alteration in Emax consistent with a competitive mode of antagonism.
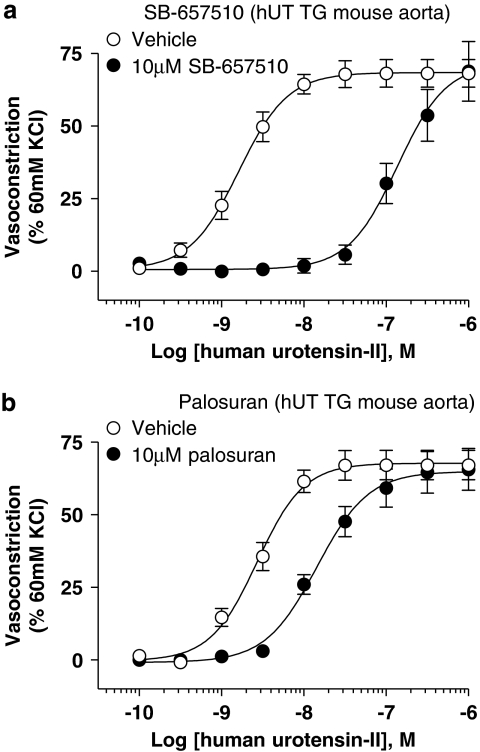
Figure 9.
Inhibition of hU-II (human urotensin-II)-induced contraction of hUT TG (human urotensin-II transgenic mice) mouse isolated aorta by (a) 10 μM SB-657510 and (b) 10 μM palosuran. Whereas SB-657510 inhibited hU-II contraction with a Kb (117 nM; Table 4) similar to its membrane hUT Ki (61 nM; Table 1), palosuran was a weak functional antagonist, inhibited hU-II-induced contraction with a Kb (2214 nM; Table 5) 443-fold lower than its membrane hUT Ki (5 nM; Table 1).
In contrast, however, palosuran was 19-fold weaker than SB-657510 in the same preparation (Table 5 and Figure 9b). Consistent with the differential membrane cf. intact cell hUT binding Ki, the resultant 2.2 μM Kb obtained in the mouse transgenic hUT isolated aorta was significantly lower than the hUT membrane Ki (5 nM). This corresponded to a 434-fold loss in activity in the transgenic hUT functional bioassay.
Discussion and conclusions
The present study demonstrates that palosuran is a suboptimal pharmacological tool for use in preclinical (non-primate) studies. Palosuran lacks appreciable affinity (Kb>1 μM) in numerous functional bioassays, specifically isolated arterial contractility assays from a diverse range of species, including the rat, cat, monkey and transgenic hUT mouse. Such findings, perhaps, provide insight into the disappointing clinical efficacy data reported recently in diabetic nephropathy patients (see Clozel et al., 2006). In this patient cohort, significant clinical exposure was most likely never attained, that is exposure was insufficient to provide adequate levels of hUT receptor occupancy. Specifically, the peak palosuran plasma concentration (Cmax ⩽260 nM) recorded in humans (Sidharta et al., 2006) was ⩾10-fold lower than the UT affinity of palosuran determined in mammalian isolated arteries in the present study (Kb values of ∼2 to >10 μM across species). The peak palosuran concentrations attained in man would, therefore, equate to ⩽10% total human UT receptor occupancy (estimated using the modified Langmuir–Clark equation where the fraction of total receptor occupancy is described by RT = 1/1+(KD/[A]); see Bowman and Rand, 1980). However, this is most likely an overestimate of palosuran–hUT occupancy because it assumes that
Palosuran–plasma protein binding does not restrict access from the plasma to the ‘extra-circulatory' site of action (as plasma albumin binding is 96%, the maximum ‘free fraction' of palosuran in human plasma could be as little as ∼10 nM (4% total drug concentration) resulting in ⩽1% hUT occupancy based on the Langmuir–Clark calculation described above; Sidharta et al., 2006).
Palosuran concentration is maintained at a constant 260 nM (Cmax) throughout the 12-h dosing period. This is clearly an invalid assumption (Sidharta et al., 2006) as pharmacokinetic analysis demonstrates that patients readily clear palosuran from the plasma (assuming palosuran–hUT dissociation (Koff) is not rate limiting; Copeland et al., 2006). Palosuran concentration falls rapidly to <50 nM at t12 h (this would equate to ∼2% hUT occupancy or <0.1% if plasma protein binding limits access to the extra-luminal hUT site of action).
No native ligand (such as hU-II, URP, etc.) is present to compete with palosuran for UT occupancy. It is estimated that human plasma U-II-like immunoreactivity is ⩽10 nM (Heller et al., 2002; Aiyar et al., 2004; Heringlake et al., 2004; Lapp et al., 2004; Krüger et al., 2005; Rdzanek et al., 2006), a concentration ∼10- to 100-fold above its EC50 activity as a vasoconstrictor of human blood vessels (∼0.1 nM in small coronary epicardial arteries; Maguire et al., 2004). This estimate of ‘intrinsic' UT occupancy by the native ligand(s) would equate to occupancy of ∼99% total hUT reserve (NB: hU-II is also a ‘pseudo-irreversible' ligand; Douglas et al., 2004a; Tölle and van der Giet, 2008). At this level of hUT occupancy by hU-II, Schild analysis suggests that significant UT antagonism would only be observed at ∼200 μM palosuran, plasma concentrations ∼100-fold above the 2 μM Kb determined at hUT in transgenic mice (Bowman and Rand, 1980; Kenakin, 2003). Such concentrations are clearly never attained in the clinic using a 125 mg b.i.d. dosage regimen (1000-fold below even at Cmax; Sidharta et al., 2006).
Palosuran is purported to regulate endocrine, angiogenic and, most strikingly, renal (dys)function in the rat both in vitro and in vivo (Clozel et al., 2004, 2006; Egido et al., 2005; Marco et al., 2005; Albertin et al., 2006; Spinazzi et al., 2006). Although the current study demonstrates that palosuran is indeed a high-affinity human and primate UT ligand in cell membrane assays (Ki values of 4–5 nM, in accord with Clozel et al., 2004), it was inactive at rat, mouse and cat UT membranes (Ki values >1 μM). Such observations mirror those reported previously (IC50 values >10 μM in rat UT radioligand and [Ca2+]i mobilization assays; Clozel et al., 2004). These findings are supported further by a lack of activity in the rat isolated aorta (Kb>10 μM in the present study). These observations suggest that the beneficial actions of palosuran in rat renal, endocrine, angiogenic, etc. assays (Clozel et al., 2004, 2006; Egido et al., 2005; Marco et al., 2005; Albertin et al., 2006; Spinazzi et al., 2006) reflect the use of high (⩾1 μM), ‘non-selective', palosuran concentrations in these preclinical models, that is, these beneficial actions are unrelated to UT occupancy.
To date, very little is known about the detailed pharmacological characteristics of palosuran (beyond the limited observations reported in the rat aorta using 5-HT, ET-1 and noradrenaline with 10 μM palosuran, a concentration less than twofold above the pD2 for U-II (6 μM); Clozel et al., 2004). Indeed, this is supported, for example, by recent data from Malagon et al. (2008) demonstrating that palosuran is a somatostatin receptor agonist (altering [3H]thymidine incorporation and [Ca2+]i mobilization at recombinant sst2 and sst5 subtypes) at low concentrations (10 nM). Indeed, somatostatin agonists have been shown to lower both glucose and albuminuria levels in diabetic mice (Segev et al., 2004; Strowski et al., 2006). In this context, it is noteworthy that the peptidic UT antagonist SB-710411 does not mimic the actions of palosuran in rat angiogenesis and adrenal cell assays (Albertin et al., 2006; Spinazzi et al., 2006), even though this moiety is significantly more active at rat UT than palosuran (aorta Kb 716 nM cf. >10 μM; Behm et al., 2006). Thus, it is prudent to conclude that caution must be exercised when interpreting data generated in preclinical models with palosuran due to (a) poor non-primate activity, (b) limited understanding of selectivity (particularly where responses to palosuran cannot be replicated with alternate UT antagonists such as SB-710411) and (c) the use of high-dosing regimens. It is, therefore, concluded that UT antagonists, such as urantide, GSK248451 and UFP-803, represent more suitable tools than palosuran for investigations performed in non-primate species including the rat (such moieties are ∼300- to 2000-fold more active than palosuran in the rat aortic contraction assay (5–35 nM Kb values); Patacchini et al., 2003; Behm et al., 2006; Camarda et al., 2006).
Concerns regarding palosuran selectivity also apply in vivo where similar ∼5 μM plasma concentrations (Clozel et al., 2004, 2006) were achieved in the rat ischaemia and diabetic nephropathy models. The exposures achieved in both rodent models lie below the rat aorta functional activities described both herein (>10 μM) and by Clozel et al. (2004; ∼6 μM pD2 in rat aortae, >10 μM IC50 values in rat recombinant UT CHO assays). It is difficult to envision significant, sustained UT receptor occupancy by palosuran under such conditions (yet strikingly, chronic oral palosuran administration effectively normalized both the hyper-triglyceridaemia and hyper-insulinaemia observed in the diabetic rat; Clozel et al., 2006). Considering the relative exposure and lack of rat UT affinity, an ‘off target' (non-UT) effect(s) is an equally plausible explanation for the profound metabolic effects observed in these nephropathy models.
Such considerations would have been mitigated in part had it been demonstrated that the dosage regimens employed were effective at (selectively) inhibiting the actions of an exogenous U-II challenge in vivo. Although it is acknowledged that such ‘challenge' studies have limitations (Clozel et al., 2004), they would have been illuminating with regard to pharmacokinetic cf. pharmacodynamic considerations, for example, the influence of plasma protein binding on palosuran-free fraction and/or function, etc. Indeed, in vivo challenge models have been employed successfully for peptidic UT antagonists (GSK248451, U-II-mediated pressor response in the cat; Behm et al., 2004; UFP-803, U-II-induced plasma extravasation in the mouse; Camarda et al., 2006) and for other peptide hormones (for example, ETA antagonist Ro 46-2005; Clozel et al., 1993a, 1993b). Thus, to date, there are no data that convincingly demonstrate (a) significant and (b) selective in vivo UT receptor blockade with palosuran at the dosage regimens employed in these nephropathy models.
In contrast to the present findings (Kb>10 μM), palosuran was reported to function as an ‘insurmountable' inhibitor (suppression of Emax) in the Wistar rat isolated aorta (pD2 ∼6 μM; Clozel et al., 2004). There is no obvious explanation for the disparity between the original characterization (Clozel et al., 2004) and the present investigation where both Wistar and Sprague–Dawley rats were used. Similar protocols were employed by both groups of investigators and the integrity of the palosuran was confirmed in the present study (both physically (NMR and LC-MS detection) and using pharmacological techniques (confirmation of high-affinity binding to primate UT)). Nevertheless, a lack of activity at the rat aorta described herein is in complete agreement with both (a) the rat UT radioligand-binding data (including those described by Clozel et al., 2004) and (b) the functional bioassays performed in arteries isolated from other species (poor activity in cat, monkey and transgenic mouse arteries). The disparity is unlikely to reflect hemi-equilibria considerations or allosterism as, where weak hU-II antagonism was observed, inhibition was competitive (dextral, parallel shifts in the U-II response curves in cat, monkey and hUT transgenic mouse arteries; Kb values of ∼2–9 μM).
One alternative explanation for the differences in activity in the rat aorta relates to a procedural consideration, namely randomization of rings to treatment (see Methods). Such an action is imperative to minimize ‘reactivity bias' (U-II Emax declines precipitously as one transitions to distal regions of the thoracic aorta; Itoh et al., 1988; Douglas et al., 2000; Maguire et al., 2000). Failure to randomize paired tissues to treatment could result in an apparent, yet erroneous, treatment-dependent inhibition marked by Emax suppression. However, such an argument remains speculative as it us unknown whether or not Clozel et al. (2004) performed such tissue randomization in their studies.
Both hU-II (Ki values of ∼2–3 nM) and SB-657510 (Ki values of ∼46–61 nM) inhibited [125I]hU-II binding to recombinant hUT with identical affinities (<2-fold difference) in both intact cells and membranes. In contrast, palosuran hUT affinity was attenuated 10- to 54-fold when assessed in intact cells (Ki 50–276 nM cf. Ki 5 nM in membrane formats). Similar observations were made at native hUT receptor in intact SJRH30 cells. These results are in accord with the loss in affinity originally reported by Clozel et al. (2004) in both CHO (recombinant hUT) and TE-671 (native hUT) cells. The mechanism(s) underlying the pronounced loss in affinity is, to date, unknown but could suggest that palosuran requires access to a distinct (allosteric) binding site, one that is restricted by an intact cell membrane. However, cell permeabilization (0.1–0.5% v v−1 saponin; Breivogel et al., 2004) did not alter palosuran hUT-binding affinity in whole cells (data not shown). In addition, Ki values for all three ligands in SJRH30 cells were not significantly altered by length of incubation (1–4 h), indicating that equilibrium binding was attained. Thus, a putative ‘slow' Kon was unlikely to be rate limiting under these conditions.
It should be noted that the present study did not measure the metabolic stability of palosuran (or the generation of putative metabolites) directly within tissues. High-efficiency catabolism of palosuran at the intact cell/smooth muscle site of action (but not in cell membranes) is speculative but could represent a possible explanation for the poor affinity of this moiety in functional bioassays. This seems unlikely, however, as palosuran was clearly soluble under the present experimental conditions (∼5 μM concentration was measured by LC-MS/MS in organ bath studies). In other words, there remained a large, 1000-fold excess concentration of palosuran (cf. hUT Ki 5 nM in cell membranes) in the Krebs solution in the isolated blood vessel experiments.
The loss of affinity in whole cell-binding studies is consistent with the observation that palosuran was 65-fold less active at inhibiting [Ca2+]i mobilization (IC50 323 nM) in recombinant hUT-CHO cells as compared to the membrane-binding affinity (Ki 5 nM). These results are in contrast to previous results reported by Clozel et al. (2004) where palosuran exhibited only a fivefold loss in activity at inhibiting [Ca2+]i mobilization (17 nM IC50) cf. membrane-binding affinity (4 nM). Although the reason for such a discrepancy is unclear (essentially identical protocols were employed), it is of interest to note that Clozel et al. (2004) demonstrated a 38-fold loss in activity when assessing the inhibitory effects of palosuran on pERK1/2 generation (150 nM IC50), a value consistent with the inhibitory activity of palosuran on [Ca2+]i mobilization observed in the present study. In contrast to palosuran, SB-657510 inhibited [Ca2+]i mobilization with an IC50 value consistent with membrane-binding data.
Palosuran also lacked appreciable activity at inhibiting hU-II-induced contraction of monkey arteries (Kb values of 1.6–2.8 μM) and hUT transgenic mouse aorta (Kb 2.2 μM), 392- to 690-fold and 443-fold reductions in functional activity. As in the binding assays, a similar loss in activity was not evident with SB-657510 (rat, <2-fold difference; cat, 4- to 28-fold difference; monkey, 4- to 9-fold difference; hUT transgenic mouse, <2-fold difference). Thus, such phenomena are consistent across both strains and species.
In summary, it is concluded that palosuran is a suboptimal pharmacological tool for use in preclinical (non-primate) studies as (a) it lacks affinity at ‘non-human' UT isoforms (mouse, rat and cat), (b) it is subject to 2–3 orders of magnitude loss in affinity/activity in intact cell assays and functional (tissue-based) assays and (c) insufficient information is available regarding the secondary pharmacological properties of this moiety (several actions of palosuran could not be replicated using an alternative peptidic UT antagonist, SB-701411). Finally, such findings provide a potential explanation for the disappointing clinical efficacy reported recently for palosuran in diabetic nephropathy patients (Clozel et al., 2006). As such, the (patho)physiological significance of U-II in diabetic nephropathy remains ambiguous.
Abbreviations
- CHO cells
Chinese hamster ovary cells
- DPBS
Dulbecco's phosphate-buffered saline
- palosuran
1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulphate salt
- SB-657510
2-bromo-N-[4-chloro-3-((R)-1-methyl-pyrrolidin-3-yloxy)-phenyl]-4,5-dimethoxybenzenesulphonamide HCl
- SJRH30
human rhabdomyosarcoma cell line
- SPA
scintillation proximity assay
- (h)U-II
(human) urotensin-II
- (h)UT
(human) urotensin-II receptor
Conflict of interest
DJB, JJM, JWD, MJN, HEF, CAE, RRH, KDH, SMH, CW and SAD are employees of GlaxoSmithKline.
References
- Aiyar N, Guida B, Ao Z, Disa J, Naselsky D, Behm DJ, et al. Differential levels of ‘urotensin-II-like' activity determined by radio-receptor and radioimmuno assays. Peptides. 2004;25:1339–1347. doi: 10.1016/j.peptides.2004.05.015. [DOI] [PubMed] [Google Scholar]
- Albertin G, Casale V, Ziolkowska A, Spinazzi R, Malendowicz LK, Rossi GP, et al. Urotensin-II and UII-receptor expression and function in the rat adrenal cortex. Int J Mol Med. 2006;17:1111–1115. [PubMed] [Google Scholar]
- Ames RS, Sarau HM, Chambers JK, Willette RN, Aiyar NV, Romanic AM, et al. Human urotensin-II is a potent vasoconstrictor and agonist for the orphan receptor GPR14. Nature. 1999;401:282–286. doi: 10.1038/45809. [DOI] [PubMed] [Google Scholar]
- Behm DJ, Doe CP, Johns DG, Maniscalco K, Stankus GP, Wibberley A, et al. Urotensin-II: a novel systemic hypertensive factor in the cat. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:274–280. doi: 10.1007/s00210-004-0873-1. [DOI] [PubMed] [Google Scholar]
- Behm DJ, Stankus G, Doe CP, Willette RN, Sarau HM, Foley JJ, et al. The peptidic urotensin-II receptor ligand GSK248451 possesses less intrinsic activity than the low-efficacy partial agonists SB-710411 and urantide in native mammalian tissues and recombinant cell systems. Br J Pharmacol. 2006;148:173–190. doi: 10.1038/sj.bjp.0706716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bousette N, Patel L, Douglas SA, Ohlstein EH, Giaid A. Increased expression of urotensin II and its cognate receptor GPR14 in atherosclerotic lesions of the human aorta. Atherosclerosis. 2004;176:117–123. doi: 10.1016/j.atherosclerosis.2004.03.023. [DOI] [PubMed] [Google Scholar]
- Bowman WC, Rand MJ. Textbook of Pharmacology. University Press: Cambridge; 1980. Kinetic analysis of drug–receptor interactions; pp. 39.16–39.24. [Google Scholar]
- Breivogel CS, Walker JM, Huang SM, Roy MB, Childers SR. Cannabinoid signaling in rat cerebellar granule cells: G-protein activation, inhibition of glutamate release and endogenous cannabinoids. Neuropharmacology. 2004;47:81–89. doi: 10.1016/j.neuropharm.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Camarda V, Spagnol M, Song W, Vergura R, Roth AL, Thompson JP, et al. In vitro and in vivo pharmacological characterization of the novel UT receptor ligand [Pen5,DTrp7,Dab8]urotensin II(4–11) (UFP-803) Br J Pharmacol. 2006;147:92–100. doi: 10.1038/sj.bjp.0706438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung BM, Leung R, Man YB, Wong LY. Plasma concentration of urotensin II is raised in hypertension. J Hypertens. 2004;22:1341–1344. doi: 10.1097/01.hjh.0000125452.28861.f1. [DOI] [PubMed] [Google Scholar]
- Clozel M, Binkert C, Birker-Robaczewska M, Boukhadra C, Ding SS, Fischli W, et al. Pharmacology of the urotensin-II receptor antagonist palosuran (ACT-058362: 1-[2-(4-benzyl-4-hydroxy-piperidin-1-yl)-ethyl]-3-(2-methyl-quinolin-4-yl)-urea sulfate salt): first demonstration of a pathophysiological role of the urotensin system. J Pharmacol Exp Ther. 2004;311:204–212. doi: 10.1124/jpet.104.068320. [DOI] [PubMed] [Google Scholar]
- Clozel M, Breu V, Burri K, Cassal JM, Fischli W, Gray GA, et al. Pathophysiological role of endothelin revealed by the first orally active endothelin receptor antagonist. Nature. 1993a;365:759–761. doi: 10.1038/365759a0. [DOI] [PubMed] [Google Scholar]
- Clozel M, Breu V, Gray GA, Löffler BM. In vivo pharmacology of Ro 46-2005, the first synthetic nonpeptide endothelin receptor antagonist: implications for endothelin physiology. J Cardiovasc Pharmacol. 1993b;22 Suppl 8:S377–S379. doi: 10.1097/00005344-199322008-00099. [DOI] [PubMed] [Google Scholar]
- Clozel M, Hess P, Qiu C, Ding SS, Rey M. The urotensin-II receptor antagonist palosuran improves pancreatic and renal function in diabetic rats. J Pharmacol Exp Ther. 2006;316:1115–1121. doi: 10.1124/jpet.105.094821. [DOI] [PubMed] [Google Scholar]
- Copeland RA, Pompliano DL, Meek TD. Drug-target residence time and its implications for lead optimization. Nat Rev Drug Discov. 2006;5:730–739. doi: 10.1038/nrd2082. [DOI] [PubMed] [Google Scholar]
- Dhanak D, Neeb MJ, Douglas SA. Urotensin-II receptor modulators. Ann Rep Med Chem. 2003;38:99–110. [Google Scholar]
- Douglas SA, Behm DJ, Aiyar NV, Naselsky D, Disa J, Brooks DP, et al. Nonpeptidic urotensin-II receptor antagonists I: in vitro pharmacological characterization of SB-706375. Br J Pharmacol. 2005;145:620–635. doi: 10.1038/sj.bjp.0706229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SA, Dhanak D, Johns DG. From ‘gills to pills': urotensin-II as a regulator of mammalian cardiorenal function. Trends Pharmacol Sci. 2004a;25:76–85. doi: 10.1016/j.tips.2003.12.005. [DOI] [PubMed] [Google Scholar]
- Douglas SA, Naselsky D, Ao Z, Disa J, Herold CL, Lynch F, et al. Identification and pharmacological characterization of native, functional human urotensin-II receptors in rhabdomyosarcoma cell lines. Br J Pharmacol. 2004b;142:921–932. doi: 10.1038/sj.bjp.0705743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SA, Ohlstein EH.Urotensin receptors The IUPHAR Compendium of Receptor Characterization and Classification 2000IUPHAR Media: London; 365–372.In: Girdlestone D (ed). [Google Scholar]
- Douglas SA, Sulpizio AC, Piercy V, Sarau HM, Ames RS, Aiyar NV, et al. Differential vasoconstrictor activity of human urotensin-II in vascular tissue isolated from the rat, mouse, dog, pig, marmoset and cynomolgus monkey. Br J Pharmacol. 2000;131:1262–1274. doi: 10.1038/sj.bjp.0703690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas SA, Tayara L, Ohlstein EH, Halawa N, Giaid A. Congestive heart failure and expression of myocardial urotensin II. Lancet. 2002;359:1990–1997. doi: 10.1016/S0140-6736(02)08831-1. [DOI] [PubMed] [Google Scholar]
- Egido EM, Silvestre RA, Hernandez R, Garcia P, Gutierrez E, Marco J. Potentiation of insulin secretion by antagonizing the urotensin II receptor. Study in the perfused rat pancreas. Diabetologia. 2005;48 Suppl 1:A453. [Google Scholar]
- Heller J, Schepke M, Neef M, Woitas R, Rabe C, Sauerbruch T. Increased urotensin II plasma levels in patients with cirrhosis and portal hypertension. J Hepatol. 2002;37:767–772. doi: 10.1016/s0168-8278(02)00295-7. [DOI] [PubMed] [Google Scholar]
- Heringlake M, Kox T, Uzun O, Will B, Bahlmann L, Klaus S, et al. The relationship between urotensin II plasma immunoreactivity and left ventricular filling pressures in coronary artery disease. Regul Pept. 2004;121:129–136. doi: 10.1016/j.regpep.2004.04.012. [DOI] [PubMed] [Google Scholar]
- Itoh H, McMaster D, Lederis K. Functional receptors for fish neuropeptide urotensin II in major rat arteries. Eur J Pharmacol. 1988;149:61–66. doi: 10.1016/0014-2999(88)90042-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson DH, Barnard EA, Hoyer D, Humphrey PPA, Leff P, Shankley NP.Terms and symbols in quantitative pharmacology The IUPHAR Compendium of Receptor Characterization and Classification 1998IUPHAR Media: London; 6–20.In: Girdlestone D (ed). [Google Scholar]
- Kenakin T. A Pharmacology Primer: Theory Application and Methods. Elsevier Academic Press: Amsterdam; 2003. Chapter VI Drug antagonism; pp. 95–105. [Google Scholar]
- Krüger S, Graf J, Kunz D, Stickel T, Merx MW, Hanrath P, et al. Urotensin II in patients with chronic heart failure. Eur J Heart Fail. 2005;7:475–478. doi: 10.1016/S1388-9842(03)00106-5. [DOI] [PubMed] [Google Scholar]
- Lapp H, Boerrigter G, Costello-Boerrigter LC, Jaekel K, Scheffold T, Krakau I, et al. Elevated plasma human urotensin-II-like immunoreactivity in ischemic cardiomyopathy. Int J Cardiol. 2004;94:93–97. doi: 10.1016/j.ijcard.2003.05.008. [DOI] [PubMed] [Google Scholar]
- Li L, Miano JM, Mercer B, Olson EN. Expression of the SM22alpha promoter in transgenic mice provides evidence for distinct transcriptional regulatory programs in vascular and visceral smooth muscle cells. J Cell Biol. 1996;132:849–859. doi: 10.1083/jcb.132.5.849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE, Davenport AP. Orphan-receptor ligand human urotensin-II: receptor localization in human tissues and comparison of vasoconstrictor responses with endothelin-1. Br J Pharmacol. 2000;131:441–446. doi: 10.1038/sj.bjp.0703601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Kuc RE, Wiley KE, Kleinz MJ, Davenport AP. Cellular distribution of immunoreactive urotensin-II in human tissues with evidence of increased expression in atherosclerosis and a greater constrictor response of small compared to large coronary arteries. Peptides. 2004;25:1767–1774. doi: 10.1016/j.peptides.2004.01.028. [DOI] [PubMed] [Google Scholar]
- Malagon MM, Molina M, Gahete MD, Duran-Prado M, Martinez-Fuentes AJ, Alcain FJ, et al. Urotensin II and urotensin II-related peptide activate somatostatin receptor subtypes 2 and 5. Peptides. 2008;29:711–720. doi: 10.1016/j.peptides.2007.12.015. [DOI] [PubMed] [Google Scholar]
- Marco J, Egido EM, Hernandez R, Silvestre RA. Evidence for endogenous urotensin-II as an inhibitor of insulin secretion: study in the perfused rat pancreas. Diabetes. 2005;54:A409. doi: 10.1016/j.peptides.2007.08.025. [DOI] [PubMed] [Google Scholar]
- Moessler H, Mericskay M, Li Z, Nagl S, Paulin D, Small JV. The SM22 promoter directs tissue-specific expression in arterial but not in venous or visceral smooth muscle cells in transgenic mice. Development. 1996;122:2415–2425. doi: 10.1242/dev.122.8.2415. [DOI] [PubMed] [Google Scholar]
- Patacchini R, Santicioli P, Giuliani S, Grieco P, Novellino E, Rovero P, et al. Urantide: an ultrapotent urotensin II antagonist peptide in the rat aorta. Br J Pharmacol. 2003;140:1155–1158. doi: 10.1038/sj.bjp.0705555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rdzanek A, Filipiak KJ, Karpiñski G, Grabowski M, Opolski G. Exercise urotensin II dynamics in myocardial infarction survivors with and without hypertension. Int J Cardiol. 2006;110:175–178. doi: 10.1016/j.ijcard.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Richards AM, Nicholls MG, Lainchbury JG, Fisher S, Yandle TG. Plasma urotensin II in heart failure. Lancet. 2002;360:545–546. doi: 10.1016/s0140-6736(02)09709-x. [DOI] [PubMed] [Google Scholar]
- Segev Y, Eshet R, Rivkis I, Hayat C, Kachko L, Phillip M, et al. Comparison between somatostatin analogues and ACE inhibitor in the NOD mouse model of diabetic kidney disease. Nephrol Dial Transplant. 2004;19:3021–3028. doi: 10.1093/ndt/gfh528. [DOI] [PubMed] [Google Scholar]
- Sidharta PN, Wagner FD, Bohnemeier H, Jungnik A, Halabi A, Krähenbühl S, et al. Pharmacodynamics and pharmacokinetics of the urotensin II receptor antagonist palosuran in macroalbuminuric, diabetic patients. Clin Pharmacol Ther. 2006;80:246–256. doi: 10.1016/j.clpt.2006.05.013. [DOI] [PubMed] [Google Scholar]
- Spinazzi R, Albertin G, Nico B, Guidolin D, Di Liddo R, Rossi GP, et al. Urotensin-II and its receptor (UT-R) are expressed in rat brain endothelial cells, and urotensin-II via UT-R stimulates angiogenesis in vivo and in vitro. Int J Mol Med. 2006;18:1107–1112. [PubMed] [Google Scholar]
- Strowski MZ, Cashen DE, Birzin ET, Yang L, Singh V, Jacks TM, et al. Antidiabetic activity of a highly potent and selective nonpeptide somatostatin receptor subtype-2 agonist. Endocrinology. 2006;147:4664–4673. doi: 10.1210/en.2006-0274. [DOI] [PubMed] [Google Scholar]
- Tölle M, van der Giet M. Cardiorenovascular effects of urotensin II and the relevance of the UT receptor. Peptides. 2008;29:743–763. doi: 10.1016/j.peptides.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Totsune K, Takahashi K, Arihara Z, Sone M, Ito S, Murakami O. Increased plasma urotensin-II levels in patients with diabetes mellitus. Clin Sci. 2003;104:1–5. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- Totsune K, Takahashi K, Arihara Z, Sone M, Murakami O, Ito S, et al. Elevated plasma levels of immunoreactive urotensin II and its increased urinary excretion in patients with type 2 diabetes mellitus: association with progress of diabetic nephropathy. Peptides. 2004;25:1809–1814. doi: 10.1016/j.peptides.2004.06.024. [DOI] [PubMed] [Google Scholar]
- Wenyi Z, Suzuki S, Hirai M, Hinokio Y, Tanizawa Y, Matsutani A, et al. Role of urotensin II gene in genetic susceptibility to type 2 diabetes mellitus in Japanese subjects. Diabetologia. 2003;46:972–976. doi: 10.1007/s00125-003-1145-1. [DOI] [PubMed] [Google Scholar]