Abstract
Human embryonic stem cells are pluripotent cells derived from the inner cell mass of preimplantation stage embryos. Their unique potential to give rise to all differentiated cell types has generated great interest in stem cell research and the potential that it may have in developmental biology, medicine and pharmacology. The main focus of stem cell research has been on cell therapy for pathological conditions with no current methods of treatment, such as neurodegenerative diseases, cardiac pathology, retinal dysfunction and lung and liver disease. The overall aim is to develop methods of application either of pure cell populations or of whole tissue parts to the diseased organ under investigation. In the field of pulmonary research, studies using human embryonic stem cells have succeeded in generating enriched cultures of type II pneumocytes in vitro. On account of their potential of indefinite proliferation in vitro, embryonic stem cells could be a source of an unlimited supply of cells available for transplantation and for use in gene therapy. Uncovering the ability to generate such cell types will expand our understanding of biological processes to such a degree that disease understanding and management could change dramatically.
Keywords: human embryonic stem cells, human lung, regeneration
Introduction
Despite extensive progress being made over recent years in both science and medicine, there are a large number of pulmonary diseases with inefficient therapeutic applications. Cystic fibrosis (CF) is one of the most prevalent of such conditions. It is most commonly caused by an autosomal-recessive deletion (ΔF508) in the CF transmembrane regulator (CFTR) gene, which results in inefficient ion transport to the apical side of the epithelial cell membrane (Bannykh et al., 2000). Diseases with no available therapies, which count only on the management of symptoms or rely on lung transplantation as a means of therapy, have attracted the interest of stem cell research as a means of providing alternative approaches for the correction of malfunctioning or damaged tissues. In the case of pulmonary conditions, the complex structure of the pulmonary epithelium, together with an incomplete understanding of its development and pathology, has made this an area of particular interest for stem cell biology, regenerative medicine, developmental biology and pharmacology.
Development of the human lung
The human lung has a complex three-dimensional structure featuring a large variety of endoderm-derived epithelial cells along its proximal–distal axis. Human lung development is divided into four stages, they are: embryonic stage, 0–5 weeks post-fertilization; (pseudo)glandular stage, 6–16 weeks; canalicular stage, 17–25 weeks and terminal sac (or saccular) stage, 26 weeks to term (Bishop et al., 2006). Each stage is determined by structural changes in the formation of the airway tree. The lung bud, which originates from the embryonic gut, gives rise to the trachea and the two main bronchi (cartilaginous airways) by the end of the embryonic stage. The two main bronchi follow a regulated process, known as branching morphogenesis, to form distal bronchioles and the alveoli as development continues. Distal bronchioles consist of bronchioles, terminal bronchioles and respiratory bronchioles. The terminal structures, the alveoli, are clusters of air spaces in the shape of saccules, which facilitate gas exchange.
Each domain is characterized by a unique type of airway epithelium. The primordial epithelium found at the embryonic stage gives rise to a pseudostratified epithelium during the glandular stage. This lines the trachea and the bronchi and consists mainly of basal, goblet, ciliated, intermediate, neuroendocrine and submucosal gland epithelial cells. As branching progresses, a simple columnar epithelium forms, consisting mainly of Clara cells, neuroendocrine and ciliated cells, covering the bronchioles. The distal part of the respiratory airways is lined by a thin epithelium consisting of flattened squamous (type I) and cuboidal (type II) pneumocytes (Bishop et al., 2006). This highly specialized organization of lung morphogenesis is regulated through a temporal and spatial distribution of a large number of signalling molecules, the interactions of which are mediated by growth factors expressed in the lung mesenchyme and endoderm (reviewed in Kumar et al., 2005).
Transcription factors involved in the development of the airway epithelium
A number of evolutionary conserved transcription factors have been shown to be important for the regulation of the various stages of lung development and epithelial cell differentiation. Morrisey et al. (1998) have demonstrated that the transcription factor GATA-binding factor 6 (GATA6), a member of the family of zinc-finger domain-containing nuclear proteins, is implicated in lung endoderm specification, and it was subsequently shown that GATA6 interacts with genes such as the human thyroid transcription factor 1 (TITF-1) and surfactant protein C (SPC), thus controlling the late stage of lung branching morphogenesis and distal epithelial cell differentiation (Yang et al., 2002). In particular, GATA6 and TITF-1 have overlapping temporal and spatial expressions in the peripheral epithelial cells of the developing lung, where GATA6 activates the transcription of TITF-1 (Shaw-White et al., 1999). SPC expression is directly regulated through this synergistic action of the N-terminal and zinc-finger domains of GATA6 and the homeodomain region of TITF-1 (Liu et al., 2002). Ectopic expression of GATA6 in mouse embryonic stem (mES) cells has been shown to induce differentiation towards extraembryonic endoderm, a prerequisite for lung organogenesis (Fujikura et al., 2002).
Another factor implicated in epithelial cell differentiation is the forkhead box (f-box) transcription factor FoxJ-1, which has been shown to be expressed in ciliated cells and is required for the late-stage formation of cilia, by reorganization of the basal bodies within the apical compartment of cells previously committed to a ciliated cell phenotype (You et al., 2004). Functional analysis of the regulatory region of lung-specific genes has shown that normal promoter activity often requires the synergistic interaction of multiple cell-specific and inducible transcription factors. A highly complex interaction of the mesenchyme and the epithelium is also required for the coordination of the transcription factor interactions (reviewed in Kumar et al., 2005). The complexity of these pathways will need to be carefully considered when creating a system replicating the development of the human lung epithelium.
Investigation of the effect of the Sox17 (SRY (sex-determining region Y) box 17), a marker of definitive endoderm in mice, has revealed the important function of this factor in the differentiation of respiratory epithelial cells into the various cells of the conducting airways (Park et al., 2006a, 2006b). In particular, it was suggested that multiple respiratory epithelial cells display high phenotypic plasticity levels, which can be regulated by different levels of Sox17, resulting in cells with multiple characteristics of epithelial cells of the conducting airways (Park et al., 2006b).
Investigation of the various molecular pathways that control lung development and epithelial cell differentiation is important for understanding and regulating the mechanisms that result in the pathological phenotypes of CF and chronic obstructive pulmonary disease (Calverley and Walker, 2003).
Endogenous stem cells of the lung
The normal tracheobronchial epithelium is continuously being renewed in a specialized and highly controlled way, which maintains the complex structure of this epithelium. The replacement of the terminally specialized epithelial cells occurs through the specialized differentiation of the endogenous lung stem cell population. These stem cells are characterized by unlimited self-renewal capacity and the ability to differentiate into all required cell types of the pulmonary epithelium upon appropriate stimuli. Identifying the endogenous stem cell population of the pulmonary epithelium will allow for further understanding of the developmental processes of lung formation as well as putative use of the specific cell populations in treating a variety of pulmonary diseases.
It is suggested that there are more than one stem cell population, a hypothesis reinforced by the highly complex and heterogeneous cell composition observed along the proximal–distal axis of the tracheobronchial tree (Wu and Wei, 2004). The determination of the stem cell population of the pulmonary epithelium has been complicated further by the great variation observed in the cell type composition of the lung epithelium from different species (Jeffery, 1983; Plopper et al., 1992). Reports of candidate stem cell populations so far include the undifferentiated columnar cell type found in the early developmental stages of the tracheobronchiolar epithelium (Plopper et al., 1992), basal cells (Hong et al., 2004a, 2004b; Hajj et al., 2007), Clara cells (Hong et al., 2001, 2004b) as well as CCSP-secreting cells that localize in the bronchoalveolar junctions (Giangreco et al., 2002; Kim et al., 2005a), type II pneumocytes (Emura, 1997; Uhal, 1997) and side population cells that show both epithelial and mesenchymal differentiation potential (Majka et al., 2005; Reynolds et al., 2007). All the proposed cell types have extensive self-renewal capacity and some of these populations have been shown to be able to contribute towards the regeneration of damaged lung epithelium upon engraftment. The first report to functionally identify a stem cell population in the human pulmonary epithelium was carried out by Zepeda et al. (1995). Although the morphological and functional characteristics of the stem cell type were not identified, the existence of such cells was proven using retroviral marking of human bronchial epithelial cells that were subsequently used for establishing xenograft models (Zepeda et al., 1995).
Further studies into the development of submucosal glands in the lung epithelium have identified more than one cell type having progenitor abilities for gland development (Engelhardt et al., 1995). The same study has also demonstrated the presence of multiple endogenous cell populations with stem cell characteristics in the lung epithelium. It is generally acknowledged that both basal and secretory cells have the ability to participate in the renewal of the epithelium but the exact pathways of differentiation are under ongoing investigation. More recently, the investigation of markers specific to human airway epithelial progenitor cells has revealed the presence of aquaporin-3-positive basal cells, which show regeneration potential in both the epithelial cell layer and the submucosal glands (Avril-Delplanque et al., 2005).
Cell–cell and cell–matrix interactions of this complex epithelium appear to determine a large part of the regeneration mechanism of the pulmonary epithelium through paracrine, autocrine and endocrine pathways (reviewed in Kumar et al., 2005). The type of stimuli used to observe lung epithelial regeneration has also been found to have an important function in the determination of the particular cell type that contributes to the renewal of the lung epithelium. In particular, the nature and the degree of injury induced in the lung, whether mechanical or toxic, determine the cell type that participates in regeneration and the extent of repopulation in each case (Beckett et al., 2005; Herzog et al., 2006). In the case of ciliated cells, Rawlins et al. (2007) have shown that although the ciliated cell type of the lung shows a transient morphological change after lung epithelium injury, this terminally differentiated cell type does not contribute to the regeneration of the pulmonary epithelium.
Clarifying the regeneration properties of the various cell types of the lung epithelium will form the basis of deciding on the target cell population in engraftment studies, especially when attempting to replicate this cell type using human embryonic stem (hES) cells.
hES cells
hES cells are pluripotent cells derived from the inner cell mass of the preimplantation embryos (Thomson et al., 1998). These immortal cells can be maintained in a culture in an undifferentiated state indefinitely, maintaining their proliferating capacity and have the unique potential to give rise to cells and tissues of all three embryonic germ layers (Thomson et al., 1998) as well as trophoblast cells (Gerami-Naini et al., 2004). These characteristics make embryonic stem (ES) cells an invaluable tool in the area of developmental biology, allowing the in-depth study and understanding of the mechanisms of cell differentiation as well as normal developmental processes. Moreover, they can be potentially used as a renewable source of cell populations in the area of regenerative medicine for the treatment of degenerative human diseases.
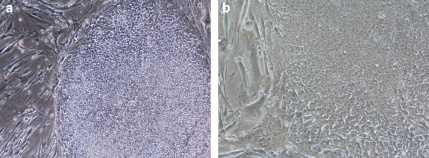
hES cells are morphologically distinct, as they exhibit a high nucleus-to-cytoplasmic ratio, with prominent nucleoli and form compact colonies with well-defined borders (Thomson et al., 1998) (Figure 1). They express high levels of telomerase activity, which is consistent with their high proliferative potential in culture (Thomson et al., 1998). Surface markers characteristic of undifferentiated hES cells are stage-specific embryonic antigen-3, -4, TRA (Trafalgar antigen)-1-60, TRA-1-81, Octamer-binding transcription factor 4 and alkaline phosphatase. hES cells can differentiate in vitro when grown in suspension and form embryoid bodies (EBs), which express markers specific to the three embryonic germ layers (Itskovitz-Eldor et al., 2000; Reubinoff et al., 2000). Further molecular characterization of differentiating hES cells and EBs demonstrated a temporal but not spatial gene expression pattern that resembles early human embryogenesis (Dvash and Benvenisty, 2004; Dvash et al., 2004). When injected in SCID (severe combined immunodeficient) mice, hES cells form teratomas, which include cells from all three embryonic germ layers (Thomson et al., 1998). Clonal hES lines have been derived that exhibited markers of undifferentiated hES cells and maintained the ability to differentiate into cells from all three germ layers (Amit et al., 2000). This demonstrated the pluripotency of single hES cell.
Figure 1.
hES cell lines (a) KCL-003-CF1 passage 38 on MEFs, × 10 (b) KCL-002 passage 59 on MEFs, × 20. The KCL-003-CF1 hES cell line is homozygous for the ΔF508 mutation, a 3 bp deletion in the coding region of the CFTR gene.
An important growth factor that has been shown to be able to maintain the undifferentiated state of hES cells when added to the culture medium is basic fibroblast growth factor (FGF) also known as FGF2. FGF2 is a member of the FGF family of ligands that signal through binding and subsequent dimerization to four different FGF receptors (FGFR1–4) on the cell surface inducing intrinsic tyrosine kinase activity. In particular, FGF binding to FGF receptor is followed by the activation of the Ras-mitogen associated protein kinase, PI3 kinase and phospholipase C-γ pathways (Reviewed in (Dailey et al., 2005; Eswarakumar et al., 2005; Mohammadi et al., 2005)). The activation of the PI3 kinase pathway by FGF2 has also been connected with the expression of extracellular matrix molecules that support hES cell proliferation (Kim et al., 2005b).
Another family of ligands that has been shown to mediate signalling involved in the regulation of the undifferentiated growth of hES cells is the transforming growth factor-β (TGF-β) superfamily (reviewed in Massague, 2003; Shi and Massague, 2003). More recently, TGF-β1 and Activin A (both members of the TGF-β superfamily) have been shown to be able to support the undifferentiated growth of hES cells both in the absence and in the presence of feeders (Amit et al., 2004; Beattie et al., 2005; Xiao et al., 2006). Nevertheless, the growth rate of hES cells appears to be reduced as compared with cultures where FGF2 is added to the growth medium, indicating a putative mitogenic action of FGF2 on hES cells (Amit et al., 2004). The exact signalling pathways that these growth factors act through are under ongoing investigation and new possible pathways are often suggested. An example is the recent report of a suggested mechanism of FGF2 initiating the release of insulin growth factor II and TGF-β factors from the microenvironment of hES cells, which prove sufficient for the maintenance of the undifferentiated state of hES cells (Bendall et al., 2007). A precise understanding of the molecular pathways involved will further aid in the establishment of a medium able to sustain more effectively the undifferentiated growth of hES cells. In addition to the molecular aspects and exogenous factors that control the undifferentiated hES cell growth, there is research into the effects of the microenvironment, such as cell density, in hES cultures (Peerani et al., 2007).
hES cell culture
There are a number of technical and methodological issues with the long-term culturing of human ES cells that currently are under intense investigation. These include derivation and efficient propagation of hES cells in defined culture conditions and the establishment of universal protocols for the reproducible differentiation of hES cells into enriched populations of cell types of interest. Traditionally, hES cells have been propagated on a substrate of inactivated mouse embryonic fibroblasts (Thomson et al., 1998), either by mechanical or by enzymatic means. Nevertheless, there have been reports that prolonged passaging by enzymatic dissociation encourages the appearance of chromosomal abnormalities (Buzzard et al., 2004; Draper et al., 2004; Mitalipova et al., 2005; Thomson et al., 2008). Hence, new alternatives have been developed, such as recombinant trypsin and Accutase, that show increased cell survival upon passaging, while maintaining the undifferentiated phenotype (Bajpai et al., 2008).
hES cells show low survival rate in single cell suspension (∼1%) (Thomson et al., 1998; Amit et al., 2000). This has been a great obstacle throughout cell culture maintenance techniques and gene transfer studies where clonal selection is of great importance. The recently discovered Y-27632 Rho-associated kinase inhibitor appears to increase the survival rate of single cell-dissociated hES cells to ∼27% (Watanabe et al., 2007). Also it has been reported that ES cells exhibit decreased growth rate and reduced differentiation efficiency when cultured with antibiotics such as gentamicin and combined penicillin–streptomycin (Cohen et al., 2006). Although the latter study was on mES cells, it provides further evidence that culture conditions of hES cells are still partly unexplored.
One of the most high-profile future uses of hES cells is in therapeutic applications, which dictates the need for well-defined derivation and culture systems devoid of animal sera and animal-derived components and substrates (that is, xeno-free conditions). There is ongoing research to create a well-defined culture media with a combination of extracellular matrices, such as laminin and fibonectin (Xu et al., 2001; Amit et al., 2004), growth factors (Beattie et al., 2005; Wang et al., 2005b; Xu et al., 2005) as well as autogeneic feeder systems (Stojkovic et al., 2005). Even though newly developed cell maintenance techniques and experimental assay setups are reported frequently, their validity remains to be confirmed and their potential long-term effects on hES cell characteristics are still to be examined.
In the recent years, numerous hES cell lines have been generated, most of them genetically normal. Nevertheless, through the donation of embryos shown to harbour known genetic disorders after preimplantation genetic diagnosis, many hES cell lines that carry mutations for disorders, such as Myotonic dystrophy, Huntington's disease (Mateizel et al., 2006) and CF (KCL-003-CF1) (Pickering et al., 2005; Mateizel et al., 2006) (Figure 1), have been established. hES cell lines that carry common mutations of monogenic diseases can be used as an in vitro model of the disease, bypassing the need for animal models and providing new tools for analysing and understanding the molecular mechanisms of the disease as well as for drug screening.
Current progress in lung regeneration
Endoderm differentiation
The great differentiation potential of hES cells is a very important factor for their use in therapeutic applications. Current research is directed towards the investigation of the various differentiation pathways of hES cells. Of particular interest is the direction of hES cells towards definitive endoderm, which in turn gives rise to organs, such as the thyroid, thymus, liver, pancreas and lung, as well as the epithelial lining of the digestive and respiratory tract. Studies so far have demonstrated that Nodal, a member of the TGF superfamily, is one of the main pathways essential for the specification of endoderm, whereas lower levels of Nodal result in the mesoderm formation (Vincent et al., 2003). On the basis of these observations, it has been shown that using activin A, another member of the TGFβ family, in a combination with low serum levels in cultures of ES cells, results in enriched cultures of hES-derived definitive endoderm (D'Amour et al., 2005). Two of the hES cell lines used for the assays were cultured on Matrigel and the rest on fibroblast feeders. The developmental competency of these hES-derived definitive endoderm cells was accessed in vivo by transplantation into SCID mice, followed by histological examination of the resulting grafts. This revealed that these cells have the ability to progress towards further endodermal differentiation in vivo (D'Amour et al., 2005). The production of definitive endoderm cells from hES cells would be the first step towards generating cells of the definitive endoderm lineage, such as pancreatic and lung epithelial cells.
Differentiation into lung cells
So far, investigation into the mechanisms that control the formation of the various epithelial cell types of the lung has included the use of mouse and human ES cells. The first report of derivation of a lung-specific cell type from stem cells was through studies of the effect of small airway growth medium (SAGM) in differentiating mES cells (Ali et al., 2002). The cultures obtained were heterogeneous, the differentiation efficiency was not quantified and the need for further investigation into the factors that direct differentiation was highlighted. Further investigation into the identification of a defined medium for the differentiation of ES cells into alveolar epithelium revealed that retinoic acid (RA) and triiodothyronine may have an inhibitory role in the formation of type II pneumocytes (Rippon et al., 2004). When compared with real-time (RT) PCR, differentiating mES cells transferred in SAGM showed a 20-fold increase in the expression of type II pneumocyte marker SPC to mES cells grown in basic Dulbecco's modified Eagle's medium (differentiation medium) (Rippon et al., 2004). The same study suggests that RA may promote maturation of proximal cell lineages during the final stages of mES cell differentiation. The main disadvantages of directing ES cell differentiation using a defined medium are the extensive assay time required and the low yields of target cells obtained.
More extensive studies into the effects of each of the growth factors found in SAGM (bovine serum albumin, insulin, transferring, bovine pituitary extract, epinephrine, triiodothyronine, RA, hydrocortisone and human epidermal growth factor) in the differentiation of mES cells into type II pneumocytes indicated that the initial effects observed were due to a serum-free environment rather than the growth factor combination in the SAGM. In particular, it was found that each growth factor individually reduced the efficiency of ES cell differentiation when compared with the results obtained in assays using the SAGM with the complete range of growth factors included. From these results, it is concluded that the effects of growth factors on ES cell differentiation, such as EGF, RA and triiodothyronine, cannot be predicted according to their role in lung development (Samadikuchaksaraei and Bishop, 2007).
At the same time, it was shown that it is possible to direct cell differentiation towards alternative phenotypes through ES cell exposure to differentiated cell extracts (Hakelien et al., 2002; Qin et al., 2005). This led to new attempts of directing mES cells towards a respiratory epithelial phenotype using murine lung epithelial cell extract (Wikenheiser et al., 1993). It was demonstrated that the exposure of mES cells to an alveolar epithelial cell extract can result in cell types positive for type II pneumocyte-specific markers (Qin et al., 2005). In particular, cells were monitored for signs of differentiation by prior stable transfection of mES with a 4.8-kb murine SPC promoter/green fluorescent protein construct, followed by immunocytochemistry and electron microscopy. The maintenance of hES characteristics after their transfection with an enhanced (E) green fluorescent protein transgene and the advantages of such a system for hES studies were demonstrated prior to this study (Liu et al., 2004). The transfected cells were used for the production of EBs, which were then exposed to murine lung epithelial-12 cell extract and cultured under differentiation-inducing conditions for a further 14 days. The resulting type II pneumocytes were shown to subsequently differentiate into type I pneumocytes. This study draws attention to the putative use of cell extract-mediated differentiation in generating lung epithelial cells from ES cells, despite the lack of understanding of the underlying regulatory mechanisms of this process. It is suggested that transcription regulators from the cell extract contribute in the differentiation process and the need for further investigation into the many variables of the extract-based ES cell culture is emphasized.
On the basis of the observations that ES cell fate is affected by the microenvironment surrounding the cells as well as the well-known property of mammalian cells to adapt and remodel in response to their environmental stimuli, Van Vranken et al. (2005) examined the effects of pulmonary mesenchyme on the differentiation capacities of mES cells. Previous studies on the effect of mesenchyme type on pulmonary epithelium differentiation had shown the significant plasticity of this epithelium, which depends on the type of mesenchyme used in each assay (Shannon et al., 1998). When murine EBs were grown in direct or indirect co-cultures with lung mesenchyme, there was evidence of the formation of lung epithelial cells originating from mES cells (Van Vranken et al., 2005).
In 2005, Coraux et al. demonstrated the generation of Clara cells as well as a fully differentiated airway epithelium from mES cells. The differentiation pathways that were examined included the differentiation of mES cells either by initial formation of EBs or by direct culture of undifferentiated mES cells on various substrates, such as collagen type I, collagen type IV, collagen type VI and gelatine. Both experimental routes examined the effects of the different substrates, keratinocyte growth factor and RA on the differentiation potential of mES cells, in both submerged cultures and air–liquid interface. Mouse ES cells grown on collagen type I show differentiation into Clara cells as early as day 8 in culture, with or without keratinocyte growth factor and RA. Ciliated cells can also be obtained after air–liquid interface culture of the type I collagen-induced mES cells. When other substrates were used, Clara cells were obtained from day 15 in culture. Differentiation of mES cells induced by the EB formation appeared not to lead to ciliated cells (Coraux et al., 2005).
Lung cell generation from adult stem cells
In parallel to research on the differentiation abilities of murine and human ES cells, there has been considerable investigation on the contribution of adult stem cells to the repair of airway epithelium as well as the possibility of generating a pulmonary epithelium cell population from adult stem cells. Bone marrow (BM)-derived stem cells are the best characterized adult stem cell population (Bonnet, 2003). They include haematopoietic stem cells and mesenchymal stem cells (MSCs). It has been reported that human MSCs (hMSCs) have the ability to differentiate into epithelial-like cells when co-cultured with small airway epithelial cells (Spees et al., 2003). Nevertheless, it was observed that up to 1% of the hMSCs obtained the epithelial phenotype after fusion with the small airway epithelial cells, which accounts for approximately 25% of all hMSCs that obtained an epithelial phenotype and an overall ∼10−2 frequency of cell fusion per hMSC plated in co-culture (Spees et al., 2003). Further studies on cell fusion as a mechanism of developmental plasticity of stem cells partially contradicted previous data and concluded that epithelia from BM-derived cells are not the result of cell–cell fusion, with the exception of severe tissue injury that appears to promote cell fusion (Harris et al., 2004). Despite conflicting data on the mechanism through which BM-derived stem cells obtain an airway epithelial phenotype, MSCs carrying the CFTR mutation have been subjected to gene correction and their ability to correct CFTR function was subsequently tested in co-cultures with airway epithelial cells (Wang et al., 2005a). It was found that gene-corrected MSCs from CF patients are able to contribute to apical chloride secretion in response to cyclic AMP stimulation (Wang et al., 2005a). Cell fusion was also observed in this study, although it was a rare event. There have been reports of cell fate alteration through spontaneous fusion and the concern for higher malignancy potential (Ying et al., 2002). Nevertheless, the resulting cells can display some functional correctness of the damaged epithelium, hence making this path an important one for clinical applications, despite the overall low efficiency of re-epithelization. In vivo studies using mouse models of CF and transplantation of MSCs carrying the wild-type CFTR gene have confirmed this observation (Loi et al., 2006). As a more efficient alternative to BM-derived MSCs, the regeneration and repair of injured airway epithelium were investigated using umbilical cord blood-derived MSCs (Sueblinvong et al., 2008). It was found that like BM-derived MSCs, cord blood-derived MSCs also have the ability to contribute to the airway epithelial regeneration in vivo.
An additional factor that appears to have an important role in the degree of epithelial repair is the type and the extent of injury induced to the epithelium. The correlation between injury threshold and BM-derived epithelia engraftment was examined in the case of irradiation-induced injury (Herzog et al., 2006). The results showed that there is a critical relationship between the extent of lung injury and the differentiation of BM cells into lung epithelia, drawing attention to the possibility of taking into account the type and extent of lung injury when considering the clinical applications of stem cells. Additional investigations into the effects the microenvironment may have on the differentiation abilities of stem cells have shown a direct correlation of the matrix elasticity to the acquired phenotype (Engler et al., 2006).
Differentiation into lung progenitor cells
Despite the progress made in identifying specific lung cell types arising from ES cell differentiation in culture, it would be very useful to be able to generate an airway epithelial progenitor cell population from ES cells. This would facilitate the study of development and cell type differentiation of the human lung as well as generate the source of various lung cell types depending on individual requirement. There are numerous studies in this field, with many different cell types being identified as putative airway progenitor cells (Wu and Wei, 2004). This is mainly due to the complex structural organization of the lung in combination with its large variety of cell types. Thus, the lung has been subdivided into regions with their own progenitor cell type (Otto, 2002). Recently, progenitor cells of the mouse lung epithelium have been generated in culture (Rippon et al., 2006). This was achieved by a combination of factors for directed endoderm differentiation, such as Activin A and knockout serum replacement and small airway basal medium.
Differentiation assays using hES cells
Studies using hES cells have succeeded in directing them towards type II pneumocytes, utilizing the same techniques used with mES cells (Samadikuchaksaraei et al., 2006). Despite the fact that the differentiation efficiency of this first attempt was low, ∼2% of the total cell population, a 99% pure population of type II pneumocytes from hES cultures was later obtained (Wang et al., 2007a). This was achieved by using Matrigel as a substrate to plate hES cells without the intermediate step of EB formation and enriching the resulting cultures using antibiotic selection.
Current problems and future applications
The potential of stem cells to generate such a diverse number of cell types is of interest to scientists in development biology, medicine and pharmacology. A fundamental question at the core of stem cell biology is whether it will be possible to fully understand cell cycle control pathways from simple survival to the development of an alternative phenotypic state. Numerous factors that are implicated in these basic biological principles are being continuously uncovered, and the complex network of interactions between the microenvironment surrounding the stem cells as well as the one created by them is under extensive investigation. The nature of stem cell research is such that technical obstacles are encountered far more frequently than in the culture of other cell lines. This forms a large part of the coordinated efforts to establish efficient, reproducible and standard protocols for their undifferentiated propagation.
The genetic makeup of these pluripotent cells is also under extensive investigation. A better understanding of the transcriptional pathways involved for the maintenance of their undifferentiated state will allow the development of more efficient culture conditions as well as differentiation protocols. The latter currently differ according to the stem cell line used, the differentiated cell type that is sought and the previous reports on the field. A typical example of such variations observed in differentiation assays is the intermediate step of EB formation that is often omitted from the protocols (Wang et al., 2007a) and the diversity of growth factors and substrates used to direct differentiation.
In addition to the technical challenges that surround the propagation of and experimentation with stem cells, particularly in the case of hES cells, there is an additional factor of assessing the biology of the resulting cells. Although techniques, such as immunocytochemistry and real-time PCR, are routinely used for the analysis of resulting differentiated cells, they are often proven to be incomplete or even misleading. An example of reported inconsistencies regarding the stem cell contribution to the regenerated tissue is described in the case of MSC-mediated lung epithelium regeneration (Spees et al., 2003; Harris et al., 2004). Although this could simply be an example specific to MSCs, it points out the possibility of false speculations that can be easily made in this emerging field of research.
Developmental biology of the lung
Of particular interest is the on-going debate about the determination of a stem cell population in the pulmonary epithelium, despite the fact that over the years a large number of cell types have been identified that display the core properties of stem cells. The main problem is that currently there is no system that allows for a continuous investigation of the developmental pathways during the formation of the lung epithelium. Hence, the information obtained is fractioned and the resulting image incomplete, allowing for speculations as to the exact type of endogenous stem cell population. A potential use of hES cells would be in expanding our understanding of airway epithelial development. It has been shown that mES cells can generate a fully differentiated and functional airway epithelial tissue (Coraux et al., 2005). Using such a model from hES cells will allow for the in vitro observation of the developmental pathways and cell lineage hierarchy in the human lung, which would in turn assist current investigations of potential endogenous lung epithelial stem cells.
Regenerative medicine and gene therapy in the lung
Owing to their potential of indefinite proliferation in vitro, hES cells could be a source of an unlimited supply of cells available for transplantation studies and for use in gene therapy. hES cells could be appropriately manipulated in vitro by directed differentiation towards the cell type of interest, which could be subsequently grafted to the appropriate tissue and contribute to its regeneration. This can be of great importance in the development of therapies for pulmonary diseases that currently rely on lung transplantation as the only means of treatment. The generation of lung cell types from hES cells has already been documented (Samadikuchaksaraei et al., 2006; Wang et al., 2007a), and a more extensive range of proximal and distal lung epithelium cell types have been generated from mES cells (Rippon et al., 2004, 2006; Coraux et al., 2005).
In addition to hES cells providing a treatment by direct transplantation and repopulation of the pathological or damaged tissue, they could be used as vectors for gene therapies. Of particular interest is the possible therapeutic use of hES in CF patients. Although there has been extensive research on the correction of CTFR gene function through gene therapy, there has been a number of obstacles, such as delivery failure of the gene carrier vector, immune reaction as well as cases of insertional mutagenesis (Davies et al., 2001; Davies, 2006). MSCs have already been shown to be possible carriers of the corrected CFTR gene (Wang et al., 2005a) that can engraft in the malfunctioning pulmonary epithelium. hES cells show greater plasticity, hence they could be a more efficient model for replicating the gene transfer with higher possibilities of integration.
Current advances towards clinical applications
The nature of ES cells is such that their potential applications in medicine were recognized very early on. The developmental pathways underlying the differentiation abilities of hES cells are under continuous and extensive study and new aspects of these mechanisms are rapidly being uncovered. The differentiation protocols published result in more enriched populations of the differentiated cell type of interest and the characterization of the molecular identity of the resulting cells becomes more extensive and accurate over the years (Synnergren et al., 2008).
The main interest has focused on pathological conditions with no current methods of treatment, such as neurodegenerative diseases, cardiac pathology, retinal dysfunction, lung and liver pathology. The overall aim is to develop methods of application either of pure cell populations or of whole tissue parts to the diseased organ under investigation, thus resulting in a reversal of the pathological condition and treatment of the cause of the disease rather than the management of symptoms. Progress of various degrees has been noted in most of the areas of interest for regenerative medicine. The common advancement is the generation of the desired cell population and at the same time the common hindrance has been the isolation of the pure progenitor population capable of regenerating the organ of interest and its expansion and survival in vitro. Despite those problems, there has been considerable progress in the field of heart regeneration (reviewed in Laflamme and Murry, 2005), retinal regeneration (Osakada et al., 2008) and neurodegenerative diseases such as Parkinson's disease (Wang et al., 2007b; Newman and Bakay, 2008).
An example of recent advances that demonstrate the putative therapeutic use of hES cells is the field of retinal regeneration. Over the last few years, retinal progenitor cells produced in vitro from hES cells and that are able to differentiate into cells that express the desired photoreceptor markers have been reported (Lamba et al., 2006). Nevertheless, they still required culture conditions that were not ideal for the widespread application of this technique, such as their co-culture with adult retinal cells. More recently, hES cells have been differentiated into putative photoreceptors in a stepwise protocol focusing on the initial production of retinal progenitor cells that are subsequently subjected to a specific cocktail of factors and over long time in culture they are able to produce cells that express many of the genes involved in phototransduction (Osakada et al., 2008). Similar positive results are being reported in studies into Parkinson's disease, where hES cells have been successfully differentiated towards dopaminergic neurons both in vitro and in vivo (Iacovitti et al., 2007). A more efficient method for large-scale production of functional dopaminergic neurons has been recently reported and their therapeutic potential in animal models has also been demonstrated (Cho et al., 2008).
Even though there are many examples of the great potential in utilizing the physiological properties of hES cells in regenerative medicine, there are still aspects that need to be examined and thoroughly understood. The most typical one is overcoming the risks of tumour formation. This is an area under current investigation, and markers that can be helpful in minimizing the number of pluripotent and hence putative carcinogenous cells are being identified (Choo et al., 2008). Other challenges to the clinical application of hES cells are the method of cell delivery, which often proves to be inefficient, the survival of the transplanted cell population and, perhaps more importantly, the control of proliferation and maintenance of karyotypic stability of the engrafted stem cells (Maitra et al., 2005). With time, a more detailed understanding of hES cell biology will allow for their safe, efficient and widespread use in medicine.
Drug testing/pharmacology
The creation of a fully differentiated and functional airway epithelium from mES cells (Coraux et al., 2005) shows the potential of creating such a system from hES cells. This would allow for the detailed study of the developmental pathways and cell lineage correlations during the formation of the human epithelium. Such an understanding could provide valuable information on the series of events preceding the formation of a pathological state on the lung, hence increasing the possibilities of finding efficient targets for the development of treatments.
Another very appealing aspect of developing a pulmonary epithelium system from hES cells is reducing the need for animal models of human pulmonary disease. This would be of great benefit as hES cells could be derived from pathological blastocysts or subsequently manipulated to replicate the disease phenotype. This would create a study system that is more accurate than the animal models due to the large variations observed among the lung epithelia of the various species.
Conclusions
Overall, the future applications of knowledge acquired in the field of hES cells can be of great benefit to both science and medicine. Uncovering the secrets of such a cell type will expand our understanding of biological processes to such a degree that disease understanding and management could change dramatically. Technical advances remain to be made for the full benefits of hES cell biology to be revealed and applied effectively.
Acknowledgments
A Varanou is supported by an MRC Case PhD studentship in collaboration with the Novartis Foundation.
Abbreviations
- BM
bone marrow
- CCSP
Clara cell secreting protein
- CF
cystic fibrosis
- CFTR
cystic fibrosis transmembrane regulator
- EB
embryoid body
- ES cell
embryonic stem cell
- FGF
fibroblast growth factor
- GATA6
GATA-binding factor 6
- hES cell
human embryonic stem cell
- IVF
in vitro fertilisation
- mES cell
mouse embryonic stem cell
- MSC
mesenchymal stem cell
- RA
retinoic acid
- SAGM
small airway growth medium
- SPC
surfactant protein C
- TGF-β
transforming growth factor-β
- TITF-1
thyroid transcription factor 1
Conflict of interest
The authors state no conflict of interest.
References
- Ali NN, Edgar AJ, Samadikuchaksaraei A, Timson CM, Romanska HM, Polak JM, et al. Derivation of type II alveolar epithelial cells from murine embryonic stem cells. Tissue Eng. 2002;8:541–550. doi: 10.1089/107632702760240463. [DOI] [PubMed] [Google Scholar]
- Amit M, Carpenter MK, Inokuma MS, Chiu CP, Harris CP, Waknitz MA, et al. Clonally derived human embryonic stem cell lines maintain pluripotency and proliferative potential for prolonged periods of culture. Dev Biol. 2000;227:271–278. doi: 10.1006/dbio.2000.9912. [DOI] [PubMed] [Google Scholar]
- Amit M, Shariki C, Margulets V, Itskovitz-Eldor J. Feeder layer- and serum-free culture of human embryonic stem cells. Biol Reprod. 2004;70:837–845. doi: 10.1095/biolreprod.103.021147. [DOI] [PubMed] [Google Scholar]
- Avril-Delplanque A, Casal I, Castillon N, Hinnrasky J, Puchelle E, Peault B. Aquaporin-3 expression in human fetal airway epithelial progenitor cells. Stem Cells. 2005;23:992–1001. doi: 10.1634/stemcells.2004-0197. [DOI] [PubMed] [Google Scholar]
- Bajpai R, Lesperance J, Kim M, Terskikh AV. Efficient propagation of single cells accutase-dissociated human embryonic stem cells. Mol Reprod Dev. 2008;75:818–827. doi: 10.1002/mrd.20809. [DOI] [PubMed] [Google Scholar]
- Bannykh SI, Bannykh GI, Fish KN, Moyer BD, Riordan JR, Balch WE. Traffic pattern of cystic fibrosis transmembrane regulator through the early exocytic pathway. Traffic. 2000;1:852–870. doi: 10.1034/j.1600-0854.2000.011105.x. [DOI] [PubMed] [Google Scholar]
- Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, et al. Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells. 2005;23:489–495. doi: 10.1634/stemcells.2004-0279. [DOI] [PubMed] [Google Scholar]
- Beckett T, Loi R, Prenovitz R, Poynter M, Goncz KK, Suratt BT, et al. Acute lung injury with endotoxin or NO2 does not enhance development of airway epithelium from bone marrow. Mol Ther. 2005;12:680–686. doi: 10.1016/j.ymthe.2005.05.007. [DOI] [PubMed] [Google Scholar]
- Bendall SC, Stewart MH, Menendez P, George D, Vijayaragavan K, Werbowetski-Ogilvie T, et al. IGF and FGF cooperatively establish the regulatory stem cell niche of pluripotent human cells in vitro. Nature. 2007;448:1015–1021. doi: 10.1038/nature06027. [DOI] [PubMed] [Google Scholar]
- Bishop AE, Polak JM, Klimanskaya I, Robert L.Pulmonary epithelium Methods in Enzymology 2006Elsevier Academic Press: San Diego; 333–349.vol. 418. [DOI] [PubMed] [Google Scholar]
- Bonnet D. Biology of human bone marrow stem cells. Clin Exp Med. 2003;3:140–149. doi: 10.1007/s10238-003-0017-9. [DOI] [PubMed] [Google Scholar]
- Buzzard JJ, Gough NM, Crook JM, Colman A. Karyotype of human ES cells during extended culture. Nat Biotech. 2004;22:381–382. doi: 10.1038/nbt0404-381. [DOI] [PubMed] [Google Scholar]
- Calverley PMA, Walker P. Chronic obstructive pulmonary disease. Lancet. 2003;362:1053–1061. doi: 10.1016/s0140-6736(03)14416-9. [DOI] [PubMed] [Google Scholar]
- Cho MS, Lee Y-E, Kim JY, Chung S, Cho YH, Kim D-S, et al. Highly efficient and large-scale generation of functional dopamine neurons from human embryonic stem cells. Proc Natl Acad Sci USA. 2008;105:3392–3397. doi: 10.1073/pnas.0712359105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choo AB, Tan HL, Ang SN, Fong WJ, Chin A, Lo J, et al. Selection against undifferentiated human embryonic stem cells by a cytotoxic antibody recognizing podocalyxin-like protein-1. Stem Cells. 2008;26:1454–1463. doi: 10.1634/stemcells.2007-0576. [DOI] [PubMed] [Google Scholar]
- Cohen S, Samadikuchaksaraei A, Polak JM, Bishop AE. Antibiotics reduce the growth rate and differentiation of embryonic stem cell cultures. Tissue Eng. 2006;12:2025–2030. doi: 10.1089/ten.2006.12.2025. [DOI] [PubMed] [Google Scholar]
- Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, et al. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32:87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- D'Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE. Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat Biotechnol. 2005;23:1534–1541. doi: 10.1038/nbt1163. [DOI] [PubMed] [Google Scholar]
- Dailey L, Ambrosetti D, Mansukhani A, Basilico C. Mechanisms underlying differential responses to FGF signaling. Cytokine Growth Factor Rev. 2005;16:233–247. doi: 10.1016/j.cytogfr.2005.01.007. [DOI] [PubMed] [Google Scholar]
- Davies JC. Gene and cell therapy for cystic fibrosis. Paediatr Respir Rev. 2006;7 Suppl 1:S163–S165. doi: 10.1016/j.prrv.2006.04.214. [DOI] [PubMed] [Google Scholar]
- Davies JC, Geddes DM, Alton EW. Gene therapy for cystic fibrosis. J Gene Med. 2001;3:409–417. doi: 10.1002/jgm.200. [DOI] [PubMed] [Google Scholar]
- Draper JS, Smith K, Gokhale P, Moore HD, Maltby E, Johnson J, et al. Recurrent gain of chromosomes 17q and 12 in cultured human embryonic stem cells. Nat Biotech. 2004;22:53–54. doi: 10.1038/nbt922. [DOI] [PubMed] [Google Scholar]
- Dvash T, Benvenisty N. Human embryonic stem cells as a model for early human development. Best Pract Res Clin Obstet Gynaecol. 2004;18:929–940. doi: 10.1016/j.bpobgyn.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Dvash T, Mayshar Y, Darr H, McElhaney M, Barker D, Yanuka O, et al. Temporal gene expression during differentiation of human embryonic stem cells and embryoid bodies. Hum Reprod. 2004;19:2875–2883. doi: 10.1093/humrep/deh529. [DOI] [PubMed] [Google Scholar]
- Emura M. Stem cells of the respiratory epithelium and their in vitro cultivation. In Vitro Cell Dev Biol Anim. 1997;33:3–14. doi: 10.1007/s11626-997-0015-4. [DOI] [PubMed] [Google Scholar]
- Engelhardt JF, Schlossberg H, Yankaskas JR, Dudus L. Progenitor cells of the adult human airway involved in submucosal gland development. Development. 1995;121:2031–2046. doi: 10.1242/dev.121.7.2031. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Eswarakumar VP, Lax I, Schlessinger J. Cellular signaling by fibroblast growth factor receptors. Cytokine Growth Factor Rev. 2005;16:139–149. doi: 10.1016/j.cytogfr.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Fujikura J, Yamato E, Yonemura S, Hosoda K, Masui S, Nakao K, et al. Differentiation of embryonic stem cells is induced by GATA factors. Genes Dev. 2002;16:784–789. doi: 10.1101/gad.968802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerami-Naini B, Dovzhenko OV, Durning M, Wegner FH, Thomson JA, Golos TG. Trophoblast differentiation in embryoid bodies derived from human embryonic stem cells. Endocrinology. 2004;145:1517–1524. doi: 10.1210/en.2003-1241. [DOI] [PubMed] [Google Scholar]
- Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–182. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajj R, Baranek T, Le Naour R, Lesimple P, Puchelle E, Coraux C. Basal cells of the human adult airway surface epithelium retain transit-amplifying cell properties. Stem Cells. 2007;25:139–148. doi: 10.1634/stemcells.2006-0288. [DOI] [PubMed] [Google Scholar]
- Hakelien A-M, Landsverk HB, Robl JM, Skalhegg BS, Collas P. Reprogramming fibroblasts to express T-cell functions using cell extracts. Nat Biotech. 2002;20:460–466. doi: 10.1038/nbt0502-460. [DOI] [PubMed] [Google Scholar]
- Harris RG, Herzog EL, Bruscia EM, Grove JE, Van Arnam JS, Krause DS. Lack of a fusion requirement for development of bone marrow-derived epithelia. Science. 2004;305:90–93. doi: 10.1126/science.1098925. [DOI] [PubMed] [Google Scholar]
- Herzog EL, Van Arnam J, Hu B, Krause DS. Threshold of lung injury required for the appearance of marrow-derived lung epithelia. Stem Cells. 2006;24:1986–1992. doi: 10.1634/stemcells.2005-0579. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004a;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. In vivo differentiation potential of tracheal basal cells: evidence for multipotent and unipotent subpopulations. Am J Physiol Lung Cell Mol Physiol. 2004b;286:L643–L649. doi: 10.1152/ajplung.00155.2003. [DOI] [PubMed] [Google Scholar]
- Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M. A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: studies in vitro and in vivo. Brain Res. 2007;1127:19–25. doi: 10.1016/j.brainres.2006.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itskovitz-Eldor J, Schuldiner M, Karsenti D, Eden A, Yanuka O, Amit M, et al. Differentiation of human embryonic stem cells into embryoid bodies compromising the three embryonic germ layers. Mol Med. 2000;6:88–95. [PMC free article] [PubMed] [Google Scholar]
- Jeffery PK. Morphologic features of airway surface epithelial cells and glands. Am Rev Respir Dis. 1983;128 2 Part 2:S14–S20. doi: 10.1164/arrd.1983.128.2P2.S14. [DOI] [PubMed] [Google Scholar]
- Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchoalveolar stem cells in normal lung and lung cancer. Cell. 2005a;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Cheon SH, Yoo SJ, Kwon J, Park JH, Kim CG, et al. Contribution of the PI3K/Akt/PKB signal pathway to maintenance of self-renewal in human embryonic stem cells. FEBS Lett. 2005b;579:534–540. doi: 10.1016/j.febslet.2004.12.024. [DOI] [PubMed] [Google Scholar]
- Kumar VH, Lakshminrusimha S, El Abiad MT, Chess PR, Ryan RM. Growth factors in lung development. Adv Clin Chem. 2005;40:261–316. doi: 10.1016/s0065-2423(05)40007-4. [DOI] [PubMed] [Google Scholar]
- Laflamme MA, Murry CE. Regenerating the heart. Nat Biotech. 2005;23:845–856. doi: 10.1038/nbt1117. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc Natl Acad Sci USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Morrisey EE, Whitsett JA. GATA-6 is required for maturation of the lung in late gestation. Am J Physiol Lung Cell Mol Physiol. 2002;283:L468–L475. doi: 10.1152/ajplung.00044.2002. [DOI] [PubMed] [Google Scholar]
- Liu Y-P, Dovzhenko OV, Garthwaite MA, Dambaeva SV, Durning M, Pollastrini LM, et al. Maintenance of pluripotency in human embryonic stem cells stably over-expressing enhanced green fluorescent protein. Stem Cells Dev. 2004;13:636–645. doi: 10.1089/scd.2004.13.636. [DOI] [PubMed] [Google Scholar]
- Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173:171–179. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maitra A, Arking DE, Shivapurkar N, Ikeda M, Stastny V, Kassauei K, et al. Genomic alterations in cultured human embryonic stem cells. Nat Genet. 2005;37:1099–1103. doi: 10.1038/ng1631. [DOI] [PubMed] [Google Scholar]
- Majka SM, Beutz MA, Hagen M, Izzo AA, Voelkel N, Helm KM. Identification of novel resident pulmonary stem cells: form and function of the lung side population. Stem Cells. 2005;23:1073–1081. doi: 10.1634/stemcells.2005-0039. [DOI] [PubMed] [Google Scholar]
- Massague J. Integration of Smad and MAPK pathways: a link and a linker revisited. Genes Dev. 2003;17:2993–2997. doi: 10.1101/gad.1167003. [DOI] [PubMed] [Google Scholar]
- Mateizel I, De Temmerman N, Ullmann U, Cauffman G, Sermon K, Van de Velde H, et al. Derivation of human embryonic stem cell lines from embryos obtained after IVF and after PGD for monogenic disorders. Hum Reprod. 2006;21:503–511. doi: 10.1093/humrep/dei345. [DOI] [PubMed] [Google Scholar]
- Mitalipova MM, Rao RR, Hoyer DM, Johnson JA, Meisner LF, Jones KL, et al. Preserving the genetic integrity of human embryonic stem cells. Nat Biotech. 2005;23:19–20. doi: 10.1038/nbt0105-19. [DOI] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Ibrahimi OA. Structural basis for fibroblast growth factor receptor activation. Cytokine Growth Factor Rev. 2005;16:107–137. doi: 10.1016/j.cytogfr.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Morrisey EE, Tang Z, Sigrist K, Lu MM, Jiang F, Ip HS, et al. GATA6 regulates HNF4 and is required for differentiation of visceral endoderm in the mouse embryo. Genes Dev. 1998;12:3579–3590. doi: 10.1101/gad.12.22.3579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MB, Bakay RAE. Therapeutic potentials of human embryonic stem cells in Parkinson's disease. Neurotherapeutics. 2008;5:237–251. doi: 10.1016/j.nurt.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osakada F, Ikeda H, Mandai M, Wataya T, Watanabe K, Yoshimura N, et al. Toward the generation of rod and cone photoreceptors from mouse, monkey and human embryonic stem cells. Nat Biotech. 2008;26:215–224. doi: 10.1038/nbt1384. [DOI] [PubMed] [Google Scholar]
- Otto WR. Lung epithelial stem cells. J Pathol. 2002;197:527–535. doi: 10.1002/path.1160. [DOI] [PubMed] [Google Scholar]
- Park K-S, Wells JM, Zorn AM, Wert SE, Laubach VE, Fernandez LG, et al. Transdifferentiation of ciliated cells during repair of the respiratory epithelium. Am J Respir Cell Mol Biol. 2006a;34:151–157. doi: 10.1165/rcmb.2005-0332OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K-S, Wells JM, Zorn AM, Wert SE, Whitsett JA. Sox17 influences the differentiation of respiratory epithelial cells. Dev Biol. 2006b;294:192–202. doi: 10.1016/j.ydbio.2006.02.038. [DOI] [PubMed] [Google Scholar]
- Peerani R, Rao BM, Bauwens C, Yin T, Wood GA, Nagy A, et al. Niche-mediated control of human embryonic stem cell self-renewal and differentiation. EMBO J. 2007;26:4744–4755. doi: 10.1038/sj.emboj.7601896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickering SJ, Minger SL, Patel M, Taylor H, Black C, Burns CJ, et al. Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis. Reprod Biomed Online. 2005;10:390–397. doi: 10.1016/s1472-6483(10)61801-9. [DOI] [PubMed] [Google Scholar]
- Plopper C, St George J, Cardoso W, Wu R, Pinkerton K, Buckpitt A. Development of airway epithelium. Patterns of expression for markers of differentiation. Chest. 1992;101 3 Suppl:2S–5S. [PubMed] [Google Scholar]
- Qin M, Tai G, Collas P, Polak JM, Bishop AE. Cell extract-derived differentiation of embryonic stem cells. Stem Cells. 2005;23:712–718. doi: 10.1634/stemcells.2004-0195. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci USA. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reubinoff BE, Pera MF, Fong C-Y, Trounson A, Bongso A. Embryonic stem cell lines from human blastocysts: somatic differentiation in vitro. Nat Biotech. 2000;18:399–404. doi: 10.1038/74447. [DOI] [PubMed] [Google Scholar]
- Reynolds SD, Shen H, Reynolds PR, Betsuyaku T, Pilewski JM, Gambelli F, et al. Molecular and functional properties of lung SP cells. Am J Physiol Lung Cell Mol Physiol. 2007;292:L972–L983. doi: 10.1152/ajplung.00090.2006. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Ali NN, Polak JM, Bishop AE. Initial observations on the effect of medium composition on the differentiation of murine embryonic stem cells to alveolar type II cells. Cloning Stem Cells. 2004;6:49–56. doi: 10.1089/1536230041372328. [DOI] [PubMed] [Google Scholar]
- Rippon HJ, Polak JM, Qin M, Bishop AE. Derivation of distal lung epithelial progenitors from murine embryonic stem cells using a novel three-step differentiation protocol. Stem Cells. 2006;24:1389–1398. doi: 10.1634/stemcells.2005-0465. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Bishop AE. Effects of growth factors on the differentiation of murine ESC into type II pneumocytes. Cloning Stem Cells. 2007;9:407–416. doi: 10.1089/clo.2006.0008. [DOI] [PubMed] [Google Scholar]
- Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, et al. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12:867–875. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- Shannon JM, Nielsen LD, Gebb SA, Randell SH. Mesenchyme specifies epithelial differentiation in reciprocal recombinants of embryonic lung and trachea. Dev Dyn. 1998;212:482–494. doi: 10.1002/(SICI)1097-0177(199808)212:4<482::AID-AJA2>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Shaw-White JR, Bruno MD, Whitsett JA. GATA-6 activates transcription of thyroid transcription factor-1. J Biol Chem. 1999;274:2658–2664. doi: 10.1074/jbc.274.5.2658. [DOI] [PubMed] [Google Scholar]
- Shi Y, Massague J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Ylostalo J, Lynch PJ, Smith J, Perry A, et al. Differentiation, cell fusion, and nuclear fusion during ex vivo repair of epithelium by human adult stem cells from bone marrow stroma. Proc Natl Acad Sci USA. 2003;100:2397–2402. doi: 10.1073/pnas.0437997100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stojkovic P, Lako M, Stewart R, Przyborski S, Armstrong L, Evans J, et al. An autogeneic feeder cell system that efficiently supports growth of undifferentiated human embryonic stem cells. Stem Cells. 2005;23:306–314. doi: 10.1634/stemcells.2004-0137. [DOI] [PubMed] [Google Scholar]
- Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, et al. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2008;177:701–711. doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Synnergren J, Akesson K, Dahlenborg K, Vidarsson H, Ameen C, Steel D, et al. Molecular signature of cardiomyocyte clusters derived from human embryonic stem cells. Stem Cells. 2008;26:1831–1840. doi: 10.1634/stemcells.2007-1033. [DOI] [PubMed] [Google Scholar]
- Thomson A, Wojtacha D, Hewitt Z, Priddle H, Sottile V, Di Domenico A, et al. Human embryonic stem cells passaged using enzymatic methods retain a normal karyotype and express CD30. Cloning Stem Cells. 2008;10:89–106. doi: 10.1089/clo.2007.0072. [DOI] [PubMed] [Google Scholar]
- Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282:1145–1147. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- Uhal BD. Cell cycle kinetics in the alveolar epithelium. Am J Physiol. 1997;272 6 Part 1:L1031–L1045. doi: 10.1152/ajplung.1997.272.6.L1031. [DOI] [PubMed] [Google Scholar]
- Van Vranken BE, Romanska HM, Polak JM, Rippon HJ, Shannon JM, Bishop AE. Coculture of embryonic stem cells with pulmonary mesenchyme: a microenvironment that promotes differentiation of pulmonary epithelium. Tissue Eng. 2005;11:1177–1187. doi: 10.1089/ten.2005.11.1177. [DOI] [PubMed] [Google Scholar]
- Vincent SD, Dunn NR, Hayashi S, Norris DP, Robertson EJ. Cell fate decisions within the mouse organizer are governed by graded Nodal signals. Genes Dev. 2003;17:1646–1662. doi: 10.1101/gad.1100503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Natl Acad Sci USA. 2007a;104:4449–4454. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Bunnell BA, Painter RG, Quiniones BC, Tom S, Lanson NA, Jr, et al. Adult stem cells from bone marrow stroma differentiate into airway epithelial cells: potential therapy for cystic fibrosis. Proc Natl Acad Sci USA. 2005a;102:186–191. doi: 10.1073/pnas.0406266102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Li L, Menendez P, Cerdan C, Bhatia M. Human embryonic stem cells maintained in the absence of mouse embryonic fibroblasts or conditioned media are capable of hematopoietic development. Blood. 2005b;105:4598–4603. doi: 10.1182/blood-2004-10-4065. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen S, Yang D, Le W-d. Stem cell transplantation: a promising therapy for Parkinson's disease. J Neuroimmune Pharmacol. 2007b;2:243–250. doi: 10.1007/s11481-007-9074-2. [DOI] [PubMed] [Google Scholar]
- Watanabe K, Ueno M, Kamiya D, Nishiyama A, Matsumura M, Wataya T, et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat Biotechnol. 2007;25:681–686. doi: 10.1038/nbt1310. [DOI] [PubMed] [Google Scholar]
- Wikenheiser KA, Vorbroker DK, Rice WR, Clark JC, Bachurski CJ, Oie HK, et al. Production of immortalized distal respiratory epithelial cell lines from surfactant protien C/Simian virus 40 large tumor antigen transgenic mice. Proc Natl Acad Sci USA. 1993;90:11029–11033. doi: 10.1073/pnas.90.23.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M, Wei Y-Q. Development of respiratory stem cells and progenitor cells. Stem Cells Dev. 2004;13:607–613. doi: 10.1089/scd.2004.13.607. [DOI] [PubMed] [Google Scholar]
- Xiao L, Yuan X, Sharkis SJ. Activin A maintains self-renewal and regulates fibroblast growth factor, Wnt, and bone morphogenic protein pathways in human embryonic stem cells. Stem Cells. 2006;24:1476–1486. doi: 10.1634/stemcells.2005-0299. [DOI] [PubMed] [Google Scholar]
- Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, et al. Feeder-free growth of undifferentiated human embryonic stem cells. Nat Biotechnol. 2001;19:971–974. doi: 10.1038/nbt1001-971. [DOI] [PubMed] [Google Scholar]
- Xu C, Rosler E, Jiang J, Lebkowski JS, Gold JD, O'Sullivan C, et al. Basic fibroblast growth factor supports undifferentiated human embryonic stem cell growth without conditioned medium. Stem Cells. 2005;23:315–323. doi: 10.1634/stemcells.2004-0211. [DOI] [PubMed] [Google Scholar]
- Yang H, Lu MM, Zhang L, Whitsett JA, Morrisey EE. GATA6 regulates differentiation of distal lung epithelium. Development. 2002;129:2233–2246. doi: 10.1242/dev.129.9.2233. [DOI] [PubMed] [Google Scholar]
- Ying QL, Nichols J, Evans EP, Smith AG. Changing potency by spontaneous fusion. Nature. 2002;416:545–548. doi: 10.1038/nature729. [DOI] [PubMed] [Google Scholar]
- You Y, Huang T, Richer EJ, Schmidt J-EH, Zabner J, Borok Z, et al. Role of f-box factor foxj1 in differentiation of ciliated airway epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2004;286:L650–L657. doi: 10.1152/ajplung.00170.2003. [DOI] [PubMed] [Google Scholar]
- Zepeda ML, Chinoy MR, Wilson JM. Characterization of stem cells in human airway capable of reconstituting a fully differentiated bronchial epithelium. Somat Cell Mol Genet. 1995;21:61–73. doi: 10.1007/BF02255823. [DOI] [PubMed] [Google Scholar]