Abstract
Background and purpose:
Ketanserin, a selective 5-HT receptor antagonist, prolongs the QT interval of ECG in patients. The purpose of the present study was to determine whether ketanserin would block human cardiac ether-à-go-go-related gene (hERG) potassium channels.
Experimental approach:
Whole-cell patch voltage-clamp technique was used to record membrane currents in HEK 293 cells expressing wild type or mutant hERG channel genes.
Key results:
Ketanserin blocked hERG current (IhERG) in a concentration-dependent manner (IC50=0.11 μM). The drug showed an open channel blocking property, the block increasing significantly at depolarizing voltages between +10 to +60 mV. Voltage-dependence for inactivation of hERG channels was negatively shifted by 0.3 μM ketanserin. A 2.8 fold attenuation of inhibition by elevation of external K+ concentration (from 5.0 to 20 mM) was observed, whereas the inactivation-deficient mutants S620T and S631A had the IC50s of 0.84±0.2 and 1.7±0.4 μM (7.6 and 15.4 fold attenuation of block). In addition, the hERG mutants in pore helix and S6 also significantly reduced the channel block (2–59 fold) by ketanserin.
Conclusions and implications:
These results suggest that ketanserin binds to and blocks the open hERG channels in the pore helix and the S6 domain; channel inactivation is also involved in the blockade of hERG channels. Blockade of hERG channels most likely contributes to the prolongation of QT intervals in ECG observed clinically at therapeutic concentrations of ketanserin.
Keywords: ketanserin, human ether-à-go-go-related gene (hERG) potassium channels, open channel block, long QTs
Introduction
Ketanserin is an antihypertensive drug used in the management of pre-eclampsia (Bolte et al., 2001; Banga et al., 2004; Duley et al., 2006) and in the treatment of chronic ulcers in leprosy patients (Salazar et al., 2001) and diabetic patients (Martinez-de Jesus et al., 1997; Quatresooz et al., 2006). Ketanserin acts pharmacologically as an antagonist of the 5-HT2 receptor in blood vessels to counteract the vasoconstrictive response to 5-HT (Bolte et al., 2001; Banga et al., 2004; Duley et al., 2006). However, ketanserin was found to have a potentially adverse effect of prolonging the cardiac QT interval of the ECG (Aldariz et al., 1986; Zehender et al., 1989; Frishman and Grewall, 2000). Experimental studies demonstrated that ketanserin prolonged cardiac action potential duration (Zaza et al., 1989; Le Grand et al., 1995a) by inhibiting the rapidly delayed rectifier potassium current IKr in guinea pig ventricular myocytes (Le Grand et al., 1995a).
Human ether-à-go-go-related gene (hERG or Kv11.1) encodes the α-subunit of cardiac IKr channels (Sanguinetti et al., 1995; Trudeau et al., 1995). It is well known that suppression of hERG function, either because of genetic defects or drug side effects, can lead to long-QT syndrome. Therefore, hERG channels have been widely used to examine the pro-arrhythmic potential of potential therapeutic agents during drug development. The present study was designed to determine in detail the electrophysiological properties of hERG channel blockade by ketanserin and to investigate the molecular determinants of hERG channel block. In addition, the effect of ketanserin on human cardiac slowly delayed rectifier potassium current (IKs) was determined in HEK 293 cells stably expressing hKCNQ1/hKCNE1 genes. Our results indicate that ketanserin is an open channel blocker of hERG K+ channels and does not block human cardiac IKs.
Materials and methods
HEK 293 cells expressing hERG channels or hKCNQ1/hKCNE1 genes
The established HEK 293 cell line stably expressing hERG channels (Tang et al., 2007) was cultured in Dulbecco's modified Eagle's medium (DMEM, Invitrogen, Hong Kong) supplemented with 10% foetal bovine serum, 400 μg mL−1 G418 (Invitrogen). The HEK 293 cell line (Dong et al., 2006) stably expressing recombinant human cardiac KCNQ1/KCNE1 channel current (IKs) was maintained in DMEM containing 10% foetal bovine serum and 100 μg mL−1 hygromycin (Invitrogen). Cells used for electrophysiology were seeded on a glass coverslip.
The mutant hERG channels were constructed as described previously (Gang and Zhang, 2006; Guo et al., 2006), and were transiently expressed in HEK 293 cells using 10 μL of Lipofectamine 2000 with 4 μg of hERG mutant cDNA in pCDNA3 vector (Tang et al., 2007).
Solutions
Tyrode solution contained (in mM): NaCl 140, KCl 5.0, MgCl2 1.0, CaCl2 1.8, NaH2PO4 0.33, HEPES 10.0, glucose 10 and pH adjusted to 7.3 with NaOH. When external K+ concentration ([K+]o) was increased, equimolar external Na+ was reduced to maintain osmolality. The pipette solution contained (in mM): KCl 130, MgCl2 1.0, HEPES 10, EGTA 5.0 and GTP 0.1, Na2-phosphocreatine 5.0, Mg2-ATP 5.0, with pH adjusted to 7.2 with KOH.
Electrophysiological recordings
Cells on a coverslip were transferred to an open cell chamber (0.5 mL) mounted on the stage of an inverted microscope, and superfused at 2–3 mL min−1 with Tyrode solution. The whole cell patch-clamp technique was used as previously described (Tian et al., 2006) with an EPC-10 amplifier and Pulse software (HEKA, Lambrecht, Germany). A 3 M KCl-agar bridge was used as the reference electrode. The tip potential was zeroed before the patch pipette touched the cell. After the gigaohm seal was obtained, the cell membrane was ruptured by applying gentle negative pressure to establish the whole cell configuration. Series resistance was 3–5 MΩ, and compensated by 50–70% to minimize voltage errors. The liquid junction potential (9–12 mV) was not corrected in the experiment. Leakage subtraction was automatically made with Pulse software. Data with a large leakage current (>500 pA) were not included for data analysis. The current signal was low-pass filtered at 5 kHz and stored in the hard disk of an IBM compatible computer. All experiments were conducted at room temperature (22–23 °C).
Data analysis
Nonlinear curve fitting was performed using Pulsefit (HEKA) and/or Sigmaplot (SPSS Science, Chicago, IL, USA). Paired and/or unpaired Student's t-tests were used to evaluate the statistical significance of differences between two group means. Data were presented as mean±s.e.mean. ANOVA was used for statistical significance among multiple groups. Values of P<0.05 were considered to be statistically significant.
Drugs
Ketanserin (Sigma-Aldrich, St Louis, MO, USA) was dissolved in DMSO to produce a stock solution of 100 mM. Ketanserin stock was diluted in experimental solutions to achieve the final concentrations. Receptor and ion channel nomenclature follow that recommended by Alexander et al (2008).
Results
Time-dependent effects of ketanserin on IKs and IhERG
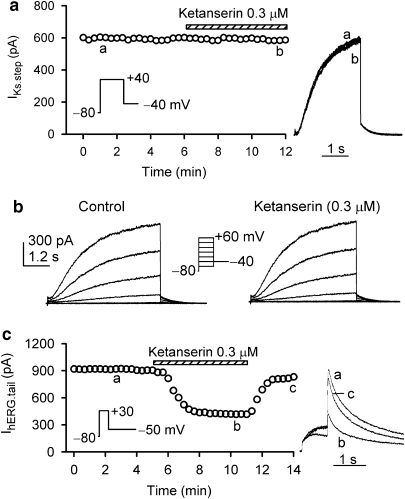
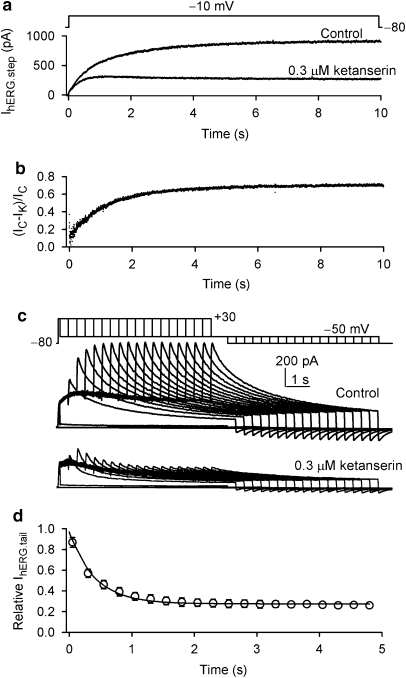
The effect of ketanserin on human cardiac IKs channels was studied in HEK 293 cells stably expressing hKCNQ1/hKCNE1 genes (Dong et al., 2006). Figure 1a illustrates the time course of IKs step current recorded in a representative cell. IKs was determined using a 3-s voltage step to +40 mV from −80 mV, then back to −40 mV (inset). Ketanserin at 0.3 μM had no effect on IKs step or tail current as the original current traces are shown on the right of the panel. Figure 1b shows voltage-dependent IKs, which is not affected by 0.3 μM ketanserin. IKs step current was 487±50 pA for control and 468±47 pA for ketanserin (n=6, P=NS). Figure 1c displays the time course of hERG tail current recorded in a representative cell with the voltage protocol shown in the inset. The current was rapidly inhibited by application of 0.3 μM ketanserin in bath solution, the effect significantly recovered on washout. Original current traces at corresponding time points are shown on the right side of the panel.
Figure 1.
Effects of ketanserin on IKs and IhERG. (a) Time course of IKs step current recorded in a HEK 293 cell stably expressing hKCNQ1/hKCNE1 genes in the absence and presence of 0.3 μM ketanserin. Membrane current was elicited by a 3 s voltage step to +40 mV from a holding potential of −80 mV, then back to −40 mV (left inset) every 20 s. Ketanserin had no effect on IKs. Original current traces at corresponding time points are shown in the right of the panel. (b) Voltage-dependent IKs was recorded in another cell using the protocol shown in the inset. No effect of ketanserin on voltage-dependent IKs was observed in this and five other cells. (c) Time course of hERG tail current recorded in a HEK 293 cell stably expressing hERG channels. Membrane current was elicited by a 1 s voltage step to +30 mV from a holding potential of −80 mV, then back to −50 mV (left inset) every 20 s. Ketanserin at 0.3 μM reversibly suppressed hERG channel current.
Voltage-dependent effects of ketanserin on hERG channels
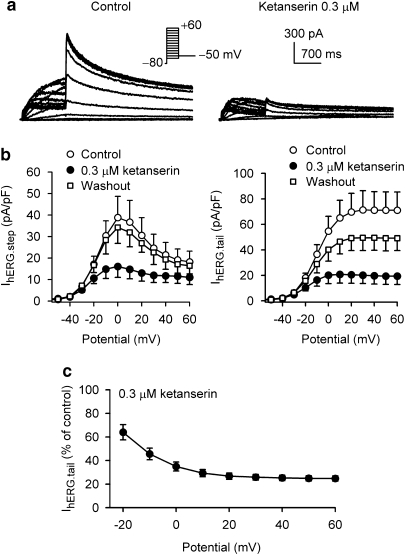
Voltage-dependent effects of ketanserin on hERG channels were determined with a voltage protocol as shown in the inset of Figure 2a. Ketanserin at 0.3 μM inhibited the voltage-dependent step current (IhERG.step) and tail current (IhERG.tail) of hERG channels with a 5-min exposure (Figure 2a). Figure 2b illustrates current-voltage (I-V) relationships of IhERG.step and IhERG.tail during control and after application of 0.3 μM ketanserin. Both IhERG.step and IhERG.tail were remarkably suppressed by 0.3 μM ketanserin, and the effect was largely reversible on washout. The fractions of ketanserin-induced hERG tail current inhibition at each depolarizing voltage were shown in Figure 2c, which indicates a voltage-dependent inhibition of hERG channels by ketanserin. The inhibition was stronger at positive potentials between +10 and +60 mV than at potentials between −20 to 0 mV (Figure 2c, n=6, P<0.01 or P<0.05).
Figure 2.
Voltage-dependence of hERG channel block. (a) Voltage-dependent hERG channel current was recorded in a typical experiment with 1 s voltage steps to between −60 and +60 mV from a holding potential of −80 mV, then back to −50 mV (inset, 20 s interval) in the absence and presence of ketanserin. Ketanserin at 0.3 μM inhibited the step current (IhERG.step) and tail current (IhERG.tail). (b) Current-voltage (I-V) relationships of IhERG.step (left) and IhERG.tail (right) before and after application of 0.3 μM ketanserin and washout. IhERG was reversibly suppressed by ketanserin at −20 mV to +60 mV for IhERG.step and for IhERG.tail (n=7, P<0.05 or P<0.01 vs control). IhERG.step was measured at the end of step from the zero current, and IhERG.tail was measured at the peak of the tail current. (c) Voltage-dependent block of hERG channel current by ketanserin. The inhibition of IhERG.tail by ketanserin was stronger at positive potentials between +10 and +60 mV than that at potentials between −20 and 0 mV (n=6, P<0.01 or P<0.05).
Concentration-dependent inhibition of hERG channels by ketanserin
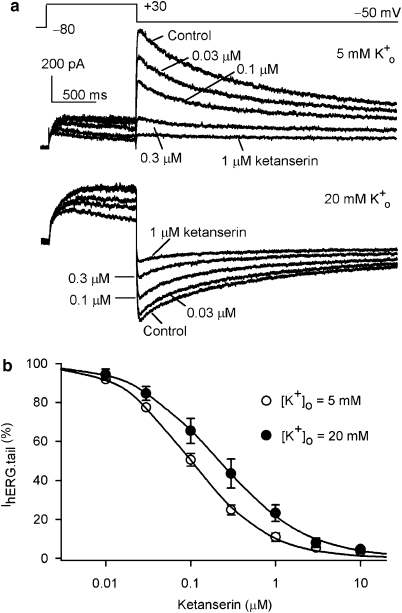
Figure 3 illustrates the concentration-dependent suppression of hERG channels by ketanserin at normal [K+]o (5 mM) and high [K+]o (20 mM). IhERG was elicited with the protocol shown on the top of the panel a. Ketanserin at 0.03, 0.1, 0.3 and 1 μM inhibited hERG channel current at 5 mM [K+]o in a concentration-dependent manner. Ketanserin also blocked hERG channel current in a concentration-dependent manner at 20 mM [K+]o (lower panel of Figure 3a); however, the effect was slightly weaker than that at 5 mM [K+]o. Concentration-dependent relationships for IhERG.tail inhibition are summarized in Figure 3b. As increasing [K+]o is known to decrease hERG inactivation, it suggests that hERG inactivation plays a role in the binding of ketanserin to hERG channels.
Figure 3.
Concentration-dependent inhibition of hERG channel current. (a) Ketanserin blocked hERG channel current in a concentration-dependent manner in 5 mM K+o and 20 mM K+o. Membrane current was recorded by 1 s voltage step to +30 mV from −80 mV, then back to −50 mV (upper of the panel, pulse interval: 20 s). The blocking effect of hERG channel current was weaker at 20 mM K+o than that at 5 mM K+o. (b) Concentration response relationship of ketanserin for inhibiting IhERG.tail in 5 and 20 mM K+o. The data from six cells were fitted to the Hill equation: E=Emax/[1+(IC50/C)b], where E is the percentage inhibition of current at concentration C, Emax is the maximum inhibition, IC50 is the concentration for a half-maximum inhibitory effect, and b is the Hill coefficient. The IC50 of ketanserin for inhibiting IhERG.tail in 5 mM K+o was 0.11±0.02 μM (n=6) and the Hill coefficient was 1.07±0.03. The IC50 for inhibiting IhERG.step in 20 mM K+o was 0.31±0.08 μM (n=6, P<0.05 vs 5 mM K+o) and the Hill coefficient was 0.96±0.05 (P=NS).
Ketanserin effects on kinetics of deactivation, inactivation and reactivation of hERG currents
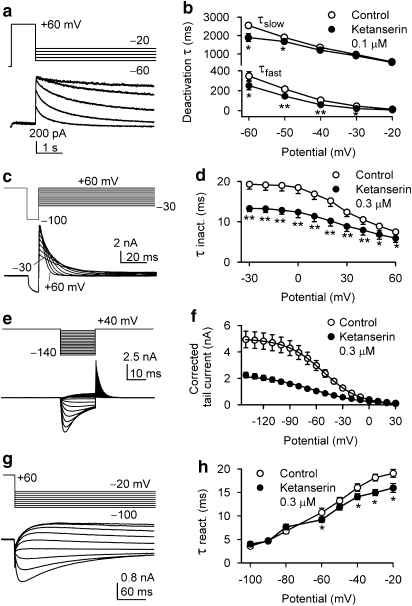
The effect of ketanserin on deactivation kinetics of hERG channels was determined by fitting the time course of the deactivating tail currents with a bi-exponential function during repolarization to between −60 and −20 mV after depolarization to +60 mV from a holding potential of −80 mV (Figure 4a). Both the rapid and slow components of the deactivating tail currents at −60 to −40 mV were quicker in the presence of 0.1 μM ketanserin (Figure 4b), suggesting a re-block of the channels in response to repolarization to −60 to −40 mV (Sanchez-Chapula et al., 2002).
Figure 4.
Effects of ketanserin on hERG channel kinetics. (a) Current traces recorded by the voltage protocol were used to evaluate deactivation time constant of hERG channel. (b) The mean values of fast and slow deactivation time constants (τfast and τslow) before and after application of 0.1 μM ketanserin (n=6, *P<0.05, **P<0.01 vs control). (c) Currents recorded by the protocol were used to evaluate inactivation time constant of hERG channel. (d) Mean values of voltage-dependent inactivation time constants (τ inact.) in control and after application of 0.3 μM ketanserin (n=7, *P<0.05, **P<0.01 vs control). (e) Current traces obtained with the protocol were used to assess steady-state inactivation. After a 1 s inactivation step to +40 mV, the rapid inactivation of hERG channels was relieved by application of 20 ms test pulses to potentials ranging from −140 to +30 mV. (f) Steady-state inactivation curves were fitted to a Boltzmann distribution. The V0.5 of hERG channel inactivation was negatively shifted by 15.3 mV with 0.3 μM ketanserin (from −38.4±2.98 mV of control to −53.7±3.6 mV, n=7, P<0.01). The slope factor was −21.1±2.1 mV for control and −28.6±1.7 mV for ketanserin (P<0.05). (g) Currents recorded with the protocol were used to evaluate recovery time constant of hERG channel. The recovery phase of the current was fitted to a mono-exponential function. (h) Time constant of recovery from inactivation (τ react.) was slightly reduced by 0.3 μM ketanserin at voltages between −60 and −20 mV compared with that of control at the same voltage range (n=7, *P<0.05 vs control).
The inactivation of hERG channels is believed to play an important role in high-affinity drug binding to hERG channels (Zhang et al., 1999). To examine whether ketanserin would affect inactivation time course of hERG channels, hERG current was fully activated and inactivated by a depolarizing step to +60 mV for 500 ms. The cell was then repolarized to −100 mV for 10 ms to allow channel recovery from inactivation to open state but not enough for channel deactivation (Smith et al., 1996; Spector et al., 1996; Guo et al., 2006). A test step was then applied to different voltages to observe inactivation time courses (Figure 4c). The inactivation time constant was obtained by fitting the current decay to a single exponential function. Figure 4d shows the mean values of voltage dependence of inactivation time constant (τ inact., n=7) before and after application of ketanserin. Ketanserin at 0.3 μM significantly reduced inactivation time constant at all voltages (P<0.01 vs control), that is, the inactivation of hERG channels was accelerated by ketanserin, suggesting that block of hERG channel is most likely dependent on channel inactivation.
The voltage protocol and recorded currents in Figure 4e were used to determine steady-state inactivation (availability) of hERG channels as previously described (Smith et al., 1996; Tang et al., 2007). Figure 4f shows the corrected current curves in the absence (control) and presence of 0.3 μM ketanserin by extrapolating the exponential decay phase back to the start of the negative voltage step and applying the same relative correction to the initial outward tail current as described previously (Smith et al., 1996). The curves were fitted to a Boltzmann distribution. The half potential (V0.5) of hERG channel inactivation was negatively shifted (from −38.4±3.0 mV of control to −53.7±3.6 mV, n=7, P<0.01).
The recovery time from inactivation of hERG channels was examined using the standard dual-pulse protocol (Figure 4g) as previously described (Spector et al., 1996). The rising phase of the current was fitted to a mono-exponential function, and the recovery time constant (τ react.) was plotted against the repolarization potentials (Figure 4h). The recovery time constant was slightly affected by 0.3 μM ketanserin.
Time-dependent block of hERG channels by ketanserin
The time-dependent block of hERG channel by ketanserin was evaluated by holding the potential at −80 mV to ensure that all hERG channels were in a closed state, followed by a long duration (10 s) voltage step to −10 mV (Tang et al., 2007). Ketanserin (0.3 μM) suppressed IhERG less at the beginning of the current activation than at the end of the depolarization step, consistent with an open channel blocking effect (Figure 5a). The onset of open channel block was analysed using the drug-sensitive current (Gao et al., 2004) formula: ((IC−IB)/IC), where IC and IB are the currents in the absence and presence of ketanserin. The drug-induced block was plotted as a function of time of the pulse. The block developed in a time-dependent manner, with an exponential time constant of 843±93 ms (n=7) with 0.3 μM ketanserin by the curve-fit shown in Figure 5b, consistent with ketanserin being an open channel blocker.
Figure 5.
Development of hERG channel current block by ketanserin using a long step pulse protocol and an envelope of tails protocol. (a) Voltage-clamp pulse protocol and representative recordings of hERG current before and after exposure of the cell to 0.3 μM ketanserin. The current was substantially inhibited by 0.3 μM ketanserin, and similar results were obtained in a total of seven cells. (b) Drug-sensitive current expressed as a proportion of the current in the absence and presence of 0.3 μM ketanserin. Raw data (points) were fitted to a single exponential function with a time constant of 852 ms. (c) Cells were held at a holding potential of −80 mV and pulsed to depolarizing voltage (+30 mV) for variable durations from 50 to 4950 ms in 250 ms increments. IhERG.tail was recorded upon repolarization to −50 mV in the absence (control) and presence of 0.3 μM ketanserin. (d) A plot of relative tail current with 0.3μM ketanserin vs the depolarizing duration. The time-dependent decay in relative tail current was fitted to a single exponential function.
The time course for the development of ketanserin block of hERG channels was also assessed using an envelope of tail test (Kamiya et al., 2001). Envelope tail protocol and representative hERG current before and after application of 0.3 μM ketanserin (Figure 5c) were used for analysing the onset of hERG channel block. The amplitude of envelope tail current with 0.3 μM ketanserin was normalized relative to control (Figure 5d), and the relative tail current decayed in a pulse duration-dependent manner. The time course of this decay was fitted to a single exponential function with a time constant of 456±77 ms (n=5).
Tonic block of hERG channel current by ketanserin was estimated by the initial value of the relative tail current activated by the envelope protocol. The initial relative tail current (at 50 ms) with 0.3 μM ketanserin was 87±5%; therefore actual tonic block of hERG channels by 0.3 μM ketanserin was about 13%. This suggests that there was only limited ketanserin binding to hERG channels in the resting state and that activation was required for ketanserin to block hERG channels.
Use-dependent effect of ketanserin on hERG channels
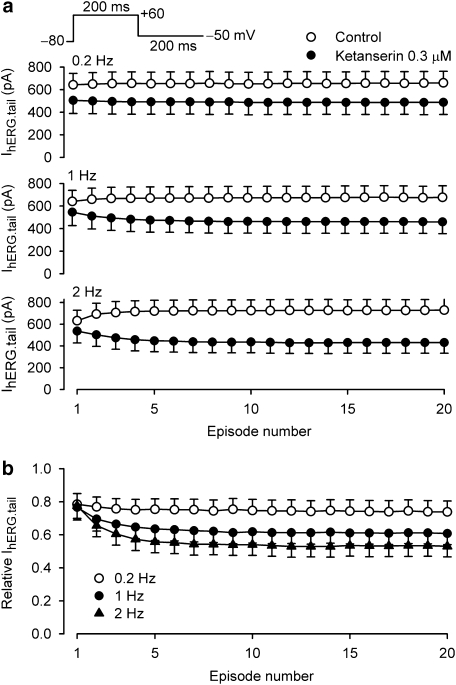
The use-dependent block of hERG channel by ketanserin was determined using a train of 20-episodes of short voltage steps as shown in the inset of Figure 6a. IhERG.tail amplitudes upon repolarization to −50 mV were measured at each pulse in the absence and presence of 0.3 μM ketanserin (Figure 6a). Consistent with the previous observation (Caballero et al., 2003), a slight increase of steady-state IhERG.tail was observed at 1 and 2 Hz, compared with that of the first episode. Ketanserin exposure (5 min) induced a substantial reduction of IhERG.tail at each frequency, and use-dependent block was observed at 1 and 2 Hz (Figure 6a). Relative IhERG.tail showed that the block of IhERG.tail (1 and 2 Hz) by ketanserin was slightly stronger at the 20th episode (61±7 and 53±6%) than the block at the first episode (77±6 and 78±6%, n=11, P<0.01). These results suggest that ketanserin has a use-dependent block of hERG channels.
Figure 6.
Frequency-dependence of hERG channel block by ketanserin. (a) A train of 200-ms voltage pulses from −80 to +60 mV, then to −50 mV (inset) were applied in control and in the presence of 0.3 μM ketanserin at 0.2, 1.0 and 2.0 Hz to record IhERG.tail. The hERG channel block by ketanserin appeared to be use-dependent at 1 and 2 Hz relative to control. (b) Ratio of IhERG.tail in the presence of 0.3 μM ketanserin relative to the control values at each pulse were plotted against pulse number. hERG Inhibition by ketanserin is use-dependent (n=6, P<0.01, vs first pulse) and frequency-dependent at 1 or 2 Hz, but not at 0.2 Hz (P<0.01 vs 0.2 Hz).
Molecular determinants of hERG channel block by ketanserin
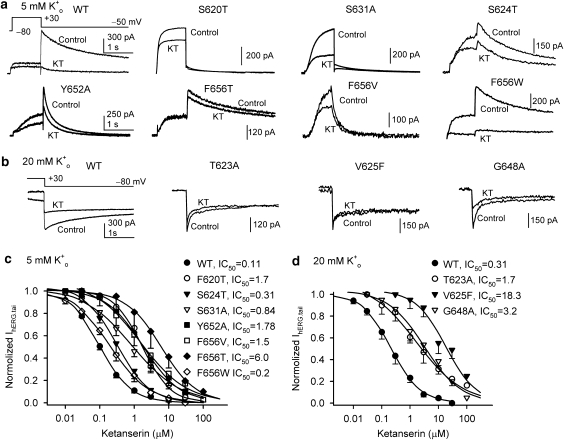
The molecular determinants of ketanserin block of hERG channels were investigated using the following mutants: S620T, T623A, S624T, V625F, S631A Y652A, F656T, F656V and F656W. S620, T623, S624 and V625 are located on the pore helix, and G648, Y652 and F656 are located on the S6 transmembrane domain. It has been shown that the binding site for MK-499, a methanesulphonanilide antiarrhythmic drug, to block hERG channel consists of amino acids located on the S6 domain (G648, Y652 and F656) and pore helix (T623 and V625) of the HERG channel subunit that face the cavity of the channel (Mitcheson et al., 2000). We used various mutants at F656 to evaluate the physicochemical feature of the amino acid side chain for ketanserin binding to hERG channels. S620T and S631A are well-documented inactivation-deficient mutants of hERG channel that have been used for investigating the role of inactivation in hERG block (Zhang et al., 1999). In addition to affecting inactivation, S620T has been suggested to be directly involved in cocaine binding to hERG channels (Guo et al., 2006).
These mutants attenuated IhERG block by ketanserin (Figure 7), which is similar to the previous observations with other hERG channel blockers (Suessbrich et al., 1997; Wang et al., 1997; Zhang et al., 1999; Mitcheson et al., 2000). The inactivation-deficient mutants S620T and S631A attenuated the channel block of ketanserin (7.6- and 15.5-fold, IC50s=0.84 and 1.7 μM) (Figure 7c). It is noted that although most mutant hERG currents were recorded in 5 mM [K+]o, the currents for T623A, V625F and G648A were recorded using a high [K+]o (20 mM) external solution because of their extremely fast inactivation gating kinetics. Their effects on ketanserin-induced block were compared with WT hERG channels under identical conditions (Figures 7b and d). These mutants markedly attenuated the hERG channel-blocking efficacy of ketanserin (1 μM). The IC50s of these mutants by ketanserin were higher than that of WT (Figures 7c and d).
Figure 7.
Effects of hERG channel mutations on ketanserin-induced block. (a) Current traces recorded under conditions of 5 mM K+o in HEK 293 cells expressing wild-type (WT), or mutant hERG channels: S620T, S624T, S631A Y652A, F656T, F656V and F656W, respectively, in the absence (control) and presence of 1 μM ketanserin (KT). The current was elicited with the protocol shown in the inset of the left panel. (b) Representative current traces recorded under conditions of 20 mM K+o in HEK 293 cells expressing wild-type hERG channel or hERG mutants T623A, V625F and G648A, respectively, before (control) and after 1 μM ketanserin (KT). The current was elicited with the protocol shown in the inset of the left panel. (c) Concentration-dependent effects of ketanserin on IhERG.tail of WT and various hERG mutants determined using 5 mM K+o (each data point represents 4–10 experiments). The IC50s of hERG mutants by ketanserin were increased by 2- to 58-fold, relative to that of WT hERG channel. (d) Concentration-dependent effects of ketanserin on IhERG.tail of WT and hERG mutants in 20 mM K+o. Each data point represents 4–9 experiments. The IC50s of hERG mutants by ketanserin were increased by 4.5- to 58-fold, relative to that of WT hERG channel.
The hydrophobicity of the side chain at position 656 has been shown to dictate the potency for block of hERG channels by several hERG channel blockers (for example, MK-499, cisapride and terfenadine) (Fernandez et al., 2004). Interestingly, F656T and F656V, but not F656W, attenuated the hERG channel block by ketanserin. The IC50s of F656T, F656V and F656W by ketanserin were 6.0, 1.5 and 0.2 μM, respectively.
Discussion
The present study demonstrates for the first time that the 5-HT2 receptor antagonist ketanserin directly blocks hERG channels stably expressed in HEK 293 cells in a concentration-dependent manner. The drug shows an open channel blocking property, enhances the inactivation degree of the channel, and negatively shifts the voltage dependence of inactivation of hERG channels. Elevation of [K+]o attenuates the binding affinity of ketanserin to hERG channels. However, ketanserin has no effect on human cardiac IKs channels.
Ketanserin is believed to be a useful medicine in the treatment of severe hypertension in pregnancy (van Schie et al., 2002) owing to its beneficial effects on platelet aggregation and thrombus formation in patients with the haemolysis, elevated liver enzymes, low-platelet syndrome (Steyn and Odendaal, 2000; Glennon et al., 2002; Hanff et al., 2005). Ketanserin acts pharmacologically as an antagonist of the 5-HT2 receptors in blood vessels to counteract the vasoconstrictive response to 5-HT (Steyn and Odendaal, 2000; Bolte et al., 2001). In addition, ketanserin showed a significant healing of local ulcers when it was topically used in diabetic patients (Martinez-de Jesus et al., 1997; Quatresooz et al., 2006) and leprosy patients (Salazar et al., 2001). Moreover, ketanserin was also applied for pharmacological management of complex regional pain syndrome (Luca-Vinhas et al., 2006; Rowbotham, 2006). However, ketanserin was found to have the cardiac toxicity that prolongs cardiac QTc interval in the ECG (Aldariz et al., 1986; Zehender et al., 1989; Frishman and Grewall, 2000).
Earlier experimental studies demonstrated that ketanserin prolonged cardiac action potential duration (Saman et al., 1985; Zaza et al., 1989), and a later study in guinea pig ventricular myocytes showed that these effects were related to the direct inhibition of the rapid delayed rectifier potassium channel current IKr (Le Grand et al., 1995b). Moreover, ketanserin decreased transient outward K+ current in rabbit (Le Grand et al., 1995a) and rat (Zhang et al., 1994) ventricular myocytes as well as ATP-sensitive K+ channels in mouse ventricular myocytes (Ju et al., 2006), but had no effect on cardiac inward rectifier K+ current (that is IK1) (Le Grand et al., 1995b) and Ca2+ current (Ouadid et al., 1992). Our present study demonstrated the additional novel information that ketanserin showed a potent and rapid block of hERG channels expressed in HEK 293 cells.
The maximum therapeutic blood plasma concentration when used in early pregnancy can reach 899 ng mL−1. The calculated free blood concentration of ketanserin is about 0.102 μM based on 94% protein-binding of the drug (Reimann et al., 1983). This concentration is close to the IC50 (0.11 μM) of ketanserin for inhibiting hERG channels expressed in HEK 293 cells (Figure 3), which indicates that therapeutic blood concentration of ketanserin could delay cardiac repolarization via inhibition of the rapid delayed rectifier K+ current IKr. Our previous study demonstrated the existence of both IKr and IKs in human ventricular myocytes (Li et al., 1996). The prolonged QTc interval of the ECG observed in clinical patients (Aldariz et al., 1986; Zehender et al., 1989; Frishman and Grewall, 2000) is likely to be related to the block of cardiac IKr and/or IKs. We found, however, that ketanserin blocked hERG channels, but not IKs channels. The blocking effect was significantly recovered on washout (Figure 1). Therefore, the cardiac toxicity can be reversed by withdrawing the drug.
Ketanserin blocked open hERG channels with features similar to those of other open-channel blockers, including dofetilide, vesnarinone, ambasilide, chloroquine and mesoridazine (Walker et al., 2000; Kamiya et al., 2001; Weerapura et al., 2002; Su et al., 2004). The blocking effect increased significantly at voltages positive to +10 mV at which hERG channel activation is maximal and/or complete (Figure 2c), and the block developed in response to a longer voltage pulse and/or envelope voltage protocol (Figure 5). This indicates that channel opening is required for the block of hERG channels by ketanserin. Tonic block of IhERG by ketanserin (0.3 μM) was only 13% (Figure 5d), which suggests that ketanserin has a small affinity with hERG channels in the closed or resting states.
The voltage dependence is essentially caused by the binding feature of ketanserin. This can be viewed as the voltage dependence is most prominent in voltages between −20 and +20 mV (Figure 2c). Between +10 and +60 mV, the voltage dependence is less prominent and the mechanism may come from the fact that the inactivation is voltage-dependent and inactivation gating facilitates ketanserin block.
The V0.5 of steady-state inactivation was negatively shifted by ketanserin (Figure 4f), suggesting that the inactivation gating of hERG channels is also likely to be affected by ketanserin. These properties are similar to those of azimilide (Walker et al., 2000). However, significant use- and frequency-block of hERG channels by ketanserin (Figure 6) differed from the hERG channel blocker azimilide (Jiang et al., 1999), but this effect is consistent with that of amiodarone (Kiehn et al., 1999).
The reduced blocking effect in the inactivation-deficient mutants S620T and S631A of hERG channels (Figure 7) suggests that the channel inactivation is required for ketanserin block of the channels, as observed in several studies using cardiac or non-cardiac agents (Su et al., 2004; Yang et al., 2004). The reduction of inactivation by elevating [K+]o, induced a decreased inhibition of IhERG.tail by ketanserin (Figure 3), though the degree of diminished block was not as strong as those observed in several cardiac active agents (Yang et al., 2004). Previous studies demonstrated that mutations interfering with hERG C-type inactivation significantly reduce blocking potency of various drugs (Zhang et al., 1999; Ficker et al., 2001).
The channel inhibition by ketanserin was significantly attenuated in the mutants T623A, S624T, V625F, G648A, Y652A and F656T (Figure 7), indicating that the pore helix and the S6 transmembrane domain contribute to the drug binding, as observed for several other drugs such as MK499, cisapride, terfenadine and cocaine (Mitcheson et al., 2000; Fernandez et al., 2004; Guo et al., 2006). F656T and F656V significantly decreased ketanserin sensitivity of the channel, suggesting that the binding of ketanserin is involved in Phe-656. However, F656W only showed a very slight decrease of sensitivity to ketanserin (Figure 7). This phenomenon is similar to that observed in cocaine (Guo et al., 2006). The possible explanation is that the magnitude of two-dimensional van der Waals hydrophobic surface area of these mutant side chains follows the order of F≈W>V and T as described previously (Fernandez et al., 2004; Guo et al., 2006). Our results suggest that the hydrophobicity of the side chain of residue 656 is also related to the channel sensitivity to ketanserin and therefore Phe656 is likely to be a site for ketanserin binding to hERG channels.
To conclude, ketanserin preferentially binds to and blocks activated hERG channels. Blockade of hERG channels is most likely to contribute to the prolongation of QT intervals of ECG observed in clinical patients using ketanserin at therapeutic doses.
Acknowledgments
This study was supported by a grant from Sun Chieh Yeh Heart Foundation of Hong Kong. We thank Dr G Robertson (University of Wisconsin-Madison, WI, USA) for providing the vector of hERG/pcDNA3.
Abbreviations
- DMEM
Dulbecco's modified Eagle's medium
- HERG
ether-à-go-go-related gene
- IC50
the concentration for a half-maximum inhibitory effect
- IhERG
hERG channel current
- LQTs
long-QT syndrome
- IKr
rapidly delayed rectifier potassium current
- IKs
slowly delayed rectifier potassium current
Conflict of interest
The authors state no conflict of interest.
References
- Alexander SPH, Mathie A, Peters JA.Guide to receptors and channels (GRAC) Br J Pharmacol 2008153Suppl. 2S1–S209.3rd edn. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldariz AE, Romero H, Baroni M, Baglivo H, Esper RJ. QT prolongation and torsade de pointes ventricular tachycardia produced by Ketanserin. Pacing Clin Electrophysiol. 1986;9:836–841. doi: 10.1111/j.1540-8159.1986.tb06633.x. [DOI] [PubMed] [Google Scholar]
- Banga FR, Bolte AC, Dekker GA, van Geijn HP. Ketanserin in women with chronic hypertension and underlying thrombophilia. Obstet Gynecol. 2004;103:1084–1087. doi: 10.1097/01.AOG.0000117085.65925.f2. [DOI] [PubMed] [Google Scholar]
- Bolte AC, van Eyck J, Gaffar SF, van Geijn HP, Dekker GA. Ketanserin for the treatment of preeclampsia. J Perinat Med. 2001;29:14–22. doi: 10.1515/JPM.2001.002. [DOI] [PubMed] [Google Scholar]
- Caballero R, Moreno I, Gonzalez T, Arias C, Valenzuela C, Delpon E, et al. Spironolactone and its main metabolite, canrenoic acid, block human ether-a-go-go-related gene channels. Circulation. 2003;107:889–895. doi: 10.1161/01.cir.0000048189.58449.f7. [DOI] [PubMed] [Google Scholar]
- Dong MQ, Lau CP, Gao Z, Tseng GN, Li GR. Characterization of recombinant human cardiac KCNQ1/KCNE1 channels (I(Ks)) stably expressed in HEK 293 cells. J Membr Biol. 2006;210:183–192. doi: 10.1007/s00232-006-0006-5. [DOI] [PubMed] [Google Scholar]
- Duley L, Henderson-Smart DJ, Meher S. Drugs for treatment of very high blood pressure during pregnancy. Cochrane Database Syst Rev. 2006;3:CD001449. doi: 10.1002/14651858.CD001449.pub2. [DOI] [PubMed] [Google Scholar]
- Fernandez D, Ghanta A, Kauffman GW, Sanguinetti MC. Physicochemical features of the HERG channel drug binding site. J Biol Chem. 2004;279:10120–10127. doi: 10.1074/jbc.M310683200. [DOI] [PubMed] [Google Scholar]
- Ficker E, Jarolimek W, Brown AM. Molecular determinants of inactivation and dofetilide block in ether a-go-go (EAG) channels and EAG-related K(+) channels. Mol Pharmacol. 2001;60:1343–1348. doi: 10.1124/mol.60.6.1343. [DOI] [PubMed] [Google Scholar]
- Frishman WH, Grewall P. Serotonin and the heart. Ann Med. 2000;32:195–209. doi: 10.3109/07853890008998827. [DOI] [PubMed] [Google Scholar]
- Gang H, Zhang S. Na+ permeation and block of hERG potassium channels. J Gen Physiol. 2006;128:55–71. doi: 10.1085/jgp.200609500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Lau CP, Chiu SW, Li GR. Inhibition of ultra-rapid delayed rectifier K+ current by verapamil in human atrial myocytes. J Mol Cell Cardiol. 2004;36:257–263. doi: 10.1016/j.yjmcc.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Glennon RA, Metwally K, Dukat M, Ismaiel AM, De los AJ, Herndon J, et al. Ketanserin and spiperone as templates for novel serotonin 5-HT(2A) antagonists. Curr Top Med Chem. 2002;2:539–558. doi: 10.2174/1568026023393787. [DOI] [PubMed] [Google Scholar]
- Guo J, Gang H, Zhang S. Molecular determinants of cocaine block of human ether-a-go-go-related gene potassium channels. J Pharmacol Exp Ther. 2006;317:865–874. doi: 10.1124/jpet.105.098103. [DOI] [PubMed] [Google Scholar]
- Hanff LM, Visser W, Steegers EA, Vulto AG. Population pharmacokinetics of ketanserin in pre-eclamptic patients and its association with antihypertensive response. Fundam Clin Pharmacol. 2005;19:585–590. doi: 10.1111/j.1472-8206.2005.00354.x. [DOI] [PubMed] [Google Scholar]
- Jiang M, Dun W, Fan JS, Tseng GN. Use-dependent ‘agonist' effect of azimilide on the HERG channel. J Pharmacol Exp Ther. 1999;291:1324–1336. [PubMed] [Google Scholar]
- Ju JM, Hwang JH, Piao LH, Park HW, Park JS, Shin DH, et al. Ketanserin, a 5-HT2 antagonist, directly inhibits the ATP-sensitive potassium channel in mouse ventricular myocytes. J Cardiovasc Pharmacol. 2006;47:96–102. doi: 10.1097/01.fjc.0000196238.51018.e9. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Mitcheson JS, Yasui K, Kodama I, Sanguinetti MC. Open channel block of HERG K(+) channels by vesnarinone. Mol Pharmacol. 2001;60:244–253. doi: 10.1124/mol.60.2.244. [DOI] [PubMed] [Google Scholar]
- Kiehn J, Thomas D, Karle CA, Schols W, Kubler W. Inhibitory effects of the class III antiarrhythmic drug amiodarone on cloned HERG potassium channels. Naunyn Schmiedebergs Arch Pharmacol. 1999;359:212–219. doi: 10.1007/pl00005344. [DOI] [PubMed] [Google Scholar]
- Le Grand B, Marty A, Colpaert FC, John GW. Ketanserin inhibits the transient outward current in rabbit ventricular myocytes. J Cardiovasc Pharmacol. 1995a;25:341–344. doi: 10.1097/00005344-199502000-00022. [DOI] [PubMed] [Google Scholar]
- Le Grand B, Talmant JM, Rieu JP, Patoiseau JF, Colpaert FC, John GW. Investigation of the mechanism by which ketanserin prolongs the duration of the cardiac action potential. J Cardiovasc Pharmacol. 1995b;26:803–809. doi: 10.1097/00005344-199511000-00018. [DOI] [PubMed] [Google Scholar]
- Li GR, Feng J, Yue L, Carrier M, Nattel S. Evidence for two components of delayed rectifier K+ current in human ventricular myocytes. Circ Res. 1996;78:689–696. doi: 10.1161/01.res.78.4.689. [DOI] [PubMed] [Google Scholar]
- Luca-Vinhas MC, Macedo CE, Brandao ML. Pharmacological assessment of the freezing, antinociception, and exploratory behavior organized in the ventrolateral periaqueductal gray. Pain. 2006;121:94–104. doi: 10.1016/j.pain.2005.12.008. [DOI] [PubMed] [Google Scholar]
- Martinez-de Jesus FR, Morales-Guzman M, Castaneda M, Perez-Morales A, Garcia-Alonso J, Mendiola-Segura I. Randomized single-blind trial of topical ketanserin for healing acceleration of diabetic foot ulcers. Arch Med Res. 1997;28:95–99. [PubMed] [Google Scholar]
- Mitcheson JS, Chen J, Lin M, Culberson C, Sanguinetti MC. A structural basis for drug-induced long QT syndrome. Proc Natl Acad Sci USA. 2000;97:12329–12333. doi: 10.1073/pnas.210244497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouadid H, Seguin J, Dumuis A, Bockaert J, Nargeot J. Serotonin increases calcium current in human atrial myocytes via the newly described 5-hydroxytryptamine4 receptors. Mol Pharmacol. 1992;41:346–351. [PubMed] [Google Scholar]
- Quatresooz P, Kharfi M, Paquet P, Vroome V, Cauwenbergh G, Pierard GE. Healing effect of ketanserin on chronic leg ulcers in patients with diabetes. J Eur Acad Dermatol Venereol. 2006;20:277–281. doi: 10.1111/j.1468-3083.2006.01422.x. [DOI] [PubMed] [Google Scholar]
- Reimann IW, Okonkwo PO, Klotz U. Pharmacokinetics of ketanserin in man. Eur J Clin Pharmacol. 1983;25:73–76. doi: 10.1007/BF00544018. [DOI] [PubMed] [Google Scholar]
- Rowbotham MC. Pharmacologic management of complex regional pain syndrome. Clin J Pain. 2006;22:425–429. doi: 10.1097/01.ajp.0000194281.74379.01. [DOI] [PubMed] [Google Scholar]
- Salazar JJ, Serrano GG, Leon-Quintero GI, Torres-Mendoza BM. Use of topical ketanserin for the treatment of ulcers in leprosy patients. Indian J Lepr. 2001;73:103–110. [PubMed] [Google Scholar]
- Saman S, Thandroyen F, Opie LH. Serotonin and the heart: effects of ketanserin on myocardial function, heart rate, and arrhythmias. J Cardiovasc Pharmacol. 1985;7 Suppl 7:S70–S75. [PubMed] [Google Scholar]
- Sanchez-Chapula JA, Navarro-Polanco RA, Culberson C, Chen J, Sanguinetti MC. Molecular determinants of voltage-dependent human ether-a-go-go related gene (HERG) K+ channel block. J Biol Chem. 2002;277:23587–23595. doi: 10.1074/jbc.M200448200. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Jiang C, Curran ME, Keating MT. A mechanistic link between an inherited and an acquired cardiac arrhythmia: HERG encodes the IKr potassium channel. Cell. 1995;81:299–307. doi: 10.1016/0092-8674(95)90340-2. [DOI] [PubMed] [Google Scholar]
- Smith PL, Baukrowitz T, Yellen G. The inward rectification mechanism of the HERG cardiac potassium channel. Nature. 1996;379:833–836. doi: 10.1038/379833a0. [DOI] [PubMed] [Google Scholar]
- Spector PS, Curran ME, Zou A, Keating MT, Sanguinetti MC. Fast inactivation causes rectification of the IKr channel. J Gen Physiol. 1996;107:611–619. doi: 10.1085/jgp.107.5.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyn DW, Odendaal HJ. Serotonin antagonism and serotonin antagonists in pregnancy: role of ketanserin. Obstet Gynecol Surv. 2000;55:582–589. doi: 10.1097/00006254-200009000-00024. [DOI] [PubMed] [Google Scholar]
- Su Z, Martin R, Cox BF, Gintant G. Mesoridazine: an open-channel blocker of human ether-a-go-go-related gene K+ channel. J Mol Cell Cardiol. 2004;36:151–160. doi: 10.1016/j.yjmcc.2003.10.017. [DOI] [PubMed] [Google Scholar]
- Suessbrich H, Schonherr R, Heinemann SH, Lang F, Busch AE. Specific block of cloned Herg channels by clofilium and its tertiary analog LY97241. FEBS Lett. 1997;414:435–438. doi: 10.1016/s0014-5793(97)01030-2. [DOI] [PubMed] [Google Scholar]
- Tang Q, Jin MW, Xiang JZ, Dong MQ, Sun HY, Lau CP, et al. The membrane permeable calcium chelator BAPTA-AM directly blocks human ether a-go-go related gene potassium channels stably expressed in HEK 293 cells. Biochem Pharmacol. 2007;74:1596–1607. doi: 10.1016/j.bcp.2007.07.042. [DOI] [PubMed] [Google Scholar]
- Tian M, Dong MQ, Chiu SW, Lau CP, Li GR. Effects of the antifungal antibiotic clotrimazole on human cardiac repolarization potassium currents. Br J Pharmacol. 2006;147:289–297. doi: 10.1038/sj.bjp.0706590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau MC, Warmke JW, Ganetzky B, Robertson GA. HERG, a human inward rectifier in the voltage-gated potassium channel family. Science. 1995;269:92–95. doi: 10.1126/science.7604285. [DOI] [PubMed] [Google Scholar]
- van Schie DL, de Jeu RM, Steyn DW, Odendaal HJ, van Geijn HP. The optimal dosage of ketanserin for patients with severe hypertension in pregnancy. Eur J Obstet Gynecol Reprod Biol. 2002;102:161–166. doi: 10.1016/s0301-2115(01)00611-x. [DOI] [PubMed] [Google Scholar]
- Walker BD, Singleton CB, Tie H, Bursill JA, Wyse KR, Valenzuela SM, et al. Comparative effects of azimilide and ambasilide on the human ether-a-go-go-related gene (HERG) potassium channel. Cardiovasc Res. 2000;48:44–58. doi: 10.1016/s0008-6363(00)00155-3. [DOI] [PubMed] [Google Scholar]
- Wang S, Morales MJ, Liu S, Strauss HC, Rasmusson RL. Modulation of HERG affinity for E-4031 by [K+]o and C-type inactivation. FEBS Lett. 1997;417:43–47. doi: 10.1016/s0014-5793(97)01245-3. [DOI] [PubMed] [Google Scholar]
- Weerapura M, Hebert TE, Nattel S. Dofetilide block involves interactions with open and inactivated states of HERG channels. Pflugers Arch. 2002;443:520–531. doi: 10.1007/s004240100720. [DOI] [PubMed] [Google Scholar]
- Yang BF, Xu DH, Xu CQ, Li Z, Du ZM, Wang HZ, et al. Inactivation gating determines drug potency: a common mechanism for drug blockade of HERG channels. Acta Pharmacol Sin. 2004;25:554–560. [PubMed] [Google Scholar]
- Zaza A, Malfatto G, Rosen MR. Electrophysiologic effects of ketanserin on canine Purkinje fibers, ventricular myocardium and the intact heart. J Pharmacol Exp Ther. 1989;250:397–405. [PubMed] [Google Scholar]
- Zehender M, Meinertz T, Hohnloser S, Geibel A, Hartung J, Seiler KU, et al. Incidence and clinical relevance of QT prolongation caused by the new selective serotonin antagonist ketanserin. Am J Cardiol. 1989;63:826–832. doi: 10.1016/0002-9149(89)90051-9. [DOI] [PubMed] [Google Scholar]
- Zhang S, Zhou Z, Gong Q, Makielski JC, January CT. Mechanism of block and identification of the verapamil-binding domain to HERG potassium channels. Circ Res. 1999;84:989–998. doi: 10.1161/01.res.84.9.989. [DOI] [PubMed] [Google Scholar]
- Zhang ZH, Boutjdir M, el-Sherif N. Ketanserin inhibits depolarization-activated outward potassium current in rat ventricular myocytes. Circ Res. 1994;75:711–721. doi: 10.1161/01.res.75.4.711. [DOI] [PubMed] [Google Scholar]