Abstract
Specialized tissues that sense acute changes in the local oxygen tension include type 1 cells of the carotid body, neuroepithelial bodies in the lungs, and smooth muscle cells of the resistance pulmonary arteries and the ductus arteriosus (DA). Hypoxia inhibits outward potassium current in carotid body type 1 cells, leading to depolarization and calcium entry through L-type calcium channels. Increased intracellular calcium concentration ([Ca++]i) leads to exocytosis of neurotransmitters, thus stimulating the carotid sinus nerve and respiration. The same K+ channel inhibition occurs with hypoxia in pulmonary artery smooth muscle cells (PASMCs), causing contraction and providing part of the mechanism of hypoxic pulmonary vasoconstriction (HPV). In the SMCs of the DA, the mechanism works in reverse. It is the shift from hypoxia to normoxia that inhibits K+ channels and causes normoxic ductal contraction. In both PA and DA, the contraction is augmented by release of Ca++ from the sarcoplasmic reticulum, entry of Ca++ through store-operated channels (SOC) and by Ca++ sensitization. The same three ‘executive' mechanisms are partly responsible for idiopathic pulmonary arterial hypertension (IPAH). While vasoconstrictor mediators constrict both PA and DA and vasodilators dilate both vessels, only redox changes mimic oxygen by having directly opposite effects on the K+ channels, membrane potential, [Ca++]i and tone in the PA and DA. There are several different hypotheses as to how redox might alter tone, which remain to be resolved. However, understanding the mechanism will facilitate drug development for pulmonary hypertension and patent DA.
Keywords: pulmonary hypertension, patent ductus arteriosus, oxygen sensing, ion channels, calcium sensitivity, carotid body
Introduction
In 1604, Joseph Acosta traversed a pass in the Andes and wrote ‘I hold this place to be one of the highest parts of land in the world, … I therefore persuade myself, that the element of the air is there so subtle and delicate, as it is not proportional with the breathing of man, which requires a more gross and temperate air' (West, 1981). He clearly understood, about two centuries before the discovery of oxygen, that the air high in the mountains was diminished in something and consequently he was short of breath. Other than being curious about the nature of breathlessness, why should we care about the mechanisms by which the body senses oxygen? At birth, directly related to the breathing of air and the consequent increase in oxygen, the small resistance PAs dilate and blood flow dramatically increases in the lungs. In a diametrically opposite response to the increase in oxygen, the ductus arteriosus (DA) contracts and the switch from the foetal to the neonatal circulation is established. Thus, the sensing of oxygen is critically important in normal birth. In the foetus, the PAs and the ductus are exposed to the same blood pressure and the same low oxygen tension in the blood. Consequently, their opposite response to increased oxygen is not the result of different conditioning prior to that point; it is intrinsic to the vessels. Perhaps we should try to understand the mechanisms that are responsible for ‘normoxic pulmonary vasodilatation' (Weir, 1978), rather than think about hypoxic pulmonary vasoconstriction (HPV). Failure of the PAs to relax and remodel at birth leads to pulmonary hypertension and the condition known as persistent pulmonary hypertension of the newborn, whereas failure of the ductus to contract results in patent DA. Both are serious neonatal conditions with significant morbidity and mortality. The first clues to the mechanisms underlying acute oxygen sensing came from the study of the type I cells of the carotid body.
Carotid body
The carotid body lies at the bifurcation of the carotid arteries. It senses the oxygen tension in the arterial blood and, if the oxygen level drops, signals to the respiratory centre in the brain by increasing traffic in the carotid sinus nerve, causing a sensation of breathlessness. The cellular mechanism by which hypoxia is detected in the type I, or glomus, cell of the carotid body was first described 20 years ago (Lopez-Barneo et al., 1988). The outward flow of potassium through voltage-gated (Kv), Ca++-sensitive (KCa) and two-pore domain acid-sensitive potassium channels is inhibited by hypoxia, leading to membrane depolarization and Ca++ entry through L-type calcium channels (Ganfornina and Lopez-Barneo, 1992; Wyatt et al., 1995b; Buckler, 1997). It is important to note that the increase in [Ca++]i, which triggers the exocytosis of vesicles from within the type I cell, does not occur in the absence of extracellular Ca++ or without membrane depolarization (Buckler and Vaughan-Jones, 1994; Urena et al., 1994). The increase in [Ca++]i is proportional to the severity of hypoxia (Dasso et al., 2000). The exocytosis causes the release of neurotransmitters, such as ACh and ATP, which stimulate sensory nerve endings in the carotid sinus nerve. This signalling mechanism, which connects K+ channel inhibition with increased respiration, has been exploited pharmacologically. The respiratory stimulant doxapram mimics hypoxia in that it inhibits both Kv and KCa currents in type I cells (Peers, 1991).
Hypoxic pulmonary vasoconstriction: K+ channels
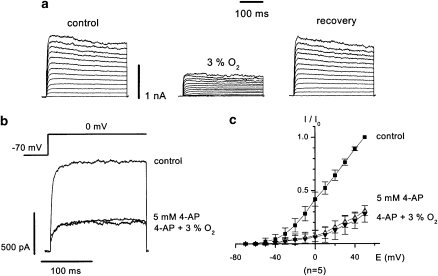
In the adult, HPV fulfils the important function of matching perfusion to ventilation in the lungs. If there is a localized area of atelectasis, then alveolar collapse and the associated hypoxia causes vasoconstriction in the small PAs that course through the parenchyma. The HPV directs the desaturated blood to better ventilated/oxygenated segments of the lung. If HPV is inhibited, systemic arterial oxygen tension is reduced, even in normal subjects (Hales and Westphal, 1978). Although it is clear that the endothelium produces vasoconstrictor (such as endothelin and thromboxane A2) and vasodilator (such as nitric oxide and prostacyclin) substances, which modulate HPV, the pulmonary arterial smooth muscle contracts to hypoxia in the absence of the endothelium (Madden, 1992). This discussion focuses on the mechanisms in the smooth muscle cell (SMC) that change in response to acute changes in oxygen tension. Based on the role of K+ channel inhibition in insulin secretion in the pancreatic islet cell, it was suggested that HPV might involve K+ channel inhibition in the SMCs of the small PAs (Archer et al., 1986a). Insulin secretion is initiated by ATP-induced blockade of KATP channels in the islet cells, whereas hypoxia causes inhibition of other K+ channels, such as the Kv, KCa and two-pore domain acid-sensitive potassium channels, in the pulmonary arterial SMCs (PASMCs) (Post et al., 1992; Yuan et al., 1993; Cornfield et al., 1996; Gurney et al, 2003; Olschewski et al., 2006). This, in turn, results in membrane depolarization and Ca++ entry through L-type calcium channels, as in the carotid body type I cell. The two-pore domain acid-sensitive potassium-1 channel carries a non-inactivating background K+ current that sets the resting membrane potential in PASMCs (Gurney et al., 2003). Hypoxic inhibition of this channel may cause sufficient depolarization to bring the membrane potential into the range where other K+ channels, such as KCa and Kv, are active (Gurney et al., 2003; Olschewski et al 2006; Gurney and Manoury, 2008). It seems likely that there is a maturational shift from oxygen sensitivity of the KCa channels in the foetus to several Kv channels in the adult PASMCs (Reeve et al., 1998; Michelakis et al., 2004). There is also geographical localization of the channels, that is, Kv channels predominate in the small resistance PAs and KCa channels predominate in the conduit PAs (Archer et al., 1996). Importantly, hypoxia does not inhibit K+ current in systemic arterial SMCs, such as renal or mesenteric (Post et al., 1992; Yuan et al., 1993). In the PASMCs, the degree of K+ current inhibition and membrane depolarization is proportional to the severity of hypoxia (Olschewski et al., 2002). Hypoxia seems to act primarily on the Kv current in rat PASMCs, as after inhibition by 4-aminopyridine (5 mM), hypoxia does not reduce the current further (Figure 1). These observations make it likely that K+ channels play a role in the mechanism underlying HPV.
Figure 1.
Effects of hypoxia on whole-cell K+ current in pulmonary artery smooth muscle cells. (a) 300 ms traces showing K+ currents elicited under normoxic conditions (control and recovery) and after a 4 min exposure to hypoxia, 3% O2. (b) K+ current stimulated by a step depolarization from −70 to 0 mV, showing that after inhibition by 4-aminopyridine, hypoxia (4 min at 3% O2) does not reduce the current further. (c) Current–voltage plots of steady-state outward K+ currents recorded under the same conditions as in (b). The current is normalized to the maximum current measured during normoxia (measured current divided by maximum current at +50 mV, I/I0). Data is shown as means±s.e.mean. The figure is adapted and modified from reference Olschewski et al (2002), with permission.
The most important oxygen-sensitive Kv channels include Kv 1.2, 1.5, 2.1, 3.1b and 9.3, as reviewed in Moudgil et al (2005). Hypoxia inhibits Kv 1.5, which has been cloned from human PAs (Archer et al., 2004b), and HPV is diminished in mice that lack this channel (Archer et al., 2001). It is interesting that not all PASMCs show the same response to hypoxia in terms of inhibition of K+ current or increase in [Ca++]i (Platoshyn et al., 2007). Using single-cell reverse transcription-PCR, it was demonstrated that the level of expression of Kv 1.5 correlates with the sensitivity of the potassium current in the individual PASMC to hypoxia. This paper also raises the important concept that there are ‘pacemaker' PASMCs, responsive to hypoxia, which communicate with other PASMCs through their gap junctions. Chronic hypoxia causes a decrease in mRNA and protein for oxygen-sensitive Kv channels and this results in membrane depolarization in PASMCs (Smirnov et al., 1994b; Osipenko et al., 1998; Reeve et al., 2001). For Kv 1.2, 1.5 and 2.1, the decrease in mRNA occurs within 6 h of the onset of hypoxia (Hong et al., 2004). The decreased expression of K+ channels in chronic hypoxia reflects the activity of the transcription factor hypoxia-inducing factor (HIF)-1α (Shimoda et al., 2001). In concert with the decrease in oxygen-sensitive K+ channels, acute HPV is diminished in rats that have been exposed to chronic hypoxia (McMurtry et al., 1978). However, HPV can be restored by aerosol transfection of Kv 1.5, again underlining the role of Kv channels in this mechanism (Pozeg et al., 2003).
Hypoxic pulmonary vasoconstriction: sarcoplasmic reticulum/store-operated channel
Although most of the Ca++ involved in HPV comes from outside the PASMC, some is released from intracellular stores (Olschewski et al., 2002). The release of Ca++ by hypoxia from the sarcoplasmic reticulum (SR) was first reported in 1993 (Salvaterra and Goldman, 1993). Since that time, evidence has been published for hypoxic release of Ca++ from both inositol triphosphate and ryanodine-sensitive SR stores in PASMCs (Jabr et al., 1997; Dipp et al., 2001a; Morio and McMurtry, 2002). The Ca++ stores are repleted by Ca++ entry through store-operated (capacitative) Ca++ channels, and subsequent sequestration of the Ca++ into the SR by the Ca++-Mg++-ATPase (Robertson et al., 2000b; Wang et al., 2005; Weigand et al., 2005; Ng et al., 2005). Blockers of capacitative Ca++ entry prevent HPV at concentrations that do not block the L-type Ca++ channels (Weigand et al., 2005). It is thought that transient receptor potential (TRP) genes may code for the store-operated channels (SOCs). Isolated mouse lungs that are deficient in TRPC6 lack the modest acute HPV, which is demonstrated in the wild-type lungs (Weissmann et al., 2006). From 1 h of hypoxia onward, the pressor response is the same in the wild-type and TRPC6-deficient mice. This suggests that different mechanisms may play more important roles at different times. Other TRP channels (TRPCs) may also participate. As mentioned earlier for Kv channels, the expression of TRPCs 1, 4 and 6 is greater in the SMCs of the distal resistance PAs than in those of the conduit main PA (Lu et al., 2008). The store-operated Ca++ entry elicited by hypoxia is also greater in the resistance PASMCs. Emptying of the inositol triphosphate-sensitive Ca++ stores in the SR of rat PASMCs using cyclopiazonic acid (an inhibitor of Ca++-Mg++-ATPase) prevents the release of Ca++ from the SR by hypoxia (Platoshyn et al., 2007), again illustrating the importance of this component of HPV. More discussions on the mechanisms of capacitative Ca++ entry and its role in HPV are given in recent reviews (Ward et al., 2005; Putney, 2007).
Hypoxic pulmonary vasoconstriction: calcium sensitization
In addition to the two mechanisms outlined above, which lead to an increase in [Ca++]i in PASMCs during hypoxia, there is a third player, Ca++ sensitization. This mechanism permits prolonged interaction of actin and myosin at any given Ca++ level. Contraction is initiated by phosphorylation of myosin light chain by myosin light-chain kinase and dephosphorylation is mediated by myosin light-chain phosphatase. As a result, vascular tone is modulated by the balance between myosin light-chain kinase and phosphatase. In 1995, Robertson et al. (1995) pointed out that when HPV is studied in small PAs, the force of contraction may gradually increase while the [Ca++] level remains constant. Hypoxia increases the GTP-bound (active) form of the small G-protein RhoA at the cell membrane of the PASMCs. This in turn stimulates Rho kinase, which inhibits myosin light-chain phosphatase, thereby prolonging phosphorylation of the light chain and augmenting contraction (Wang et al., 2001). As Rho kinase activation increases HPV (Ward et al., 2004), therefore Rho kinase inhibition reduces it (Robertson et al., 2000a; Fagan et al., 2004). In the fawn-hooded rat, which develops spontaneous pulmonary hypertension, activated RhoA is increased and, related to this, Rho kinase inhibition largely prevents the development of pulmonary hypertension (Nagaoka et al., 2006). After 4 weeks of hypoxia, pulmonary hypertension is established in the Sprague–Dawley rat model. Somewhat surprisingly, the pulmonary hypertension can be nearly normalized by Rho kinase inhibition (Nagaoka et al., 2005). This suggests that although remodelling of the small PAs has occurred by this point, the pulmonary hypertension in this model is more a product of vasoconstriction (Stenmark and McMurtry, 2005). When the Rho kinase inhibitor is administered by inhalation, the effect on the pulmonary circulation, relative to the systemic, is specific.
Clinical significance of the HPV mechanisms
From the literature reviewed above, it is evident that there are at least three components to the ‘executive' side of the mechanism of HPV: (1) K+ channel inhibition, membrane depolarization and Ca++ entry through the L-type, voltage-gated Ca++ channels; (2) release of Ca++ from the SR, associated with repletion by capacitative Ca++ entry through SOCs; and (3) Ca++ sensitization. Interestingly, the pathophysiology of idiopathic pulmonary arterial hypertension (IPAH) involves the same three pathways. In PASMCs of patients with IPAH, expression of the channels Kv 1.5 and 2.1 is reduced, the membrane potential is depolarized and cytosolic calcium is increased compared with PASMCs of patients with secondary pulmonary hypertension (Yuan et al., 1998a, 1998b). Seventeen single-nucleotide polymorphisms of the gene coding Kv 1.5 have been identified in IPAH patients (Remillard et al., 2007). However, it is not yet clear whether these polymorphisms alter the function of the channel. The anorectic drug fenfluramine has been shown to increase the incidence of IPAH. Experimentally it causes pulmonary vasoconstriction and inhibits potassium current in PASMCs (Weir et al., 1996a; Perchenet et al., 2001). These observations of a decrease in PASMC potassium current in the aetiologic mechanism of pulmonary hypertension and pulmonary vasoconstriction have led to studies of a possible therapy. Overexpression of Kv 1.5 in PASMCs increases potassium current, leading to hyperpolarization and more apoptosis (Brevnova et al., 2004). Greater apoptosis would be expected to reduce the number of PASMCs in the media of the resistance PAs. In the chronic hypoxic model of pulmonary hypertension in rats, inhalation of the gene for Kv 1.5 reduces pulmonary vascular remodelling and hypertension (Pozeg et al., 2003).
If lack of expression or function of Kv channels leads to membrane depolarization and activation of the L-type Ca++ channels, then why are inhibitors of these Ca++ channels (for example, dihydropyridines, such as nifedipine) now found to be successful in fewer than 20% of IPAH patients (Rich and Brundage, 1987)? It could be that other components of the executive mechanism become more important, that remodelling becomes irreversible or that the lack of expression of K+ channels results in an increase in cytosolic K+, which in turn inhibits apoptosis (Krick et al., 2001). Inhibition of the L-type Ca++ channel would not affect the increase in cytosolic K+ or prevent the membrane depolarization, which may cause Ca++ release from the SR, unrelated to calcium entry (Ganitkevich and Isenberg, 1993; Valle-Rodriguez et al., 2006).
The pathophysiology of IPAH, again mimicking HPV, also involves increased expression/function of store-operated Ca++ channels (Yu et al., 2004). Although there are blockers of store-operated Ca++ channels that are used in animal studies (2-APB and SKF96365), there are no specific blockers in clinical use.
The third component of the executive mechanism of HPV is Ca++ sensitization, which depends on the activation of RhoA/Rho kinase. Inhibition of Rho kinase by fasudil has been reported to cause some acute pulmonary vasodilatation in a group of patients with severe pulmonary hypertension unresponsive to nifedipine or 100% oxygen (Fukumoto et al., 2005). A clinical trial of the efficacy of fasudil in IPAH is being conducted. As both HPV and IPAH affect only the pulmonary circulation, perhaps it is not surprising that they share these three executive mechanisms. Insights into the pathophysiology of one increase our understanding of the other.
Normoxic contraction of the ductus arteriosus
As stated earlier, the DA behaves exactly opposite to the resistance PAs, in that it contracts during normoxia and relaxes in hypoxia. As a consequence, failure of the DA to close after birth is a much more common problem in populations living at high altitude (Alzamora-Castro et al., 1960). Normoxic contraction of the DA involves the same three executive mechanisms as HPV. An increase in oxygen tension from foetal to neonatal levels inhibits Kv channels in the DASMCs, resulting in membrane depolarization and Ca++ entry through the L-type Ca++ channels (Tristani-Firouzi et al., 1996b; Michelakis et al., 2000), Failure of the DA to close is common in premature infants and this may be related to a decreased expression of oxygen-sensitive Kv channels preterm (Thebaud et al., 2004). Transfection of Kv 1.5 and 2.1 in the premature rabbit DA can restore oxygen sensitivity.
In addition to the influx of Ca++ into DASMCs stimulated by membrane depolarization, Ca++ is released from the SR and repleted through store-operated Ca++ channels (Hong et al., 2006). Finally, as in HPV, normoxic contraction of the DA is enhanced by Ca++ sensitization (Hong et al., 2006; Kajimoto et al., 2007). The main difference is that normoxia increases RhoB in the DA, rather than the RhoA that is increased in PA (Kajimoto et al., 2007). RhoB then increases the activity of Rho kinase.
Dilator prostaglandins help to keep the DA open in the foetus and the therapeutic use of indomethacin in the neonate with a patent DA is designed to remove this dilator influence. However, the normoxic DA contraction discussed above occurs irrespective of the presence or absence of prostaglandins or nitric oxide (Michelakis et al., 2000).
Oxygen sensing
If the mechanisms responsible for smooth muscle contraction in HPV and normoxic contraction of the DA are virtually the same, why is the response to a change in oxygen diametrically opposite? It might be thought that a subtle difference in the α-subunit of a K+ channel would provide the explanation. However, when the same human Kv 1.5 gene is transfected into rat PA and mesenteric artery SMCs, the whole-cell potassium current is only inhibited by hypoxia in the PASMC and not in the mesenteric cells (Platoshyn et al., 2006). The conclusion is that there must be signalling upstream to the potassium channel (and presumably the SR/SOC and Rho kinase), which is specific for the PA. This could be a different beta subunit of the potassium channels in the PASMCs compared with the DASMCs, or could be a switch between two potential signalling cascades. A clue came from the observation that oxidants could cause pulmonary vasodilatation (Weir and Will, 1982). This led to the hypothesis that hypoxia would cause a change in the redox status of the PASMC to a more reduced state and inhibit the K+ current, leading to membrane depolarization and Ca++ entry (Archer et al., 1986a). Subsequent observations supported this concept in the PA (Archer et al., 1993), whereas in the DA, the redox gating of the K+ current was opposite, such that an oxidant stimulus would inhibit the K+ current (Reeve et al., 2001b). It is important to note that vasoconstrictors, such as endothelin, phenylephrine or prostaglandin F2α, contract both PA and DA, whereas vasodilators, such as prostaglandins E1 and E2, dilate both. Hypoxia is unusual in that it constricts the PA and dilates the DA. Redox status has the same effect as changes in oxygen tension; a reducing agent, such as dithiotreitol, will mimic hypoxia as it inhibits K+ current, depolarizes membrane potential, increases cytosolic calcium in PASMCs and causes PA constriction, whereas it has the opposite effects in DASMCs and relaxes the DA (Olschewski et al., 2004). Conversely, an oxidizing agent, such as dithionitrobenzoic acid increases K+ current in PASMCs and relaxes PAs, while doing the opposite in the DA. In both vessels, a reducing agent has the same effect as hypoxia and an oxidizing agent as normoxia. Recently, it has been reported that the oxidizing agent diamide also reduces capacitative Ca++ entry in the PA, again mimicking normoxia (Schach et al., 2007).
The redox status of the SMCs is determined by the activity of the mitochondria and enzymes (for example, NADPH oxidase). The relevant components are the generation of reactive oxygen species (ROS), such as superoxide anion and hydrogen peroxide, and the ratios of redox couples. Hypoxia increases the ratio of reduced to oxidized redox pairs NAD(P)H/NAD(P) and glutathione (reduced, GSH)/glutathione (oxidized, GSSG) in the lung (Chander et al., 1980; Shigemori et al., 1996; Leach et al., 2001; Reeve et al., 2001) and carotid body (Biscoe and Duchen, 1990). Using the patch-clamp technique to study PASMCs, and to alter the intracellular milieu, inclusion of GSH in the patch pipette reduces the potassium current and inclusion of GSSG increases it (Weir and Archer, 1995). An elegant experiment reported by Tipparaju et al. (2005) illustrates the redox effect of not only pyridine nucleotides but also the associated β-subunits. When Kv 1.5 is expressed in COS-7 cells, the current is not altered by inclusion of the oxidized pyridine nucleotides, NAD or NADP, in the patch pipette. However, if Kvβ 1.3 is also transfected into the cell, then there is marked inactivation of the K+ current, which can be prevented by inclusion of NAD or NADP. In single-channel studies, NAD also increased the mean open time of the Kv 1.5 channel. These reports illustrate how a change in redox status induced by hypoxia might alter K+ channel gating in PASMCs. A more complex role for NAD and NADH has been proposed for the release of Ca++ from the SR, through an increase in cADP-ribose (Dipp and Evans, 2001b; Wilson et al., 2001); Evans et al., 2005).
Clearly, the generation of ROS is a determinant of the cellular redox status. Given the large number of papers focused on the specialized tissues that make up the body's ‘homeostatic oxygen-sensing system' (Weir et al., 2005), it might be thought that there would be a consensus as to whether ROS levels increase or decrease during physiological hypoxia. Unfortunately, this is not the case as illustrated in a recent Point:Counterpoint debate (Ward, 2006; Weir and Archer, 2006). Additional papers have provided arguments on both sides (Bonnet et al., 2006; Waypa et al., 2006; Wang et al., 2007; Archer et al., 2008; Waypa and Schumacker, 2008). It seems likely that the opposite conclusions arise from the use of different techniques for measuring ROS; the study of cultured cells, vessels and isolated perfused lungs; and differences in the severity and duration of hypoxia. In addition to measurement of ROS per se, insight may be gained by integrating changes in ROS with function (for example, the pressure response to hypoxia in the isolated lung or of Ca++ in PASMCs); with signalling mechanisms (for example, HIF1α, K+ current or RhoA/Rho kinase activity); with structure (for example, differences in mitochondrial morphology); with models of pulmonary hypertension (for example, the fawn-hooded rat or chronic hypoxia) and with treatment (for example, dichloroacetate or antioxidant enzymes, such as catalase). One example is illustrated by the fawn-hooded rat, which spontaneously develops pulmonary hypertension. In this rat, even under normoxic circumstances, the normal filamentous network of mitochondria in the PASMCs is disrupted, there is loss of electron transport chain complexes, especially complex 1, and less ROS production (Bonnet et al., 2006). As a result, there is increased activation of the transcription factor HIF1α, normally increased by hypoxia, and decreased expression of Kv 1.5 and decreased K+ current. These changes mimic the changes seen in the pulmonary hypertension associated with chronic hypoxia and in IPAH. The application of the oxidant t-butyl hydrogen peroxide (cell permeable form of H2O2), inhibits HIF1α activation and restores Kv 1.5 expression in fawn-hooded PASMCs. Treatment of the fawn-hooded rats with dichloroacetate, a mitochondrial pyruvate dehydrogenase kinase inhibitor that would make the PASMCs more oxidized, reduces HIF1 activation and the spontaneous pulmonary hypertension (Bonnet et al., 2006). These observations support the conclusion that the fawn-hooded rat behaves as if it was in a hypoxic environment. The effects in the fawn-hooded model of t-butyl H2O2, which is also known to decrease acute HPV (Weir and Will, 1982), and dichloroacetate, which has also been shown to decrease chronic hypoxic pulmonary hypertension (Michelakis et al., 2002c), strengthens the concept that in the PASMC, hypoxia is signalled by a more reduced environment.
In the DAMSCs, redox moves in the same direction with hypoxia as in PASMCs, becoming more reduced (Michelakis et al., 2002b; Kajimoto et al., 2007). An increase in oxygen and a more oxidized DASMC leads to the inhibition of K+ current and membrane depolarization, an effect that is mimicked by extracellular t-butyl H2O2 (Michelakis et al., 2002b) or intracellular H2O2 (Reeve et al., 2001b). The normoxic inhibition of K+ current can be prevented by increasing intracellular catalase, indicating that the increase in oxygen is signalled by an increase in endogenous H2O2.
The discussion so far has concerned the potential role of redox changes in controlling K+ channels and, to a lesser extent, store-operated Ca++ channels. The executive component of both HPV and normoxic DA contraction has the third element of Ca++ sensitization, involving Rho kinase. Both an increase in oxygen and the addition of H2O2 to DASMCs have been shown to increase Rho kinase expression and activity (Kajimoto et al., 2007). The implication is that H2O2 might be a critical link in the signalling cascade between a rise in oxygen and ductal contraction. This conclusion is strengthened by the finding that the proximal mitochondrial inhibitor rotenone, which, like hypoxia, increases potassium current and relaxes the normoxic DA, also like hypoxia, decreases H2O2 production in the DA (Michelakis et al., 2002c). In PASMCs and PAs, it does exactly the opposite (Archer et al., 1993; Michelakis et al., 2002a).
The papers cited above indicate that hypoxia, reducing agents and mitochondrial inhibitors can cause inhibition of potassium current, membrane depolarization and contraction of the PA. The same interventions cause an increase in potassium current, membrane hyperpolarization and relaxation in the DA. Most of those who study oxygen sensing would agree that a change in the redox status of the SMCs in the PA and DA initiates the change in vascular tone. Exactly what the redox signal may be and the subsequent signalling sequence remains to be determined. When the mechanism is defined, it will open the path for the development of new treatments for pulmonary hypertension, patent DA and perhaps for conditions such as sleep apnoea.
Acknowledgments
Supported by NIH ROI HL 65322 (EKW). The development of the K+ channel and redox hypotheses of HPV and normoxic contraction of the ductus arteriosus owe much to collaboration with Stephen L. Archer and Evangelos Michelakis.
Abbreviations
- DA
ductus arteriosus
- HPV
hypoxic pulmonary vasoconstriction
- IPAH
idiopathic pulmonary arterial hypertension
- KCa
calcium-sensitive potassium
- Kv
voltage-gated potassium
- PA
pulmonary artery
- ROS
reactive oxygen species
- SMC
smooth muscle cell
- SOC
store-operated channel
- SR
sarcoplasmic reticulum
- TRP
transient receptor potential
Conflict of interest
The authors state no conflict of interest.
References
- Alzamora-Castro V, Battilana G, Abigattas R, Sailer S. Patent ductus arteriosus and high altitude. Am J Cardiol. 1960;5:761–763. doi: 10.1016/0002-9149(60)90052-7. [DOI] [PubMed] [Google Scholar]
- Archer S, Gomberg-Maitland M, Maitland M, Rich S, Garcia JG, Weir E. Mitochondrial metabolism, redox signaling, and fusion: a mitochondria-ROS-HIF-1α-Kv1.5 O2-sensing pathway at the intersection of pulmonary hypertension and cancer. Am J Physiol. 2008;294:H570–H578. doi: 10.1152/ajpheart.01324.2007. [DOI] [PubMed] [Google Scholar]
- Archer S, Huang J, Henry T, Peterson D, Weir E. A redox-based O2 sensor in rat pulmonary vasculature. Circ Res. 1993;73:1100–1112. doi: 10.1161/01.res.73.6.1100. [DOI] [PubMed] [Google Scholar]
- Archer S, Huang J, Reeve H, Hampl V, Tolarova S, Michelakis E, et al. The differential distribution of electrophysiologically distinct myocytes in conduit and resistance arteries determines their response to nitric oxide and hypoxia. Circ Res. 1996;78:431–442. doi: 10.1161/01.res.78.3.431. [DOI] [PubMed] [Google Scholar]
- Archer S, London B, Hampl V, Wu X, Nsair A, Puttagunta L, et al. Impairment of hypoxic pulmonary vasoconstriction in mice lacking the voltage-gated potassium channel Kv1.5. FASEB. 2001;10:1096. doi: 10.1096/fj.00-0649fje. [DOI] [PubMed] [Google Scholar]
- Archer S, Will J, Weir E. Redox status in the control of pulmonary vascular tone. Hertz. 1986a;11:127–141. [PubMed] [Google Scholar]
- Archer SL, Wu XC, Thebaud B, Nsair A, Bonnet S, Tyrrell B, et al. Preferential expression and function of voltage-gated, 02-sensitive K+ channels in resistance pulmonary arteries explains regional heterogeneity in hypoxic pulmonary vasoconstriction: ionic diversity in smooth muscle cells. Circ Res. 2004b;95:308–318. doi: 10.1161/01.RES.0000137173.42723.fb. [DOI] [PubMed] [Google Scholar]
- Biscoe T, Duchen M. Responses of type I cells dissociated from the rabbit carotid body to hypoxia. J Physiol. 1990;428:39–59. doi: 10.1113/jphysiol.1990.sp018199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonnet S, Michelakis E, Porter C, Thebaud B, Bonnet S, Haromy A, et al. An abnormal mitochondrial-HIF1α-Kv channel pathway disrupts oxygen-sensing and triggers pulmonary arterial hypertension (PAH) in fawn-hooded rats: similarities to human PAH. Circulation. 2006;113:2630–2641. doi: 10.1161/CIRCULATIONAHA.105.609008. [DOI] [PubMed] [Google Scholar]
- Brevnova E, Platoshyn O, Zhang S, Yuan J. Overexpresion of human KCNA5 increases IK(V) and enhances apoptosis. Am J Physiol. 2004;287:C715–C722. doi: 10.1152/ajpcell.00050.2004. [DOI] [PubMed] [Google Scholar]
- Buckler K. A novel oxygen-sensitive potassium current in rat carotid body type I cells. J Physiol. 1997;498:649–662. doi: 10.1113/jphysiol.1997.sp021890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckler K, Vaughan-Jones R. Effects of hypoxia on membrane potential and intracellular calcium in rat neonatal carotid body type I cells. J Physiol. 1994;476:423–428. doi: 10.1113/jphysiol.1994.sp020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chander A, Dhariwal K, Viswanathan R. Pyridine nucleotides in lung and liver of hypoxic rats. Life Sci. 1980;26:1935–1945. doi: 10.1016/0024-3205(80)90624-4. [DOI] [PubMed] [Google Scholar]
- Cornfield D, Reeve H, Tolarova S, Weir E, Archer S. Oxygen causes fetal pulmonary vasodilation through activation of a calcium-dependent potassium channel. Proc Natl Acad Sci USA. 1996;93:8089–8094. doi: 10.1073/pnas.93.15.8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dasso L, Buckler K, Vaughan-Jones R. Interactions between hypoxia and hypercapnic acidosis on calcium signaling in carotid body type I cells. Am J Physiol. 2000;279:L36–L42. doi: 10.1152/ajplung.2000.279.1.L36. [DOI] [PubMed] [Google Scholar]
- Dipp M, Evans A. Cyclic ADP-ribose is the primary trigger for hypoxic pulmonary vasoconstriction in the rat lung in situ. Circ Res. 2001b;89:77–83. doi: 10.1161/hh1301.093616. [DOI] [PubMed] [Google Scholar]
- Dipp M, Nye P, Evans A. Hypoxic release of calcium from the sarcoplasmic reticulum of pulmonary artery smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2001a;281:L314–L317. doi: 10.1152/ajplung.2001.281.2.L318. [DOI] [PubMed] [Google Scholar]
- Evans A, Mustard K, Wyatt C, Peers C, Dipp M, Kumar P, et al. Does AMP-activated protein kinase couple inhibition of mitochondrial oxidative phosphorylation by hypoxia to calcium signaling in O2-sensing cells. J Biol Chem. 2005;280:41504–41511. doi: 10.1074/jbc.M510040200. [DOI] [PubMed] [Google Scholar]
- Fagan K, Oka M, Bauer N, Gebb S, Ivy D, Morris K, et al. Attenuation of acute hypoxic pulmonary vasoconstriction and hypoxic pulmonary hypertension in mice by inhibition of rho-kinase. Am J Physiol Lung Cell Mol Physiol. 2004;287:L656–L664. doi: 10.1152/ajplung.00090.2003. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y, Matoba T, Ito A, Tanaka H, Kishi T, Hayashidani S, et al. Acute vasodilator effects of a rho-kinase inhibitor, fasudil, in patients with severe pulmonary hypertension. Heart. 2005;91:391–392. doi: 10.1136/hrt.2003.029470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganfornina M, Lopez-Barneo J. Potassium channel types in arterial chemoreceptor cells and their selective modulation by oxyen. J Gen Physiol. 1992;100:401–426. doi: 10.1085/jgp.100.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganitkevich Vya, Isenberg G. Membrane potential modulates inositol 1,4,5-trisphosphate-mediated Ca2+ transients in guinea-pig coronary myocytes. 1993. [DOI] [PMC free article] [PubMed]
- Gurney AM, Osipenko ON, MacMillan D, McFarlane KM, Tate RJ, Kempsill FEJ. Two-pore domain K channel TASK-1, in pulmonary artery smooth muscle cells. Circ Res. 2003;93:957–964. doi: 10.1161/01.RES.0000099883.68414.61. [DOI] [PubMed] [Google Scholar]
- Gurney A, Manoury B.Two-pore potassium channels in the cardiovascular system Eur Biophys J 2008(in press) [DOI] [PubMed]
- Hales C, Westphal D. Hypoxemia following the administration of sublingual nitroglycerin. Am J Med. 1978;65:911–918. doi: 10.1016/0002-9343(78)90742-8. [DOI] [PubMed] [Google Scholar]
- Hong Z, Hong F, Olschewski A, Cabrera J, Varghese A, Nelson D, et al. Role of store-operated calcium channels and calcium sensitization in normoxic contraction of the ductus arteriosus. Circulation. 2006;114:1372–1379. doi: 10.1161/CIRCULATIONAHA.106.641126. [DOI] [PubMed] [Google Scholar]
- Hong Z, Weir E, Nelson D, Olschewski A. Subacute hypoxia decrease voltage-activated potassium channel expresion and function in pulmonary artery myocytes. Am J Respir Cell Mol Biol. 2004;31:1–7. doi: 10.1165/rcmb.2003-0386OC. [DOI] [PubMed] [Google Scholar]
- Jabr R, Toland H, Gelband C, Wang X, Hune J. Prominent role of intracellular Ca2+ release in hypoxic vasoconstriction of canine pulmonary artery. Br J Pharmacol. 1997;122:21–30. doi: 10.1038/sj.bjp.0701326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimoto H, Hashimoto K, Bonnet S, Harmony A, Harry G, Moudgil R, et al. Oxygen activates the rho/rho-kinase pathway and induces RhoB and ROCK-1 expression in human and rabbit ductus arteriosus by increasing mitochondria-derived reactive oxygen species. Circulation. 2007;115:1777–1788. doi: 10.1161/CIRCULATIONAHA.106.649566. [DOI] [PubMed] [Google Scholar]
- Krick S, Platoshyn O, McDaniel SS, Rubin LJ, Yuan JX. Augmented K(+) currents and mitochondrial membrane depolarization in pulmonary artery myocyte apoptosis. Am J Physiol Lung Cell Mol Physiol. 2001;281:L887–L894. doi: 10.1152/ajplung.2001.281.4.L887. [DOI] [PubMed] [Google Scholar]
- Leach R, Hill H, Snetkov V, Robertson T, Ward J. Divergent roles of glycolysis and the mitochondrial electron transport chain in hypoxic pulmonary vasoconstriction of the rat: identity of the hypoxic sensor. J Physiol. 2001;536:211–224. doi: 10.1111/j.1469-7793.2001.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo J, Lopez-Lopez J, Urena J, Gonzalez C. Chemotransduction in the carotid body: K+ current modulated by Po2 in type I chemoreceptor cells. Science. 1988;242:580–582. doi: 10.1126/science.2456613. [DOI] [PubMed] [Google Scholar]
- Lu W, Wang J, Shimoda A, Sylvester JT.Differences in STIM1 and TRPC expression in proximal and distal pulmonary arterial smooth muscle are associated with differences in Ca2+ responses to hypoxia 2008(in press) [DOI] [PMC free article] [PubMed]
- Madden JA, Vadula MS, Kurup VP. Effects of hypoxia and other vasoactive agents on pulmonary and cerebral artery smooth muscle cells. Am J Physiol. 1992;263:L384–L393. doi: 10.1152/ajplung.1992.263.3.L384. [DOI] [PubMed] [Google Scholar]
- McMurtry I, Petrun M, Reeves J. Lungs from chronically hypoxic rats have decreased pressor response to acute hypoxia. Am J Physiol. 1978;235:H104–H109. doi: 10.1152/ajpheart.1978.235.1.H104. [DOI] [PubMed] [Google Scholar]
- Michelakis E, Hampl V, Nsair A. Diversity in mitochondrial function explains differences in vascular oxygen sensing. Circ Res. 2002a;90:1307–1315. doi: 10.1161/01.res.0000024689.07590.c2. [DOI] [PubMed] [Google Scholar]
- Michelakis E, McMurtry M, Wu X, Dyck J, Moudgil R, Hopkins T, et al. Dichloroacetate, a metabolic modulator, prevents and reverses chronic hypoxic pulmonary hypertension in rats. Role of increased expression and activity of voltage-gated potassium channels. Circulation. 2002c;105:244–250. doi: 10.1161/hc0202.101974. [DOI] [PubMed] [Google Scholar]
- Michelakis E, Rebeyka I, Bateson J, Olley P, Puttagunta L, Archer S. Voltage-gated potassium channels in human ductus arteriosus. Lancet. 2000;356:134–137. doi: 10.1016/S0140-6736(00)02452-1. [DOI] [PubMed] [Google Scholar]
- Michelakis E, Rebeyka I, Wu X, Msaor A, Tjebaid B, Hashimoto K, et al. O2 sensing in the human ductus arteriosus regulation of voltage-gated K+ channels in smooth muscle cells by a mitochondrial redox sensor. Circ Res. 2002b;91:478–486. doi: 10.1161/01.res.0000035057.63303.d1. [DOI] [PubMed] [Google Scholar]
- Michelakis E, Thebaud B, Weir E, Archer S. Hypoxic pulmonary vasoconstriction: redox regulation of O(2)-sensitive K(+) channels by a mitochondrial O(2)-sensor in resistance artery smooth muscle cells. J Mol Cell Cardiol. 2004;37:1119–1136. doi: 10.1016/j.yjmcc.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Moudgil R, Michelakis ED, Archer SL. Hypoxic pulmonary vasoconstriction. J Appl Physiol. 2005;98:390–403. doi: 10.1152/japplphysiol.00733.2004. [DOI] [PubMed] [Google Scholar]
- Morio Y, McMurtry I. Ca2+ release from ryanodine-sensitive store contributes to mechanism of hypoxic vasoconstriction in rat lungs. J Appl Physiol. 2002;92:527–534. doi: 10.1152/jappl.2002.92.2.527. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Fagan K, Gebb S, Morris K, Suzuki T, Shimokawa H, et al. Inhaled rho kinase inhibitors are potent and selective vasodilators in rat pulmonary hypertension. Am J Respir Crit Care Med. 2005;171:494–499. doi: 10.1164/rccm.200405-637OC. [DOI] [PubMed] [Google Scholar]
- Nagaoka T, Gebb S, Karoor V, Homma N, Morris K, McMurtry I, et al. Involvement of RhoA/Rho kinase signaling in pulmonary hypertension of the fawn-hooded rat. J Appl Physiol. 2006;100:996–1002. doi: 10.1152/japplphysiol.01028.2005. [DOI] [PubMed] [Google Scholar]
- Ng L, Wilson S, Hume J. Mobilization of sarcoplasmic reticulum stores by hypoxia leads to consequent activation of capacitative Ca2+ entry in isolated canine pulmonary arterial smooth muscle cells. J Physiol. 2005;563:409–419. doi: 10.1113/jphysiol.2004.078311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olschewski A, Hong Z, Nelson D, Weir E. Graded response of K+ current, membrane potential and [Ca2+]i to hypoxia in pulmonary arterial smooth muscle. Am J Physiol. 2002;283:L1143–L1150. doi: 10.1152/ajplung.00104.2002. [DOI] [PubMed] [Google Scholar]
- Olschewski A, Hong Z, Peterson D, Nelson D, Porter V, Weir E. Opposite effects of redox status on membrane potential, cytosolic calcium, and tone in pulmonary arteries and ductus arteriosus. Am J Physiol. 2004;286:L15–L22. doi: 10.1152/ajplung.00372.2002. [DOI] [PubMed] [Google Scholar]
- Olschewski A, Li Y, Bi T, Hanze J, Eul B, Bohle R, et al. Impact of TASK-1 in human pulmonay artery smooth muscle cells. Circ Res. 2006;98:1072–1080. doi: 10.1161/01.RES.0000219677.12988.e9. [DOI] [PubMed] [Google Scholar]
- Osipenko O, Alexander D, MacLean M, Gurney A. Influence of chronic hypoxia on the contributions of non-inactivating and delayed rectifier K currents to the resting potential and tone of rat pulmonary artery smooth muscle. Br J Pharmacol. 1998;124:1335–1337. doi: 10.1038/sj.bjp.0702006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peers C. Effects of doxapram on ionic currents recorded in isolated type 1 cells of the neonatal rat carotid body. Brain Res. 1991;568:116–122. doi: 10.1016/0006-8993(91)91386-f. [DOI] [PubMed] [Google Scholar]
- Perchenet L, Hilfiger L, Mizrahi J, Clement-Chomienne O. Effects of anorexinogen agent on cloned voltage-gated K+ channel hKv1.5. J Pharmacol Exp Ther. 2001;298:1108–1119. [PubMed] [Google Scholar]
- Platoshyn O, Brevnova E, Burg E, Yu Y, Remillard C, Yuan J. Acute hypoxia selectively inhibits KCNA5 channels in pulmonary artery smooth muscle cells. Am J Physiol. 2006;290:C907–C916. doi: 10.1152/ajpcell.00028.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platoshyn O, Yu Y, Ko E, Remillard C, Yuan J. Heterogeneity of hypoxia-mediated decrease in IK(v) and increase in [Ca2+]cyt in pulmonary artery smooth muscle cells. Am J Physiol. 2007;293:L402–L416. doi: 10.1152/ajplung.00391.2006. [DOI] [PubMed] [Google Scholar]
- Post J, Hume J, Archer S, Weir E. Direct role for potassium channel inhibition in hypoxic pulmonary vasoconstriction. Am J Physiol. 1992;262:C882–C890. doi: 10.1152/ajpcell.1992.262.4.C882. [DOI] [PubMed] [Google Scholar]
- Pozeg Z, ED M, McMurtry M, Thebaud B, Wu X, Dyck J, et al. In vivo gene transfer of the O2-sensitive potassium channel Kv1.5 reduces pulmonary hypertension and restores hypoxic pulmonary vasoconstriction in chronically hypoxic rats. Circulation. 2003;107:2037–2044. doi: 10.1161/01.CIR.0000062688.76508.B3. [DOI] [PubMed] [Google Scholar]
- Putney J., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve H, Archer S, Weir E, Cornfield D. A maturational shift in pulmonary K+ channel from Ca2+ sensitive to voltage-dependent. Am J Physiol. 1998;5:L1019–L1025. doi: 10.1152/ajplung.1998.275.6.L1019. [DOI] [PubMed] [Google Scholar]
- Reeve H, Michelakis E, Nelson D, Weir E, Archer S. Alterations in a redox oxygen sensing mechanism in chronic hypoxia. J Appl Physiol. 2001;90:2249–2256. doi: 10.1152/jappl.2001.90.6.2249. [DOI] [PubMed] [Google Scholar]
- Reeve H, Tolarova S, Nelson D, Archer S, Weir E. Redox control of oxygen sensing in the rabbit ductus arteriosus. J Physiol. 2001b;533:253–261. doi: 10.1111/j.1469-7793.2001.0253b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remillard C, Tigno D, Platoshyn O, Burg D, Brevnova E, Conger D, Nicholson A, et al. Function of Kv 1.5 channels and genetic variations of KCNA5 in patients with idiopathic pulmonary arterial hypertension. Am J Physiol. 2007;292:C1837–C1853. doi: 10.1152/ajpcell.00405.2006. [DOI] [PubMed] [Google Scholar]
- Rich S, Brundage BH. High-dose calcium channel-blocking therapy for primary pulmonary hypertension: evidence for long-term reduction in pulmonary arterial pressure and regression of right ventricular hypertrophy. Circulation. 1987;76:135–141. doi: 10.1161/01.cir.76.1.135. [DOI] [PubMed] [Google Scholar]
- Robertson T, Aaronson P, Ward J. Hypoxic vasoconstriction and intracellular Ca2+ in pulmonary arteries: evidence for PKC-independent Ca2+ sensitization. Am J Physiol. 1995;268:301–307. doi: 10.1152/ajpheart.1995.268.1.H301. [DOI] [PubMed] [Google Scholar]
- Robertson T, Dipp M, Ward J, Aaronson P, Evans A. Inhibition of sustained hypoxic vasoconstriction by Y-27632 in isolated intrapulmonary arteries and perfused lung of the rat. Br J Pharmacol. 2000a;131:5–9. doi: 10.1038/sj.bjp.0703537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson T, Hague D, Aaronson P, Ward J. Voltage-independent calcium entry in hypoxic pulmonary vasoconstriction of intrapulmonary arteries of the rat. J Physiol. 2000b;525:669–680. doi: 10.1111/j.1469-7793.2000.t01-1-00669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvaterra C, Goldman W. Acute hypoxia increases cytosolic calcium in cultured pulmonary arterial myocytes. Am J Physiol. 1993;264:L323–L328. doi: 10.1152/ajplung.1993.264.3.L323. [DOI] [PubMed] [Google Scholar]
- Schach C, Xu M, Platoshyn O, Kelller S, Yuan J. Thiol oxidation causes pulmonary vasodilation by activating K+ channels and inhibiting store-operated Ca2+ channels. Am J Physiol. 2007;292:L685–L698. doi: 10.1152/ajplung.00276.2006. [DOI] [PubMed] [Google Scholar]
- Shigemori K, Ishizaki T, Matsukawa S, Sakai A, Nakai T, Miyabo S. Adenine nucleotides via activation of ATP-sensitive K+ channels modulate hypoxic response in rat pulmonary arteries. Am J Physiol. 1996;270:L803–L809. doi: 10.1152/ajplung.1996.270.5.L803. [DOI] [PubMed] [Google Scholar]
- Shimoda LA, Manalo DJ, Sham JS, Semenza GL, Sylvester JT. Partial HIF-1alpha deficiency impairs pulmonary arterial myocyte electrophysiological responses to hypoxia. Am J Physiol. 2001;281:L202–L208. doi: 10.1152/ajplung.2001.281.1.L202. [DOI] [PubMed] [Google Scholar]
- Smirnov S, Robertson T, Ward J, Aaronson P. Chronic hypoxia is associated with reduced delayed rectifier K+ current in rat pulmonary artery muscle cells. Am J Physiol. 1994b;266:H365–H370. doi: 10.1152/ajpheart.1994.266.1.H365. [DOI] [PubMed] [Google Scholar]
- Stenmark K, McMurtry I. Vascular remodeling versus vasoconstriction in chronic hypoxic pulmonary hypertension a time for reappraisal. Circ Res. 2005;97:95–98. doi: 10.1161/01.RES.00000175934.68087.29. [DOI] [PubMed] [Google Scholar]
- Thebaud B, Michelakis E, Wu X, Moudgil R, Kuzyk M, Dyck J, et al. Oxygen-sensitive Kv channel gene transfer confers oxygen responsiveness to preterm rabbit and remodeled human ductus arteriosus: implications for infants with patient ductus arteriosus. Circulation. 2004;110:1372–1379. doi: 10.1161/01.CIR.0000141292.28616.65. [DOI] [PubMed] [Google Scholar]
- Tipparaju S, Saxena N, Liu S, Kumar R, Bhatnagar A. Differential regulation of voltage-gated K+ channels by oxidized and reduced pyridine nucleotide coenzymes. Am J Physiol. 2005;288:C366–C376. doi: 10.1152/ajpcell.00354.2004. [DOI] [PubMed] [Google Scholar]
- Tristani-Firouzi M, Reeve H, Tolarova S, Weir E, Archer S. Oxygen-induced constriction of rabbit ductus arteriosus occurs via inhibition of a 4-aminopyridine-, voltage-sensitive potassium channel. J Clin Invest. 1996b;98:1959–1965. doi: 10.1172/JCI118999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urena J, Fernadez-Chacon R, Benot A, Alvarez de Toledo G, Lopez-Barneo J. Hypoxia induces voltage-dependent Ca2+ entry and quantal dopamine secretion in carotid body glomus cells. Proc Natl Acad Sci USA. 1994;91:10208–10211. doi: 10.1073/pnas.91.21.10208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle-Rodriguez A, Calderon E, Ruiz M, Ordonez A, Lopez-Barneo J, Urena J. Metabotropic Ca2+ channel-induced Ca2+ release and ATP-dependent facilitation of arterial myocyte contraction. Proc Natl Acad Sci USA. 2006;103:4316–4321. doi: 10.1073/pnas.0508781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Shimoda L, Weigand L, Wang W, Sun D, Sylvester JT. Acute hypoxia increases intracellular [Ca2+] entry. Am J Physiol Lung Cell Mol Physiol. 2005;288:L1059–L1069. doi: 10.1152/ajplung.00448.2004. [DOI] [PubMed] [Google Scholar]
- Wang Q, Zheng Y, Dong L, Ho Y, Guo Z, Wang Y. Role of mitochondrial reactive oxygen species in hypoxia-dependent increase in intracellular calcium in pulmonary artery myocytes. Free Radic Biol Med. 2007;42:642–653. doi: 10.1016/j.freeradbiomed.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Jin N, Garguli S, Swartz D, Li L, Rhoades R. Rho-kinase activation is involved in hypoxia-induced pulmonary vasoconstriction. Am J Respir Cell Mol Biol. 2001;25:628–635. doi: 10.1165/ajrcmb.25.5.4461. [DOI] [PubMed] [Google Scholar]
- Ward J.Hypoxic pulmonary vasoconstriction is mediated by increased production of reactive oxygen species J Appl Phsyiol 2006101933–995.and 1004 [DOI] [PubMed] [Google Scholar]
- Ward J, Knock G, Snetkov V, Aaronson P. Protein kinases in vascular smooth muscle tone—role in the pulmonary vasculature and hypoxic pulmonary vasoconstriction. Pharmacol Ther. 2004;104:207–231. doi: 10.1016/j.pharmthera.2004.08.009. [DOI] [PubMed] [Google Scholar]
- Ward J, Robertson T, Aaronson P. Capacitative calcium entry: a central role in hypoxic pulmonary vasoconstriction. Am J Physiol Lung Cell Mol Physiol. 2005;289:2–4. doi: 10.1152/ajplung.00101.2005. [DOI] [PubMed] [Google Scholar]
- Waypa G, Guzy R, Mungai P, Mack M, Marks J, Roe M, et al. Increases in mitochondrial reactive oxygen species trigger hypoxia-induced calcium responses in pulmonary artery smooth muscle cells. Circ Res. 2006;99:970–978. doi: 10.1161/01.RES.0000247068.75808.3f. [DOI] [PubMed] [Google Scholar]
- Waypa G, Schumacker P. Oxygen sensing in hypoxic pulmonary vasoconstriction: using new tools to answer and age-old question. Exp Physiol. 2008;93:133–138. doi: 10.1113/expphysiol.2007.041236. [DOI] [PubMed] [Google Scholar]
- Weigand L, Foxson J, Wang J, Schimoda L, Sylvester J. Inhibition of hypoxic pulmonary vasoconstriction by store-operated Ca2+ and nonselective cation channel antagonists. Am J Physiol Lung Cell Mol Physiol. 2005;289:L5–L13. doi: 10.1152/ajplung.00044.2005. [DOI] [PubMed] [Google Scholar]
- Weir E. Does normoxic pulmonary vasodilatation rather than hypoxic vasoconstriction account for the pulmonary pressor response to hypoxia. Lancet. 1978;1:476–477. doi: 10.1016/s0140-6736(78)90138-1. [DOI] [PubMed] [Google Scholar]
- Weir E, Archer S. The mechanism of acute hypoxic pulmonary vasoconstriction: the tale of two channels. FASEB J. 1995;9:183–189. doi: 10.1096/fasebj.9.2.7781921. [DOI] [PubMed] [Google Scholar]
- Weir E, Archer S. Counterpoint: hypoxic pulmonary vasoconstriction is not mediated by increased production of reactive oxygen species. J Appl Physiol. 2006;101:995–998. doi: 10.1152/japplphysiol.00480a.2006. [DOI] [PubMed] [Google Scholar]
- Weir E, Lopez-Barneo J, Buckler K, Archer S. Acute oxygen-sensing mechanisms. N Engl J Med. 2005;353:2042–2055. doi: 10.1056/NEJMra050002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weir E, Reeve H, Huang J, Michelakis E, Nelson D, Hampl V, et al. The anorexic agents, aminorex, fenfluramine and dexfenfluramine inhibit potassium current in rat pulmonary vascular smooth muscle and cause pulmonary vasoconstriction. Circulation. 1996a;94:2216–2220. doi: 10.1161/01.cir.94.9.2216. [DOI] [PubMed] [Google Scholar]
- Weir E, Will J. Oxidants: a new group of pulmonary vasodilators. Clin Res Physiol. 1982;18:81–85. [Google Scholar]
- Weissmann N, Dietrich A, Fuchs B, Kalwa H, Ay M, Dumitrascu R, et al. Classical transient receptor potential channel 6 (TRPC6) is essential for hypoxic pulmonary vasoconstriction and alveolar gas exchange. Proc Natl Acad Sci USA. 2006;103:19093–19098. doi: 10.1073/pnas.0606728103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. High Altitude Physiology. Hutchinson Ross Publishing Company: Stroudsburg, PA; 1981. [Google Scholar]
- Wilson H, Dipp M, Thomas J, Lad C, Galione A, Evans A. ADP-ribosyl cyclase and cyclic ADP-ribose hydrolase act as a redox sensor: a primary role for cADPR in hypoxic pulmonary vasoconstriction. J Biol Chem. 2001;276:11180–11188. doi: 10.1074/jbc.M004849200. [DOI] [PubMed] [Google Scholar]
- Wyatt C, Wright C, Bee D, Peers C. O2-sensitive K+ currents in carotid body chemoreceptor cells from normoxic and chronically hypoxic rats and their role in hypoxic chemotransduction. Proc Natl Acad Sci USA. 1995b;92:295–299. doi: 10.1073/pnas.92.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y, Fantozzii I, Remillard C, Langsberg J, Kunichika N, Platoshyn O, et al. Enhanced expression of transient receptor potential channels in idiopathic pulmonary arterial hypertension. Proc Natl Acad Sci USA. 2004;10:13861–13866. doi: 10.1073/pnas.0405908101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J, Aldinger B, Juhaszova M, Wang J, Conte J, Gaine S, et al. Dysfunctional voltage-gated K+ channels in pulmonary artery smooth muscle cells of patients with primary pulmonary hypertension. Circulation. 1998a;98:1400–1406. doi: 10.1161/01.cir.98.14.1400. [DOI] [PubMed] [Google Scholar]
- Yuan J, Wang J, Juhaszova M, Gaine S, Rubin L. Attenuated K+ channel gene transcription in primary pulmonary hypertension. Lancet. 1998b;351:726–727. doi: 10.1016/S0140-6736(05)78495-6. [DOI] [PubMed] [Google Scholar]
- Yuan X-J, Goldman W, Tod M, Rubin L, Blaustein M. Hypoxia reduces potassium currents in cultured rat pulmonary but not mesenteric arterial myocytes. Am J Physiol. 1993;264:L116–L123. doi: 10.1152/ajplung.1993.264.2.L116. [DOI] [PubMed] [Google Scholar]