Abstract
Immunomodulatory effects of alcohol use involve regulation of innate immune cell function leading to liver disease. Alteration of inflammatory responses by alcohol is linked to dysregulated TNF-α production. Alcohol-induced oxidative stress also contributes to alterations in inflammatory cell activity. Heat shock proteins (hsps) and the heat shock transcription factor-1 (HSF-1) induced by oxidative stress regulate NF-κB activation and TNF-α gene expression in monocytes and macrophages. Here, we report that in vitro alcohol treatment induced and augmented LPS-induced HSF-1 nuclear translocation and DNA-binding activity in monocytes and macrophages. Supershift analysis revealed that alcohol regulated HSF-1- and not HSF-2-binding activity. Hsp70, a target gene induced by HSF-1, was transiently increased within 24 h by alcohol, but extended alcohol exposure decreased hsp70 in macrophages. The alcohol-induced alteration of hsp70 correlated with a concomitant change in hsp70 promoter activity. Hsp90, another HSF-1 target gene, was decreased during short-term alcohol but increased after prolonged alcohol exposure. Decreased hsp90-HSF-1 complexes after short-term alcohol indicated dissociation of HSF-1 from hsp90. On the other hand, hsp90 interacted with client protein IκB kinase β, a signaling intermediate of the LPS pathway, followed by IκBα degradation and increased NF-κB activity after chronic alcohol exposure, indicating that hsp90 plays an important role in supporting inflammatory cytokine production. Inhibition of hsp90 using geldanamycin prevented prolonged alcohol-induced elevation in LPS-induced NF-κB and TNF-α production. These results suggest that alcohol exposure differentially regulates hsp70 and hsp90 via HSF-1 activation. Further, hsp90 regulates TNF-α production in macrophages contributing to alcohol-induced inflammation.
Keywords: HSF-1, ethanol, hsp70, hsp 90, IKKβ
INTRODUCTION
Alcohol consumption is associated with alterations in host immune responses manifested as increased susceptibility to bacterial and viral infections, reduced elimination of pathogens, and immunosuppression [1,2,3]. The innate immune system plays an important role in immune regulation owing to the ability of monocytes and macrophages to recognize invading pathogens and produce inflammatory cytokines. TNF-α, a pivotal mediator of host defenses, is essential for survival during infections and also contributes to the pathogenesis of early alcohol-induced liver injury. Acute or short-term alcohol exposure is linked to decreased production of inflammatory cytokines including TNF-α [4], whereas chronic alcohol increased TNF-α secretion by monocytes and macrophages in in vivo and in vitro models [5]. Our laboratory and work by other groups has demonstrated various mechanisms for alcohol-induced modulation of TNF-α production by macrophages ranging from alterations in NF-κB activity, increased production of reactive oxygen species radicals (ROS), augmentation of Erk1/2 activity to promote TNF-α transcription, increased p38 MAPK activity resulting in increased stability of TNF-α mRNA, and modulations in the TLR4-CD14 receptor complex and downstream signaling molecules [6,7,8,9].
Exposure of cells to a wide variety of stressors including heat shock, hydrogen peroxide, infection, inflammation, and particularly of interest, alcohol drinking results in accumulation of stress proteins known as heat shock proteins (hsps) [10, 11]. The primary function of these proteins is to operate as intracellular chaperones and provide cytoprotection against stress. Induction of hsps is initiated by activation of heat shock factors (HSF), the principal mediators of the cellular stress response, which exit as monomers in nonstressed cells and upon stress, undergo trimerization, nuclear translocation, and binding to the heat shock-binding elements (HSE) to induce a family of hsp genes [12]. Increasing evidence suggests a prominent role for hsps, particularly hsp70 and hsp90, in inflammatory responses. Specifically, hsp70 and hsp90 play an important role in regulation of the TLR4-mediated, down-stream signaling pathway, leading to activation of NF-κB [13, 14]. Hsp70 and hsp90 associate with TLR4 in lipid rafts and are essential for LPS recognition [15]. Hsp90 activity is required for constitutive and inducible IκB kinase (IKK) and NF-κB activation and increased TNF-α production [14]. On the other hand, heat shock-induced hsp70 inhibits intranuclear accumulation of NF-κB and prevents amplification of the inflammatory response [16].
The induction of hsps in the liver and brain during alcohol-related organ injury [17,18,19,20] has been reported. However, the role of hsps in alcohol-induced inflammatory response is still elusive. We have shown earlier that acute alcohol exposure impairs NF-κB activation [6], whereas chronic alcohol increases NF-κB-binding activity in primary human monocytes (P. Mandrekar et al., unpublished). Oxidative stress-induced ROS play an important role in induction of TNF-α production by alcohol [7, 21]. As oxidative stress injury induces HSF-1 activation [22] and expression of hsps that can regulate inflammatory responses, we wanted to determine whether alcohol exposure induces HSF-1 activation and expression of hsp70 and hsp90 in monocytes and macrophages, contributing to TNF-α production.
MATERIALS AND METHODS
Cell culture and reagents and stimulations
RAW 264.7 macrophages were purchased from American Type Culture Collection (Manassas, VA, USA) and maintained in DMEM containing 10% FBS. Monocytes from human peripheral blood were isolated by selective adherence from Ficoll-Hypaque-purified mononuclear cell preparations as described previously [4]. Healthy individuals, aged 18–60 females and males with no previous alcohol abuse history who consumed less than six drinks/week, were recruited in the study.
Human peripheral blood monocytes or macrophages were exposed to alcohol (25 mM, 50 mM, and 75 mM) for 15 min, 48 h, or 72 h and then stimulated with Escherichia coli-derived LPS (100 ng/ml) at the times indicated in the figure legends. The 25-mM in vitro ethanol concentration approximates a 0.1-g/dl blood alcohol level, which is achieved in vivo after a dose of moderate drink. The short-term or acute alcohol exposure is referred to as “acute” alcohol exposure from 15 min to 24 h by virtue of its ability to inhibit NF-κB activation and TNF-α production [4, 6]. Whereas 72 h of alcohol exposure has been designated as “chronic” as a result of its ability to increase LPS-induced NF-κB-binding activity and TNF-α, as observed in previous chronic alcohol-induced liver injury models [5, 8]. Cell viability was not affected by ethanol or LPS treatment. For positive induction of HSF, monocytes/macrophages were heat-shocked at 42°C for 45 min, and for hsp expression, heat shocking was followed by recovery at 37°C for 18 h. For inhibition of hsp90 activity, macrophages were treated with 0.2 μM geldanamycin with 72 h of alcohol exposure, followed by LPS treatment as described in the figure legends.
ELISA
TNF-α was determined in culture supernatants in an ELISA (BD PharMingen, San Diego, CA, USA).
Preparation of nuclear and cytoplasmic extracts
Nuclear and cytoplasmic extracts from cells with or without stimulation at 37°C were performed by the method of Schatzle et al. [23]. Briefly, at the end of the stimulation period, cells were scraped and washed in ice-cold PBS. Cells were then resuspended in cold hypotonic buffer A (10 mM HEPES, pH 7.9, 10 mM KCl, 0.1 mM EDTA, 0.1 mM EGTA, 1 mM DTT, 1 mM PMSF, and 10 μg/ml protease inhibitors such as aprotinin, antipain, and leupeptin, Sigma-Aldrich Co., St Louis, MO, USA) and incubated on ice for 20 min. Cells were then lysed in 0.6% Nonidet P-40 by vortexing for 20 s. The lysate was then centrifuged at 12,000 g for 30 s to pellet the nuclei, and the supernatant was stored at −80°C as the cytoplasmic extract. The nuclear pellet was then resuspended in ice-cold buffer B [20 mM HEPES (pH 7.9), 400 mM KCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, 1 mM PMSF, and 20% glycerol]. All tubes were kept on a shaker at 4°C for 30 min. The lysate was then centrifuged at 12,000 g for 15 min, and the supernatant was stored at −80°C as the nuclear extract. Protein content was determined in the cytoplasmic and nuclear extract by the Bio-Rad dye reagent assay (Bio-Rad Laboratories, Hercules, CA, USA).
EMSA
A consensus double-stranded HSE (5′GCCTCGAATGTTCGCGAAGTT3′) or NF-κB (5′AGTTAGGGGACTTTCCCAGGC3′) consensus sequence was used for EMSA [6]. End-labeling was accomplished by treatment with T4 polynucleotide kinase in the presence of γ32P-ATP (Dupont-NEN, Boston, MA, USA). Labeled oligonucleotide was purified on a polyacrylamide copolymer column (Bio-Rad Laboratories). Nuclear protein (5 μg) was added to a binding reaction mixture containing 20 mM HEPES (pH 7.9), 50 mM KCl, 0.1 mM EDTA, 1 mM DTT, 5% glycerol, 200 μg/ml BSA, 2 μg polydeoxyinosinic:polydeoxycytidylic acid, and 50,000 cpm γ32P-labeled HSE or NF-κB oligonucleotide. Samples were incubated at room temperature for 30 min. All reactions were run on a 4% polyacrylamide gel, and the dried gel was exposed to an X-ray film at −80°C overnight. For the cold competition reaction, a 20-fold excess of a specific, unlabeled, double-stranded probe was added to the reaction mixture before adding the labeled oligonucleotide. Supershift analysis was carried out by addition of 2 μl HSF-1 or HSF-2 antibody (Stressgen Bioreagents, Ann Arbor, MI, USA) 30 min after addition of the labeled HSE, followed by 30 min incubation at room temperature.
Immunoblotting and immunoprecipitation
Whole cell extracts or cytoplasmic proteins (20 μg) were loaded onto each well, separated on 10% SDS-polyacrylamide gel, and electroblotted onto nitrocellulose membranes. Nonspecific binding was blocked by incubation of the membranes in TBS/1% nonfat dried milk/0.1% Tween-20, followed by antibodies indicated in the figure legends. The antibodies against HSF-1, hsp70, and hsp90 were purchased from Stressgen Bioreagents. β-Tubulin and TATA-binding protein (TBP) antibodies were from Abcam (Cambridge, MA, USA). The antibodies were detected using HRP-conjugated secondary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and chemiluminescence assay reagents from Cell Signaling (Danvers, MA, USA).
For immunoprecipitations, samples were precleared with 50 μl TrueBlot anti-rabbit IgG IP beads (eBioscience Inc., San Diego, CA, USA) for 1 h, and then the precleared samples were incubated with the 5-μg anti-IKKβ antibody (Santa Cruz Biotechnology) or hsp90 antibody overnight at 4°C. An aliquot of the precleared sample (1/20th vol) before the immunoprecipitation reaction was designated as the input sample. The next day, 50 μl TrueBlot anti-rabbit IgG IP beads were added to each sample for 1 h. The beads were washed three times with lysing buffer and then eluted with sample buffer. Subsequently, Western blotting was performed, and blots were probed with anti-hsp90 or HSF-1 antibody (Stressgen Bioreagents), respectively.
RNA analysis
Total RNA was extracted using the RNeasy mini kit (Qiagen Sciences, Germantown, MD, USA) according to the manufacturer’s instructions (Qiagen GmbH, Hilden, Germany). cDNA synthesis was performed by RT of total RNA using the RT system (Promega, Madison, WI, USA). Real-time quantitative PCR was performed using the iCycler iQ real-time detection system (Bio-Rad Laboratories). Primers were synthesized by IDT, Inc. (Coralville, IA, USA), and the hsp70 primer sequences are forward 5′ ttgtccatgttaaggttttgtggtata 3′ and reverse 5′ gtttttttcattagtttgtagtgatgcaa 3′. The reaction mixture for real-time PCR contained 12.5 μL QuantiTect SYBR Green PCR master mix (Qiagen, Valencia, CA, uSA), 0.25 μM each forward and reverse primers, and 0.5 μL cDNA (corresponding to 25 ng RNA) for a total reaction volume of 25 μL. All amplifications and detections were carried out in the iCycler iQ PCR plates with optical tapes (Bio-Rad Laboratories). At each amplification cycle, accumulation of PCR products was detected by monitoring the increase in fluorescence by double-stranded DNA-binding SYBR Green. Relative gene expression was determined using the standard curve method. The expression level of target genes was normalized to the housekeeping gene, 18S, in each sample and expressed as fold change in the target gene expression between experimental groups. Melt-curve analysis and agarose-gel electrophoresis were used to confirm the authenticity of the PCR products.
Transient transfection
The hsp70 promoter-driven luciferase reporter plasmid [p(hsp70)-Luc] was a kind gift from Dr. Richard Morimoto (Northwestern University, Chicago, IL, USA) [24]. RAW264.7 macrophages exposed to alcohol for 72 h or grown in the absence of alcohol were transfected with the reporter plasmids hsp70-firefly luciferase and Renilla-luciferase as control (Promega) using the transfection agent FuGENE 6 (Roche Applied Science, Indianapolis, IN, USA). After 24 h of incubation, cells were treated with 50 mM alcohol for acute alcohol studies in the presence or absence of LPS (100 ng/ml) for 24 h, and luciferase activity was assessed with Dual Glo luciferase assay reagent (Promega), according to the manufacturer’s instructions. hsp70 promoter-driven transcriptional activity, as detected by firefly luciferase activity, was normalized with the Renilla-luciferase activity. The relative light units (RLU) represent an average of triplicate samples.
Statistical analysis
All data are expressed as means ± se. Results between treatment groups were compared by the Wilcoxon-signed rank, nonparametric data analysis.
RESULTS
Activation of HSF-1 in alcohol-treated monocytes and macrophages
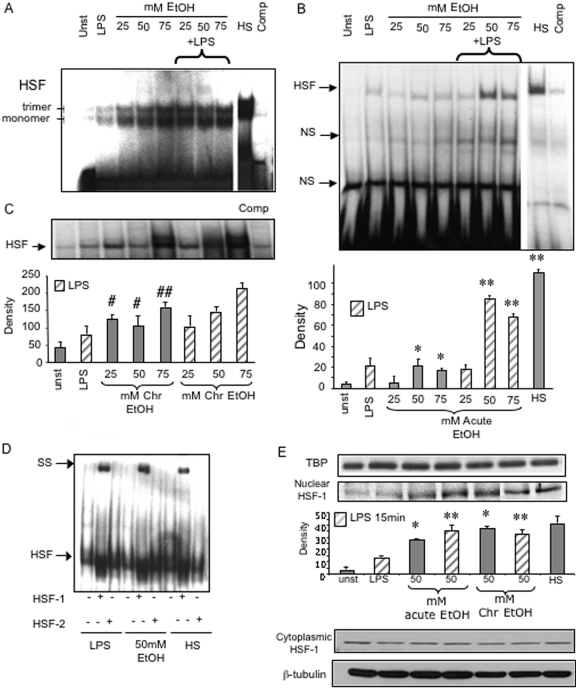
During the early stages of alcoholic liver disease (ALD), circulating monocytes and resident hepatic macrophages are activated in response to bacterial endotoxin derived from the gut, resulting in inflammatory cytokine production [25, 26]. Earlier studies have shown that although prolonged alcohol treatment increases TNF-α, acute alcohol exposure decreases TNF-α production in monocytic cells [4,5,6, 8]. Oxidative stress-regulated hsp expression is tightly controlled by binding of the HSF to its HSE in the promoter region of hsps [10,11,12, 22]. Activation of HSF is regulated by oxidative stress caused by various environmental insults such as heat and alcohol [22, 27]. Here, we first determined whether alcohol by itself or in combination with LPS affects HSF DNA-binding activity and nuclear translocation. In the current study, alcohol treatment for 15 min–24 h represents “short-term or acute” alcohol exposure on the basis of decreased, LPS-induced TNF-α production [4, 6], whereas 72 h alcohol treatment in vitro represents “prolonged or chronic” exposure, determined by increased, LPS-induced TNF-α production, described previously by in vivo and in vitro studies [5, 8]. Figure 1A shows that treatment of 1 μg/ml LPS induces HSF DNA-binding activity in human peripheral blood monocytes. Exposure of different concentrations of alcohol (25 mM, 50 mM, and 75 mM) for 60 min or 100 ng/ml LPS induced significant HSF-binding activity as compared with unstimulated human monocytes. The upper band represents the trimeric, functional HSF-1, and the lower band is the nonfunctional, monomeric HSF-1, as described in the literature [28]. A combination of LPS plus alcohol at various concentrations for 60 min augmented the LPS-induced, HSF-binding activity in monocytes compared with LPS treatment alone (Fig. 1A). These results indicate that alcohol modulates HSF-1 activation in human monocytes.
Fig. 1.
Alcohol exposure increases HSF-1 nuclear translocation and binding activity in monocytes and macrophages. (A) Human monocytes were exposed to 25 mM, 50 mM, and 100 mM alcohol [ethanol (EtOH)] for 60 min followed by 100 ng/ml LPS for 60 min. HSF was detected in the nuclear extracts by EMSA using a 32P-labeled, double-stranded HSE oligonucleotide. (Upper band) Trimeric HSF. (Lower band) Monomeric HSF. HS, Heat shock treatment at 40°C for 45 min served as a positive control for HSF induction. A 20-fold excess of unlabeled oligonucleotide was included as a cold competitor (Comp). Unst, Unstimulated. (B) RAW macrophages were stimulated with LPS (100 ng/ml) in the presence or absence of 25 mM, 50 mM, and 75 mM ethanol for 15 min (Acute). HSF was detected in the nuclear extracts by EMSA using a 32P-labeled, double-stranded HSE oligonucleotide. A representative EMSA shown in the upper panel and the bar graph in the lower panel show mean density ± se of five experiments (*, P<0.02, compared with unstimulated; **, P<0.001, compared with LPS). NS, Nonspecific. (C) Cells were exposed to 25 mM and 50 mM alcohol for 72 h alone (Chr) or followed by LPS (100 ng/ml) for 15 min. HSF was detected in the nuclear extracts by EMSA using a 32P-labeled, double-stranded HSE oligonucleotide. A 20-fold excess of unlabeled oligonucleotide was included as a cold competitor. A representative EMSA shown in the upper panel and the bar graph in the lower panel show mean density ± se of five experiments. (#, P<0.05; ##, P<0.02, compared with unstimulated). (D) Supershift analysis of HSF-binding activity in alcohol-treated macrophages. RAW macrophages were heat-shocked at 42°C for 45 min and treated with 50 mM ethanol for 15 min or with LPS (100 ng/ml) for 15 min, and nuclear extracts were subjected to gel shift analysis. All reactions were admixed with HSF-1 or HSF-1 antibody (2 μg) after addition of the 32P-HSF oligonucleotide and incubated for 30 min. Shifted bands were detected by their retarded mobility. SS, Supershifted. (E) Macrophages were exposed to 50 mM alcohol for 15 min or 72 h alone or followed by LPS (100 ng/ml) for 15 min. HSF-1 was detected in the nuclear (30 μg; second gel from top) and cytoplasmic (30 μg; third gel from top) extracts by Western blotting using an anti-mouse HSF-1 antibody. Antibodies to TBP for nuclear extracts and β-tubulin for cytoplasm were used as internal loading control. A representative gel from a total of three experiments for nuclear and cytoplasmic extracts and the bar graph show mean density ± se of three experiments (*, P<0.01, compared with unstimulated; **, P<0.05, compared with LPS).
To extend our observations in monocytes to macrophages, we performed subsequent studies using RAW 264.7 macrophages, which when treated with LPS for 15 min, induced HSF-binding activity (Fig. 1B), however similar but to a lesser extent than in human monocytes (Fig. 1A). Treatment of macrophages with physiologically relevant doses (25 mM, 50 mM, 75 mM) of alcohol for 15 min significantly increased HSF-binding activity (Fig. 1B). A combination of LPS with alcohol (50 mM and 75 mM) resulted in a further augmentation of HSF DNA binding as indicated in the bar graph (Fig. 1B, lower panel), as compared with LPS or alcohol treatment alone (Fig. 1B). Heat shock at 42°C for 45 min, used as a positive control, showed the highest HSF-binding activity in monocytes (Fig. 1A) and RAW macrophages (Fig. 1B). These data suggest that HSF-binding activity was induced by short-term alcohol exposure and augmented by a combination of alcohol with LPS in monocytes and macrophages.
Prolonged alcohol intoxication enhances monocyte and macrophage production of inflammatory cytokines, particularly TNF-α in humans and in experimental animal models of chronic alcohol abuse [5, 8, 29]. Here, we investigated whether prolonged alcohol treatment of macrophages for 72 h in vitro affects HSF-binding activity. The 72-h in vitro treatment of alcohol was selected on the basis of a “classical” increase in LPS-induced NF-κB activation and TNF-α production in RAW macrophages, similar to previous in vivo and in vitro studies [5, 8, 29, 30]. Here, we found that prolonged exposure of alcohol for 72 h significantly increased HSF-binding activity in RAW macrophages (Fig. 1C). However, LPS treatment after chronic alcohol exposure of macrophages did not significantly further augment the HSF-binding activity as compared with alcohol treatment alone (Fig. 1C), as shown in the bar graph (Fig. 1C, lower panel). These data ascertain that prolonged alcohol exposure increases HSF-binding activity by itself with no further augmentation by LPS in monocytic cells and macrophages.
In mammals, two predominant isoforms of HSF, HSF-1 and HSF-2, have been identified [12, 26]. In supershift analysis, our results show that HSF-1 and not HSF-2 antibodies supershifted the HSF complex in LPS-treated, acute alcohol-treated, and heat-shocked macrophages, indicating the predominance of HSF-1 in the binding complex (Fig. 1D). Similar results were obtained for monocytes (data not shown), where HSF-1 antibodies and not HSF-2 shifted the bands (monomeric and dimeric forms of HSF-1) observed in the EMSA in Figure 1A. Furthermore, supershift analysis of chronic alcohol-exposed macrophages also indicated the presence of HSF-1 and not HSF-1 in the binding complex (data not shown). Previous studies have shown that although the monomeric and trimeric forms bind to DNA, only the trimeric form induces transactivation of target genes [28]. Our data suggest that alcohol increases DNA binding of HSF-1 and not HSF-2.
In the absence of stress stimuli, inactive HSF-1 is sequestered in the cytoplasm by the constitutive hsp90 in a monomeric form [31]. Upon activation, HSF-1 trimerizes and traverses to the nucleus to bind to the promoter region and induces transcription of hsp genes in a stimulus and cell type-specific manner [32]. As we observed increased HSF-1 DNA-binding activity in acute and chronic alcohol-exposed monocytes and macrophages, we wanted to determine whether alcohol exposure promotes HSF-1 nuclear translocation. Based on our findings in Figure 1, B and C, that 50 mM alcohol induced most pronounced effects in HSF-1-binding activity, all subsequent experiments were performed at this alcohol concentration. Figure 1E shows that 50 mM alcohol exposure of macrophages for 15 min (acute) and 72 h (chronic) increases HSF-1 levels in the nucleus, as determined by Western blotting using a HSF-1 antibody. Although LPS treatment induced an increase in nuclear HSF-1 levels, a combination of 50 mM alcohol and LPS significantly augmented the HSF-1 nuclear levels (Fig. 1E). Cytoplasmic levels of HSF-1 were unaltered in all treatment groups (Fig. 1E). These results suggest that alcohol exposure, acute or chronic, induces nuclear translocation of HSF-1 and facilitates DNA binding to influence target gene expression.
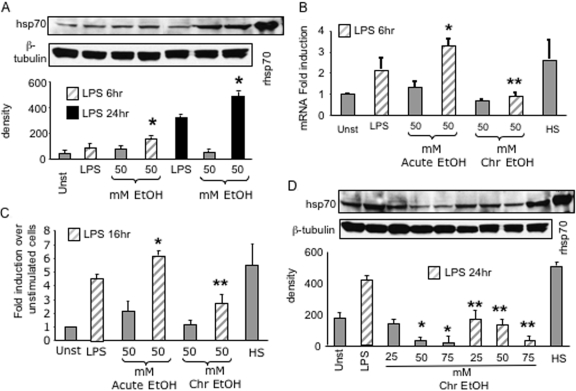
Acute alcohol transiently induces hsp70, whereas prolonged alcohol decreases hsp70 in macrophages
The HSF induces expression of various hsps, particularly hsp70 and hsp90, of significance to regulation of inflammatory responses [12, 27, 33]. Here, we investigated whether alcohol-induced HSF-1 activation modulates hsp70 expression in macrophages. Figure 2A shows that LPS at 100 ng/ml slightly increases cellular hsp70 levels by 6 h but reaches a maximum at 24 h. Acute alcohol exposure for 6 h and 24 h by itself did not affect hsp70. However, a combination of alcohol plus LPS significantly increased hsp70 levels at 6 h and 24 h in macrophages. Real-time PCR analysis revealed that macrophages exposed to LPS for 6 h showed an increase in hsp70 mRNA levels, whereas alcohol alone did not increase hsp70 mRNA significantly (Fig. 2B). A combination of alcohol and LPS showed a significant up-regulation of hsp70 mRNA levels (Fig. 2B). These results mirrored our findings on hsp70 protein levels after acute alcohol exposure in macrophages. In addition, an acute alcohol-induced increase in hsp70 in macrophages could be a result of augmentation of HSF-1 activation (Fig. 1B). To determine whether acute alcohol-induced HSF-1-binding activity correlates with hsp70 expression, transient transfection studies using a hsp70 promoter-driven luciferase construct in RAW macrophages were performed. LPS stimulation of macrophages transfected with the hsp70 promoter reporter construct induced a significant increase in hsp70 promoter-driven luciferase activity (Fig. 2C). Acute alcohol exposure (50 mM) for 16 h did not significantly increase hsp70 promoter-driven luciferase activity; however, a combination of LPS plus alcohol showed an augmentation of luciferase activity compared with LPS alone. Collectively, these results suggest that acute alcohol induces HSF-1 activation, leading to increased hsp70 promoter-driven transcriptional activity, endogenous hsp70 mRNA, and protein in macrophages.
Fig. 2.
Acute alcohol transiently increases, but chronic alcohol decreases, LPS-induced hsp70 in macrophages. (A) RAW macrophages were stimulated with 50 mM ethanol in the presence or absence of LPS (100 ng/ml) for 6 h or 24 h. Whole cell lysates (30 μg) were then subjected to immunoblotting using the hsp70 antibody and visualized by chemiluminescence. β-Tubulin antibody was used as an internal loading control. Representative gel and bar graph show mean density ± se of four individual experiments (*, P<0.02, compared with LPS). (B) Macrophages were exposed to 50 mM alcohol for 72 h (Chr) alone, followed by LPS (100 ng/ml) or LPS plus alcohol (Acute) for 6 h. Total RNA extracts were subjected to real-time PCR using the hsp70 primers. Results are shown as the bar graph of mean fold induction of hsp70 mRNA over unstimulated cells ± se of three individual experiments (*, P<0.009; **, P<0.02, compared with LPS). rhsp70, Recombinant hsp70. (C) RAW 264.7 macrophages with or without 50 mM alcohol exposure for 72 h were transiently transfected with hsp70 promoter luciferase reporter construct and the Renilla-luciferase construct. Twenty-four hours after transfection, cells were exposed to 50 mM ethanol for acute exposure, and all cells were stimulated with or without LPS (100 ng/ml) for 16 h. Cells were then lysed to determine firefly luciferase activity and normalized to the Renilla-luciferase activity. The bar graph represents the fold induction of the luciferase activity of a total of three experiments (*, P<0.03; **, P<0.02, compared with LPS). (D) Macrophages were exposed to 25 mM, 50 mM, and 75 mM alcohol for 72 h alone (Chr) or followed by LPS (100 ng/ml) for 24 h. Whole cell extracts (30 μg) were then subjected to immunoblotting using the hsp70 antibody and visualized by chemiluminescence. β-Tubulin antibody was used as an internal loading control. Representative gel and bar graph show mean density ± se of four individual experiments (*, P<0.02, compared with unstimulated; **, P<0.01, compared with LPS).
Similar to acute alcohol, prolonged alcohol exposure in vitro significantly increased HSF-1-binding activity in macrophages. Hence, we wanted to evaluate whether prolonged alcohol treatment affects hsp70 expression. Figure 2D illustrates that macrophages exposed to LPS for 24 h had increased cellular hsp70 levels significantly compared with unstimulated cells. Intriguingly, prolonged alcohol exposure alone for 72 h significantly decreased baseline hsp70 levels in a dose-dependent manner (Fig. 2D). Real-time PCR analysis showed a trend for decrease in hsp70 mRNA levels on exposure of 50 mM alcohol for 72 h in macrophages (Fig. 2B). Additionally, exposure of 50 mM alcohol for 72 h followed by LPS treatment showed a more pronounced down-regulation in hsp70 mRNA (Fig. 2B) and protein (Fig. 2D) levels as compared with LPS alone. Heat shock at 42°C for 45 min, followed by recovery for 16 h used as a positive control for hsp70 induction, showed significant up-regulation of hsp70 mRNA and protein in macrophages. As prolonged alcohol induces HSF-1 activation but decreases hsp70, we wanted to determine whether hsp70 promoter-driven luciferase activity was altered. Transient transfection studies show that 72 h chronic alcohol exposure alone did not affect luciferase activity but decreased LPS-stimulated luciferase activity (Fig. 2C). These results indicate that there is lack of correlation between increased HSF-1-binding activity and decreased hsp70 promoter activity, mRNA, and protein levels after prolonged alcohol exposure in macrophages.
Prolonged alcohol treatment increases hsp90, stabilizes the hsp90-IKK complex, and induces NF-κB
Hsp90 chaperones various components of the LPS-inducible TLR signaling pathway [14, 34, 35] and is important in inflammatory cytokine production. Constitutive hsp90 negatively regulates HSF-1 activity by sequestration of HSF-1 in an inactive complex in the cytoplasm [31]. As acute and prolonged alcohol exposure induces HSF-1-binding activity, we studied whether alcohol exposure affects hsp90 levels in macrophages. Additionally, as a result of the lack of correlation between HSF-1 activation and hsp70 induction in prolonged alcohol-exposed macrophages, we sought to investigate whether alcohol-induced HSF-1 activation correlates with hsp90 protein induction after prolonged alcohol exposure. Hsp90 protein levels were assessed in the total cellular lysates as well as in the cytoplasmic compartment in macrophages, where hsp90 binds to HSF-1 and other client proteins. We found that LPS treatment by itself did not increase hsp90 levels in macrophages (Fig. 3A), but 50 mM (not 25 mM) alcohol for 72 h increased hsp90 significantly in macrophages. When LPS was added 72 h after alcohol treatment, hsp90 levels were increased significantly in total cellular lysates compared with LPS treatment alone (Fig. 3A). Furthermore, macrophages treated with 50 mM alcohol for 72 h followed by LPS showed significantly increased accumulation of hsp90 in the cytoplasm (Fig. 3B), despite concomitant HSF-1 activation (Fig. 1C). Analysis of hsp90 after acute alcohol exposure in combination with LPS showed reduced cytoplasmic hsp90, indicating a correlation between HSF-1 activation and loss of hsp90 in the cytoplasm. These results indicate that increased hsp90 in the cytoplasm of chronic alcohol macrophages is not associated with HSF-1, whereas acute alcohol exposure decreases hsp90 levels and its association with HSF-1.
Fig. 3.
Prolonged alcohol treatment increases hsp90. (A) Macrophages were exposed to 25 mM and 50 mM alcohol for 72 h alone (Chr) or followed by LPS (100 ng/ml) for 24 h. Whole cell extracts (30 μg) were then subjected to immunoblotting using the hsp90 antibody and visualized by chemiluminescence. β-Tubulin antibody was used as an internal loading control. Representative gel and bar graph show mean density ± se of four individual experiments (*, P<0.05, compared with unstimulated; **, P<0.02, compared with LPS). (B) Macrophages were exposed to 50 mM alcohol for 72 h (Chr) followed by LPS (100 ng/ml) or in the presence of alcohol plus LPS for 24 h (acute). Cytoplasmic extracts (30 μg) were then subjected to immunoblotting using the hsp90 antibody and visualized by chemiluminescence. β-Actin antibody was used as an internal loading control. Representative gel and bar graph show mean density ± se of four individual experiments (*, P<0.02; **, P<0.001, compared with LPS). (C) RAW macrophages exposed to 50 mM alcohol for 72 h (Chr) followed by LPS (100 ng/ml) or in the presence of alcohol plus LPS for 15 min (acute). Cytoplasmic extracts (300 μg) were then subjected to immunoprecipitation (IP) using the hsp90 antibody (Stressgen Biotechnologies), followed by immunoblotting using the HSF-1 antibody and visualized by chemiluminescence (lower gel). Immunoblotting with the hsp90 antibody showed successful immunoprecipitation of hsp90 (upper gel). A representative gel and the bar graph with mean density of four individual experiments are shown (*, P<0.02, compared with unstimulated). WB, Western blot.
Under normal condition, hsp90 binds and retains HSF-1 in an inactive state in the cytoplasm [31]. To test whether the association of hsp90 and HSF-1 is altered in alcohol-exposed macrophages, we performed immunoprecipitation analysis of cytoplasmic extracts using the anti-hsp90 and HSF-1 antibodies. Figure 3C shows that although unstimulated macrophages show maximal HSF-1 in hsp90 immunoprecipitates, acute as well chronic alcohol exposure shows decreased HSF-1 in the hsp90-immunoprecipitated complexes. As expected, heat shocking of macrophages at 42°C resulted in a decrease in HSF-1-hsp90 complexes as a result of dissociation of the hsp90-HSF-1 during heat stress (Fig. 3C). Thus, decreased association of hsp90 HSF-1 with increased HSF-1 DNA-binding activity suggests a hsp90-dependent HSF-1 activation in acute alcohol-exposed macrophages. However, it appears that increased hsp90 in the cytoplasm of prolonged alcohol-exposed macrophages does not associate with HSF-1, suggesting that hsp90 may interact with other client proteins in the cytoplasm and not HSF-1.
In the cytoplasm, hsp90 interacts with other proteins including IKK to regulate maintenance of constitutive and inducible IKK kinase activity and NF-κB induction [14, 33, 35]. As we observed increased hsp90, which was not associated with HSF-1 in the cytoplasm of prolonged alcohol-exposed macrophages, we postulated that hsp90 could interact with IKK to promote stabilization of the IKK complex and promote NF-κB activation and TNF-α production. Figure 4A demonstrates that although short-term alcohol (50 mM) exposure decreased hsp90-IKKβ complexes, prolonged alcohol increased association of hsp90 and IKKβ induced by LPS. Concomitant analysis of IKKβ kinase activity revealed that prolonged alcohol exposure increased, whereas acute alcohol decreased, LPS-induced IKKβ kinase activity (Fig. 4B). Reduced hsp90-IKKβ complexes were accompanied by decreased IκBα-independent, NF-κB-binding activity in nuclear extracts of short-term, alcohol-exposed macrophages (Fig. 4C). On the other hand, increased hsp90-IKKβ complexes in prolonged alcohol-exposed macrophages culminated in IκBα degradation (Fig. 4D, upper gel), hence, increased NF-κB DNA-binding activity (Fig. 4D; lower gel). These results support the contention that the increased hsp90 levels after prolonged alcohol exposure could play a role in IKK stabilization and increased NF-κB acitivity, leading to increased TNF-α production in macrophages.
Fig. 4.
Alcohol regulates hsp90-IKKβ complexes in macrophages. (A) RAW macrophages were exposed to 50 mM alcohol alone for 24 h (Acute) with LPS or 72 h (Chr) followed by LPS (100 ng/ml) for 24 h. Cytoplasmic extracts (300 μg) were then subjected to immunoprecipitation using the IKKβ antibody followed by immunoblotting using the hsp90 antibody and visualized by chemiluminescence (lower gel). Immunoblotting with the IKKβ antibody after immunoprecipitation with IKKβ showed equal loading (upper gel). A representative gel and the bar graph with mean density ± se of four individual experiments are shown. (B) IKK kinase assay. RAW macrophages were exposed to 50 mM alcohol for 72 h followed by LPS (100 ng/ml) or in the presence of alcohol plus LPS for 24 h. Cytoplasmic extracts (300 μg) were then subjected to immunoprecipitation using the IKKβ antibody followed by in vitro kinase assay using the GST-IκBα as a substrate. Results are shown as mean cpm incorporated in the substrate ± se of four individual experiments (*, P<0.001; **, P<0.05, compared with LPS). (C) RAW macrophages were stimulated with LPS (100 ng/ml) in the presence or absence of 25 mM, 50 mM, and 75 mM ethanol for 15 min. IκBα was estimated in the cytoplasmic extracts by Western blotting (upper representative gel of a total of five). NF-κB was detected in the nuclear extracts by EMSA using a 32P-labeled, double-stranded NF-κB oligonucleotide (lower representative gel of a total of five). A 20-fold excess of unlabeled oligonucleotide was included as a cold competitor. The bar graph (NF-κB) in the lower panel shows mean density ± se of five experiments (*, P<0.001, compared with LPS). (D) RAW macrophages were exposed to 25 mM and 50 mM alcohol for 72 h alone or followed by LPS (100 ng/ml) for 15 min. IκBα was estimated in the cytoplasmic extracts by Western blotting (upper representative gel of a total of five). NF-κB was detected in the nuclear extracts by EMSA using a 32P-labeled, double-stranded NF-κB oligonucleotide (lower representative gel of a total of five). A 20-fold excess of unlabeled oligonucleotide was included as a cold competitor. A representative EMSA shown in the upper panel and the bar graph in the lower panel show mean density ± se of five experiments (*, P<0.02, compared with unstimulated; **, P<0.001, compared with LPS).
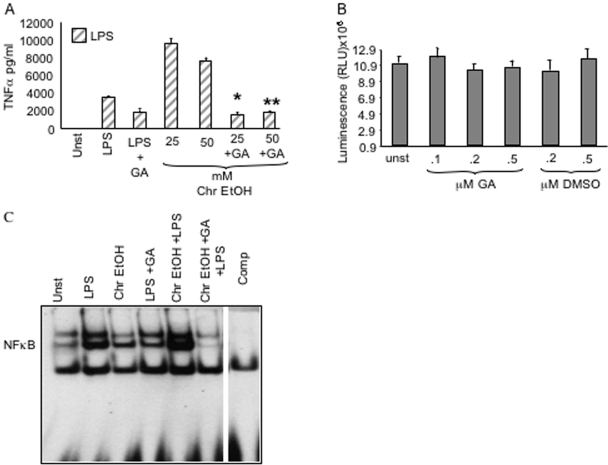
Inhibition of hsp90 prevents a prolonged alcohol-induced increase in LPS-mediated TNF-α production
Geldanamycin A (GA), a specific antagonist of hsp90, is a benzoquinone ansamycin that alters hsp90 function by inhibition of ATPase activity [36]. Inhibition of hsp90 function results in loss of IKK kinase activity and DNA-binding activity of NF-κB by preventing polyubiquitination of the IKK complex [14, 33, 35] and reduction of TNF-α production. Here, we investigated whether GA prevents a prolonged, alcohol-mediated increase in LPS-induced NF-κB activation and TNF-α production in RAW macrophages. Figure 5A shows that RAW macrophages treated with LPS (100 ng/ml) for 16 h induced TNF-α production. Prolonged alcohol (25 mM and 50 mM) exposure of macrophages for 72 h followed by LPS resulted in a significant augmentation of the LPS-induced TNF-α production (Fig. 5A). However, pretreatment of macrophages with suboptimal doses of GA (0.2 μM) for 72 h during alcohol exposure decreased the LPS-induced TNF-α production (Fig. 5A). Furthermore, GA pretreatment prevented the chronic alcohol-induced augmentation of LPS-induced TNF-α (Fig. 5A) without affecting cellular viability (Fig. 5B), indicating that inhibition of hsp90 down-regulates TNF-α production after chronic alcohol exposure.
Fig. 5.
Inhibition of hsp90 by geldanamycin blocks alcohol-induced NF-κB activation and TNF-α increase without affecting cell viability. (A) RAW macrophages were treated with 25 mM and 50 mM ethanol for 72 h (Chr) in the presence or absence of 0.2 μM GA, followed by LPS treatment for 8 h. Cell supernatants were collected and subject to ELISA to determine TNF-α levels. The bar graph represents TNF-α mean pg/ml ± se of a total of eight experiments (*, **, P<0.001, compared with LPS). (B) RAW macrophages were treated with various concentrations (0.5 μM, 0.2 μM, and 0.1 μM) of GA or DMSO control for 72 h. Viability of cells was determined using the CellTiter-Glo luminescent cell viability assay (Promega). The RLU represent mean ± se of three individual experiments for each treatment. (C) RAW macrophages were treated with 50 mM alcohol for 72 h (Chr) in the presence or absence of 0.2 μM GA, followed by LPS treatment for 15 min. NF-κB activation was detected in the nuclear extracts by EMSA using a 32P-labeled, double-stranded NF-κB oligonucleotide. A 20-fold excess of unlabeled oligonucleotide was included as a cold competitor. A representative EMSA is shown of a total of three experiments.
As hsp90 facilitates NF-κB-binding activity via stability of IKK kinase, we also determined whether restoration of LPS-induced TNF-α levels in geldanamycin-treated chronic alcohol exposed macrophages could be a result of inhibition of NF-κB-binding activity by geldanamycin. Figure 5C shows that whereas chronic alcohol exposure significantly increases LPS-induced NF-κB-binding activity, geldanamycin treatment significantly inhibits chronic alcohol-induced, NF-κB-binding activity in macrophages, indicating that hsp90 plays an important role in the induction of NF-κB-binding activity after chronic alcohol exposure. Thus, our results suggest that hsp90 inhibition may prevent the alcohol-induced augmentation of LPS-induced NF-κB-binding activity and TNF-α and subsequently reduce inflammation in ALD. Hence, we propose that hsp90 plays an important role in alcohol-induced inflammation.
DISCUSSION
In this study, we show for the first time that exposure of macrophages to in vitro alcohol results in induction of hsp70 and hsp90 by its key regulator, the HSF, and that these factors play an important role in alcohol-induced proinflammatory cytokine production. Here, we propose novel mechanisms involving hsp70 and hsp90 in contribution to alcohol-induced TNF-α production. Specifically, we show that in monocytes and macrophages, short-term alcohol increases HSF-1-binding activity and induces hsp70 mRNA and protein. On the other hand, prolonged alcohol exposure increases HSF-1-binding activity without hsp70 induction but instead, resulting in hsp90 expression. Our studies also show that the increased hsp90 is associated with IKKβ in the cytoplasm in prolonged alcohol-exposed macrophages, thereby supporting IKK kinase activity, IκBα degradation, and increased NF-κB activation. Importantly, geldanamycin, a specific hsp90 inhibitor, confirmed that blocking of hsp90 results in inhibition of LPS-induced NF-κB activation and TNF-α production after prolonged alcohol exposure. Collectively, our results strongly suggest that alcohol regulates hsp70 and hsp90 based on the duration of exposure, and prolonged alcohol-induced hsp90 contributes to LPS-induced TNF-α production in macrophages.
Hsps are a group of highly conserved proteins that are induced rapidly in response to a variety of environmental stresses including high temperatures, trauma, glucose deprivation, and ethanol exposure [10, 11]. Previous studies about hsps have revealed that during stress, hsps contribute to cell survival by chaperoning various kinases and misfolded proteins to facilitate their correct folding and assembly. Induction of hsp genes is regulated by the HSF [12, 27]. Studies showing induction of hsps by alcohol in the liver and brain have speculated that hsp expression is a cellular, adaptive response to ethanol-induced oxidative stress [17,18,19,20]. In neuronal cells, acute and chronic alcohol exposure induces hsp genes such as hsp70, hsp90, glucose-regulated protein (grp)78, and grp94 via HSF-1 activation [20, 37, 38]. Increased hsp70 gene expression was also observed in livers of ethanol-fed rats [17]. Long-term alcohol exposure also affects hsp27 and hsp90 mRNA in skeletal muscle in rats [39]. Whereas at first interpreted as a signal of cellular adaptation to stress and protection from the injury, it is now being recognized that hsps and its transcription factor HSF-1 can also contribute to cellular injury [40]. Our studies here point to a role for hsp90 in regulating alcohol-induced inflammation and thus, contributing to alcoholic cellular injury.
Activation of HSF is required for hsp induction [10,11,12]. Here, we show that regardless of the duration of exposure, alcohol induces nuclear translocation and DNA-binding activity of HSF-1. Several HSFs (HSF-1–4) have been described in vertebrate cells [12, 27]. In human cells, however, HSF-1 and HSF-2 share a common, functional region and are regulated differently. Although HSF-1 is the predominant type activated during stress, HSF-2 plays an important role in early development and also seems to participate in transcriptional regulation of hsps during stress [12, 41]. Our supershift experiments identify HSF-1 and not HSF-2 as the principle form affected by alcohol. In nonstressed cells, monomeric HSF-1 remains inactive in the cytoplasm in a multichaperone complex containing hsp90 and other cochaperone proteins [31]. Here, we show that acute and prolonged alcohol exposure decreased association of hsp90 and HSF-1 in the cytoplasm and increased HSF-1 nuclear translocation and DNA-binding activity in macrophages.
HSF-1 activation results in induction of target genes such as the family of hsps [12]. Earlier reports have shown that oxidative stress-induced hsps, particularly hsp70 and hsp90, play an important role in regulating inflammatory cytokines such as TNF-α. For instance, hsp70 participates in post-transcriptional control of TNF-α release [42] and inhibition of nuclear translocation of NF-κB [43, 44]. Here, we show that although short-term alcohol alone does not affect hsp70, LPS-induced hsp70 promoter activity, mRNA, and protein are increased in macrophages. Hsp70 induction is regulated by cooperation from other transcription factors such as STAT1, which interacts directly with HSF-1 to induce hsp70 [45]. Recent studies show that acute alcohol decreases cytokine-induced STAT1 activity in human monocytes [46]. Although it is likely that a chronic alcohol-mediated decrease in LPS-induced hsp70 could be a result of a lack of cooperation of STAT1, the role of STAT1 in induction of hsp70 by acute alcohol is still unclear.
Previous studies show that acute alcohol decreases LPS-induced NF-κB activation and TNF-α production in monocytes and macrophages [6]. Here, it is likely that acute alcohol directly increases transcription of hsp70, which in turn could repress TNF-α gene expression. Hsp70 could act via sequestration of NF-κB in the cytoplasm [43, 44] or direct binding to TNF-α and inhibition of its release [42] in acute alcohol exposure. Further investigations of the molecular interactions of components of the NF-κB pathway and hsp70 will provide insights into the role of hsp70 in TNF-α down-regulation after acute alcohol exposure.
On the other hand, chronic alcohol exposure decreased hsp70 mRNA and protein in macrophages. Addition of LPS to prolonged alcohol-exposed macrophages down-regulated hsp70 promoter-driven reporter activity and hsp70 mRNA. Studies have shown that the hsp70 mRNA is more stable in cells treated with protein synthesis inhibitors [47]. It is likely that prolonged alcohol treatment induces new protein synthesis to affect turnover rate of hsp70 mRNA, resulting in decreased hsp70 expression. In addition to HSF-1, hsp70 induction is modulated by STAT1 and specificity of protein 1 (Sp1) DNA-binding activity [19, 45, 48]. Previous studies have shown that chronic alcohol decreases STAT1 activity [49]. Decreased hsp70 levels after chronic alcohol exposure could be a result of decreased STAT1 activity. Alcohol could also alter Sp1-binding activity in macrophages and contribute to alteration in hsp70 induction.
Aside from hsp70, HSF-1 also induces hsp90, another target gene [12, 27]. Induction of hsp90 is common in various forms of cancer and tissue injury [50]. Our studies demonstrate that acute alcohol exposure showed no significant differences in total cellular hsp90 but decreased cytoplasmic hsp90 and correlated with HSF-1 activation in these cells. Chemotherapeutic agents such as taxotere cause ubiquitination and subsequent proteasomal degradation of hsp90 [51]. It is likely that acute alcohol-induced reduction in cytoplasmic hsp90 levels occurs as a result of proteasomal degradation. In nonstressed cells, hsp90 sequesters monomeric HSF-1 in the cytoplasm and upon stress, releases HSF-1 from this chaperone complex with hsp90 [31]. In our studies, acute alcohol exposure decreased cytoplasmic hsp90-HSF-1 complexes. Hsp90 binds to monomeric HSF-1 as well as trimeric HSF-1 in the nucleus [52]. The possibility that acute alcohol exposure results in localization of hsp90 to the nucleus to facilitate HSF-1 DNA binding is likely.
Prolonged alcohol exposure and in combination with LPS increased total cellular and cytoplasmic hsp90 levels in RAW macrophages. Interestingly, hsp90 did not form complexes with HSF-1 in the cytoplasm of prolonged alcohol-exposed cells. Hsp90 is required for efficient function and stability of kinases induced by the LPS pathway [14, 34, 35]. A requirement of hsp90 for activation of NF-κB was suggested by studies that use GA, an efficient and specific hsp90 inhibitor [14, 36, 53]. GA inhibits LPS-induced NF-κB activity and TNF-α production in macrophages [36, 53, 54]. Subsequent studies showed that hsp90 interacts with LPS-induced IKK kinase and is required for constitutive and inducible IKK and NF-κB activation [14]. Prolonged alcohol exposure increases LPS-induced NF-κB activation and TNF-α production in macrophages [5, 30]. Here, we show that increased hsp90 after prolonged alcohol exposure binds to IKKβ to facilitate IKK kinase activity and NF-κB activation. Immunoprecipitation experiments revealed that hsp90 formed stable complexes with IKKβ similar to LPS treatment of RAW macrophages after prolonged alcohol exposure. Using suboptimal doses of geldanamycin, a specific inhibitor of hsp90 ATPase activity, we confirmed that GA prevents chronic alcohol-induced IKKβ kinase activity and NF-κB DNA-binding activity. Our results thus suggest that hsp90 plays an important role in alcohol-induced NF-κB activation and TNF-α production. From these studies, we propose that hsp90 could be an attractive target in vivo for inhibition of TNF-α production and attenuation of alcohol-induced liver injury.
Collectively, our data suggest an important checkpoint role for hsps and their regulator HSF-1 in alcohol-induced TNF-α regulation. Although HSF-1-induced hsp70 in acute alcohol exposure could play an important role in regulation of inhibition of inflammation, hsp90 is supportive to induction of proinflammatory cytokines in alcoholic liver injury. Future in vivo pharmacologic hsp90 inhibitor studies will help provide evidence about the possibility of hsp90 as a target for treatment strategies in alleviating alcohol-induced liver injury.
Acknowledgments
This work was supported by National Institute on Alcohol Abuse and Alcoholism grant AA14238.
References
- Nelson S, Bagby G J, Bainton B G, Summer W R. The effects of acute and chronic alcoholism on tumor necrosis factor and inflammatory response. J Infect Dis. 1989;160:422–429. doi: 10.1093/infdis/160.3.422. [DOI] [PubMed] [Google Scholar]
- Nelson S, Kolls J K. Alcohol, host defence and society. Nat Rev Immunol. 2002;2:205–209. doi: 10.1038/nri744. [DOI] [PubMed] [Google Scholar]
- Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol. 1999;34:830–841. doi: 10.1093/alcalc/34.6.830. [DOI] [PubMed] [Google Scholar]
- Szabo G, Mandrekar P, Newman L, Catalano D. Regulation of human monocyte function by acute ethanol treatment: decreased tumor necrosis factor-α, interleukin-1 β and elevated IL-10 and transforming growth factor-β production. Alcohol Clin Exp Res. 1996;20:900–907. doi: 10.1111/j.1530-0277.1996.tb05269.x. [DOI] [PubMed] [Google Scholar]
- Nagy L E. Recent insights into the role of the innate immune system in the development of alcoholic liver disease. Exp Biol Med (Maywood) 2003;228:882–890. doi: 10.1177/153537020322800803. [DOI] [PubMed] [Google Scholar]
- Mandrekar P, Catalano D, Szabo G. Inhibition of LPS-mediated NFκB activation by ethanol in human monocyte. Int Immunol. 1999;11:1781–1790. doi: 10.1093/intimm/11.11.1781. [DOI] [PubMed] [Google Scholar]
- Hoek J B, Pastorino J G. Ethanol, oxidative stress and cytokine-induced liver injury. Alcohol. 2002;27:63–68. doi: 10.1016/s0741-8329(02)00215-x. [DOI] [PubMed] [Google Scholar]
- Kishore R, McMullen M R, Cocuzzi E, Nagy L E. Lipopolysaccharide-mediated signal transduction: stabilization of TNF-α mRNA contributes to increased lipopolysaccharide-stimulated TNF-α production by Kupffer cells after chronic ethanol feeding. Comp Hepatol. 2004;3:S31–S37. doi: 10.1186/1476-5926-2-S1-S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oak S, Mandrekar P, Catalano D, Kodys K, Szabo G. TLR2- and TLR4-mediated signals determine attenuation or augmentation of inflammation by acute alcohol in monocytes. J Immunol. 2006;176:7628–7635. doi: 10.4049/jimmunol.176.12.7628. [DOI] [PubMed] [Google Scholar]
- Morimoto R I. Cells in stress: transcriptional activation of heat shock genes. Science. 1993;259:1409–1410. doi: 10.1126/science.8451637. [DOI] [PubMed] [Google Scholar]
- Schlesinger M J. How the cell copes with stress and the function of heat shock proteins. Pediatr Res. 1994;36:1–6. doi: 10.1203/00006450-199407001-00001. [DOI] [PubMed] [Google Scholar]
- Morimoto R I. Regulation of heat shock transcriptional response: cross talk between a family of heat shock factors, molecular chaperones, and negative regulators. Genes Dev. 1998;12:3788–3796. doi: 10.1101/gad.12.24.3788. [DOI] [PubMed] [Google Scholar]
- Vabulas R M, Ahmad-Nejad P, Ghose S, Kirschning C J, Issels R D, Wagner H. hsp70 as endogenous stimulus of the Toll/interleukin-1 receptor signal pathway. J Biol Chem. 2002;277:15107–15112. doi: 10.1074/jbc.M111204200. [DOI] [PubMed] [Google Scholar]
- Broemer M, Krappmann D, Scheidereit C. Requirement of hsp90 activity for IκB kinase (IKK) biosynthesis and for constitutive IKK and NFκB activation. Oncogene. 2004;23:5378–5386. doi: 10.1038/sj.onc.1207705. [DOI] [PubMed] [Google Scholar]
- Triantafilou M, Triantafilou K. Heat shock protein 70 and heat shock protein 90 associate with Toll-like receptor 4 in response to bacterial lipopolysaccahride. Biochem Soc Trans. 2004;32:636–639. doi: 10.1042/BST0320636. [DOI] [PubMed] [Google Scholar]
- Meng X, Harken A H. The interaction between hsp70 and TNF-α expression: a novel mechanism for protection of the myocardium against post-injury depression. Shock. 2002;17:345–353. doi: 10.1097/00024382-200205000-00001. [DOI] [PubMed] [Google Scholar]
- Nanji A A, Griniuviene B, Yacoub L K, Sadrzadeh S M, Levitsky S, McCully J D. Heat-shock gene expression in alcoholic liver disease in the rat is related to the severity of liver injury and lipid peroxidation. Proc Soc Exp Biol Med. 1995;210:12–19. doi: 10.3181/00379727-210-43918. [DOI] [PubMed] [Google Scholar]
- Omar R, Pappolla M, Saran B. Immunocytochemical detection of the 70-kd heat shock protein in alcoholic liver disease. Arch Pathol Lab Med. 1990;114:589–592. [PubMed] [Google Scholar]
- Wilke N, Sganga M W, Gayer G G, Hsieh K P, Miles M F. Characterization of promoter elements mediating ethanol regulation of hsc70 gene transcription. J Pharmacol Exp Ther. 2000;292:173–180. [PubMed] [Google Scholar]
- Pignataro L, Miller A N, Ma L, Midha S, Protiva P, Herrera D G, Harrison N L. Alcohol regulates gene expression in neurons via activation of heat shock factor 1. J Neurosci. 2007;27:12957–12966. doi: 10.1523/JNEUROSCI.4142-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Wang L, Song Z, Lambert J C, McClain C J, Kang Y J. A critical involvement of oxidative stress in acute alcohol-induced hepatic TNF-α production. Am J Pathol. 2003;163:1137–1146. doi: 10.1016/s0002-9440(10)63473-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finkel T, Holbrook N J. Oxidants, oxidative stress and ageing. Nature. 2000;408:239–247. doi: 10.1038/35041687. [DOI] [PubMed] [Google Scholar]
- Schatzle J D, Kralova J, Bose H R., Jr Avian IκBα is transcriptionally induced by c-Rel and v-Rel with different kinetics. J Virol. 1995;69:5383–5390. doi: 10.1128/jvi.69.9.5383-5390.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams G T, Morimoto R I. Maximal stress-induced transcription from the human hsp70 promoter requires interactions with the basal promoter elements independent of rotational alignment. Mol Cell Biol. 1990;10:3125–3136. doi: 10.1128/mcb.10.6.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurman R G, Bradford B U, Iimuro Y, Frankenberg M V, Knecht K T, Connor H D, Adachi Y, Wall C, Arteel G E, Raleigh J A, Forman D T, Mason R P. Mechanisms of alcohol-induced hepatotoxicity: studies in rats. Front Biosci. 1999;4:e42–e46. doi: 10.2741/A478. [DOI] [PubMed] [Google Scholar]
- Thurman R G. II. Alcoholic liver injury involves activation of Kupffer cells by endotoxin. Am J Physiol. 1998;275:G605–G611. doi: 10.1152/ajpgi.1998.275.4.G605. [DOI] [PubMed] [Google Scholar]
- Christians E S, Liang-Jun Y, Benjamin I J. Heat shock factor 1 and heat shock proteins: critical partners in protection against acute cell injury. Crit Care Med. 2002;30:S43–S50. [PubMed] [Google Scholar]
- Wigmore S J, Sangster K, McNally S J, Harrison E M, Ross J A, Fearon K C, Garden O J. De-repression of heat shock transcription factor-1 in interleukin-6 treated hepatocytes is mediated by down-regulation of glycogen synthase kinase 3β and MAPK/ERK-1. Int J Mol Med. 2007;19:413–420. [PubMed] [Google Scholar]
- McClain C, Barve S, Joshi-Barve S, Song Z, Deaciuc I, Chen T, Hill D. Dysregulated cytokine metabolism, altered hepatic methionine metabolism and proteasome dysfunction in alcoholic liver disease. Alcohol Clin Exp Res. 2005;29:180S–188S. doi: 10.1097/01.alc.0000189276.34230.f5. [DOI] [PubMed] [Google Scholar]
- Gobejishvili L, Barve S, Joshi-Barve S, Uriarte S, Song Z, McClain C. Chronic ethanol-mediated decrease in camp primes macrophages to enhanced LPS-inducible NF-κB activity and TNF expression: relevance to alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol. 2006;291:G681–G688. doi: 10.1152/ajpgi.00098.2006. [DOI] [PubMed] [Google Scholar]
- Zou J, Guo Y, Guettouche T, Smith D F, Voellmy R. Repression of heat shock transcription factor HSF1 activation by hsp90 (hsp90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- Sarge K D, Murphy S P, Morimoto R I. Activation of heat shock gene transcription by heat shock factor 1 involves oligomerization, acquisition of DNA binding activity and nuclear localization and can occur in the absence of stress. Mol Cell Biol. 1993;13:1392–1407. doi: 10.1128/mcb.13.3.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salminen A, Paimela T, Suurone T, Kaarniranta K. Innate immunity meets with cellular stress at the IKK complex: regulation of the IKK complex by hsp70 and hsp90. Immunol Lett. 2008;117:9–15. doi: 10.1016/j.imlet.2007.12.017. [DOI] [PubMed] [Google Scholar]
- De Nardo D, Masendycz P, Ho S, Cross M, Fleetwood A J, Reynolds E C, Hamilton J A, Scholz G M. A central role for the hsp90.cdc37 molecular chaperone module in interleukin-1 receptor associated-kinase-dependent signaling by Toll-like receptors. J Biol Chem. 2005;280:9813–9822. doi: 10.1074/jbc.M409745200. [DOI] [PubMed] [Google Scholar]
- Pittet J-F, Lee H, Pespeni M, O'Mahony A, Roux J, Welch W J. Stress-induced inhibition of the NFκB signaling pathway results from the insolubilization of the IκB kinase complex following its dissociation from heat shock protein 90. J Immunol. 2005;174:384–394. doi: 10.4049/jimmunol.174.1.384. [DOI] [PubMed] [Google Scholar]
- Ochel H J, Eichhorn K, Gademann G. Geldanamycin: the prototype of a class of anti-tumor drugs targeting the heat shock protein 90 family of molecular chaperones. Cell Stress Chaperones. 2001;6:105–112. doi: 10.1379/1466-1268(2001)006<0105:gtpoac>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunici P, Schiaffonati L, Rabellotti E, Tiberio L, Perin A, Sessa A. In vivo modulation of 73 kDa heat shock cognate and 78 kDa glucose-regulating protein gene expression in rat liver and brain by ethanol. Alcohol Clin Exp Res. 1999;23:1861–1867. [PubMed] [Google Scholar]
- Miles M F, Wilke N, Eliot M, Tanner W, Shah S. Ethanol responsive genes in neural cells include the 78-kilodalton glucose-regulated protein (GRP78) and 94-kilodalton glucose-regulated protein (GRP94) molecular chaperones. Mol Pharmacol. 1994;46:873–879. [PubMed] [Google Scholar]
- Nakahara T, Hunter R, Hirano M, Uchimura H, McArdle A, Broome C S, Koll M, Martin C R, Preedy V R. Alcohol alters skeletal muscle heat shock protein gene expression in rats: these effects are moderated by sex, raised endogenous acetaldehyde and starvation. Metabolism. 2006;55:843–851. doi: 10.1016/j.metabol.2006.02.009. [DOI] [PubMed] [Google Scholar]
- Whitesell L, Bagatell R, Falsey R. The stress response implications for the clinical development of hsp90 inhibitors. Curr Cancer Drug Targets. 2003;3:349–358. doi: 10.2174/1568009033481787. [DOI] [PubMed] [Google Scholar]
- Ostling P, Bjork J K, Roos-Mattjus P, Mezger V, Sistonen L. Heat shock factor 2 (HSF2) contributes to inducible expression of hsp genes through interplay with HSF1. J Biol Chem. 2007;282:7077–7086. doi: 10.1074/jbc.M607556200. [DOI] [PubMed] [Google Scholar]
- Ribeiro S P, Cahill C M, Chu B, Prevelige R, Bickford K, Stevenson M A, Calderwood S K. Effects of the stress response in septic rats and LPS-stimulated alveolar macrophages: evidence for TNFα post-translational regulation. Am J Respir Crit Care Med. 1996;154:1843–1850. doi: 10.1164/ajrccm.154.6.8970379. [DOI] [PubMed] [Google Scholar]
- Malhotra V, Wong H R. Interactions between the heat shock response and the nuclear factor-κB signaling pathway. Crit Care Med. 2002;30:S89–S95. [PubMed] [Google Scholar]
- Feinstein D L, Galea E, Aquino D A, Li G C, Xu H, Reis D J. Heat shock protein 70 suppresses astroglial-inducible nitric oxide synthase expression by decreasing NFkB activation. J Biol Chem. 1996;271:17724–17732. doi: 10.1074/jbc.271.30.17724. [DOI] [PubMed] [Google Scholar]
- Stephanou A, Isenberg D A, Nakajima K, Latchman D S. Signal transducer and activator of transcription-1 and heat shock factor-1 interact and activate the transcription of the hsp70 and hsp90β gene promoters. J Biol Chem. 1999;274:1723–1728. doi: 10.1074/jbc.274.3.1723. [DOI] [PubMed] [Google Scholar]
- Norkina O, Dolganiuc A, Catalano D, Kodys K, Mandrekar P, Syed A, Efors M, Szabo G. Acute alcohol intake induces SOCS1 and SOCS 3 and inhibits cytokine induced STAT1 and STAT3 signaling in human monocytes. Alcohol Clin Exp Res. 2008 doi: 10.1111/j.1530-0277.2008.00726.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theodorakis N G, Morimoto R I. Post-transcriptional regulation of hsp70 expression in human cells: effects of heat shock, inhibition of protein synthesis and adenovirus infection on translation and mRNA stability. Mol Cell Biol. 1987;7:4357–4368. doi: 10.1128/mcb.7.12.4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W D. Transcription factor Sp1 binds to and activates a human hsp70 gene promoter. Mol Cell Biol. 1989;9:4099–4104. doi: 10.1128/mcb.9.9.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaruga B, Hong F, Kim W H, Sun R, Fan S, Gao B. Chronic alcohol consumption accelerates liver injury in T cell-mediated hepatitis: alcohol disregulation of NF-κB and STAT3 signaling pathways. Am J Physiol Gastrointest Liver Physiol. 2004;287:G471–G479. doi: 10.1152/ajpgi.00018.2004. [DOI] [PubMed] [Google Scholar]
- Pratt W B. The hsp90-based chaperone system: involvement in signal transduction from a variety of hormone and growth factor receptors. Proc Soc Exp Biol Med. 1998;217:420–434. doi: 10.3181/00379727-217-44252. [DOI] [PubMed] [Google Scholar]
- Murtagh J, Lu H, Schwatrz E L. Taxotere-induced inhibition of human endothelial cell migration is a result of heat shock protein 90 degradation. Cancer Res. 2006;66:8192–8199. doi: 10.1158/0008-5472.CAN-06-0748. [DOI] [PubMed] [Google Scholar]
- Bharadwaj S, Ali A, Ovsenek N. Multiple components of the HSP90 chaperone complex function in regulation of heat shock factor 1 in vivo. Mol Cell Biol. 1999;19:8033–8041. doi: 10.1128/mcb.19.12.8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wax S, Piecyk M, Maritim B, Anderson P. Geldanamycin inhibits the production of inflammatory cytokines in activated macrophages by reducing the stability and translation of cytokine transcripts. Arthritis Rheum. 2003;48:541–550. doi: 10.1002/art.10780. [DOI] [PubMed] [Google Scholar]
- Vega V L, DeMaio A. Geldanamycin treatment ameliorates the response to LPS in murine macrophages by decreasing CD14 surface expression. Mol Biol Cell. 2003;14:764–773. doi: 10.1091/mbc.E02-08-0498. listed for * and ** in Panels B and E.) throughout? [DOI] [PMC free article] [PubMed] [Google Scholar]