Abstract
Upon stimulation, activation of NADPH oxidase complexes in neutrophils produces a burst of superoxide anions contributing to oxidative stress and the development of inflammatory process. Store-operated calcium entry (SOCE), whereby the depletion of intracellular stores induces extracellular calcium influx, is known to be a crucial element of NADPH oxidase regulation. However, the mechanistic basis mediating SOCE is still only partially understood, as is the signal-coupling pathway leading to modulation of store-operated channels. This review emphasizes the role of calcium influx in the control of the NADPH oxidase and summarizes the current knowledge of pathways mediating this extracellular calcium entry in neutrophils. Such investigations into the cross-talk between NADPH oxidase and calcium might allow the identification of novel pharmacological targets with clinical use, particularly in inflammatory diseases.
Keywords: store-operated Ca2+ entry, NADPH oxidase, granulocytes
INTRODUCTION
Many cellular signal transduction pathways such as fertilization, proliferation, and development are modulated by a spatial and temporal elevation of the cytosolic-free calcium concentration ([Ca2+]c) [1]. In nonexcitable cells, the [Ca2+]c increase is predominantly a result of a Ca2+ influx from the extracellular medium through the opening of Ca2+-permeable channels subsequent to the emptying of intracellular Ca2+ stores. This store depletion is mediated by the synthesis of inositol 1,4,5 trisphosphate (InsP3), a Ca2+-mobilizing, second messenger, causing the activation of channels located in the endoplasmic reticulum (ER) membranes [2]. The intracellular signal resulting from this mechanism, known as store-operated Ca2+ entry (SOCE), is characterized by the formation of a transient Ca2+ spike (with a substantial amplitude), which returns to a basal level of [Ca2+]c, dependent on the activity of the plasma membrane and sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) pumps. Over the years, it became clear that Ca2+ mobilization plays an important role in the regulation of superoxide anion secretion by phagocytic cells [3,4,5]. Originally, it was believed that NADPH oxidase, the most important generator of superoxide anions, was only assembled and activated in the plasma membrane or phagosome. Now, it is well established that this enzymatic complex can be activated in specific granules, and produced oxidants are intracellularly retained (see refs. [6, 7] for review). Evidence for the requirement of extracellular Ca2+ entry for NADPH oxidase activation is supported by a significant decrease of superoxide anion production when extracellular Ca2+ is suppressed or chelated by EGTA [3, 8, 9]. Although oxidants are released from neutrophils upon chemoattractant stimulation, ionomycin-induced oxidant production is largely intracellular [10]. In the latter case, Ca2+ influx through ionophore activity is sufficient to activate NADPH oxidase in the granule fraction [11].
Like Ca2+ ionophores, thapsigargin, an inhibitor of Ca2+ re-uptake by SERCA, permits a slow ER emptying, followed by SOCE [12], which is unable to activate plasma membrane-localized NADPH oxidase in neutrophils. This suggests that NADPH oxidase regulation requires a second signal, Ca2+-independent pathway, acting in synergy with Ca2+ influx from SOCE [3].
During the last decade, SOCE has been studied extensively; many questions remain unanswered about the mechanism linking [Ca2+]c elevation to superoxide anion production. This review summarizes the present knowledge about Ca2+ influx-regulated NADPH oxidase activity induced by external stimuli in neutrophils.
NEUTROPHIL NADPH OXIDASE
Activated state
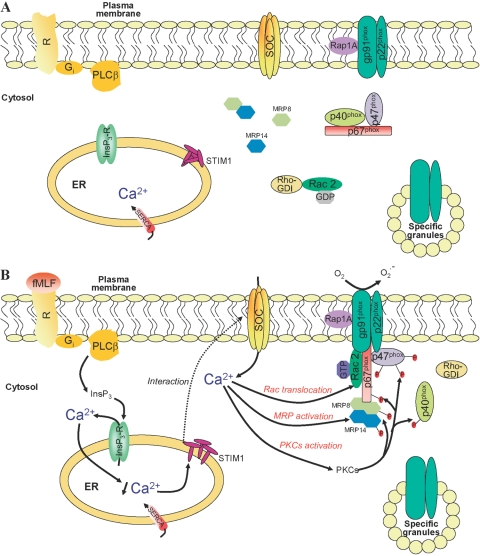
NADPH oxidase was first described in neutrophils, where it is normally inactive. This complex is a multicomponent enzyme, including cytosolic and membrane-bound proteins, which is unassembled in resting cells. Membrane components include a stable, heterodimeric flavocytochrome b558 composed of two subunits: gp91phox (known as Nox2 in the new terminology) and p22phox. Cytosolic components include four soluble factors: p67phox, p47phox, p40phox, and a small G-protein Rac (Rac1 and Rac2 isoforms). Upon cell surface receptor activation by soluble inflammatory mediators (such as fMLF), cytosolic components translocate to the plasma or phagosomal membrane, where NADPH oxidase is assembled (Fig. 1). A large quantity of superoxide anions, which are precursors of a variety of reactive oxygen species (ROS) used for microbial killing, is released. The precise mechanism governing NADPH oxidase assembly has been excellently reviewed previously (see refs. [13,14,15,16,17,18,19]) and is therefore not detailed here. Cellular responses induced by opsonized zymosan differ from those induced by chemoattractant-activated G-protein-coupled receptors. Cytosolic components translocate to the granular membrane, and oxygen metabolites are formed intracellularly, probably for the intracellular destruction of bacteria [6, 20] within the phagosome. Oxidant-producing granules could bind to the plasma membrane or fuse to form larger structures that eventually associate with the plasma membrane to release superoxide anions extracellularly [7]. Therefore, it has to be concluded that common agents used for activation (fMLF, opsonized zymosan) trigger different signaling pathways, resulting in NADPH oxidase activation.
Fig. 1.
Model depicting proposed mechanisms of NADPH oxidase regulation by SOCE in human neutrophils activated by chemoattractants. (A) Resting membrane of human neutrophils: NADPH oxidase components are compartmentalized between cytosol and plasma (granules) membrane. Ca2+ channels, including store-operated channels (SOCs) and non-SOCs, are likely to be in the plasma membrane, and stromal-interacting molecule 1 (STIM1) is in the ER. Myeloid-related protein 8 (MRP8 or S100A8) and MRP14 (or S100A9) are present in the cytosol as monomers or as one of one noncovalent heterodimers. (B) Activated membrane of human neurophils: Interaction of fMLF with G-protein-coupled membrane receptors results in the generation of InsP3 by phospholipase Cβ (PLCβ), which activates the release of Ca2+ from intracellular stores through its channel receptor (InsP3-R). Upon Ca2+ store depletion, STIM1 interacts with SOC channels, and extracellular Ca2+ entry occurs (which might allow the direct reloading in Ca2+ of ER through the SERCA pump). Resulting elevation in cytosolic Ca2+ is a prerequisite to regulate the assembly of all NADPH oxidase components at the plasma membrane (or granular membrane upon opsonized particle stimulation). SOCE mediates the translocation of Rac, activates MRP proteins, which enhance the organization and recruitment of cytosolic factors to the membrane-bound flavoCytochrome b558, and stimulates protein kinase Cs (PKCs) involved in the phosphorylation of cytosolic phox proteins. Rho-GDI, Rho-GDP inhibitor.
Primed state
Complete NADPH oxidase assembly results in a large superoxide anion production, which can be increased in response to a second activating stimulus during a process known as priming [21,22,23,24]. Priming agents per se do not cause NADPH oxidase activation. Many studies about different agents able to induce the neutrophil priming suggest that several complementary signal transductions are involved in this mechanism. For example, [Ca2+]c elevation and phosphorylation of p47phox by different protein kinases (PKC, MAPK, PKA, p21-activated kinase) allowing conformational changes of p47phox may promote oxidase activation in response to GM-CSF [25]. Changes in PLC, PLD, PLA2, and phosphoinositide 3-hydroxykinase activity, protein phosphorylation, modulation of the expression of chemotactic peptide receptors, sequential phosphorylation of p47phox and p67phox, and assembly of the NADPH oxidase in lipid rafts (reviewed by Sheppard et al. [26]) also seem to be implicated in the “priming” phenomenon. Indeed, the great diversity of substances inducing priming suggests that a variety of signaling pathways, alone or in combination, is responsible for NADPH oxidase up-regulation.
It is found that Ca2+ ionophores, such as ionomycin, might be considered as priming activators and that their effects are correlated with [Ca2+]c elevation [27]. However, the relation between [Ca2+]c and priming seems to be dependent on the nature of priming agents. For example, TNF-α [28] is unable to mobilize Ca2+ in contrast to lysophosphatidylcholine [29] or platelet-activating factor (PAF) [30], which can enhance [Ca2+]c in response to other agonists. Similarly, several studies have reported that IL-8 stimulates a [Ca2+]c rise in response to other agonists, as demonstrated by Wozniak et al. [23]. These authors suggest that the stimulation of neutrophils with IL-8 increases [Ca2+]c by mobilizing Ca2+ from internal stores and by increasing the Ca2+ influx. Our own recent work confirms that IL-8 regulates extracellular Ca2+ entry and that NADPH oxidase priming could be mediated by this [Ca2+]c elevation [31]. MacKinnon et al. [32] suggested that [Ca2+]c elevation upon stimulation by priming agents such as PAF or a substance P analog may be dependent on the generation of sphingosine 1-phosphate, described previously as the “calcium influx factor” by Itagaki and Hauser [33]. Thus, priming agents might be implicated in sphingosine 1-phosphate synthesis and subsequently, through SOCE in the elevation of [Ca2+]c. However, it is clear that other mechanisms, independent of intracellular Ca2+ signaling pathways, are required for the priming of the NADPH oxidase [31].
A signaling role of Ca2+ in priming has been established in the majority with neutrophils in suspension. However, NADPH oxidase activation of neutrophils adherent to extracellular matrix proteins differs considerably from that of neutrophils in suspension. Effectively, adherence of neutrophils can apparently capacitate them to respond to stimuli that are not effective when in suspension [34]. For example, although neutrophils in suspension show no oxidant production in response to soluble cytokines such as TNF-α, adhesion of neutrophils to substrates via leukocyte β2 integrin is able to trigger a massive oxidative response from neutrophils [34, 35]. As [Ca2+]c elevation is prevented by treatment with cytochalasin B, and antibodies against the adhesion receptor, β2 integrin-mediated adhesion is thought to be responsible for [Ca2+]c elevation [36, 37]. This conclusion is supported by rapid-time confocal scanning experiments in which Pettit and Hallett [38] observed multiple elevations in [Ca2+]c as a result of Ca2+ influx during β2 integrin-dependent adhesion. Generation of β2 integrin-induced Ca2+ influx seems dependent on the formation of InsP3 [39]. Although precise mechanisms remain to be determined, [Ca2+]c, elevation may be the link to adhesion-dependent priming [40].
MECHANISMS OF Ca2+ ENTRY
SOCE
In neutrophils, changes in [Ca2+]c are often associated with InsP3-R-mediated, rapid Ca2+ release of intracellular stores followed by SOCE as a result of the activation of channels in the plasma membrane [2, 41, 42] (Fig. 1). STIM1 has recently been identified as the sensor for ER Ca2+ content, which initiates a process resulting in a signal being sent from the stores to SOCs [43,44,45]. Three models have been put forward to link store-emptying to SOCs activation (reviewed by Putney et al. [46]): a dynamic, conformational coupling [41] (Fig. 1) involving a direct protein–protein interaction between InsP3-Rs and SOCs; an unidentified, diffusible messenger, termed calcium influx factor [33, 46,47,48], generated and released in response to store depletion; and a fusion of secretory vesicles [49, 50] with the plasma membrane causing preformed SOCs protein insertion into the plasma membrane through exocytosis or regulatory molecules. Although the conformational coupling may not apply to all cell types, we cannot rule out a combination of these different concepts, as considerable evidence exists to support an involvement of conformational changes in SOCE.
At this point, it is necessary to introduce proteins that could be considered as candidates for SOCs in neutrophils. During the last decade, research focused on mammalian homologs of Drosophila transient receptor potential canonical (TRPC) that are activated by an active and passive Ca2+ store depletion. Mammalian TRPC, closely related to Drosophila TRP, are divided into several subfamilies: TRPC1, TRPC3-6-7, and TRPC4-5 based on their sequence homologies and functional similarities [51]. Human neutrophils are known to express mRNAs for TRPC1, TRPC3, TRPC4, and TRPC6 [52,53,54], and corresponding proteins are found to be expressed in the cell membrane [53]. First, Itagaki et al. [53] provided evidence that TRPC proteins might participate in SOCE in human neutrophils after internalization of TRPC proteins by calyculin A. Cytoskeletal reorganization induced by this phosphatase inhibitor causes the displacement of TRPC proteins from the cell surface into a diffuse cytosolic pattern followed by the inhibition of SOCE induced by physiological and pharmacological stimuli [53]. Considerable efforts have been devoted to elucidate the function of TRPC proteins in SOCE, but few studies tackled the role that these channels play in neutrophils. Overexpression of TRPC in other mammalian cells [human embryo kidney (HEK)-293, DT40 B cells, platelets, salivary gland cells, adrenal cells] has been reported to result in an enhancement of SOCE, and reduction of TRPC expression using antisense strategies was shown to decrease SOCE. More precisely, it is generally accepted that TRPC1, TRPC4, and TRPC5 are activated by Ca2+ store depletion [55,56,57,58,59,60,61], and TRPC3, TRPC6, and TRPC7 are directly activated by diacylglycerol and its cell-permeant analogs in an InsP3-R-independent manner [62,63,64,65,66]. However, some studies have focused on the role of TRPC3 and TRPC6 as SOCs in human neutrophils. Indeed, we have found that endogenous TRPC6 channels are sensitive to Ca2+ store depletion in neutrophil-like, DMSO-differentiated HL-60 cells [67], and TRPC3, in combination with TRPC1 or TRPC4, might be involved in SOCs formation [53]. TRPC1 seems to be implicated in intracellular Ca2+ signaling by contributing to functional coupling between the plasma membrane and the ER. Support for this hypothesis is derived from information obtained in human platelets and B lymphocytes, which suggests that TRPC1 is activated upon interaction with InsP3-Rs [57] and that it acts not only as a component of SOCs but also as a regulatory subunit of InsP3-Rs [58]. In addition to these studies, electrotransjection of a STIM1 antibody into platelets inhibited SOCE by reducing coupling between TRPC1 and InsP3-Rs [68]. Further, Ca2+ store depletion stimulated rapid STIM1 surface expression and its association with TRPC1. However, TRPC channel functions might be dependent on their mutual association in forming heteromeric complexes, a feature that may be typical of certain myeloid lineages.
A novel, essential regulator of SOCE, Orai1, has been identified [69,70,71]. Mutations of two conserved acidic residues in transmembrane segments of Orai1 [72] as well as RNA interference knockdown [73] result in a decrease of Ca2+ influx in mammalian cells leading to three hypothesis: Orai1 is a pore subunit of SOCs; Orai1 forms the channel itself; or Orai1 is an accessory protein of the SOC signaling machinery (plasma membrane acceptor or docking protein, possibly for STIM1) [69]. Experiments of Orai1 and STIM1 overexpression [74,75,76] provided some evidences for the interaction of STIM1, anchored within ER membranes, with Orai1 after intracellular Ca2+ store depletion.
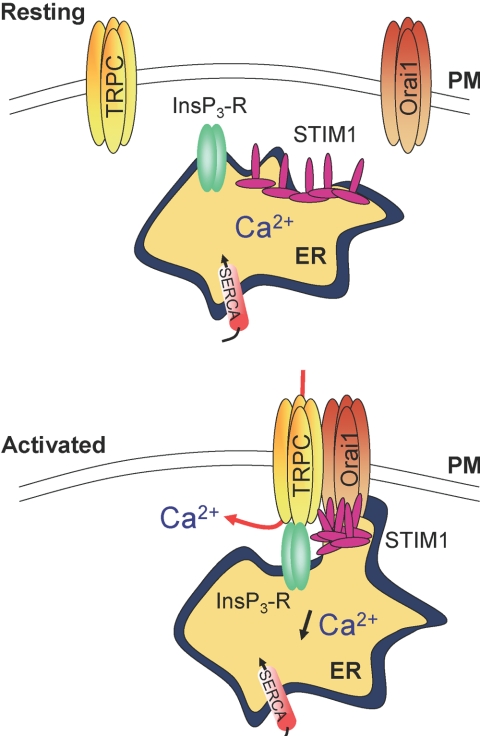
Recent studies provided evidence for the formation of a ternary complex among TRPC, Orai1, and STIM1 (Fig. 2), in which TRPC is the pore-forming component of SOCs and Orai1, the regulatory subunit that confers store depletion sensitivity to TRPC [77, 79]. Despite accumulating data that point to a preponderant role of a ternary complex formed by TRPC/Orai1/STIM1 in the activation of SOCE, conflicting findings exist about the mode of activation of TRPC channels. An expression profile appears to have an influence on the behavior of TRPC channels. To illustrate this hypothesis, it was demonstrated that individual TRPC channels could behave as a SOC or as a receptor-operated channel, depending on their interaction with other TRPC and Orai1 proteins [78, 80]. Although results obtained in other nonexcitable cells might provide an important means to ultimately resolve the molecular mystery of the SOCE pathway in neutrophils, they must be extrapolated with prudence to human neutrophils, and more studies will be necessary in these cells to define the exact arrangement of SOCs and mechanisms leading to their activation.
Fig. 2.
Potential role of STIM1, Orai1, and TRPC channels in SOCE (based on Ong et al. [77] and Liao et al. [78]). STIM1 proteins, located in the ER, redistributes into “punctae” upon internal Ca2+ store depletion and colocalizes with Orai1 and TRPC channels in the plasma membrane (PM) region. An unknown signal allows the assembly of TRPC/STIM1/Orai1 into a ternary complex and the activation of SOC channels. This reorganization might mediate communication between ER and the plasma membrane, perhaps by facilitating coupling between TRPC and InsP3-R. By interacting with TRPC channels, Orai1 proteins might act as regulatory subunits and confer a sensitivity of these channels to Ca2+ store depletion mediated by STIM1.
Non-SOCE
SOCE is considered the prominent mechanism for Ca2+ influx into neutrophils after Ca2+ pool discharge. However, it is becoming increasingly apparent that stimulation of cells not only activates SOCE but also promotes additional Ca2+ entry pathways. In addition to the InsP3 pathway, cyclic ADP-ribose (cADPr) appears to be required for sustained extracellular Ca2+ influx in neutrophils stimulated by fMLF [81, 82]. cADPr is formed in neutrophil granulocytes by the transmembrane glycoprotein CD38, which acts as an ADP ribosyl cyclase, converting NAD+ into cADPr [83]. Ca2+ influx is severely impaired by the specific cADPr antagonist, 8-bromo-cADPr, and in CD38 knockout mice [81]. Based on these observations and additional experiments, Partida-Sanchez et al. [81] postulated that cADPr directly activates Ca2+ influx by mobilizing the intracellular Ca2+ store through the ryanodine receptor. Later, the same group found functional differences in human neutrophils [82]. The high-affinity, fMLF-binding receptor induces a cADPr-independent Ca2+ response, whereas the low-affinity receptor related to the fMLF receptor [84] can trigger a Ca2+ response regulated by cADPr, which is primarly a result of extracellular Ca2+ influx [82]. Based on functional experiments with neutrophil-like HL-60 cells, another interpretation is provided by our lab: cADPr could support intracellular Ca2+ release by regulating extracellular Ca2+ entry through non-SOCE [85]. There is no consensus about mechanisms by which cADPr induces Ca2+ influx, but the possibility that cADPr is first hydrolyzed to ADPr [86] and then gates channels has recently attracted attention. Patch-clamp experiments in neutrophils provided evidence for a channel activation of the TRP family TRPM2 by intracellular ADPr [87, 88]. NAD+ is reported as an alternative stimulus for TRPM2 in addition to ADPr [88], where redox state interestingly mediates independent activation of this channel [89].
Other messengers resulting from diacylglycerol metabolism, including arachidonic acid [90], can cause a Ca2+ influx clearly distinct from SOCE in some cell types, which involves Orai channels [90]. As it has been demonstrated that diacylglycerol analogs cannot activate TRPC3 and TRPC6 channels through stimulation of PKC, diacylglycerol could itself regulate TRPC3 and TRPC6 directly [91]. Although there are an increasing number of reports supporting significant Ca2+ influx via non-SOCE (which are closely related to receptor-operated calcium entry) in neutrophils, intracellular signaling molecules that initiate this response have not formally been identified.
Ca2+ SIGNAL-MEDIATED NADPH OXIDASE ACTIVITY
As we described previously, the localization of superoxide anion production induced by antibody-opsonized Escherichia coli or zymosan particles differs from the one induced by G-protein-coupled chemoattractant receptors (fMLF). Although both pathways are Ca2+-dependent, the mechanism of Ca2+ entry required for intraphagosomal and extracellular superoxide anion production seems different. Complexities to concomitantly measure NADPH oxidase activity and [Ca2+]c variations with a high resolution make it difficult to analyze the link between both phenomena.
Quantitative measurements of NADPH oxidase activity and Ca2+ change
Different methods and instrumentations (spectroscopy, electrophysiology, chemiluminescence, Raman resonance imaging, electron spin resonance) have been developed to detect the production of ROS (see refs. [92,93,94] for review). Fluorescent dyes have an exquisite spatio-temporal resolution as well as a high dynamic range and are easy to use and cheap. NADPH oxidase activity is routinely measured by the conversion of nonfluorescent compounds to fluorescent counterparts. Dihydrorhodamine 123 (DHR) and more recently, Amplex Red are presented as the most sensitive and stable indicators of ROS production and are often used to assess NADPH oxidase activity. Although Amplex Red is membrane-impermeant, DHR can traverse the membrane and distributes in multiple cellular subcompartments. Although Amplex and DHR allow for reliable measurements of extracellular and global intracellular H2O2 production, both dyes are not suitable for measuring ROS formation restricted to phagosomes and their accumulation in granules during phagocytosis [31, 95,96,97,98]. Another probe, 2′,7′-dichlorodihydrofluorescein (DCDHF), does not redistribute between compartments and therefore, is an effective probe for monitoring kinetics of localized release of oxidative products within the forming phagocytic vacuoles in activated neutrophils [99]. The probe is firmly attached to microorganisms to target it to the specific phagosome. To monitor the oxidative activity during phagocytosis, DCDHF-labeled zymosan has been developed [100]. The major problem with this strategy is that myeloperoxidase, delivered to the phagosome, affects DCDHF-zymosan oxidation. This effect influences the non-linear relationship between fluorescence in the phagosome and NADPH oxidase activity. To overcome the dependence of peroxidase degranulation in the phagosome and to increase the specificity of the assays, measurement of DCDHF-zymosan oxidation during phagocytosis in the presence of extracellular peroxidase is required [100].
Similar techniques based on fluorescence indicators are also widely used to measure quantitative changes in [Ca2+]c [101, 102]. However, these probes cannot be selectively targeted to a specific cellular compartment without microinjection, and [Ca2+]c elevation can be generated artifactually as a result of the rapid diffusion of the probe-Ca2+ complex away from a more restricted region of the cytosol. Currently, genetically encoded indicators are developed to measure local Ca2+ changes occurring within specific cell compartments. In a detailed review, Demaurex [103] discusses design and use of these genetic Ca2+ indicators.
Despite some disadvantages characteristic to each probe, fluorescent dyes have remained the most reliable tools to monitor oxidative activity and [Ca2+]c elevation. In this context, Hallett’s group [100] provided convincing data about temporal and spatial Ca2+ signal-regulated phagocytosis in using DCDHF-labeled zymosan and fura-2 microinjection. Furthermore, several reports based on the use of fluorescent dyes underlined the requirement of Ca2+ influx in plasma membrane NADPH oxidase activity. Progress in the generation of new indicators will further improve the approach to image Ca2+ and NADPH oxidase; this will be utmostly conclusive in deciphering the calcium-related phenomena observed in specific cellular compartments of regions.
Regulation of chemoattractant-induced NADPH oxidase by Ca2+ influx
Strong evidence for Ca2+ dependence of NADPH oxidase activation came from deprivation of extracellular Ca2+ and depletion of intracellular Ca2+ stores [3,4,5,6,7]. Although the precise nature of [Ca2+]c elevation in fMLF-induced NADPH oxidase activation has not been resolved conclusively, evidence, based on the use of pharmalogical blockers of SOCE, exists to support a role of SOCE in the oxidative response. Although the specificity of 1-{β-[3-(4-methoxy-phenyl)propoxy]-4-methoxyphenethyl}-1H-imidazole hydrochloride (SK&F) 96365 and 2-aminoethoxydiphenyl borate (2-APB) remains questionable, N-propargylnitrendipine, MRS1845, represents a promising compound for the development of selective SOCs inhibitors. It possesses micromolar potency at SOCs in HL-60 cells [104] and is far more potent than traditional SOCE inhibitors. Unlike currently used SOC blockers, MRS1845 does not activate intracellular Ca2+ release at concentrations required to block SOCE. MRS1845 is able to suppress Ca2+ uptake triggered by fMLF and thapsigargin as well as NADPH oxidase activation at concentrations similar to those needed to suppress SOCE [105]. This apparent link between SOCE and NADPH oxidase is supported by data of Lee et al. [106], relating a modulation of Ca2+ entry and attenuation of NADPH oxidase activity in neutrophils that have been subjected to MRS1845. NADPH oxidase activity is also inhibited by the pyrazole derivative N-{4-[3,5-bis(trifluoromethyl)-1H-pyrazol-1-yl]phenyl}-4-methyl-1,2,3-thiadiazole-5-carboxamide (BTP2), a new inhibitor of SOCE described in neutrophils [107], to the same level as in the absence of extracellular Ca2+. BTP2 has no effect on PMA-stimulated NADPH oxidase, indicating a specific effect of BTP2 for the Ca2+ dependence of NADPH oxidase activation. These findings confirm that Ca2+ uptake via SOCE is required for NADPH oxidase activation.
On the other hand, Itagaki et al. [108] suggest that Ca2+ influx occurring through a mechanism other than SOCE could be a relevant event to activate the oxidative response. This conclusion is based on multiple observations made in this study. For example, when lysophosphatidic acid is applied to HL-60 cells in Ca2+-free medium, no store depletion is observed, but Ca2+ influx is detected immediately after readdition of external Ca2+. Further, lysophosphatidic acid has stimulatory effects on NADPH oxidase in a concentration-dependent manner. Taken together, these results provide strong evidence for the involvement of two separate Ca2+ signaling pathways in NADPH oxidase regulation but no direct correlation between non-SOCE or SOCE, and NADPH oxidase has yet been formally established.
The contribution of transient [Ca2+]c elevation in NADPH oxidase activation varies with applied stimulus classes and within the seven transmembrane-spanning G-protein-coupled receptor family. Activation of neutrophils with PAF and fMLF is accompanied by a transient [Ca2+]c elevation, which is of similar magnitude for each activator. In both cases, the peak of [Ca2+]c is followed by a gradual decrease in [Ca2+]c [109]. Although fMLF and the PAF allow a Ca2+ mobilization, the functional response elicited by these chemoattractants is distinctly different. Although fMLF provokes NADPH oxidase activation, exposure to PAF does not induce ROS generation. This difference is attributed to the coupling of both chemoattractants to two distinct G proteins and consequently, to different signals of transduction [110]. Therefore, a transient [Ca2+]c elevation is not in any way linked to the generation of a NADPH oxidase-activating signal from G-protein-coupled receptors. An increase of [Ca2+]c is accordingly not sufficient to initiate NADPH oxidase activation in the plasma membrane; an additionnal signal in combination with Ca2+ influx is almost certainly required for NADPH oxidase activation [3].
Ca2+-regulated NADPH oxidase during phagocytosis
Sources of Ca2+ involved in superoxide anion production in the phagosome are not yet determined. As elevation of periphagosomal Ca2+ is not affected by BTP2, SOCs do not seem to participate in phagocytic stimuli-mediated Ca2+ influx in contrast to those induced by chemoattractants (Fig. 1).
Through binding to β2integrin molecules, complement component, C3bi-opsonized zymosan particles mediate Ca2+ signals and subsequent NADPH oxidase activation during phagocytosis in neutrophils. The work of Hallett’s group [100] permitted resolving of Ca2+ signals into two temporally separated phases and clarified the role of [Ca2+]c change in NADPH oxidase activation during phagocytosis. First, the global Ca2+ signal is occurring during integrin engagement at the point of contact between the particle and the cell and is subsequent to an intracellular Ca2+ store release near the plasma membrane. Based on their observations, Dewitt et al. [100, 111] proposed that a Ca2+ influx, triggering a global [Ca2+]c change, is responsible for increased mobility of β2 integrins distant from the contact site, which in turn activates calpain. Subsequently, activated calpain releases distant β2 integrin from their tethers and allows their diffusion to the contact site to complete the phagocytic event.
Some Ca2+ stores are located at the periphery of phagocytic vesicles [112] and are distinct from those implicated by G-protein-coupled receptors, such as fMLF [113, 114]. The identity of the intracellular messenger responsible for the release of these peripheral Ca2+ stores remains unknown. Although InsP3 can diffuse rapidly in the cytosol [115], it is not certain that this fMLF-mediated phosphoinositide metabolite can trigger integrin-mediated store depletion. A second global [Ca2+]c change is occurring at the time of phagosomal closure and is temporally correlated with the activation of NADPH oxidase. Such a conclusion has been proposed by Dewitt and Hallett [111] following the development of a micromanipulation technique in which C3bi-opsonized particles are presented to neutrophils, coupled to a microscopic detection of intraphagosomal particles labeled with an oxidant-sensitive probe. Activation of the NADPH oxidase is triggered locally in the phagosome, but the second Ca2+ signal is not restricted to the region of the phagosome. Indeed, localized store depletion may generate a diffusible signal (calcium influx factor), gating Ca2+ channels in the plasma membrane distant from the initial contact site, and Ca2+ influx may thus occur across the entire neutrophil surface. In the same study, the authors demonstrated that extracellular Ni2+, used as a blocker of Ca2+ influx, and a phosphoinositide-3-kinase inhibitor (LY294002) prevented the β2 integrin-triggered global Ca2+signal. Blockade of Ca2+ influx is accompanied by a slowed phagocytosis and a decrease of NADPH oxidase activity. Thus, global changes of Ca2+ resulting from Ca2+ influx are necessary but insufficient to activate the NADPH oxidase during phagocytosis; other slower events are required, probably including phosphatidylinositol 3,4,5 triphosphate (PIP3) formation in the phagosomal membrane and its binding to the phox homology domain of phox proteins [116]. Furthermore, it is demonstrated that PIP3 accumulation and anchoring at the phagocytic membrane are prerequisites for the generation of Ca2+ signaling [117]. Exact mechanisms linking PIP3 formation to Ca2+ signals are not yet known, but PIP3 may activate a PLC isoform triggering InsP3 production [118]. It is also possible, as described in platelets, that PIP3 regulates a novel pathway of Ca2+ entry, which is independent of an increase of PLC activity [119] and which would sustain NADPH oxidase activity during phagocytosis.
Potential targets regulated by Ca2+ changes
Ca2+-regulated PKC
PKC, a phospholipid-dependent family of serine-threonine protein kinases, acts in multiple signal transduction pathways including the regulation of the NADPH oxidase. At least 12 different PKC isoforms have been characterized so far, and these can be grouped into the following three subgroups: conventional PKCs (α, βI, βII, and γ); novel PKCs (δ, ε, μ, θ, and η), and atypical PKCs (ζ and τ/λ), on the basis of their molecular structure and the requirement of Ca2+ for activation (conventional) and diacylglycerol-binding activity (conventional and novel). Conventional and novel PKCs are directly activated by phorbol esters (PMA), potent activators of NADPH oxidase acting as analogs of diacylglycerol, and atypical isoforms are insensitive to PMA [120,121,122,123]. Most of the data supports the contention that [Ca2+]c does not change during PMA stimulation, and NADPH oxidase activation is independent of the extracellular Ca2+ concentration [124,125,126,127,128]. In disagreement with the fact, largely reported, that PMA stimulates NADPH oxidase independently of Ca2+ influx, some studies demonstrate that PMA-activated NADPH oxidase is decreased significantly in the absence of extracellular Ca2+ [129]. However, this Ca2+ dependency is only observed on adherent neutrophils stimulated at low cell density, reinforcing the plausible impact of the granulocyte cellular environment in selecting distinct, Ca2+-dependent transduction pathways.
In neutrophils and HL-60 cells differentiated into neutrophil-like cells, α, β, δ, and ζ appear to be the main PKC isotypes involved in NADPH oxidase activation by most physiologic agonists, as shown by studies using antisense strategy, pharmacological inhibitors, or knockout [105, 123, 122, 130,131,132]. However, their respective contribution in regulating NADPH oxidase has not been well documented yet. Conventional PKCs are pointed out as participants for regulating NADPH oxidase in neutrophils. By down-regulating the enzyme activity through an antisense oligonucleotide strategy, Korchak et al. [105] established that PKCβ and -α are required for a full fMLF- and PMA-mediated superoxide anion production in neutrophil-like HL-60 cells. Consistent with this assumption, PKCβ antisense inhibits phosphorylation and translocation of p47phox induced by fMLF, providing evidence for the involvement of PKCβ in the signaling pathway leading to fMLF- and PMA-mediated NADPH oxidase activation. In addition to PKCα, PKCβII expressed in human neutrophils can phosphorylate p47phox and induce its translocation and NADPH oxidase activation [133]. Thus, downstream effects of conventional, Ca2+-activated PKCs include a direct phosphorylation of p47phox, which leads to membrane translocation of cytosolic components (Fig. 1).
Ca2+-regulated cytosolic PLA2 (cPLA2)
Many agonists that stimulate superoxide anion production in phagocytic cells cause the release of arachidonic acid from membrane phospholipids via the hydrolysis of fatty acids from the sn-2 position of membrane phospholipids by PLA2 [134,135,136]. Several PLA2 isoforms have been described including a cPLA2, which is activated rapidly by increased [Ca2+]c. Stimulation of cells induces an immediate and transient translocation of cPLA2 to nuclear membranes [137]. Evidence that cPLA2 is recruited at the plasma membrane comes from an intracellular distribution study of cPLA2 in neutrophils and granulocyte-like PLB-985 cells [138]. Although underlying mechanisms are not totally understood, it is known that cPLA2 is involved in the regulation of phagocytic cell superoxide anion production. In response to a variety of soluble and particulate stimuli, NADPH oxidase activation is impaired in cPLA2-deficient, differentiated PLB-985, but addition of arachidonic acid is able to rescue NADPH oxidase activity [139, 140]. Subsequently, Pessach et al. [141] described a normal translocation of oxidase cytosolic components in activated, differentiated PLB-985 cells lacking cPLA2 or in neutrophils treated with cPLA2 inhibitors [141]. Thus, cPLA2 is not required for translocation of cytosolic factors to membranes [139, 140]. Taken together, these data have a substantial implication: cPLA2 serves a critical role in oxidase activation after the assembly of enzyme complex in neutrophils. Arachidonic acid may mediate structural changes in NADPH oxidase component triggering interaction between oxidase subunits or affecting the function of flavocytochrome b558 [142]. Another alternative idea puts forward the fact that arachidonic acid might be a cofactor enhancing the affinity of the assembled NADPH oxidase for NADPH, probably via induction of structural changes [138].
As cPLA2 deficiency is associated with profound effects on NADPH oxidase activity, cPLA2 activation mechanisms by Ca2+ mobilization need to be investigated further. A limited number of studies focused on this signaling pathway in a variety of cells. Indeed, it is shown that translocation of cPLA2 is dependent on [Ca2+]c elevation [143, 144], and PKCα is required for cPLA2 activity [145], supporting evidence that Ca2+ regulates NADPH oxidase through the PKC/cPLA2/arachidonic acid pathway. However, no clear proof exists for such a regulation in neutrophils, and further studies are needed to elucidate the exact involvement of cPLA2 in NADPH oxidase activity of these cells.
Ca2+-regulated S100 proteins
In the present nomenclature, gp91phox homologous NADPH oxidase found in non-phagocytic cells is referred to as Nox enzymes (gp91phox is specified as Nox2). Five Nox proteins are described in humans with distinct tissue distribution. Nox5, essentially expressed in lymphoid tissues and in testis [146], is distinguished from the other Nox proteins by an additional N-terminal extension containing three canonical and one noncanonical EF-hand motifs. These four calcium-binding sites allow Nox5 to be sensitive to the presence of Ca2+. A recent study demonstrated that [Ca2+]c elevation triggers a conformational change in the Nox5 N terminus, leading to NADPH oxidase activation through an interaction between the regulatory N terminus and C terminal catalytic domain [147]. In contrast to Nox5, no EF-hand domains have been found in Nox2. However, a potential role of Ca2+ in NADPH oxidase activation via S100A8 and S100A9 has been found in granulocytes [148]. In human neutrophils, Lemarchand et al. [149] described that the translocation of myeloid-related proteins, belonging to the family of S100 proteins, namely S100A8 and S100A9, is dependent on extracellular Ca2+. Moreover, the absence of S100A8 recruitment to the plasma membrane correlated with a decrease in superoxide anion production, which supports a role for this S100 protein in the potentialization of the oxidative response [149]. Elevation of [Ca2+]c by ionomycin results in a change of the phosphorylation level of S100A9, and identical observations have been made following neutrophil exposure to different stimuli (chemotactic peptide or phorbol ester) [150], hence the translocation process in relation to the phosphorylation status of Thr113 (penultimate amino acid of the C-terminal part in S100A9) [151] is questionable. Recently, attention has been directed to p38 MAPK as a potential protein kinase mediating S100A9 phosphorylation, and some experimental findings support this suggestion: Stimulation with fMLF markedly enhances phosphorylation of S100A9, and pretreatment with p38 MAPK inhibitor SB203580 abolishes fMLF-induced phosphorylation, indicating that S100A9 (although in a heterodimeric complex with S100A8) is a direct substrate of p38 MAPK in human neutrophils; and in the presence of p38 MAPK, S100A9 peptides that contain Thr113 show an increase in mass [152]. As fMLF stimulation mediates a significant increase in S100A9 localized to the base of lamellipodia and as phosphorylated S100A8/S100A9 associates with F-actin, it can be assumed that phosphorylation results in S100A9 translocation. Biochemical analysis and confocal microscopic analysis established that S100A9 translocates to the plasma membrane, secretory vesicle, and gelatinase granules dependently on p38 MAPK-mediated phosphorylation. Although the mechanism of S100 protein translocation is not yet fully understood, phosphorylation is clearly involved in this process [152]. When an extracellular, Ca2+-independent stimulus is used, S100A9 proteins, which are markedly phosphorylated, and S100A8 proteins, which are phosphorylated weakly, do not translocate [153], indicating that phosphorylation is strictly dependent on Ca2+ influx. Subsequent to Ca2+ fixation, a heterocomplex is formed by a noncovalent association of Ca2+-binding proteins S100A8 and S100A9 [154, 155]. Using an immunofluorescence approach, it has been reported that in bovine neutrophils stimulated by PMA [156], these S100 proteins are concentrated under the plasma membrane with cytosolic phox proteins. A similar observation is made for S100A8 in human neutrophils [155]. S100A8/S100A9 heterodimers appear to interact preferentially with p67phox and might favor the organization of the cytosolic factors and enhance their recruitment to the membrane-bound flavocytochrome b558, acting as scaffold proteins (Fig. 1). Berthier et al. [157] confirmed, in a semirecombinant, cell-free system, increased affinity of p67phox for flavocytochrome b558 inferred by S100A8/S100A9. According to these authors, S100 proteins interact directly with flavocytochrome b558 and mediate its transition from an inactive to an active conformation state, resulting in NADPH oxidase activation. Preincubation of S100A8/S100A9 in the absence of Ca2+ led to an interaction with flavocytochrome b558 but not to a conformational change, supporting the fact that Ca2+, through S100 binding, is required for NADPH oxidase activity [157]. Determination of specific interactions of the S100 complex with flavocytochrome b558 by atomic force microscopy [157] has been confirmed later by the same group using different experimental systems. A combination of ex-vivo and in-vitro methods followed by flavocytochrome b558 purification provides evidence for the activation of flavocytochrome b558 by the recombinant S100 complex in the absence of phox cytosolic factors and arachidonic acid [158]. On the other hand, protein–protein interaction studies based on pull-down assays in a semirecombinant system similar to the one of Berthier et al. [157] reveal that p67phox and Rac proteins might interact with the S100 complex [159]. This controversy about the site of S100 complex interaction could be explained by the use of different neutrophil model systems and different methods to investigate protein interactions. However, the transition of cytochrome b558 to an activated state appears to be a result of the binding of the S100 complex.
NADPH oxidase is also dependent on cPLA2-mediated arachidonic acid formation, which binds reversibly with high affinity to a heterocomplex formed by S100A8 and S100A9 [156]. By adding S100A8/S100A9 complexes to a semi-recombinant system comprising bovine neutrophil membranes, cytosolic phox proteins, GTPγS-loaded Rac2, and the optimal amount of arachidonic acid, Kerkhoff’s and Doussière’s groups [159, 160] demonstrated that binding of arachidonic acid to the S100 heterodimer is a critical step to promote NADPH oxidase activation. Mutant S100A8/S100A9 complexes, unable to bind arachidonic acid, only exhibit a slight variation of oxidase activity in comparison with wild-type recombinant S100 complexes. S100A8/S100A9 complexes facilitate NADPH oxidase activation by transferring arachidonic acid to the NADPH oxidase complex [159]. In fact, S100 proteins binding to arachidonic acid may increase NADPH oxidase activity by decreasing the deactivation rate [160]. Thus, S100 protein translocation to the plasma membrane together with p67phox, p47phox, and Rac supports the hypothesis that these proteins might deliver bound arachidonic acid in a Ca2+-dependent manner to the NADPH oxidase [157].
Ca2+-regulated Rac activation
First, evidence that activation of the monomeric G protein in neuronal cells is dependent on [Ca2+]c has been reported by Farnsworth et al. [161]. They proposed that the exchange factor Ras-guanine nucleotide-releasing factor activity is regulated by Ca2+ mobilization through a mechanism involving the Ca2+-calmodulin complex. Later, Valentin et al. [4] determinated that Ca2+ influx plays a primordial role in plasma membrane translocation of Rac (Fig. 1) by demonstrating that extracellular Ca2+ deprivation abolishes Rac membrane recruitment stimulated by fMLF in differentiated HL-60 cells. A calmodulin antagonist does not induce variations of Rac translocation stimulated by fMLF; in these conditions, the mechanism might be different than the one described by Farnsworth et al. [161]. By studying the localization of Rac in human prostate carcinoma cells following activation with thapsigargin, Price et al. [162] established that [Ca2+]c elevation induces a temporary increase in Rac translocation to the plasma membrane, emphasizing the fact that this phenomenon is promoted by extracellular Ca2+ entry (Fig. 1). These authors proposed that the [Ca2+]c rise triggers PKC-dependent Rho-GDI phosphorylation, leading to Rac dissociation of the Rac-Rho-GDI complex and subsequent translocation of Rac to the plasma membrane. Rac translocation is correlated with its activation [163], but it appears likely that both events are regulated independently. The activation of Rac by a chemoattractant has been reported to be independent on [Ca2+]c changes, but relevant experiments have been performed in Ca2+-free buffer [164].
Using bone marrow-derived neutrophils from RhoG knockout mice, Condliffe et al. [165] showed a reduction of fMLF-induced oxidant production but an unaltered response to PMA and opsonized zymosan. This reduction of oxidase activation is associated with a partial decrease of Rac protein activation. The authors hypothesized that RhoG acts on a subset of Rac required for targeted oxidase assembly or for determining its precise cellular localization. In spite of this, the precise target of calcium ions remains unknown, but it is possible that Ca2+ influx may impact on Rac activity through the regulation of RhoG.
REGULATION OF Ca2+ INFLUX BY ACTIVATION OF THE NADPH OXIDASE
Activation of the NADPH oxidase is associated with the electrogenic transfer of electrons to molecular oxygen across the plasma membrane, generating a decrease in membrane potential. Accumulation of negative charges on one side of the membrane by activated neutrophils would turn off further electron transfer, prematurely interrupting the killing process of pathogens. To preserve electroneutrality and to allow for the extrusion of the intracellular acid released in the cytosol during the hydrolysis of NADPH and its resynthesis by the hexose monophosphate shunt, the most efficient mechanism would be to extrude H+ ions from the cells through a proton pathway [166, 167]. Proton channels responsible for H+ efflux were originally proposed by the group of Lydia Henderson [168] to be contained within the gp91phox subunit of NADPH oxidase. However, the experimental evidence regarding the H+ channel function of gp91phox remains contradictory, and a body of evidence indicates that a protein other than the transmembrane oxidase subunit gp91phox could act as a voltage-gated proton channel (for a review, see ref. [169]).
As two depolarizing agents, KCl and a pore-forming ionophore gramicidin D, reduced the [Ca2+]c increase caused by fMLF, it has been suggested that plasma membrane depolarization in human neutrophils is a physiological feedback mechanism inhibiting [Ca2+]c changes [170]. Later, effects of fMLF on SOCE were investigated in neutrophil granulocytes of patients suffering from chronic granulomatous disease, who possess a deficient NADPH oxidase activity and an attenuation of depolarization following fMLF stimulation. By using an indirect Mn2+/fura-2 fluorescence-quenching procedure, accelerated Ca2+ influx has been observed in these cells, suggesting that depolarization impairs uncontrolled Ca2+ influx in triggering early attenuation of store-operated Ca2+ uptake and restricting Ca2+ influx [171, 172]. Failure of depolarization in chronic granulomatous disease is associated with Ca2+ overload as a result of accelerated influx of the cation and hyperactivity of several proinflammatory activities of these cells [173]. The correlation between inhibition of depolarization and Ca2+ influx through opened SOCs [174] is supported by experiments in genetically modified neutrophilic cell lines unable to produce superoxide anions but possessing SOCE mechanism. More evidence involving NADPH oxidase activation in the restriction of SOCE in fMLF-activated neutrophils is derived from experiments using a selective inhibitor of NADPH oxidase, diphenyleneiodonium. This agent accelerates the rates of membrane repolarization and SOCE by preventing plasma membrane depolarization [173,174,175]. Furthermore, K+ ionophore valinomycin, which allowed increased charge compensation, decreased depolarization-potentiated, chemoattractant-mediated [Ca2+]c. The enhanced Ca2+ permeability across the plasma membrane could be explained again by an increase of the driving force for Ca2+. This notion is supported by experiments where a decrease in the concentration of external Ca2+ added to thapsigargin-pretreated cells from 500 to 50 μM resulted in a measurable impairment of Ca2+ entry [171]. The general hypothesis is that when the cells are depolarized, the driving force for Ca2+ influx is markedly decreased as a result of elimination of the electrical component of the electrochemical gradient for Ca2+. The driving force for Ca2+ ions as a result of the extensive depolarization following stimulation is certainly ample enough to cause detectable diminution of Ca2+ influx by itself.
NADPH oxidase regulates neutrophil Ca2+ influx not only via its electrogenic activity but also as a consequence of ROS generation. This phenomenon is well documented by the recent work of Tintinger et al. [176], which focused on the effects of neutrophil-derived ROS on SOCE. Treatment of cells with catalase potentiates the rate and the magnitude of store-operated uptake of Ca2+ [176]. Furthermore, inhibitors of myeloperoxidase (enzyme catalazing the formation of HOCl) shorten the time to onset of Ca2+ influx, prolong the linear phase of influx, and increase the magnitude of store-operated uptake of Ca2+. A putative target of derived ROS has been identified, linking the redox state to Ca2+ homeostasis. The non-selective cation channel TRPM2, a member of the TRP family, is recognized to have an oxidative sensitivity and be positively regulated by H2O2. In TRPM2-transfected HEK-293 cells, Ca2+ is increased rapidly by H2O2 stimulation, suggesting the possibility that oxidative stress mediates influx of Ca2+ through TRPM2 in granulocytes [177].
NADPH oxidase also has a physiological relevance in controlling Ca2+ influx in neutrophils. It seems to fulfill a physiologically important, anti-inflammatory function by preventing a Ca2+ overload and hyper-reactivity of neutrophils. An activation of NADPH oxidase activity could constitute an interesting pharmacological strategy. However, NADPH oxidase is also potentially involved in the activation of the Ca2+ pathway, and the potentialization of its activity may be accompanied by a risk of oxidant-mediated toxicity.
Ca2+ CHANNELS AS PHARMACOLOGICAL TARGETS
Neutrophilic lung inflammation is an essential component of host defense against various pathogens. During activation, neutrophils release ROS, triggering the inactivation of protease inhibitors, which protect lung tissue from proteolytic damage. Infiltration and persistent presence of neutrophils in response to chronic inflammatory airway diseases, such as chronic obstructive lung disease, asthma, cystic fibrosis, and bronchiolitis, aggravate lung tissue damage observed in these diseases. Few available therapeutic agents efficiently down-regulate neutrophil proinflammatory activity by reducing local neutrophil infiltration. However, neurophils are reported to be relatively insensitive to such chemotherapeutic strategies, and development of alternative treatment is undoubtely required.
Given the critical involvement of [Ca2+]c elevation in the regulation of ROS generation and thus, in the proinflammatory activities of neutrophils, Ca2+ channel inhibition holds considerable therapeutic promise. TRP and Orai channels, emerging candidates for store-operated and nonstore-operated channels, may be potential drug targets, and their inhibition might offer anti-inflammatory, therapeutical benefits for treating pulmonary diseases responsible for progressive lung degradation (see Tintinger and Steel [178] for review).
A diversity of inorganic channel inhibitors (lanthanides, divalent cations, Gd3+) or organic SOCE inhibitors, including channel blockers (SK&F 96365, 2-APB), has been described (reviewed by Putney [179]). In most instances, the exact mechanisms by which they interfere with SOCs remain to be established, and nonspecific effects as a result of interference with other mechanisms important for Ca2+ signals, such as Ca2+ pumps, mitochondrial Ca2+ homeostasis, Ca2+ release, and activity of K+ channels, cannot be excluded. For example, 2-ABP has been shown to potentiate and block Ca2+ entry into cells [180], and it inhibits InsP3 receptor-mediated Ca2+ release [181]. Similar antagonistic effects are observed with SK&F 96365 [182]; also, an activation of intracellular Ca2+ depletion in various cell types at different concentrations can be observed using those inhibitors [183]. Recently, it was been documented that a pyrazole derivative, named BTP2, inhibits SOCE in human neutrophils [107], potentially by interfering with TRPC channels from the extracellular space [184]. Preincubation of BTP2 clearly reduces superoxide anion production, having no effect on phagocytosis or intraphagosomal radical production [107]. These findings make SOCs excellent targets for down-regulating the inflammatory response without impairing bacterial killing. Similarly, MRS1845 seems to represent a new, promising candidate for selective SOC inhibitors and could be used as a lead for drug design [104, 105].
Other pharmacological drugs, such as tenidap [5-chloro-2,3-dihydro-3-(hydroxy-2-thienylmethylene)-2-oxo-1H-indole-1-carboxamide], could also be used as an inhibitor of the Ca2+ influx pathway or channels in nonexcitable cells, as they are known to modify thapsigargin-induced Ca2+ entry across the plasma membrane [185]. Given the importance of Ca2+ influx in NADPH oxidase activation, it is attractive to speculate that tenipad is able to alter the functioning of this enzyme that mediates inflammatory responses. In agreement with this hypothesis, tenidap has been shown to attenuate superoxide anion production by activated neutrophils [186]. However, tenidap has no effect on the generation of superoxide anions by NADPH oxidase reconstituted from fractionated neutrophil lysates; only inhibition of xanthine oxidase is observed [187]. Similar conclusions are provided with the imidazole antimycotic, itraconazole: Interference with SOCE into human neutrophils does not appear to affect the NADPH oxidase [188].
One of the major difficulties for the development of anti-inflammatory drugs targeting TRP channels is linked to the expression of TRPC in many different cell types. Any potential drugs might be associated with nondesired effects if not applied strictly, locally. Meaningful progress can be obtained by developing alternative strategies, in which drugs may be devolved, which are directed against the activation mechanisms upstream of TRP channels. Some of these mechanisms might be sufficiently unique to allow a specific targeting of neutrophils as observed in TRPM2 activation [189].
CONCLUSION
Evidence is provided that SOCE plays a fundamental role in host defense by regulating the oxidative response in human neutrophils. Several targets of Ca2+ ions involved in NADPH oxidase control are resolved, such as PKCα, PKCβII, S100 proteins, or cPLA2, but further insight is needed to identify other pathway-activated NADPH oxidase and apparently sensitive Ca2+ influx, particularly in view of the influence of the granulocyte cellular microenvironment. It appears that the latter may selectively modify the transduction pathways associated with NADPH oxidase, although Ca2+ influx may represent the ab initio signal for superoxide anion secretion. The discovery of a selectively targeted transduction intersection in Ca2+-dependent NADPH oxidase activation might allow for selection of specific inhibitors that modify this signaling process and thus, might be therapeutically useful in chronic and acute inflammatory diseases engendered by an excessive activation of the NADPH oxidase. However, it must be stressed that the mechanism of SOCE is surrounded by multiple contradictions, but some important Ca2+ routes have been resolved, where TRPC, Orai1, and STIM1 are important actors that demonstrated their clear involvement in NADPH oxidase circuitous control. However, as a result of some of these inconsistencies, therapeutic approaches could not be exploited adequately for now. Dissection of the mechanism linking Ca2+ store depletion to SOCs channel activation as well as the nature of these Ca2+ channels might help to characterize the regulation of superoxide anion release and serve as targets for pharmacological drug development in inflammatory diseases.
Acknowledgments
This study was supported by the University of Luxembourg.
References
- Berridge M J, Lipp P, Bootman M D. The versatility and universality of calcium signaling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- Putney J W., Jr Recent breakthroughs in the molecular mechanism of capacitative calcium entry (with thoughts on how we got here) Cell Calcium. 2007;42:103–110. doi: 10.1016/j.ceca.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foyouzi-Youssefi R, Petersson F, Lew D P, Krause K H, Nusse O. Chemoattractant-induced respiratory burst: increases in cytosolic Ca2+ concentrations are essential and synergize with a kinetically distinct second signal. Biochem J. 1997;322:709–718. doi: 10.1042/bj3220709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin F, Bueb J-L, Capdeville-Atkinson C, Tschirhart E. Rac-1-mediated O2– secretion requires Ca2+ influx in neutrophil-like HL-60 cells. Cell Calcium. 2001;29:409–415. doi: 10.1054/ceca.2001.0203. [DOI] [PubMed] [Google Scholar]
- Granfeldt D, Samuelsson M, Karlsson A. Capacitative Ca2+ influx and activation of the neutrophil respiratory burst. Different regulation of plasma membrane- and granule-localized NADPH-oxidase. J Leukoc Biol. 2002;71:611–617. [PubMed] [Google Scholar]
- Karlsson A, Dahlgren C. Assembly and activation of the neutrophil NADPH oxidase in granule membranes. Antioxid Redox Signal. 2002;4:49–60. doi: 10.1089/152308602753625852. [DOI] [PubMed] [Google Scholar]
- Seguchi H, Kobayashi T. Study of NADPH oxidase-activated sites in human neutrophils. J Electron Microsc (Tokyo) 2002;51:87–91. doi: 10.1093/jmicro/51.2.87. [DOI] [PubMed] [Google Scholar]
- Pozzan T, Lew D P, Wollheim C B, Tsien R Y. Is cytosolic ionized calcium regulating neutrophil activation? Science. 1983;221:1413–1415. doi: 10.1126/science.6310757. [DOI] [PubMed] [Google Scholar]
- Gallois A, Bueb J-L, Tschirhart E J. Effect of SK&F 96365 on extracellular Ca2+-dependent O2– production in neutrophil-like HL-60 cells. Eur J Pharmacol. 1998;361:293–298. doi: 10.1016/s0014-2999(98)00728-6. [DOI] [PubMed] [Google Scholar]
- Dahlgren C, Karlsson A. Ionomycin-induced neutrophil NADPH oxidase activity is selectively inhibited by the serine protease inhibitor diisopropyl fluorophosphate. Antioxid Redox Signal. 2002;4:17–25. doi: 10.1089/152308602753625816. [DOI] [PubMed] [Google Scholar]
- Lundqvist H, Karlsson A, Follin P, Dagher M C, Sjölin C, Dahlgren C. Phagocytosis following translocation of the neutrophil b-cytochrome from the specific granule to the plasma membrane is associated with an increased leakage of reactive oxygen species. Scand J Immunol. 1992;36:885–891. doi: 10.1111/j.1365-3083.1992.tb03151.x. [DOI] [PubMed] [Google Scholar]
- Thastrup O, Dawson A P, Scharff O, Foder B, Cullen P J, Drobak B K, Bjerrum P J, Christensen S B, Hanley M R. Thapsigargin, a novel molecular probe for studying intracellular calcium release and storage. Agents Actions. 1989;27:17–23. doi: 10.1007/BF02222186. [DOI] [PubMed] [Google Scholar]
- Lambeth J D. Regulation of the phagocyte respiratory burst oxidase by protein interactions. J Biochem Mol Biol. 2000;33:427–439. [Google Scholar]
- Babior B M, Lambeth J D, Nauseef W. The neutrophil NADPH oxidase. Arch Biochem Biophys. 2002;397:342–344. doi: 10.1006/abbi.2001.2642. [DOI] [PubMed] [Google Scholar]
- Bokoch G M, Diebold B A. Current molecular models for NADPH oxidase regulation by Rac GTPase. Blood. 2002;100:2692–2696. doi: 10.1182/blood-2002-04-1149. [DOI] [PubMed] [Google Scholar]
- Groemping Y, Rittinger K. Activation and assembly of the NADPH oxidase: a structural perspective. Biochem J. 2005;386:401–416. doi: 10.1042/BJ20041835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard F R, Kelher M R, Moore E E, McLaughlin N J, Banerjee A, Silliman C C. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- Nobuhisa I, Takeya R, Ogura K, Ueno N, Kohda D, Inagaki F, Sumimoto H. Activation of the superoxide-producing phagocyte NADPH oxidase requires co-operation between the tandem SH3 domains of p47phox in recognition of a polyproline type II helix and an adjacent α-helix of p22phox. Biochem J. 2006;396:183–192. doi: 10.1042/BJ20051899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueyama T, Tatsuno T, Kawasaki T, Tsujibe S, Shirai Y, Sumimoto H, Leto T L, Saito N. A regulated adaptor function of p40phox: distinct p67phox membrane targeting by p40phox and by p47phox. Mol Biol Cell. 2007;18:441–454. doi: 10.1091/mbc.E06-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaissiere C, Le Cabec V, Maridonneau-Parini I. NADPH oxidase is functionally assembled in specific granules during activation of human neutrophils. J Leukoc Biol. 1999;65:629–634. doi: 10.1002/jlb.65.5.629. [DOI] [PubMed] [Google Scholar]
- Yuo A, Kitagawa S, Kasahara T, Matsushima K, Saito M, Takaku F. Stimulation and priming of human neutrophils by interleukin-8: cooperation with tumor necrosis factor and colony-stimulating factors. Blood. 1991;78:2708–2714. [PubMed] [Google Scholar]
- Daniels R H, Finnen M J, Hill M E, Lackie J M. Recombinant human monocyte IL-8 primes NADPH-oxidase and phospholipase A2 activation in human neutrophils. Immunology. 1992;75:157–163. [PMC free article] [PubMed] [Google Scholar]
- Wozniak A, Betts W H, Murphy G A, Rokicinski M. Interleukin-8 primes human neutrophils for enhanced superoxide anion production. Immunology. 1993;79:608–615. [PMC free article] [PubMed] [Google Scholar]
- Condliffe A M, Hawkins P T, Stephens L R, Haslett C, Chilvers E R. Priming of human neutrophils superoxide generation by tumor necrosis factor-α is signaled by enhanced phosphatidylinositol 3,4,5-trisphosphate but not inositol 1,4,5-trisphosphate accumulation. FEBS Lett. 1998;439:147–151. doi: 10.1016/s0014-5793(98)01358-1. [DOI] [PubMed] [Google Scholar]
- Dang P M, Dewas C, Gaudry M, Fay M, Pedruzzi E, Gougerot-Pocidalo M A, El Benna J. Priming of human neutrophil respiratory burst by granulocyte/macrophage colony-stimulating factor (GM-CSF) involves partial phosphorylation of p47(phox) J Biol Chem. 1999;274:20704–20708. doi: 10.1074/jbc.274.29.20704. [DOI] [PubMed] [Google Scholar]
- Sheppard F R, Kelher M R, Moore E E, McLaughlin N J, Banerjee A, Silliman C C. Structural organization of the neutrophil NADPH oxidase: phosphorylation and translocation during priming and activation. J Leukoc Biol. 2005;78:1025–1042. doi: 10.1189/jlb.0804442. [DOI] [PubMed] [Google Scholar]
- Finkel T H, Pabst M J, Suzuki H, Guthrie L A, Forehand J R, Phillips W A, Johnston R B., Jr Priming of neutrophils and macrophages for enhanced release of superoxide anion by the calcium ionophore ionomycin. Implications for regulation of the respiratory burst. J Biol Chem. 1987;262:12589–12596. [PubMed] [Google Scholar]
- Lloyds D, Hallett M B. Development of oxidase “priming” in maturing HL60 cells: correlation with protein expression and tyrosine phosphorylation. Biochim Biophys Acta. 1995;1267:65–71. doi: 10.1016/0167-4889(95)00031-m. [DOI] [PubMed] [Google Scholar]
- Silliman C C, Elzi D J, Ambruso D R, Musters R J, Hamiel C, Harbeck R J, Paterson A J, Bjornsen A J, Wyman T H, Kelher M, England K M, McLaughlin-Malaxecheberria N, Barnett C C, Aiboshi J, Bannerjee A. Lysophosphatidylcholines prime the NADPH oxidase and stimulate multiple neutrophil functions through changes in cytosolic calcium. J Leukoc Biol. 2003;73:511–524. doi: 10.1189/jlb.0402179. [DOI] [PubMed] [Google Scholar]
- Koenderman L, Yazdanbakhsh M, Roos D, Verhoeven A J. Dual mechanisms in priming of the chemoattractant-induced respiratory burst in human granulocytes. A Ca2+-dependent and a Ca2+-independent route. J Immunol. 1989;142:623–628. [PubMed] [Google Scholar]
- Brechard S, Bueb J-L, Tschirhart E J. Interleukin-8 primes oxidative burst in neutrophil-like HL-60 through changes in cytosolic calcium. Cell Calcium. 2005;37:531–540. doi: 10.1016/j.ceca.2005.01.019. [DOI] [PubMed] [Google Scholar]
- MacKinnon A C, Buckley A, Chilvers E R, Rossi A G, Haslett C, Sethi T. Sphingosine kinase: a point of convergence in the action of diverse neutrophil priming agents. J Immunol. 2002;169:6394–6400. doi: 10.4049/jimmunol.169.11.6394. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Hauser C J. Sphingosine 1-phosphate, a diffusible calcium influx factor mediating store-operated calcium entry. J Biol Chem. 2003;278:27540–27547. doi: 10.1074/jbc.M301763200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C, Srimal S, Farber C, Sanchez E, Kabbash L, Asch A, Gailit J, Wright S D. Cytokine-induced respiratory burst of human neutrophils: dependence on extracellular matrix proteins and CD11/CD18 integrins. J Cell Biol. 1989;109:1341–1349. doi: 10.1083/jcb.109.3.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C F. Neutrophil activation on biological surfaces. Massive secretion of hydrogen peroxide in response to products of macrophages and lymphocytes. J Clin Invest. 1987;80:1550–1560. doi: 10.1172/JCI113241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M E E, Theler J M, Schlegel W, Appel R D, Wright S D, Lew P D. Multiple elevations of cytosolic-free Ca2+ in human neutrophils: initiation by adherence receptors of the integrin family. J Cell Biol. 1991;112:1249–1257. doi: 10.1083/jcb.112.6.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaconi M E E, Rivest W, Schlegel W, Wollheim C B, Pittet D, Lew P D. Spontaneous and chemoattractant-induced oscillations of cytosolic free calcium in single adherent human neutrophils. J Biol Chem. 1988;263:10557–10560. [PubMed] [Google Scholar]
- Pettit E J, Hallett M B. Pulsatile Ca2+ influx in human neutrophils undergoing CD11b/CD18 integrin engagement. Biochem Biophys Res Commun. 1997;230:258–261. doi: 10.1006/bbrc.1996.5931. [DOI] [PubMed] [Google Scholar]
- Hellberg C, Molony L, Zheng L, Anderson T. Ca2+ signaling mechanisms of the β 2 integrin on neutrophils: involvement of phospholipase γ 2 and Ins(1,4,5)P3. Biochem J. 1996;317:403–409. doi: 10.1042/bj3170403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter J, Andersson T, Olsson I. Effect of tumor necrosis factor and granulocyte/macrophage colony-stimulating factor on neutrophil degranulation. J Immunol. 1989;142:3199–3205. [PubMed] [Google Scholar]
- Berridge M J. Capacitative calcium entry. Biochem J. 1995;312:1–11. doi: 10.1042/bj3120001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor C W. IP3 receptors: the search for structure. Trends Biochem Sci. 2004;29:210–219. doi: 10.1016/j.tibs.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Liou J, Kim M L, Heo W D, Jones J T, Myers J W, Ferrell J E, Jr, Meyer T. STIM is a Ca2+ sensor essential for Ca2+-store-depletion-triggered Ca2+ influx. Curr Biol. 2005;15:1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S L, Yu Y, Roos J, Kozak J A, Deerinck T J, Ellisman M H, Stauderman K A, Cahalan M D. STIM1 is a Ca2+ sensor that activates CRAC channels and migrates from the Ca2+ store to the plasma membrane. Nature. 2005;437:902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spassova M A, Soboloff J, He L P, Xu W, Dziadek M A, Gill D L. STIM1 has a plasma membrane role in the activation of store-operated Ca2+ channels. Proc Natl Acad Sci USA. 2006;103:4040–4045. doi: 10.1073/pnas.0510050103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J W, Jr, Broad L M, Braun F J, Lievremont J P, Bird G S. Mechanisms of capacitative calcium entry. J Cell Sci. 2001;114:2223–2229. doi: 10.1242/jcs.114.12.2223. [DOI] [PubMed] [Google Scholar]
- Randriamampita C, Tsien R Y. Emptying of intracellular Ca2+ stores releases a novel small messenger that stimulates Ca2+ influx. Nature. 1993;364:809–814. doi: 10.1038/364809a0. [DOI] [PubMed] [Google Scholar]
- Smani T, Zakharov S I, Csutora P, Leno E, Trepakova E S, Bolotina V M. A novel mechanism for the store-operated calcium influx pathway. Nat Cell Biol. 2004;6:113–120. doi: 10.1038/ncb1089. [DOI] [PubMed] [Google Scholar]
- Fasolato C, Hoth M, Penner R. A GTP-dependent step in the activation mechanism of capacitative calcium influx. J Biol Chem. 1993;268:20737–20740. [PubMed] [Google Scholar]
- Patterson R L, van Rossum D B, Gill D L. Store-operated Ca2+ entry: evidence for a secretion-like coupling model. Cell. 1999;98:487–499. doi: 10.1016/s0092-8674(00)81977-7. [DOI] [PubMed] [Google Scholar]
- Montell C. The TRP superfamily of cation channels. Sci STKE. 2005;2005:re3. doi: 10.1126/stke.2722005re3. [DOI] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Halaszovich C R, Wehage E, Jüngling E, Zitt C, Lückhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itagaki K, Kannan K B, Singh B B, Hauser C J. Cytoskeletal reorganization internalizes multiple transient receptor potential channels and blocks calcium entry into human neutrophils. J Immunol. 2004;172:601–607. doi: 10.4049/jimmunol.172.1.601. [DOI] [PubMed] [Google Scholar]
- McMeekin S R, Dransfiel I, Rossi A G, Haslett C, Walker T R. E-selectin permits communication between PAF receptors and TRPC channels in human neutrophils. Blood. 2006;107:4938–4945. doi: 10.1182/blood-2005-09-3803. [DOI] [PubMed] [Google Scholar]
- Philipp S, Trost C, Warnat J. TRP4 (CCE1) protein is part of native calcium release-activated Ca2+-like channels in adrenal cells. J Biol Chem. 2000;275:23965–23972. doi: 10.1074/jbc.M003408200. [DOI] [PubMed] [Google Scholar]
- Wu X, Babnigg G, Zagranichnaya T, Villereal M L. The role of endogenous human Trp4 in regulating carbachol-induced calcium oscillations in HEK-293 cells. J Biol Chem. 2002;277:13597–13608. doi: 10.1074/jbc.M110881200. [DOI] [PubMed] [Google Scholar]
- Rosado J A, Brownlow S L, Sage S O. Endogenously expressed Trp1 is involved in store-mediated Ca2+ entry by conformational coupling in human platelets. J Biol Chem. 2002;277:42157–42163. doi: 10.1074/jbc.M207320200. [DOI] [PubMed] [Google Scholar]
- Mori Y, Wakamori M, Miyakawa T, Hermosura M, Hara Y, Nishida M, Hirose K, Mizushima A, Kurosaki M, Mori E, Gotoh K, Okada T, Fleig A, Penner R, Iino M, Kurosaki T. Transient receptor potential 1 regulates capacitative Ca(2+) entry and Ca(2+) release from endoplasmic reticulum in B lymphocytes. J Exp Med. 2002;195:673–681. doi: 10.1084/jem.20011758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanden Abeele F, Lemonnier L, Thebault S. Two types of store-operated Ca2+ channels with different activation modes and molecular origin in LNCaP human prostate cancer epithelial cells. J Biol Chem. 2004;279:30326–30337. doi: 10.1074/jbc.M400106200. [DOI] [PubMed] [Google Scholar]
- Wang X, Pluznick J L, Wei P, Padanilam B J, Sansom S C. TRPC4 forms store-operated Ca2+ channels in mouse mesangial cells. Am J Physiol Cell Physiol. 2004;287:C357–C364. doi: 10.1152/ajpcell.00068.2004. [DOI] [PubMed] [Google Scholar]
- Trebak M, Lemonnier L, Smyth J T, Vazquez G, Putney J W., Jr Phospholipase C-coupled receptors and activation of TRPC channels. Handb Exp Pharmacol. 2007;179:593–614. doi: 10.1007/978-3-540-34891-7_35. [DOI] [PubMed] [Google Scholar]
- Gamberucci A, Giurisato E, Pizzo P, Tassi M, Giunti R, McIntosh D P, Benedetti A. Diacylglycerol activates the influx of extracellular cations in T-lymphocytes independently of intracellular calcium-store depletion and possibly involving endogenous TRP6 gene products. Biochem J. 2002;364:245–254. doi: 10.1042/bj3640245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiselyov K, Xu X, Mozhayeva G. Functional interaction between InsP3 receptors and store-operated Htrp3 channels. Nature. 1998;396:478–482. doi: 10.1038/24890. [DOI] [PubMed] [Google Scholar]
- Hofmann T, Obukhov A G, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Halaszovich C R, Zitt C, Jungling E, Luckhoff A. Inhibition of TRP3 channels by lanthanides. Block from the cytosolic side of the plasma membrane. J Biol Chem. 2000;275:37423–37428. doi: 10.1074/jbc.M007010200. [DOI] [PubMed] [Google Scholar]
- Ma H T, Patterson R L, van Rossum D B, Birnbaumer L, Mikoshiba K, Gill D L. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287:1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 67.Brechard, S., Melchior, C., Plançon, S., Tschirhart, E. J. (2008) Store-operated Ca2+ channels formed by TRPC1, TRPC6 and Orai1 and non-store-operated channels formed by TRPC3 are involved in the regulation of NADPH oxidase in HL-60 granulocytes. Cell Calcium, Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Lopez J J, Salido G M, Pariente J A, Rosado J A. Interaction of STIM1 with endogenously expressed hTRPC1 upon depletion of intracellular Ca2+ stores. J Biol Chem. 2006;281:28254–28264. doi: 10.1074/jbc.M604272200. [DOI] [PubMed] [Google Scholar]
- Vig M, Peinelt C, Beck A, Koomoa D L, Rabah D, Koblan-Huberson M, Kraft S, Turner H, Fleig A, Penner R, Kinet J P. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312:1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S L, Yeromin A V, Zhang X H, Safrina O, Penna A, Roos J, Stauderman K A, Cahalan M D. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc Natl Acad Sci USA. 2006;103:9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feske S, Gwack Y, Prakriya M, Srikanth S, Puppel S H, Tanasa B, Hogan P G, Lewis R S, Daly M, Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- Prakriya M, Feske S, Gwack Y, Srikanth S, Rao A, Hogan P G. Orai1 is an essential pore subunit of the CRAC channel. Nature. 2006;443:230–233. doi: 10.1038/nature05122. [DOI] [PubMed] [Google Scholar]
- Yeromin A V, Zhang S L, Jiang W, Yu Y, Safrina O, Cahalan M D. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443:226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer J C, Dehaven W I, Smyth J T, Wedel B, Boyles R R, Bird G S, Putney J W., Jr Large-store operated calcium selective currents due to co-expression of Orai1 or Orai2 with the intracellular calcium sensor, Stim1. J Biol Chem. 2006;281:24979–24990. doi: 10.1074/jbc.M604589200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peinelt C, Vig M, Koomoa D L, Beck A, Nadler M J, Koblan-Huberson M, Lis A, Fleig A, Penner R, Kinet J P. Amplification of CRAC current by STIM1 and CRACM1 (Orai1) Nat Cell Biol. 2006;8:771–773. doi: 10.1038/ncb1435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soboloff J, Spassova M A, Tang X D, Hewavitharana T, Xu W, Gill D L. Orai1 and STIM reconstitute store-operated calcium channel function. J Biol Chem. 2006;281:20661–20665. doi: 10.1074/jbc.C600126200. [DOI] [PubMed] [Google Scholar]
- Ong H L, Cheng K T, Liu X, Bandyopadhay B C, Paria B C, Soboloff J, Pani B, Gwack Y, Srikanth S, Singh B B, Gill D, Ambudkar I S. Dynamic assembly of TRPC1/STIM1/Orai1 ternary complex is involved in store operated calcium influx. J Biol Chem. 2007;282:9105–9116. doi: 10.1074/jbc.M608942200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Erxleben C, Yildirim E, Abramowitz J, Armstrong D L, Birnbaumer L. Orai proteins interact with TRPC channels and confer responsiveness to store depletion. Proc Natl Acad Sci USA. 2007;104:4682–4687. doi: 10.1073/pnas.0611692104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J P, Zeng W, Huang G N, Worley P F, Muallem S. STIM1 heteromultimerizes TRPC channels to determine their function as store-operated channels. Nat Cell Biol. 2007;9:636–645. doi: 10.1038/ncb1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagranichnaya T K, Wu X, Villereal M L. Endogenous TRPC1, TRPC3, and TRPC7 proteins combine to form native store-operated channels in HEK-293 cells. J Biol Chem. 2005;280:29559–29569. doi: 10.1074/jbc.M505842200. [DOI] [PubMed] [Google Scholar]
- Partida-Sanchez S, Iribarren P, Moreno-Garcia M E, Gao J L, Murphy P M, Oppenheimer N, Wang J M, Lund F E. Chemotaxis and calcium responses of phagocytes to formyl peptide receptor ligands is differentially regulated by cyclic ADP ribose. J Immunol. 2004;172:1896–1906. doi: 10.4049/jimmunol.172.3.1896. [DOI] [PubMed] [Google Scholar]
- Partida-Sánchez S, Cockayne D, Monard S, Jacobson E, Oppenheimer N, Garvy B, Kusser K, Goodrich S, Howard M, Harmsen A, Randall T, Lund F. Cyclic ADP-ribose production by CD38 regulates intracellular calcium release, extracellular calcium influx and chemotaxis in neutrophils and is required for bacterial clearance in vitro. Nat Med. 2001;7:1209–1216. doi: 10.1038/nm1101-1209. [DOI] [PubMed] [Google Scholar]
- Schuber F, Lund F E. Structure and enzymology of ADP-ribosyl cyclases: conserved enzymes that produce multiple calcium mobilizing metabolites. Curr Mol Med. 2004;4:249–261. doi: 10.2174/1566524043360708. [DOI] [PubMed] [Google Scholar]
- Gao J L, Murphy P M. Species and subtype variants of the N-formyl peptide chemotactic receptor reveal multiple important functional domains. J Biol Chem. 1993;268:25395–25401. [PubMed] [Google Scholar]
- Brechard S, Brunello A, Bueb J-L, Tschirhart E J. Modulation by cADPr of Ca2+ mobilization and oxidative response in dimethylsulfoxide- or retinoic acid-differentiated HL-60 cells. Biochim Biophys Acta. 2006;1763:129–136. doi: 10.1016/j.bbamcr.2005.12.003. [DOI] [PubMed] [Google Scholar]
- Howard M, Grimaldi J C, Bazan J F, Lund F E, Santos-Argumedo L, Parkhouse R M, Walseth T F, Lee H C. Formation and hydrolysis of cyclic ADP-ribose catalyzed by lymphocyte antigen CD38. Science. 1993;262:1056–1059. doi: 10.1126/science.8235624. [DOI] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Warnstedt M, Radukina N, Jüngling E, Lückhoff A. Endogenous ADP-ribose enables calcium-regulated cation currents through TRPM2 channels in neutrophil granulocytes. Biochem J. 2006;398:225–232. doi: 10.1042/BJ20060183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiner I, Eisfeld J, Halaszovich C R, Wehage E, Jüngling E, Zitt C, Lückhoff A. Expression profile of the transient receptor potential (TRP) family in neutrophil granulocytes: evidence for currents through long TRP channel 2 induced by ADP-ribose and NAD. Biochem J. 2003;371:1045–1053. doi: 10.1042/BJ20021975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. Activation of the cation channel long transient receptor potential channel 2 (LTRPC2) by hydrogen peroxide. J Biol Chem. 2002;277:23150–23156. doi: 10.1074/jbc.M112096200. [DOI] [PubMed] [Google Scholar]
- Mignen O, Thompson J L, Shuttleworth T J. Both Orai1 and Orai3 are essential components of the arachidonate-regulated Ca2+-selective (ARC) channels. J Physiol. 2008;586:185–195. doi: 10.1113/jphysiol.2007.146258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann T, Obukhov A G, Schaefer M, Harteneck C, Gudermann T, Schultz G. Direct activation of human TRPC6 and TRPC3 channels by diacylglycerol. Nature. 1999;397:259–263. doi: 10.1038/16711. [DOI] [PubMed] [Google Scholar]
- Edwards S W. The O2– generating NADPH oxidase of phagocytes: structure and methods of detection. Methods. 1996;9:563–577. doi: 10.1006/meth.1996.0064. [DOI] [PubMed] [Google Scholar]
- Tarpey M M, Wink D A, Grisham M B. Methods for detection of reactive metabolites of oxygen and nitrogen: in vitro and in vivo considerations. Am J Physiol Regul Integr Comp Physiol. 2004;286:R431–R444. doi: 10.1152/ajpregu.00361.2003. [DOI] [PubMed] [Google Scholar]
- Yeung T, Touret N, Grinstein S. Quantitative fluorescence microscopy to probe intracellular microenvironments. Curr Opin Microbiol. 2005;8:350–358. doi: 10.1016/j.mib.2005.04.004. [DOI] [PubMed] [Google Scholar]
- Jankowski A, Grinstein S. A non-invasive fluorimetric procedure for measurement of membrane potential. Quantification of the NADPH oxidase-induced depolarization in activated neutrophils. J Biol Chem. 1999;274:26098–26104. doi: 10.1074/jbc.274.37.26098. [DOI] [PubMed] [Google Scholar]
- Bassøe C F, Li N, Ragheb K, Lawler G, Sturgis J, Robinson J P. Investigations of phagosomes, mitochondria, and acidic granules in human neutrophils using fluorescent probes. Cytometry B Clin Cytom. 2003;51:21–29. doi: 10.1002/cyto.b.10003. [DOI] [PubMed] [Google Scholar]
- Mohanty J G, Jaffe J S, Schulman S E, Raible D G. A highly sensitive fluorescent micro-assay of H2O2 release from activated human leukocytes using a dihydroxyphenoxazine derivative. J Immunol Methods. 1997;202:133–141. doi: 10.1016/s0022-1759(96)00244-x. [DOI] [PubMed] [Google Scholar]
- Zhou M, Diwu Z, Panchuk-Voloshina N, Haugland R P. A stable nonfluorescent derivative of resorufin for the fluorometric determination of trace hydrogen peroxide: applications in detecting the activity of phagocyte NADPH oxidase and other oxidases. Anal Biochem. 1997;253:162–168. doi: 10.1006/abio.1997.2391. [DOI] [PubMed] [Google Scholar]
- Ryan T C, Weil G J, Newburger P E, Haugland R, Simon E R. Measurement of superoxide release in the phagovacuoles of immune complex-stimulated human neutrophils. J Immunol Methods. 1990;130:223–233. doi: 10.1016/0022-1759(90)90052-w. [DOI] [PubMed] [Google Scholar]
- Dewitt S, Laffafian I, Hallett M B. Phagosomal oxidative activity during β 2 integrin (CR3)-mediated phagocytosis by neutrophils is triggered by a non-restricted Ca2+ signal: Ca2+ controls time not space. J Cell Sci. 2003;116:2857–2865. doi: 10.1242/jcs.00499. [DOI] [PubMed] [Google Scholar]
- Tsien R Y. Intracellular signal transduction in four dimensions: from molecular design to physiology. Am J Physiol. 1992;263:C723–C728. doi: 10.1152/ajpcell.1992.263.4.C723. [DOI] [PubMed] [Google Scholar]
- 102.Putney, J. W. (2000) Calcium Signaling, Chapters 1–4, Boca Raton, FL, USA, CRC, 1–131. [Google Scholar]
- Demaurex N. Calcium measurements in organelles with Ca2+-sensitive fluorescent proteins. Cell Calcium. 2005;38:213–222. doi: 10.1016/j.ceca.2005.06.026. [DOI] [PubMed] [Google Scholar]
- Harper J L, Camerini-Otero C S, Li A H, Kim S A, Jacobson K A, Daly J W. Dihydropyridines as inhibitors of capacitative calcium entry in leukemic HL-60 cells. Biochem Pharmacol. 2003;65:329–338. doi: 10.1016/s0006-2952(02)01488-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korchak H M, Dorsey L B, Li H, Mackie D, Kilpatrick L E. Selective roles for α-PKC in positive signaling for O2– generation and calcium mobilization but not elastase release in differentiated HL60 cells. Biochim Biophys Acta. 2007;1773:440–449. doi: 10.1016/j.bbamcr.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Lee C, Xu D-Z, Feketeova E, Kannan K B, Fekete Z, Deitch E A, Livingston D H, Hauser C J. Store-operated calcium channel inhibition attenuates neutrophil function and postshock acute lung injury. J Trauma. 2005;59:56–63. doi: 10.1097/01.ta.0000171456.54921.fe. [DOI] [PubMed] [Google Scholar]
- Steinckwich N, Frippiat J P, Stasia M J, Erard M, Boxio R, Tankovic C, Doignon I, Nüße O. Potent inhibition of store-operated Ca2+ influx and superoxide production in HL60 cells and polymorphonuclear neutrophils by the pyrazole derivative BTP2. J Leukoc Biol. 2007;81:1054–1064. doi: 10.1189/jlb.0406248. [DOI] [PubMed] [Google Scholar]
- Itagaki K, Kannan K B, Hauser C J. Lysophosphatidic acid triggers calcium entry through a non-store-operated pathway in human neutrophils. J Leukoc Biol. 2005;77:181–189. doi: 10.1189/jlb.0704390. [DOI] [PubMed] [Google Scholar]
- Steel H C, Anderson R. Dissociation of the PAF-receptor from NADPH oxidase and adenylate cyclase in human neutrophils results in accelerated influx and delayed clearance of cytosolic calcium. Br J Pharmacol. 2002;136:81–89. doi: 10.1038/sj.bjp.0704685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nick J A, Avdi N J, Young S K, Knall C, Gerwins P, Johnson G L, Worthen G S. Common and distinct intracellular signaling pathways in human neutrophils utilized by platelet activating factor and fMLP. J Clin Invest. 1997;99:975–986. doi: 10.1172/JCI119263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S, Hallett M B. Cytosolic free Ca2+ changes and calpain activation are required for integrin-accelerated phagocytosis by human neutrophils. J Cell Biol. 2002;159:181–189. doi: 10.1083/jcb.200206089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O, Krause K H, Krischer J, Jerstrom P, Theler J M, Clark R A, Carpentier J L, Lew D P. Redistribution of intracellular Ca2+ stores during phagocytosis in human neutrophils. Science. 1994;265:1439–1441. doi: 10.1126/science.8073285. [DOI] [PubMed] [Google Scholar]
- Hallett M B, Davis E V, Campbell A K. Oxidase activation in individual neutrophils is dependent on the onset and magnitude of the Ca2+ signal. Cell Calcium. 1990;11:655–663. doi: 10.1016/0143-4160(90)90020-u. [DOI] [PubMed] [Google Scholar]
- Davies E V, Hallett M B, Campbell A K. Localized superoxide release by neutrophils can be provoked by a cytosolic calcium “cloud”. Immunology. 1991;73:228–234. [PMC free article] [PubMed] [Google Scholar]
- Allbritton N L, Meyer T, Stryer L. Range of messenger action of calcium ion and inositol 1,4,5-trisphosphate. Science. 1992;258:1812–1815. doi: 10.1126/science.1465619. [DOI] [PubMed] [Google Scholar]
- Ellson C, Davidson K, Anderson K, Stephens L R, Hawkins P T. PtdIns3P binding to the PX domain of p40phox is a physiological signal in NADPH oxidase activation. EMBO J. 2006;25:4468–4478. doi: 10.1038/sj.emboj.7601346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitt S, Tian W, Hallett M B. Localized PtdIns(3,4,5)P3 or PtdIns(3,4)P2 at the phagocytic cup is required for both phagosome closure and Ca2+ signaling in HL60 neutrophils. J Cell Sci. 2006;119:443–451. doi: 10.1242/jcs.02756. [DOI] [PubMed] [Google Scholar]
- Rameh L E, Rhee S G, Spokes K, Kazlauskas A, Cantley L C, Cantle L G. Phosphoinositide 3-kinase regulates phospholipase Cγ-mediated calcium signaling. J Biol Chem. 1998;273:23750–23757. doi: 10.1074/jbc.273.37.23750. [DOI] [PubMed] [Google Scholar]
- Pasquet J-M, Quek L, Stevens C, Bobe R, Huber M, Duronio V, Krystal G, Watson S P. Phosphatidylinositol 3,4,5-trisphosphate regulates Ca(2+) entry via btk in platelets and megakaryocytes without increasing phospholipase C activity. EMBO J. 2000;19:2793–2802. doi: 10.1093/emboj/19.12.2793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hug H, Sarre T F. Protein kinase C isoenzymes: divergence in signal transduction? Biochem J. 1993;291:329–343. doi: 10.1042/bj2910329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newton A C. Protein kinase C: structure, function, and regulation. J Biol Chem. 1995;270:28495–28498. doi: 10.1074/jbc.270.48.28495. [DOI] [PubMed] [Google Scholar]
- Sergeant S, McPhail L C. Opsonized zymosan stimulates the redistribution of protein kinase C isoforms in human neutrophils. J Immunol. 1997;159:2877–2885. [PubMed] [Google Scholar]
- Nishizuka Y. Protein kinase C and lipid signaling for sustained cellular responses. FASEB J. 1995;9:484–496. [PubMed] [Google Scholar]
- Di Virgilio F, Vincentini L M, Treves S, Riz G. Protein kinase C activation of physiological processes in human neutrophils at vanishingly small cytosolic Ca2+ levels. Nature. 1984;310:691–693. doi: 10.1038/310691a0. [DOI] [PubMed] [Google Scholar]
- Maridonneau-Parini I, Tringale S M, Tauber A L. Identification of distinct activation pathways of the human neutrophil NADPH-oxidase. J Immunol. 1986;137:2925–2929. [PubMed] [Google Scholar]
- Hazan I, Dana R, Granot Y, Levy R. Cytosolic phosphatase A2 and its mode of activation in human neutrophils by opsonized zymosan. Biochem J. 1997;326:867–876. doi: 10.1042/bj3260867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu T H, Bei L, Qian Z M, Shen X. Intracellular free calcium regulates the onset of the respiratory burst of human neutrophils activated by phorbol myristate acetate. Cell Signal. 1999;11:355–360. doi: 10.1016/s0898-6568(99)00007-8. [DOI] [PubMed] [Google Scholar]
- Mahomed A G, Anderson R. Activation of human neutrophils with chemotactic peptide, opsonized zymosan and the calcium ionophore A23187, but not with a phorbol ester, is accompanied by efflux and store-operated influx of calcium. Inflammation. 2000;24:559–569. doi: 10.1023/a:1007029524141. [DOI] [PubMed] [Google Scholar]
- Ishihara Y, Rosolia D L, McKenna P J, Peters S P, Albertine K H, Gee M H. Calcium is required for PMA induced superoxide release from human neutrophils. J Leukoc Biol. 1990;48:89–99. doi: 10.1002/jlb.48.1.89. [DOI] [PubMed] [Google Scholar]
- Majumdar S, Kane L H, Rossi M W, Volpp B D, Nauseef W M, Korchak H M. Protein kinase C isotypes and signal-transduction in human neutrophils: selective substrate specificity of calcium-dependent β-PKC and novel calcium-independent nPKC. Biochim Biophys Acta. 1993;1176:276–286. doi: 10.1016/0167-4889(93)90056-u. [DOI] [PubMed] [Google Scholar]
- Dang P M, Fontayne A, Hakim J, el Benna J, Perianin A. Protein kinase C ζ phosphorylates a subset of selective sites of the NADPH oxidase component p47phox and participates in formyl peptide-mediated neutrophil respiratory burst. J Immunol. 2001;166:1206–1213. doi: 10.4049/jimmunol.166.2.1206. [DOI] [PubMed] [Google Scholar]
- Brown G E, Stewart M Q, Liu H, Ha V L, Yaffe M B. A novel assay system implicates PtdIns(3,4)P(2), PtdIns(3)P, and PKC δ in intracellular production of reactive oxygen species by the NADPH oxidase. Mol Cell. 2003;11:35–47. doi: 10.1016/s1097-2765(03)00005-4. [DOI] [PubMed] [Google Scholar]
- Fontayne A, Dang P M, Gougerot-Pocidalo M A, el Benna J , Phosphorylation of p47phox sites by PKC α, β II, δ, and ζ: effect on binding to p22phox and on NADPH oxidase activation. Biochemistry. 2002;41:7743–7750. doi: 10.1021/bi011953s. [DOI] [PubMed] [Google Scholar]
- Clark J D, Milona N, Knopf J L. Purification of a 110-kilodalton cytosolic phospholipase A2 from the human monocytic cell line U937. Proc Natl Acad Sci USA. 1990;87:7708–7712. doi: 10.1073/pnas.87.19.7708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer R M, Sharp J D. Structure, function and regulation of Ca2+ sensitive cytosolic phospholipase A2 (cPLA2) FEBS Lett. 1997;410:49–53. doi: 10.1016/s0014-5793(97)00322-0. [DOI] [PubMed] [Google Scholar]
- Leslie C C. Properties and regulation of cytosolic phospholipase A2. J Biol Chem. 1997;272:16709–16712. doi: 10.1074/jbc.272.27.16709. [DOI] [PubMed] [Google Scholar]
- Marshall J, Krump E, Lindsay T, Downey G, Ford D A, Zhu P, Walker P, Rubin B. Involvement of cytosolic phospholipase A2 and secretory phospholipase A2 in arachidonic acid release from human neutrophils. J Immunol. 2000;164:2084–2091. doi: 10.4049/jimmunol.164.4.2084. [DOI] [PubMed] [Google Scholar]
- Shmelzer Z, Haddad N, Admon E, Pessach I, Leto T L, Eitan-Hazan Z, Hershfinkel M, Levy R. Unique targeting of cytosolic phospholipase A2 to plasma membranes mediated by the NADPH oxidase in phagocytes. J Cell Biol. 2003;162:683–692. doi: 10.1083/jcb.200211056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dana R, Leto T L, Malech H L, Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J Biol Chem. 1998;273:441–445. doi: 10.1074/jbc.273.1.441. [DOI] [PubMed] [Google Scholar]
- Lowenthal A, Levy R. Essential requirement of cytosolic phospholipase A2 for activation of the H+ channel in phagocyte-like cells. J Biol Chem. 1999;274:21603–21608. doi: 10.1074/jbc.274.31.21603. [DOI] [PubMed] [Google Scholar]
- Pessach I, Leto T L, Malech H L, Levy R. Essential requirement of cytosolic phospholipase A2 for stimulation of NADPH oxidase-associated diaphorase activity in granulocyte-like cells. J Biol Chem. 2001;276:33495–33503. doi: 10.1074/jbc.M011417200. [DOI] [PubMed] [Google Scholar]
- Foubert T R, Burritt J B, Taylor R M, Jesaitis A J. Structural changes are induced in human neutrophil cytochrome b by NADPH oxidase activators, LDS, SDS, and arachidonate: intermolecular resonance energy transfer between trisulfopyrenyl-wheat germ agglutinin and cytochrome b558. Biochim Biophys Acta. 2002;1567:221–231. doi: 10.1016/s0005-2736(02)00619-3. [DOI] [PubMed] [Google Scholar]
- Glover S, de Carvalho M S, Bayburt T, Jonas M, Chi E, Leslie C C, Gelb M H. Translocation of the 85-kDa phospholipase A2 from cytosol to the nuclear envelope in rat basophilic leukemia cells stimulated with calcium ionophore or IgE/antigen. J Biol Chem. 1995;270:15359–15367. doi: 10.1074/jbc.270.25.15359. [DOI] [PubMed] [Google Scholar]
- Schievella A R, Regier M K, Smith W L, Lin L L. Calcium-mediated translocation of cytosolic phospholipase A2 to the nuclear envelope and endoplasmic reticulum. J Biol Chem. 1995;270:30749–30754. doi: 10.1074/jbc.270.51.30749. [DOI] [PubMed] [Google Scholar]
- Li Q, Subbulakshmi V, Fields A P, Murray N R, Cathcart M K. Protein kinase C α regulate human monocytes O2– production and low density lipoprotein lipid oxidation. J Biol Chem. 1999;274:3764–3771. doi: 10.1074/jbc.274.6.3764. [DOI] [PubMed] [Google Scholar]
- Banfi B, Molnar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause K H. A Ca(2+)-activated NADPH oxidase in testis, spleen, and lymph nodes. J Biol Chem. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnár G Z, Krause K H, Cox J A. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- Schenten V, Bréchard S, Melchior C, Plançon S, Salsmann A, Tschirhart E J. Ca2+-dependent regulation of NOX2 activity via MRP proteins in HL-60 granulocytes. Calcium Binding Protein. 2008;3:25–27. [Google Scholar]
- Lemarchand P, Vaglio M, Mauel J, Markert M J. Translocation of a small cytosolic calcium-binding protein (MRP-8) to plasma membrane correlates with human neutrophil activation. J Biol Chem. 1992;267:19379–19382. [PubMed] [Google Scholar]
- Bengis-Garber C, Gruener N. Calcium-binding myeloid protein (P8, 14) is phosphorylated in fMet-Leu-Phe-stimulated neutrophils. J Leukoc Biol. 1993;54:114–118. doi: 10.1002/jlb.54.2.114. [DOI] [PubMed] [Google Scholar]
- Edgeworth J, Freemont P, Hogg R, Freemont N. Ionomycin-regulated phosphorylation of the myeloid calcium-binding protein p14. Nature. 1989;342:189–192. doi: 10.1038/342189a0. [DOI] [PubMed] [Google Scholar]
- Lominadze G, Madhavi J R, Merchant M, Cai J, Ward R A, McLeish K R. Myeloid-related protein-14 is a p38 MAPK substrate in human neutrophils. J Immunol. 2005;174:7257–7267. doi: 10.4049/jimmunol.174.11.7257. [DOI] [PubMed] [Google Scholar]
- Guignard F, Mauel J, Markert N. Phosphorylation of myeloid-related proteins MRP-14 and MRP-8 during human neutrophil activation. Eur J Biochem. 1996;241:265–271. doi: 10.1111/j.1432-1033.1996.0265t.x. [DOI] [PubMed] [Google Scholar]
- Edgeworth J, Gorman M, Bennett R, Freemont P, Hogg N. Identification of p8,14 as a highly abundant heterodimeric calcium binding protein complex of myeloid cells. J Biol Chem. 1991;266:7706–7713. [PubMed] [Google Scholar]
- Vogl T, Roth J, Sorg C, Hillenkamp F, Strupat K. Calcium-induced noncovalently linked tetramers of MRP8 and MRP14 detected by ultraviolet matrix-assisted laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom. 1999;10:1124–1130. doi: 10.1016/s1044-0305(99)00085-9. [DOI] [PubMed] [Google Scholar]
- Dianoux A C, Stasia M J, Garin J, Gagnon J, Vignais P V. The 23-kilodalton protein, a substrate of protein kinase C, in bovine neutrophil cytosol is a member of the S100 family. Biochemistry. 1992;31:5898–5905. doi: 10.1021/bi00140a028. [DOI] [PubMed] [Google Scholar]
- Berthier S, Paclet M H, Lerouge S, Roux F, Vergnaud S, Coleman A W, Morel F. Changing the conformation state of cytochrome b558 initiates NADPH oxidase activation: MRP8/MRP14 regulation. J Biol Chem. 2003;278:25499–25508. doi: 10.1074/jbc.M209755200. [DOI] [PubMed] [Google Scholar]
- Paclet M H, Berthier S, Kuhn L, Garin J, Morel F. Regulation of phagocyte NADPH oxidase activity: identification of two cytochrome b558 activation states. FASEB J. 2007;21:1244–1255. doi: 10.1096/fj.06-6852com. [DOI] [PubMed] [Google Scholar]
- Kerkhoff C, Nacken W, Benedyk M, Dagher M C, Sopalla C, Doussiere J. The arachidonic acid-binding protein S100A8/A9 promotes NADPH oxidase activation by interaction with p67phox and Rac-2. FASEB J. 2005;19:467–469. doi: 10.1096/fj.04-2377fje. [DOI] [PubMed] [Google Scholar]
- Bouzidi F, Doussiere J. Binding of arachidonic acid to myeloid-related proteins (S100A8/A9) enhances phagocytic NADPH oxidase activation. Biochem Biophys Res Commun. 2004;325:1060–1065. doi: 10.1016/j.bbrc.2004.10.134. [DOI] [PubMed] [Google Scholar]
- Farnsworth C L, Freshney N W, Rosen L B, Ghosh A, Greenberg M E, Feig L A. Calcium activation of Ras mediated by neuronal exchange factor Ras-GRF. Nature. 1995;376:524–527. doi: 10.1038/376524a0. [DOI] [PubMed] [Google Scholar]
- Price L S, Langeslag M, ten Klooster J P, Hordijk P L, Jalink K, Collard J G. Calcium signaling regulates translocation and activation of Rac. J Biol Chem. 2003;278:39413–39421. doi: 10.1074/jbc.M302083200. [DOI] [PubMed] [Google Scholar]
- Fleming I N, Elliott C M, Exton J H. Differential translocation of rho family GTPases by lysophosphatidic acid, endothelin-1, and platelet-derived growth factor. J Biol Chem. 1996;271:33067–33073. doi: 10.1074/jbc.271.51.33067. [DOI] [PubMed] [Google Scholar]
- Geijsen N, van Delft S, Raaijmakers J A, Lammers J W, Collard J G, Koenderman L, Coffer P J. Regulation of p21rac activation in human neutrophils. Blood. 1999;94:1121–1130. [PubMed] [Google Scholar]
- Condliffe A M, Webb L M C, Ferguson G J, Davidson K, Turner M, Vigorito E, Manifava M, Chilvers E R, Stephens L R, Hawkins P T. RhoG regulates the neutrophil NADPH oxidase. J Immunol. 2006;176:5314–5320. doi: 10.4049/jimmunol.176.9.5314. [DOI] [PubMed] [Google Scholar]
- Henderson L M, Chappell J B, Jones O T. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils. Further evidence for the presence of an HC conducting channel. Biochem J. 1988a;251:563–567. doi: 10.1042/bj2510563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson L M, Chappell J B, Jones O T. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem J. 1988b;255:285–290. [PMC free article] [PubMed] [Google Scholar]
- Henderson L M, Thomas S, Bantig G, Chappell J B. The arachidonate-activable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91-phox. Biochem J. 1997;325:701–705. doi: 10.1042/bj3250701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demaurex N, Petheo G L. Electron and proton transport by NADPH oxidases. Philos Trans R Soc London B Biol Sci. 2005;360:2315–2325. doi: 10.1098/rstb.2005.1769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio F, Lew P D, Andersson T, Pozzan T. Plasma membrane potential modulates chemotactic peptide-stimulated cytosolic free Ca2+ changes in human neutrophils. J Biol Chem. 1987;262:4574–4579. [PubMed] [Google Scholar]
- Geiszt M, Kapus A, Német K, Farkas L, Ligeti E. Regulation of capacitative Ca2+ influx in human neutrophil granulocytes. Alterations in chronic granulomatous disease. J Biol Chem. 1997;272:26471–26478. doi: 10.1074/jbc.272.42.26471. [DOI] [PubMed] [Google Scholar]
- Tintinger G R, Theron A J, Steel H C, Anderson R. Accelerated calcium influx and hyperactivation of neutrophil in chronic granulomatous disease. Clin Exp Immunol. 2001;123:254–263. doi: 10.1046/j.1365-2249.2001.01447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada B K, Geiszt M, Van Bruggen R, Német K, Roos D, Ligeti E. Calcium signaling is altered in myeloid cells with a deficiency in NADPH oxidase activity. Clin Exp Immunol. 2003;132:53–60. doi: 10.1046/j.1365-2249.2003.02138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintinger G R, Anderson R. Counteracting effects of NADPH oxidase and the Na+/Ca2+ exchanger on membrane repolarization and store-operated uptake of Ca2+ by chemoattractant-activated human neutrophils. Biochem Pharmacol. 2004;67:2263–2271. doi: 10.1016/j.bcp.2004.02.029. [DOI] [PubMed] [Google Scholar]
- Oommen J, Steel H C, Theron A J, Anderson R. Investigation into the relationship between calyculin A-mediated potentiation of NADPH oxidase activity and inhibition of store-operated uptake of calcium by human neutrophils. Biochem Pharmacol. 2004;68:1721–1728. doi: 10.1016/j.bcp.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Tintinger G R, Theron A J, Moliehi P, Anderson R. Reactive oxidants regulate membrane repolarization and store-operated uptake of calcium by formyl peptide-activated human neutrophils. Free Radic Biol Med. 2007;42:1851–1857. doi: 10.1016/j.freeradbiomed.2007.03.012. [DOI] [PubMed] [Google Scholar]
- Wehage E, Eisfeld J, Heiner I, Jüngling E, Zitt C, Lückhoff A. Reactive oxidants regulate membrane repolarization and store-operated uptake of calcium by formyl peptide-activated human neutrophils. J Biol Chem. 2002;277:23150–23156. [Google Scholar]
- Tintinger G, Steel H C. Taming the neutrophil: calcium clearance and influx mechanisms as novel targets for pharmacological control. Clin Exp Immunol. 2005;141:191–200. doi: 10.1111/j.1365-2249.2005.02800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney J W., Jr Pharmacology of capacitative calcium entry. Mol Interv. 2001;1:84–94. [PubMed] [Google Scholar]
- Prakriya M, Lewis R S. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J Physiol. 2001;536:3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J Biochem. 1997;122:498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- Leung Y M, Kwan C Y, Loh T T. Dual effects of SK&F 96365 in human leukemic HL-60 cells. Inhibition of calcium entry and activation of a novel cation influx pathway. Biochem Pharmacol. 1996;51:605–612. doi: 10.1016/s0006-2952(95)02181-7. [DOI] [PubMed] [Google Scholar]
- Merritt J E, Armstrong W P, Benham C D, Hallam T J, Jacob R, Jaxa-Chamiec A, Leigh B K, McCarthy S A, Moores K E, Rink T J. SK&F 96365, a novel inhibitor of receptor-mediated calcium entry. Biochem J. 1990;271:515–522. doi: 10.1042/bj2710515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He L P, Hewavitharana T, Soboloff J, Spassova M A, Gill D L. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J Biol Chem. 2005;280:10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- Cleveland P L, Millard P J, Showell H J, Fewtrell C M S. Tenidap: a novel inhibitor of calcium influx in a mast cell line. Cell Calcium. 1993;14:1–16. doi: 10.1016/0143-4160(93)90013-v. [DOI] [PubMed] [Google Scholar]
- Blackburn W D, Loose L D, Heck L W, Chatham W W. Tenidap, in contrast to several available nonsteroidal antiinflammatory drugs, potently inhibits the release of activated neutrophil collagenase. Arthritis Rheum. 1991;34:211–216. doi: 10.1002/art.1780340213. [DOI] [PubMed] [Google Scholar]
- Chatham W W, Baggott J E, Loose L D, Blackburn W D. Effects of tenidap on superoxide-generating enzymes. Non-competitive inhibition of xanthine oxidase. Biochem Pharmacol. 1995;50:811–814. doi: 10.1016/0006-2952(95)00204-d. [DOI] [PubMed] [Google Scholar]
- Steel H C, Anderson R. Itraconazole antagonizes store-operated influx of calcium into chemoattractant-activated human neutrophils. Clin Exp Immunol. 2004;136:255–261. doi: 10.1111/j.1365-2249.2004.02443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiner I, Radukina N, Eisfeld J, Kühn F, Lückhoff A. Regulation of TRPM2 channels in neutrophil granulocytes by ADP-ribose: a promising pharmalogical target. Naunyn Schmiedebergs Arch Pharmacol. 2005;371:325–333. doi: 10.1007/s00210-005-1033-y. [DOI] [PubMed] [Google Scholar]