Abstract
Maturation of dendritic cells (DCs) by TLR ligands induces expression of IFN-β and autocrine activation of IFN-inducible Stat1-dependent genes important for DC function. In this study, we analyzed the regulation of STAT signaling during maturation of human DCs by TNF-α and PGE2, which induced maturation of human DCs comparably with LPS but did not induce detectable IFN-β production or Stat1 tyrosine phosphorylation. Consistent with these results, TNF-α and PGE2 did not induce Stat1 DNA binding to a standard Stat1-binding oligonucleotide. Instead, TNF-α and PGE2 increased Stat1 serine phosphorylation and Stat4 tyrosine phosphorylation and activated expression of the NF-κB and Stat1 target gene IFN regulatory factor 1 (IRF1), which contributes to IFN responses. TNF-α and PGE2 induced a complex that bound an oligonucleotide derived from the IRF1 promoter that contains a STAT-binding sequence embedded in a larger palindromic sequence, and this complex was recognized by Stat1 antibodies. These results suggest that TNF-α and PGE2 activate STAT-mediated components of human DC maturation by alternative pathways to the IFN-β-mediated autocrine loop used by TLRs.
Keywords: inflammation, Jak
INTRODUCTION
Dendritic cells (DCs) play a key role in capturing foreign antigens and presenting antigens to naïve T cells [1]. Thus, DCs play a key role in the transition from innate to acquired immunity. Under steady-state, physiological conditions, immature DCs are located at sentinel regions throughout the body, such as skin and mucosal surfaces. Interaction with microbial pathogens and their products induces a maturation program in DCs, whereby they acquire enhanced and altered migratory properties and a markedly enhanced capacity to activate T cells. The altered migratory properties of mature DCs are mediated in part by de novo expression of the chemokine receptor CCR7, which allows DCs to respond to lymph node-expressed chemokines CCL19 and CCL21 and migrate from peripheral sites to lymph nodes, where they interact with and activate T cells [1]. A key component of the enhanced antigen-presenting capacity of mature DCs is their expression of costimulatory molecules such as CD80 and CD86.
Induction of DC maturation in response to microbial invasion is mediated by innate immune pattern recognition receptors, of which TLRs have been studied most extensively (reviewed in ref. [2]). TLRs recognize microbial molecules such as lipopeptides (TLR1, TLR2, TLR6), LPS (TLR4), and nucleic acids (TLR3, TLR7, TLR8, TLR9). TLRs activate complex signal transduction cascades that lead to inflammatory cytokine production and DC maturation. All TLRs (except TLR3 that recognizes dsRNA) activate a MyD88-dependent signaling pathway that leads to activation of NF-κB and MAPKs and downstream production of inflammatory cytokines. In addition, many TLRs, including TLR4 and TLR7–9, activate a parallel signaling pathway, which leads to activation of kinases, TNFR-associated factor family member-associated NF-κB activator-binding kinase 1 and IκB kinase (IKK)ε and transcription factor IFN regulatory factor 3 (IRF3), and downstream production of IFN-β. In the case of TLR4, induction of IFN-β expression does not require MyD88 and instead, depends on an alternative signaling molecule, Toll/IL-1R domain-containing adaptor-inducing IFN-β. In turn, IFN-β acts in an autocrine manner to activate the Jak-STAT pathway and expression of IFN-inducible and STAT-dependent genes. Canonical IFN-β signaling is mediated by the IFN-stimulated gene factor 3 (ISGF3) complex, which is comprised of Stat1, Stat2, and IRF9 and binds to IFN-stimulated response element (ISRE) promoter elements [3,4,5]. In addition, type I IFNs (IFN-α/β) induce expression of Stat1- and Stat4-dependent genes [6, 7]. TLR-induced activation of autocrine IFN-β and the Jak-STAT pathway is important for DC maturation, expression of costimulatory molecules, and antigen cross-presentation [4, 7,8,9,10,11,12,13,14].
Early work with human DCs demonstrated that DC maturation can be induced by endogenous inflammatory factors [15, 16]. A cocktail of inflammatory factors was required initially, of which a combination of TNF-α and PGE2 was shown to be effective [15, 16]. Maturation of DCs by endogenous inflammatory factors is important during chronic inflammation, where mature DCs can contribute to local inflammation and potential autoimmunity [17, 18]. In addition, maturation of human DCs with endogenous factors ex vivo is important for DC-based vaccines and immunotherapy [19]. However, endogenous, inflammatory mediators, including TNF-α and PGE2, do not activate an IFN-β-mediated autocrine loop in human DCs [7, 20, 21], which contrasts with our recent description of a TNF-α-induced, IFN-β-mediated autocrine loop in monocytes and macrophages [22]. Lack of IFN-β induction suggests that DC maturation by TNF-α and PGE2 is incomplete relative to maturation induced by TLRs or that TNF-α and PGE2 activate additional pathways that can lead to Jak-STAT activation and at least partially substitute for autocrine IFN-β. In this study, we compared maturation of human DCs by TNF-α and PGE2 with maturation by LPS. TNF-α and PGE2 induced comparable DC maturation with LPS, even in the absence of IFN-β production and detectable Stat1 tyrosine phosphorylation. Instead, TNF-α and PGE2 activated alternative signals in the Jak-STAT pathway that included increased Stat1 serine phosphorylation, Stat4 tyrosine phosphorylation, and induction of expression of the Stat1 target gene IRF1. IRF1 mediates gene induction downstream of Stat1 and binds to ISRE elements similar to those bound by IFN-β-induced ISGF3 and thus, can induce expression of STAT target genes. TNF-α-induced activation of NF-κB contributed to IRF1 expression and induction of DC maturation markers CD25, CD40, and CD86. These results suggest that TNF-α and PGE2 activate STAT-mediated components of DC maturation by alternative pathways to the IFN-β-ISGF3 autocrine loop used by TLRs.
MATERIALS AND METHODS
Reagents and antibodies
CD14-FITC, CD25-PE, CD40-FITC, CD80-PE, CD86-PE, and their corresponding isotype control antibodies were from BD PharMingen (San Diego, CA, USA), and HLA-DR-PE was from Immunotech (Beckman Coulter, Fullerton, CA, USA). Antibodies specific for STAT1 phosphorylated on Tyr701 or Ser727, STAT2 phosphorylated on Tyr 689, and STAT3 phosphorylated on Tyr705 were from Cell Signaling Technology (Beverly, MA, USA). Phosphorylation-specific STAT4 (Tyr693) antibody was from Zymed Laboratories, Inc. (San Francisco, CA, USA). STAT1 mAb was from BD Transduction Laboratories (Lexington, KY, USA). α-Tubulin mAb was from Sigma-Aldrich (St. Louis, MO, USA). Bay 11-7082 was from Calbiochem (Gibbstown, NJ, USA). IFN-α/β R2 antibody was from PBL (Piscataway, NJ, USA).
Human DC culture
Human monocyte-derived DCs were generated as described previously [23, 24]. Briefly, PBMC were isolated by density gradient centrifugation with Ficoll (Invitrogen, Carlsbad, CA, USA) of buffy coats purchased from the New York Blood Center (New York, NY, USA). CD14+ monocytes (>97% pure, as verified by flow cytometry) were obtained from PBMC immediately after isolation by positive selection with anti-CD14 magnetic beads, as recommended by the manufacturer (Miltenyi Biotec, Auburn, CA, USA). CD14+ cells (106/ml) were plated in six-well plates in 3 ml RPMI-1640 medium (Invitrogen), supplemented with 10% heat-inactivated FCS (Hyclone, Logan, UT, USA), recombinant human (h)GM-CSF (1000 U/ml, Leukine, Immunex, Seattle, WA, USA), and hIL-4 (25 ng/ml, R&D Systems, Minneapolis, MN, USA) to generate immature DCs. Cytokines were replenished on Days 2 and 4 of culture. TNF-α (25 ng/ml) + PGE2 (1 ng/ml) or LPS (10 ng/ml) were added to cultures on Day 5 for an additional 2 days to mature DC. The experiments using human cells were approved by the Hospital for Special Surgery Institutional Review Board (New York, NY, USA).
Immunoblotting
Total cell extracts were obtained as described [25]. Cell extracts corresponding to 3.3 × 105 cells were fractionated by 10% SDS-PAGE, transferred to polyvinylidene fluoride membranes (Millipore, Bedford, MA), and incubated with specific antibodies; ECL was used for detection.
EMSAs
Cell extracts (5 μg) were incubated for 15 min at room temperature with 0.5 ng 32P-labeled, double-stranded oligonucleotides in 15 μl of a binding reaction containing 40 mM NaCl and 2 μg polydeoxyinosinic:polydeoxycytidylic acid (Pharmacia, Piscataway, NJ, USA), and complexes were resolved on nondenaturing 4.5% polyacrylamide gels, as described previously [26]. Gels were dried and subjected to autoradiography. In competition experiments, an excess (50× or 200×) of unlabeled competitor oligonucleotide was incubated for 5 min with the cell extract before addition of labeled probe. Oligonucleotide sequences are: human sis-inducible element (hSIE), 5′ GTCGACATTTCCCGTAAATCGTCGA [27]; IRF1, 5′ CCTGATTTCCCCGAAATGAC; IRF1 mutant, 5′ CCTGATGGCCCCGCCATGAC. STAT-binding sites are underlined, and mutated bases are in italics. In supershift experiments, 1 μl of a 1:10 dilution of specific antiserum [27] was added to extracts on ice for 1 h before the addition of radiolabeled probe.
Flow cytometry
Cells were analyzed using flow cytometry as described previously [26]. Analysis was done using a FACSCalibur™ flow cytometer with CellQuest software™ (Becton Dickinson, San Jose, CA, USA).
Real-time quantitative RT-PCR (qPCR)
For real-time qPCR, total RNA was extracted using a RNeasy Mini kit (Qiagen, Valencia, CA, USA), and 1 μg total RNA was treated with RNase-free DNase before RT into cDNA using a First-Strand cDNA synthesis kit (Fermentas, Hanover, MD, USA). Real-time qPCR was performed as described previously [28] using iQ SYBR-Green Supermix and the iCycler iQ thermal cycler (Bio-Rad, Hercules, CA, USA). Relative expression was normalized relative to levels of GAPDH or β-actin. The generation of only the correct size amplification products was confirmed using agarose gel electrophoresis. Oligonucleotide primers were as follows: GAPDH: 5′-GTGAAGGTCGGAGTCAAC-3′ and 5′-TGGAATTTGCCATGGGTG-3′; IFN-β, 5′-AGCAGTTCCAGAAGGAGGAC-3′ and 5′-TGATAGACATTAGCCAGGAGGTT-3′; IFN-inducible protein 10 (IP-10), 5′-ATTTGCTGCCTTATCTTTCTG-3′ and 5′-TCTCACCCTTCTTTTTCATTGTAG-3′; CD25, 5′-CGTGGTGGGGCAGATGGTTTATTA-3′ and 5′-CTTGTCTTCCCGTGGGTCATTTTG-3′; IRF1 5′-CAAATCCCGGGGCTCATCTGG-3′ and 5′-CTGGCTCCTTTTCCCCTGCTTTGT-3′; ISG56, 5′ TTCGGAGAAAGGCATTAGA-3′ and 5′-TCCAGGGCTTCATTCATAT-3′.
RESULTS
Comparable maturation of human DCs by LPS and TNF-α plus PGE2
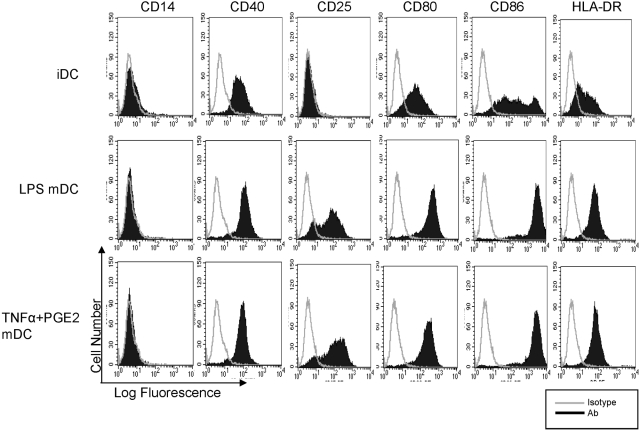
We wished to compare the ability of TNF-α and PGE2 to induce human DC maturation relative to LPS. A role for NF-κB in maturation of DCs is well established, and we wished instead to focus on the induction of costimulatory molecules whose expression is mediated by MyD88-independent and IFN-β-dependent pathways downstream of TLR4 that have been explored predominantly in murine systems [3, 8,9,10,11,12, 29]. Human monocyte-derived, immature DCs were derived as described previously [23] and then treated for 2 days with LPS or TNF-α + PGE2 or left untreated as a control. LPS and TNF-α + PGE2 induced comparable expression of costimulatory molecules CD80 and CD86, HLA-DR, CD40, and the late maturation marker CD25 [30] (Fig. 1). TNF-α or PGE2, used individually, were not effective inducers of DC differentiation (data not shown), consistent with previous reports [31, 32]. Of the mature DC marker and effector genes induced by TNF-α + PGE2, CD40, CD86, and HLA-DR are Stat1-dependent, IFN-inducible genes [33, 34] whose induction by LPS is dependent on an IFN-Stat1-mediated autocrine loop [3,4,5, 8,9,10, 29]. Thus, induction of these genes suggests that TNF-α plus PGE2 are capable of inducing an IFN-β-mediated, Stat1-like response that contributes to human DC maturation.
Fig. 1.
Cell surface phenotype of human monocyte-derived, immature DCs (iDC) and mature DCs (mDC). DCs were generated as described in Materials and Methods, and the expression of CD14, CD40, CD25, CD80, CD86, and HLA-DR was analyzed by flow cytometry after 2 days of stimulation with 10 ng/ml LPS or TNF-α (25 ng/ml) + PGE2 (1 ng/ml). Open histograms = isotype control staining; shaded histograms = staining with corresponding antibodies. One representative experiment of seven is shown.
TNF-α + PGE2 do not detectably activate Stat1 tyrosine phosphorylation
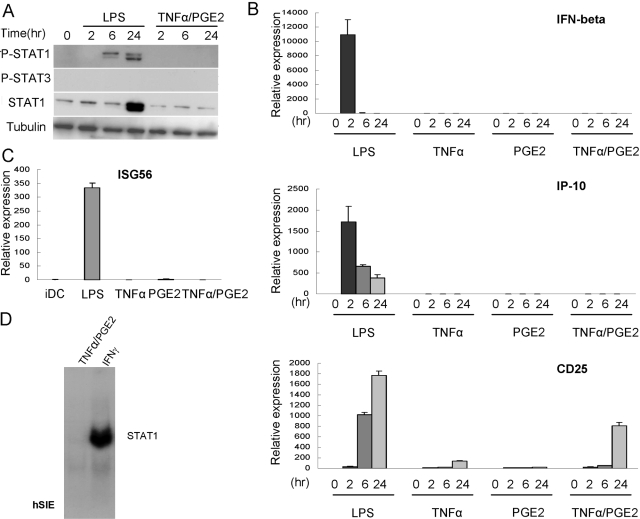
We have shown recently that TNF-α activates an IFN-β-mediated autocrine loop in macrophages [22], but TNF-α has been reported to not induce IFN-β in human DCs [7], and TNF-α and PGE2 do not typically activate STATs [20, 21]. We examined whether TNF-α and/or PGE2 activated STATs during DC maturation in our system as a possible explanation for the induction of IFN/STAT-inducible genes. As a positive control, LPS induced delayed and sustained Stat1 tyrosine phosphorylation that has been reported previously to be mediated by autocrine IFN-β action [3, 14] (Fig. 2A). Importantly, Stat1 expression increased dramatically after LPS stimulation (Fig. 2A). Stat1 expression is induced by low concentrations of type I and type II IFNs by a Stat1-dependent mechanism [28, 35], and thus, induction of Stat1 expression serves as a good reporter for endogenous IFN production. In contrast, in DCs treated with TNF-α + PGE2, there was no detectable induction of Stat1 tyrosine phosphorylation or increase in Stat1 expression (Fig. 2A). We have found previously that low concentrations of IFNs (3 pg/ml IFN-α, 15 pg/ml IFN-γ) induce substantial levels of Stat1 expression [28, 35]. Thus, these results suggest that TNF-α + PGE2 induced minimal, if any, endogenous IFN production in human DCs. This idea was supported further by experiments showing negligible, if any, expression of IFN-β or its target genes IP-10 or ISG56 (which is dependent on ISGF3) in TNF-α + PGE2-treated DCs relative to LPS-treated DCs (Fig. 2, B and C). In contrast, LPS and TNF-α + PGE2 induced CD25 mRNA expression (Fig. 2B), consistent with the flow cytometry results shown in Figure 1.
Fig. 2.
TNF-α + PGE2 do not detectably activate Stat1 or autocrine IFN-β production. Human monocyte-derived, immature DCs were stimulated with 10 ng/ml LPS, TNF-α (25 ng/ml), PGE2 (1 ng/ml), or TNF-α + PGE2 for indicated times. (A) Whole cell extracts were analyzed using immunoblotting with antibodies against tyrosine-phosphorylated (P-)Stat1 and Stat3, followed by probing the same filter with antibodies against Stat1 and tubulin. (B) mRNA levels of IFN-β, IP-10, and CD25 were measured by real-time PCR and normalized relative to GAPDH. (C) mRNA levels of ISG56 were measured by real-time PCR and normalized relative to GAPDH. (D) Cell extracts were subjected to EMSA using a radiolabeled hSIE oligonucleotide. Representative results from at least three experiments are shown.
We also looked for evidence of Stat1 activation in TNF-α + PGE2-matured DCs using EMSA assays that can be more sensitive than immunoblotting. EMSA was performed using a radiolabeled, high-affinity hSIE oligonucleotide that contains a γ-activated sequence (GAS) that effectively binds Stat1 and Stat3 and is commonly used to measure DNA binding by these factors [26, 27]. As expected, Stat1 DNA-binding activity was readily detected in control cells that had been stimulated with IFN-γ (Fig. 2D). In contrast, no DNA-binding activity was detected in TNF-α + PGE2-treated DCs (Fig. 2D). Collectively, the results support the notion that in contrast to LPS, TNF-α and PGE2 do not activate an IFN-Stat1-mediated autocrine loop during DC maturation. These results are consistent with the literature that TNF-α and PGE2 do not activate signaling pathways important for IFN production or Jak-STAT activation in DCs [7, 20, 21].
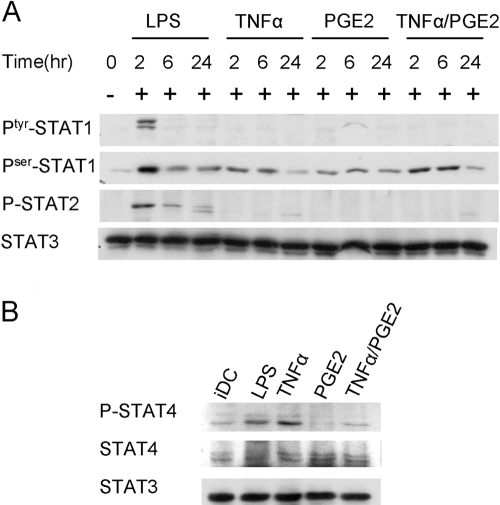
We next examined the effects of TNF-α and PGE2 when used individually and whether TNF-α and/or PGE2 activated STATs other than Stat1. Similar to the TNF-α + PGE2 combination, TNF-α or PGE2, when used individually, did not activate expression of IFN-β or IP-10 (Fig. 2B). TNF-α, but not PGE2, induced CD25 expression, which was induced further when TNF-α and PGE2 were used together (Fig. 2B). When signaling was analyzed, positive control LPS consistently induced tyrosine phosphorylation of Stat1, Stat2, and Stat4, but not Stat3, in human DCs, although the kinetics varied among different donors (Figs. 2A and 3, A and B; and data not shown). TNF-α and/or PGE2 did not induce tyrosine phosphorylation of Stat1, Stat3, or Stat5 (Figs. 2A and 3A; and data not shown). TNF-α, but not PGE2, minimally induced Stat2 tyrosine phosphorylation 24 h after stimulation, which contrasted with much stronger and earlier activation of Stat2 by LPS (Fig. 3A). On the other hand, TNF-α induced Stat4 tyrosine phosphorylation comparably with LPS (Fig. 3B), and TNF-α and PGE2 increased Stat1 serine phosphorylation (Fig. 3A). Collectively, the results show that TNF-α exhibits several activating effects on Jak-STAT signaling in human DCs but only minimally, if at all, activates the IFN-β-Stat1-ISGF3 loop implicated in TLR responses.
Fig. 3.
TNF-α + PGE2 induce serine phosphorylation of STAT1 and tyrosine phosphorylation of STAT4. (A) Human monocyte-derived, immature DCs were stimulated with 10 ng/ml LPS, TNF-α (25 ng/ml), PGE2 (1 ng/ml), or TNF-α + PGE2 for indicated times. Whole cell extracts were analyzed using immunoblotting with antibodies against tyrosine-phosphorylated (Ptyr-) and serine-phosphorylated (Pser-) Stat1 and tyrosine-phosporylated Stat2, followed by probing the same filter with antibodies against Stat3. One representative experiment of three is shown. (B) Human monocyte-derived, immature DCs were stimulated with 10 ng/ml LPS, TNF-α (25 ng/ml), PGE2 (1 ng/ml), or TNF-α + PGE2 for 48 h. Whole cell extracts were analyzed using immunoblotting with antibodies against tyrosine-phosphorylated STAT4, STAT4, and STAT3. One representative experiment of three is shown.
TNF-α + PGE2 induce IRF1 expression that is partially dependent on NF-κB but not on IFN-α/β
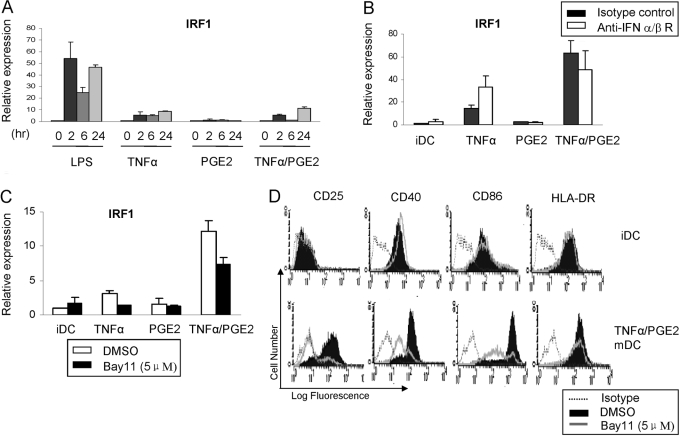
TNF-α induces expression of the IRF1 member of the IRF family of transcription factors that contributes to gene induction downstream of Stat1, binds to similar ISRE elements as does IFN-β-activated ISGF3, and has been implicated in DC maturation and function [13, 36]. IRF1 expression can be activated by NF-κB, but in many systems, induction is dependent on IFN-α/β or IFN-γ [36]. We wished to investigate mechanisms by which TNF-α and PGE2 activate IRF1 in our human DC maturation system, where IFNs are not produced. As expected, TNF-α activated sustained expression of IRF-1 (Fig. 4A). Although PGE2 alone did not increase IRF1 expression, in many donors, TNF-α + PGE2 induced greater expression of IRF1 than did TNF-α alone (Fig. 4, B and C). TNF-α-induced expression of IRF1 was not affected by a blocking antibody against the IFNAR receptor for IFN-α/β (Fig. 4B) that suppressed LPS-induced expression of IFN target genes (data not shown). In contrast, inhibition of NF-κB partially suppressed TNF-α- and TNF-α + PGE2-induced IRF1 expression (Fig. 4C). Consistent with the previous literature indicating an important role for NF-κB in TLR-induced DC maturation, inhibition of NF-κB suppressed induction of CD86, CD40, and CD25 (but not HLA-DR) during maturation of human DCs by TNF-α/PGE2 (Fig. 4D). Collectively, the data show a synergistic activation of IRF1 by TNF-α and PGE2 that is not dependent on IFN-α/β but partially dependent on NF-κB.
Fig. 4.
TNF-α + PGE2-induced IRF1 expression is partially dependent on NF-κB but not on IFN-α/β. (A) mRNA levels of IRF1 in immature DC treated with LPS, TNF-α, PGE2, or TNF-α + PGE2 for indicated times were measured by real-time PCR and normalized relative to GAPDH. (B) mRNA levels of IRF1 in immature DC treated with TNF-α, PGE2, or TNF-α + PGE2, with or without blocking anti-IFNR-α/β antibodies, were measured by real-time PCR and normalized relative to GAPDH. (C) mRNA levels of IRF1 in immature DC treated with TNF-α, PGE2, or TNF-α + PGE2, with or without the IKK inhibitor Bay11, were measured by real-time PCR and normalized relative to GAPDH. (D) The expression of CD25, CD40, CD86, and HLA-DR was analyzed by flow cytometry after 48 h of stimulation with TNF-α (25 ng/ml) + PGE2 (1 ng/ml), with or without Bay11 treatment. Open histograms with dotted line = isotype control staining; shaded histograms = staining with corresponding antibodies without Bay11 treatment; and open histograms with solid line = staining with corresponding antibodies in immature DC treated with Bay11. One representative experiment of two is shown.
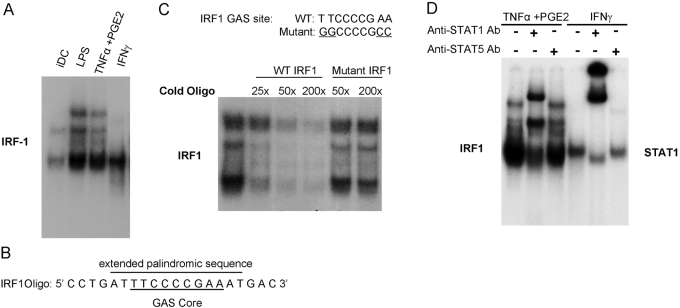
TNF-α + PGE2 induce a complex that binds to an IRF1 promoter GAS sequence
TNF-α/PGE2-induced expression of IRF1 was only partially dependent on NF-κB, and IRF1 is a well-known STAT target gene that contains a conserved STAT-binding sequence, termed a GAS sequence, in its promoter [37,38,39]. Thus, we tested whether TNF-α/PGE2 induced binding to the IRF1 promoter-derived GAS. Interestingly, TNF-α + PGE2 induced binding to the IRF1 oligonucleotide, which was comparable with that induced by LPS, and to the positive control DNA-binding activity present in IFN-γ-stimulated cells (Fig. 5A). TNF-α/PGE2-induced binding to the IRF1 GAS (Fig. 5A) contrasted with lack of binding to the hSIE oligonucleotide (Fig. 2D), which binds Stat1 dimers with high affinity [27]. These results are consistent with the lack of TNF-α/PGE2-induced Stat1 tyrosine phosphorylation (Fig. 2A) and suggest that TNF-α/PGE2 activate IRF1 expression by a mechanism that is not dependent on activation of tyrosine-phosphorylated Stat1 dimers.
Fig. 5.
TNF-α + PGE2 induce a complex that binds to an IRF1 promoter GAS sequence. (A) Cell extracts were subjected to EMSA using the radiolabeled IRF1 oligonucleotide. (B) The sequence of IRF1 oligonucleotide used in EMSA assays. The core GAS sequence and the extended palindromic sequence are identified. (C) TNF-α + PGE2 mature DC extracts were subjected to EMSA using the radiolabeled IRF1 oligonucleotide and unlabeled wild-type (WT) or mutant oligonucleotides as competitors. (D) Cell extracts were subjected to EMSA using the radiolabeled IRF1 oligonucleotide. Stat1 or Stat5 antibodies were added to extracts for 1 h prior to addition of the radiolabeled probe to supershift Stat-containing complexes.
We further investigated TNF-α/PGE2-induced binding to the IRF1 GAS and noted that the IRF1 GAS is embedded within a longer palindromic sequence that can potentially facilitate binding of additional proteins (Fig. 5B). We used competition experiments with unlabeled oligonucleotides to determine whether the TNF-α + PGE2-induced complex bound specifically to the GAS sequence contained in the IRF1 oligonucleotide. DNA binding to the IRF1 promoter sequence was competed effectively by an unlabeled, wild-type IRF1 oligonucleotide (Fig. 5C), thus confirming specificity of binding. DNA binding was affected minimally by an oligonucleotide containing mutations in the GAS site that are important for binding of STATs (Fig. 5C). This result suggests that a canonical GAS sequence, similar to that bound by STATs, is required for binding by the TNF-α + PGE2-inducd complex. This result prompted us to use supershift experiments to test if the TNF-α + PGE2-induced complex contained STAT proteins. Surprisingly, most of the DNA-binding complex was shifted by Stat1 antibodies (Fig. 5D); in our hands, this supershift assay is specific for Stat1 relative to other STATs [26]. No supershift was observed when Stat5 or Stat4 antibodies were used (Fig. 5D; and data not shown). In addition, tyrosine phosphorylation of Stat3, Stat5, and Stat6 was not observed in TNF-α + PGE2-treated DCs. These results suggest that TNF-α + PGE2 can activate a subset of Stat1 target genes, such as IRF1, by inducing a complex that binds to DNA sequences similar to the GAS sites that mediate canonical, IFN-induced, STAT-dependent gene expression. This TNF-α/PGE2-induced, GAS-binding complex contains a protein that cross-reacts with Stat1 antibodies or contains minimally tyrosine-phosphorylated Stat1. There is precedent for the latter possibility, as discussed below.
DISCUSSION
Induction of IFNs and of Stat1 target genes is an important component of DC maturation by TLRs [7, 9,10,11, 13, 14, 40]. In this study, we have shown that the endogenous, inflammatory factors TNF-α + PGE2 induce human DC maturation comparably with LPS, including activation of canonical, IFN-inducible and Stat1 target genes. TNF-α and PGE2 minimally, if at all, activated IFN production and Stat1 tyrosine phosphorylation in human DCs and thus, would require an alternative mechanism to activate STAT target genes and thereby, induce full DC maturation [20, 21]. Our results suggest that this is achieved in part by TNF-α + PGE2 induction of a protein complex that binds to a subset of GAS elements and is capable of activating IRF1 and possibly additional genes typically induced by IFNs. IRF1 plays a key role in mediating expression of many IFN-inducible genes and binds to ISRE elements [37,38,39] and thus, likely contributes to TNF-α + PGE2-induced expression of DC maturation genes. In support of this notion, IRF1 has recently been shown to induce IL-27 during DC maturation [13]. In addition, our results support and extend the findings of other groups [7, 13, 14] that DC maturation by TNF-α + PGE2 is also mediated by NF-κB and Stat4.
Neither TNF-α nor PGE2 activates signaling pathways required for expression of IFNs in human monocyte-derived DCs [7, 20, 21]. Several lines of evidence support the notion that induction of the IRF1 promoter GAS-binding complex by TNF-α + PGE2 was not mediated by autocrine action of endogenous IFNs: There was minimal induction of IFN-β expression and of the IFN-inducible gene IP-10; Stat1 tyrosine phosphorylation was below the limits of detection; expression of Stat1, which increases in response to low IFN concentrations, was not induced. Induction of the IRF1 GAS-binding complex during DC maturation occurred with delayed kinetics (Y. Hu, unpublished results), suggesting that it required de novo expression of transcription factors whose expression was induced by TNF-α + PGE2. These factors could be induced directly or indirectly via autocrine factors, similar to the manner in which LPS activates STAT pathways using an autocrine loop.
TNF-α has a maturation-inducing effect on human monocyte-derived DCs but does not induce a fully mature phenotype relative to LPS. Addition of PGE2 enhances TNF-α-mediated maturation of DCs [15, 16, 31, 32]. Our results show that TNF-α alone induced Stat1 serine phsphorylation and Stat4 tyrosine phosphorylation but only modestly increased IRF1 expression. Addition of PGE2 resulted in an additive increase in Stat1 serine phosphorylation and a synergistic increase in IRF1 expression. These results yield insights into how TNF-α and PGE2 cooperate to promote human DC maturation and suggest a role for IRF1 in integrating TNF-α and PGE2 signals.
The TNF-α + PGE2-induced complex bound to a canonical core GAS sequence, suggesting that it may contain STAT proteins. However, tyrosine phosphorylation of STATs, which is required for DNA binding, was not detected (Fig. 1 and Y. Hu, unpublished results). Surprisingly, despite the apparent absence of Stat1 tyrosine phosphorylation, the TNF-α + PGE2-induced complex reacted with Stat1 antibodies in supershift assays. It is possible that TNF-α + PGE2 induced low-level tyrosine phosphorylation of Stat1, which was below the limits of detection, and tyrosine-phosphorylated Stat1 was incorporated into the TNF-α + PGE2-induced complex. However, in this case, we would have expected to see induction of Stat1 target genes such as IP-10 and Stat1 themselves. In addition, low-level Stat1 tyrosine phosphorylation in TNF-α + PGE2-treated cells relative to LPS-treated cells does not explain why binding to the IRF1 oligonucleotide was comparable in cells treated with TNF-α + PGE2 and LPS.
It is possible that TNF-α + PGE2 induced a protein other than Stat1 that comigrates with Stat1 on gels and cross-reacts with the Stat1 antibody. However, we are most intrigued by the possibility that TNF-α + PGE2 induce formation of a complex containing nontyrosine-phosphorylated Stat1 and additional TNF-α + PGE2-induced proteins that can bind to select promoter sequences, including the extended GAS element in the IRF1 promoter. Clear precedents exist for nontyrosine-phosphorylated Stat1 or Stat3 binding to DNA and activating gene expression as part of a protein complex, including a complex that contains Stat1 and IRF1; serine phosphorylation of STATs may promote their incorporation into such complexes [41, 42]. Definitive resolution of whether the complex induced by TNF-α + PGE2 in primary human DCs contains Stat1 (or potentially other STATs) will require a genetic approach such as use of RNA interference (RNAi). Although we have been successful in using RNAi to knock down expression of other proteins in primary human myeloid cells (ref. [43] and unpublished data), Stat1 protein is stable in these cells [28], and this approach is not feasible. Interaction of Stat1 with TNF-α + PGE2-induced transcription factors could potentially be regulated by serine phosphorylation, and such an interaction, plus activation of IRF1 expression and Stat4 tyrosine phosphorylation, would provide a mechanism by which TNF-α and PGE2 activate expression of STAT target genes during human DC maturation.
Acknowledgments
This work was supported by grants from the National Institutes of Health (L. B. I.). We thank Lu Wang and George Kalliolas for critically reviewing the manuscript.
References
- Banchereau J, Steinman R M. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- Medzhitov R. Toll-like receptors and innate immunity. Nat Rev Immunol. 2001;1:135–145. doi: 10.1038/35100529. [DOI] [PubMed] [Google Scholar]
- Toshchakov V, Jones B W, Perera P Y, Thomas K, Cody M J, Zhang S, Williams B R, Major J, Hamilton T A, Fenton M J, Vogel S N. TLR4, but not TLR2, mediates IFN-β-induced STAT1α/β-dependent gene expression in macrophages. Nat Immunol. 2002;3:392–398. doi: 10.1038/ni774. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Hemmi H, Hoshino K, Kaisho T, Sanjo H, Takeuchi O, Sugiyama M, Okabe M, Takeda K, Akira S. Role of adaptor TRIF in the MyD88-independent Toll-like receptor signaling pathway. Science. 2003;301:640–643. doi: 10.1126/science.1087262. [DOI] [PubMed] [Google Scholar]
- Yamamoto M, Sato S, Mori K, Hoshino K, Takeuchi O, Takeda K, Akira S. Cutting edge: a novel Toll/IL-1 receptor domain-containing adapter that preferentially activates the IFN-β promoter in the Toll-like receptor signaling. J Immunol. 2002;169:6668–6672. doi: 10.4049/jimmunol.169.12.6668. [DOI] [PubMed] [Google Scholar]
- Tassiulas I, Hu X, Ho H, Kashyap Y, Paik P, Hu Y, Lowell C A, Ivashkiv L B. Amplification of IFN-α-induced STAT1 activation and inflammatory function by Syk and ITAM-containing adaptors. Nat Immunol. 2004;5:1181–1189. doi: 10.1038/ni1126. [DOI] [PubMed] [Google Scholar]
- Longman R S, Braun D, Pellegrini S, Rice C M, Darnell R B, Albert M L. Dendritic-cell maturation alters intracellular signaling networks, enabling differential effects of IFN-α/β on antigen cross-presentation. Blood. 2007;109:1113–1122. doi: 10.1182/blood-2006-05-023465. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Toll-like receptor control of the adaptive immune responses. Nat Immunol. 2004;5:987–995. doi: 10.1038/ni1112. [DOI] [PubMed] [Google Scholar]
- Hoebe K, Janssen E M, Kim S O, Alexopoulou L, Flavell R A, Han J, Beutler B. Upregulation of costimulatory molecules induced by lipopolysaccharide and double-stranded RNA occurs by Trif-dependent and Trif-independent pathways. Nat Immunol. 2003;4:1223–1229. doi: 10.1038/ni1010. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Kaisho T, Iwabe T, Takeuchi O, Akira S. Differential involvement of IFN-β in Toll-like receptor-stimulated dendritic cell activation. Int Immunol. 2002;14:1225–1231. doi: 10.1093/intimm/dxf089. [DOI] [PubMed] [Google Scholar]
- Honda K, Sakaguchi S, Nakajima C, Watanabe A, Yanai H, Matsumoto M, Ohteki T, Kaisho T, Takaoka A, Akira S, Seya T, Taniguchi T. Selective contribution of IFN-α/β signaling to the maturation of dendritic cells induced by double-stranded RNA or viral infection. Proc Natl Acad Sci USA. 2003;100:10872–10877. doi: 10.1073/pnas.1934678100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stetson D B, Medzhitov R. Type I interferons in host defense. Immunity. 2006;25:373–381. doi: 10.1016/j.immuni.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Remoli M E, Gafa V, Giacomini E, Severa M, Lande R, Coccia E M. IFN-β modulates the response to TLR stimulation in human DC: involvement of IFN regulatory factor-1 (IRF-1) in IL-27 gene expression. Eur J Immunol. 2007;37:3499–3508. doi: 10.1002/eji.200737566. [DOI] [PubMed] [Google Scholar]
- Severa M, Remoli M E, Giacomini E, Ragimbeau J, Lande R, Uze G, Pellegrini S, Coccia E M. Differential responsiveness to IFN-α and IFN-β of human mature DC through modulation of IFNAR expression. J Leukoc Biol. 2006;79:1286–1294. doi: 10.1189/jlb.1205742. [DOI] [PubMed] [Google Scholar]
- O'Doherty U, Steinman R M, Peng M, Cameron P U, Gezelter S, Kopeloff I, Swiggard W J, Pope M, Bhardwaj N. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–1076. doi: 10.1084/jem.178.3.1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rieser C, Bock G, Klocker H, Bartsch G, Thurnher M. Prostaglandin E2 and tumor necrosis factor α cooperate to activate human dendritic cells: synergistic activation of interleukin 12 production. J Exp Med. 1997;186:1603–1608. doi: 10.1084/jem.186.9.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R M, Hawiger D, Nussenzweig M C. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711. doi: 10.1146/annurev.immunol.21.120601.141040. [DOI] [PubMed] [Google Scholar]
- Thomas R, Davis L S, Lipsky P E. Rheumatoid synovium is enriched in mature antigen-presenting dendritic cells. J Immunol. 1994;152:2613–2623. [PubMed] [Google Scholar]
- Steinman R M, Pope M. Exploiting dendritic cells to improve vaccine efficacy. J Clin Invest. 2002;109:1519–1526. doi: 10.1172/JCI15962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldawer L. The tumor necrosis factor superfamily and its receptors. Paul W E, editor. Philadelphia, PA, USA: Lippincott Williams & Wilkins; Fundamental Immunology. 2003:749–773. [Google Scholar]
- Harris S G, Padilla J, Koumas L, Ray D, Phipps R P. Prostaglandins as modulators of immunity. Trends Immunol. 2002;23:144–150. doi: 10.1016/s1471-4906(01)02154-8. [DOI] [PubMed] [Google Scholar]
- Yarilina A, Park-Min K H, Antoniv T, Hu X, Ivashkiv L B. TNF activates an IRF1-dependent autocrine loop leading to sustained expression of chemokines and STAT1-dependent type I interferon-response genes. Nat Immunol. 2008;9:378–387. doi: 10.1038/ni1576. [DOI] [PubMed] [Google Scholar]
- Hu Y, Ivashkiv L B. Costimulation of chemokine receptor signaling by matrix metalloproteinase-9 mediates enhanced migration of IFN-α dendritic cells. J Immunol. 2006;176:6022–6033. doi: 10.4049/jimmunol.176.10.6022. [DOI] [PubMed] [Google Scholar]
- Granelli-Piperno A, Golebiowska A, Trumpfheller C, Siegal F P, Steinman R M. HIV-1-infected monocyte-derived dendritic cells do not undergo maturation but can elicit IL-10 production and T cell regulation. Proc Natl Acad Sci USA. 2004;101:7669–7674. doi: 10.1073/pnas.0402431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I H, Li W P, Hisert K B, Ivashkiv L B. Inhibition of interleukin 2 signaling and signal transducer and activator of transcription (STAT)5 activation during T cell receptor-mediated feedback inhibition of T cell expansion. J Exp Med. 1999;190:1263–1274. doi: 10.1084/jem.190.9.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta T K, Chen A, Zhong Z, Darnell J E, Jr, Ivashkiv L B. Activation of monocyte effector genes and STAT family transcription factors by inflammatory synovial fluid is independent of interferon γ. J Exp Med. 1995;181:1015–1025. doi: 10.1084/jem.181.3.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong Z, Wen Z, Darnell J E., Jr Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Science. 1994;264:95–98. doi: 10.1126/science.8140422. [DOI] [PubMed] [Google Scholar]
- Hu X, Herrero C, Li W P, Antoniv T T, Falck-Pedersen E, Koch A E, Woods J M, Haines G K, Ivashkiv L B. Sensitization of IFN-γ Jak-STAT signaling during macrophage activation. Nat Immunol. 2002;3:859–866. doi: 10.1038/ni828. [DOI] [PubMed] [Google Scholar]
- De Trez C, Pajak B, Brait M, Glaichenhaus N, Urbain J, Moser M, Lauvau G, Muraille E. TLR4 and Toll-IL-1 receptor domain-containing adapter-inducing IFN-β, but not MyD88, regulate Escherichia coli-induced dendritic cell maturation and apoptosis in vivo. J Immunol. 2005;175:839–846. doi: 10.4049/jimmunol.175.2.839. [DOI] [PubMed] [Google Scholar]
- Velten F W, Rambow F, Metharom P, Goerdt S. Enhanced T-cell activation and T-cell-dependent IL-2 production by CD83+, CD25(high), CD43(high) human monocyte-derived dendritic cells. Mol Immunol. 2007;44:1544–1550. doi: 10.1016/j.molimm.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Reddy A, Sapp M, Feldman M, Subklewe M, Bhardwaj N. A monocyte conditioned medium is more effective than defined cytokines in mediating the terminal maturation of human dendritic cells. Blood. 1997;90:3640–3646. [PubMed] [Google Scholar]
- Jonuleit H, Kuhn U, Muller G, Steinbrink K, Paragnik L, Schmitt E, Knop J, Enk A H. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–3142. doi: 10.1002/eji.1830271209. [DOI] [PubMed] [Google Scholar]
- Nguyen V T, Benveniste E N. Involvement of STAT-1 and ets family members in interferon-γ induction of CD40 transcription in microglia/macrophages. J Biol Chem. 2000;275:23674–23684. doi: 10.1074/jbc.M002482200. [DOI] [PubMed] [Google Scholar]
- Li J, Colovai A I, Cortesini R, Suciu-Foca N. Cloning and functional characterization of the 5′-regulatory region of the human CD86 gene. Hum Immunol. 2000;61:486–498. doi: 10.1016/s0198-8859(00)00099-9. [DOI] [PubMed] [Google Scholar]
- Sharif M N, Tassiulas I, Hu Y, Mecklenbrauker I, Tarakhovsky A, Ivashkiv L B. IFN-α priming results in a gain of proinflammatory function by IL-10: implications for systemic lupus erythematosus pathogenesis. J Immunol. 2004;172:6476–6481. doi: 10.4049/jimmunol.172.10.6476. [DOI] [PubMed] [Google Scholar]
- Taniguchi T, Ogasawara K, Takaoka A, Tanaka N. IRF family of transcription factors as regulators of host defense. Annu Rev Immunol. 2001;19:623–655. doi: 10.1146/annurev.immunol.19.1.623. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Kimura T, Kitagawa M, Pfeffer K, Kawakami T, Watanabe N, Kundig T M, Amakawa R, Kishihara K, Wakeham A. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell. 1993;75:83–97. [PubMed] [Google Scholar]
- Kimura T, Nakayama K, Penninger J, Kitagawa M, Harada H, Matsuyama T, Tanaka N, Kamijo R, Vilcek J, Mak T W. Involvement of the IRF-1 transcription factor in antiviral responses to interferons. Science. 1994;264:1921–1924. doi: 10.1126/science.8009222. [DOI] [PubMed] [Google Scholar]
- Darnell J E., Jr STATs and gene regulation. Science. 1997;277:1630–1635. doi: 10.1126/science.277.5332.1630. [DOI] [PubMed] [Google Scholar]
- Luft T, Pang K C, Thomas E, Hertzog P, Hart D N, Trapani J, Cebon J. Type I IFNs enhance the terminal differentiation of dendritic cells. J Immunol. 1998;161:1947–1953. [PubMed] [Google Scholar]
- Chatterjee-Kishore M, Wright K L, Ting J P, Stark G R. How Stat1 mediates constitutive gene expression: a complex of unphosphorylated Stat1 and IRF1 supports transcription of the LMP2 gene. EMBO J. 2000;19:4111–4122. doi: 10.1093/emboj/19.15.4111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Chatterjee-Kishore M, Staugaitis S M, Nguyen H, Schlessinger K, Levy D E, Stark G R. Novel roles of unphosphorylated STAT3 in oncogenesis and transcriptional regulation. Cancer Res. 2005;65:939–947. [PubMed] [Google Scholar]
- Hu X, Paik P K, Chen J, Yarilina A, Kockeritz L, Lu T T, Woodgett J R, Ivashkiv L B. IFN-γ suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]