Abstract
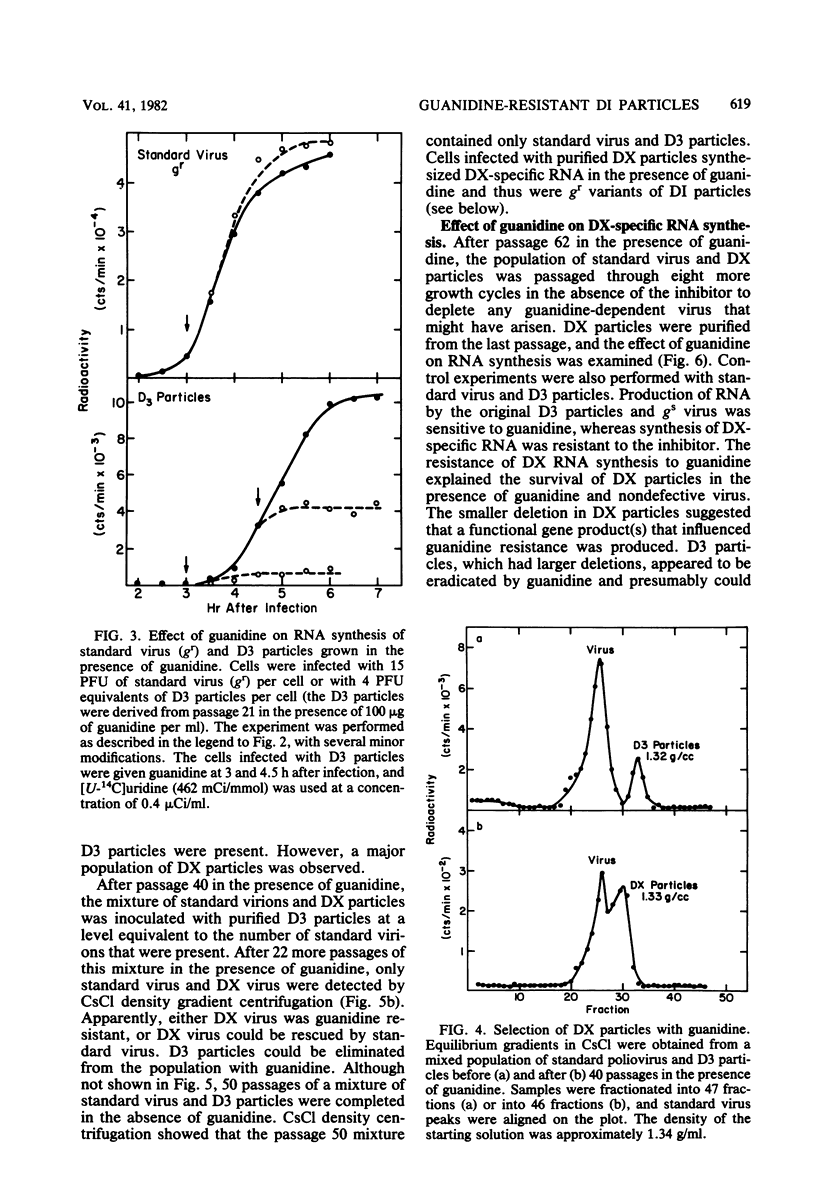
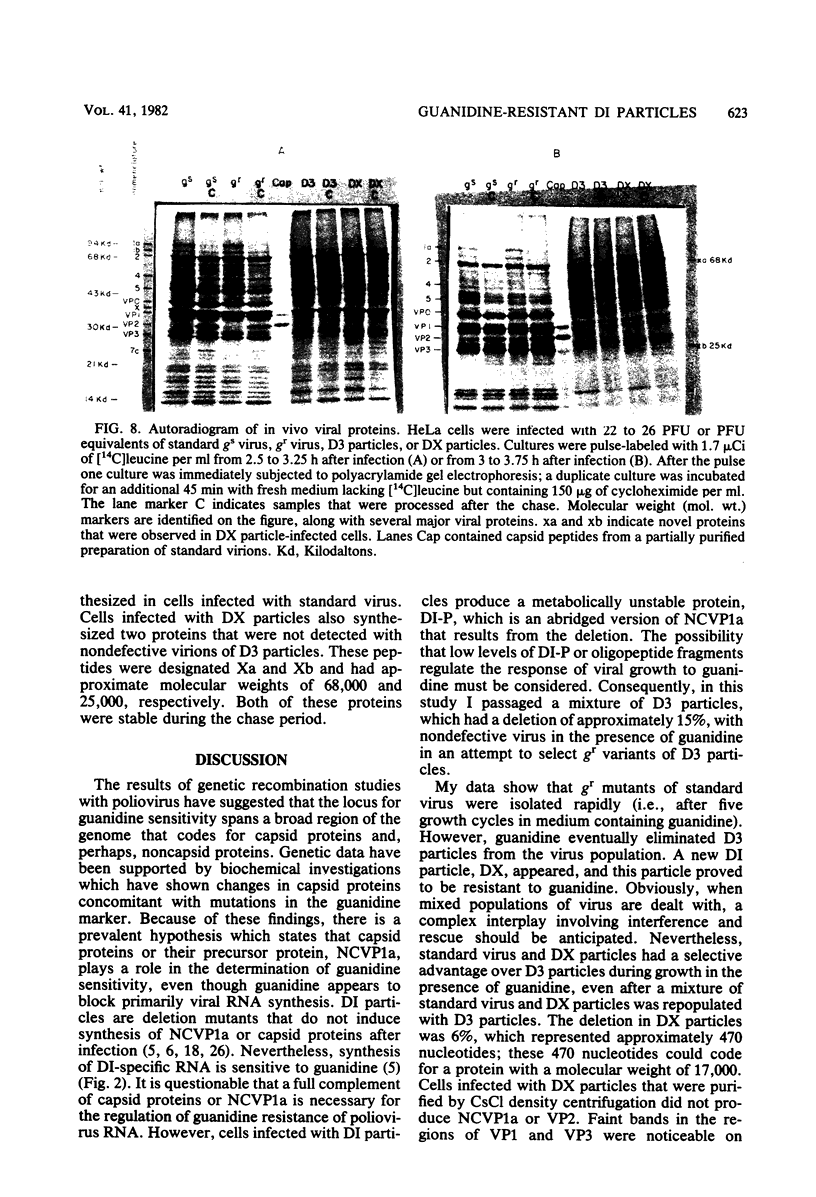
A mixture containing standard poliovirus and D3 particles (mutants with deletions in the capsid locus) was serially passaged in the presence of guanidine. Within five growth cycles, the standard virus was guanidine resistant, but the D3 particles were guanidine sensitive, even after 21 passages with the inhibitor. By passage 40 with guanidine, D3 particles were eliminated, and a new deletion mutant (DX) appeared in the virus population. D3 particles contained a 15% deletion, and DX particles contained a 6% deletion in the capsid locus. Although neither mutant induced the synthesis of NCVP1a or a complete complement of capsid proteins after infection, cells infected with DX particles produced two novel proteins, which had molecular weights of approximately 68,000 and 25,000.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CROWTHER D., MELNICK J. L. Studies of the inhibitory action of guanidine on poliovirus multiplication in cell cultures. Virology. 1961 Sep;15:65–74. doi: 10.1016/0042-6822(61)90078-2. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Action of guanidine on the replication of poliovirus RNA. Virology. 1968 Jul;35(3):408–417. doi: 10.1016/0042-6822(68)90219-5. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Baltimore D. Defective interfering particles of poliovirus. II. Nature of the defect. J Mol Biol. 1973 May 25;76(3):325–343. doi: 10.1016/0022-2836(73)90508-1. [DOI] [PubMed] [Google Scholar]
- Cole C. N., Smoler D., Wimmer E., Baltimore D. Defective interfering particles of poliovirus. I. Isolation and physical properties. J Virol. 1971 Apr;7(4):478–485. doi: 10.1128/jvi.7.4.478-485.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper P. D., Wentworth B. B., McCahon D. Guanidine inhibition of poliovirus: a dependence of viral RNA synthesis on the configuration of structural protein. Virology. 1970 Mar;40(3):480–493. doi: 10.1016/0042-6822(70)90191-1. [DOI] [PubMed] [Google Scholar]
- Dawson W. O. Guanidine inhibits tobacco mosaic virus RNA synthesis at two stages. Intervirology. 1975;6(2):83–89. doi: 10.1159/000149459. [DOI] [PubMed] [Google Scholar]
- Friedman R. M. Basis for variable response of arboviruses to guanidine treatment. J Virol. 1970 Nov;6(5):628–636. doi: 10.1128/jvi.6.5.628-636.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson M. F., Baltimore D. Morphogenesis of poliovirus. I. Association of the viral RNA with coat protein. J Mol Biol. 1968 Apr 28;33(2):369–378. doi: 10.1016/0022-2836(68)90195-2. [DOI] [PubMed] [Google Scholar]
- Koschel K., Wecker E. Early functions of poliovirus. 3. The effect of guanidine on early functions. Z Naturforsch B. 1971 Sep;26(9):940–944. doi: 10.1515/znb-1971-0917. [DOI] [PubMed] [Google Scholar]
- LODDO B., FERRARI W., BROTZU G., SPANEDDA A. In vitro inhibition of infectivity of polio viruses by guanidine. Nature. 1962 Jan 6;193:97–98. doi: 10.1038/193097a0. [DOI] [PubMed] [Google Scholar]
- LODDO B., MUNTONI S., SPANEDDA A., BROTZU G., FERRARI W. Guanidine conditioned infectivity of ribonucleic acid extracted from a strain of guanidine-dependent polio-1-virus. Nature. 1963 Jan 19;197:315–315. doi: 10.1038/197315a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Lee Y. S., Yajima Y., Nonoyama M. Mechanism of infection by Epstein-Barr virus. II. Comparison of viral DNA from HR-1 and superinfected Raji cells by restriction enzymes. Virology. 1977 Aug;81(1):17–24. doi: 10.1016/0042-6822(77)90054-x. [DOI] [PubMed] [Google Scholar]
- Lundquist R. E., Sullivan M., Maizel J. V., Jr Characterization of a new isolate of poliovirus defective interfering particles. Cell. 1979 Nov;18(3):759–769. doi: 10.1016/0092-8674(79)90129-6. [DOI] [PubMed] [Google Scholar]
- McClure M. A., Holland J. J., Perrault J. Generation of defective interfering particles in picornaviruses. Virology. 1980 Jan 30;100(2):408–418. doi: 10.1016/0042-6822(80)90532-2. [DOI] [PubMed] [Google Scholar]
- McLaren L. C., Holland J. J. Defective interfering particles from poliovirus vaccine and vaccine reference strains. Virology. 1974 Aug;60(2):579–583. doi: 10.1016/0042-6822(74)90352-3. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKANO M., IWAMI S., TAGAYA I. A GUANIDINE-DEPENDENT VARIANT OF POLIOVIRUS. ISOLATION OF A VARIANT AND SOME OF ITS BIOLOGICAL PROPERTIES. Virology. 1963 Oct;21:264–266. doi: 10.1016/0042-6822(63)90266-6. [DOI] [PubMed] [Google Scholar]
- Penman S., Summers D. Effects on host cell metabolism following synchronous infection with poliovirus. Virology. 1965 Dec;27(4):614–620. doi: 10.1016/0042-6822(65)90187-x. [DOI] [PubMed] [Google Scholar]
- Phillips B. A., Lundquist R. E., Maizel J. V., Jr Absence of subviral particles and assembly activity in HeLa cells infected with defective-interfering (DI) particles of poliovirus. Virology. 1980 Jan 15;100(1):116–124. doi: 10.1016/0042-6822(80)90557-7. [DOI] [PubMed] [Google Scholar]
- RIGHTSEL W. A., DICE J. R., McALPINE R. J., TIMM E. A., McLEAN I. W., Jr, DIXON G. J., SCHABEL F. M., Jr Antiviral effect of guanidine. Science. 1961 Aug 25;134(3478):558–559. doi: 10.1126/science.134.3478.558. [DOI] [PubMed] [Google Scholar]
- Tershak D. R. Peptide-chain initiation with Lsc poliovirus is intrinsically more resistant to hypertonic environment than is peptide-chain initiation with Mahoney virus and deletion mutants of Mahoney virus. J Virol. 1978 Dec;28(3):1006–1010. doi: 10.1128/jvi.28.3.1006-1010.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma J. P. Inhibition of tobacco necrosis virus by guanidine carbonate. Virology. 1968 Oct;36(2):305–308. doi: 10.1016/0042-6822(68)90149-9. [DOI] [PubMed] [Google Scholar]
- Yin F. H. Involvement of viral procapsid in the RNA synthesis and maturation of poliovirus. Virology. 1977 Oct 15;82(2):299–307. doi: 10.1016/0042-6822(77)90005-8. [DOI] [PubMed] [Google Scholar]





