Abstract
In this study, the early innate cytokine and chemokine response of murine dendritic cells (DCs) and macrophages to Mycobacterium tuberculosis infection was compared. The findings indicate a dissimilar gene expression pattern between the two cell types. The expression of IL-12 and IL-23, important for promoting Th1 and Th17 cells, respectively, was up-regulated only in DCs. In addition, expression of CCL1 and CCL17, which are important in recruitment of T regulatory cells, was DC-specific, as was the expression of the immunosuppressive cytokine IL-10. Macrophages, in contrast, exhibited enhanced expression for CCL2 and CXCL10, chemokines that recruit cells to sites of inflammation, and for mycobactericidal molecules NO synthase 2 and TNF. Together, the findings suggest that a component of the innate DC response is not only programmed toward Th1 priming but is also for controlling the magnitude of the Th1 response, and part of the macrophage response is intended for recruiting cells to the lung and for mycobactericidal functions.
Keywords: IL-12, IL-23, Th1, NOS, CXCL10
INTRODUCTION
Dendritic cells (DCs) and macrophages are central regulators of early innate immunity. Following pulmonary infection with Mycobacterium tuberculosis, both of these cell types are activated rapidly in the lungs [1,2,3] and produce an array of chemokines and cytokines. However, the interaction of these cells with M. tuberculosis initiates distinct signaling pathways. DCs infected with M. tuberculosis produce IL-12 [4], migrate to the draining lymph nodes [5], and participate in the development of Th1 cells [5, 6]. In contrast, following M. tuberculosis infection, macrophages do not produce IL-12 or migrate to the draining lymph nodes and consequently, are inefficient at initiating T cell responses [4, 5]. These data indicate that DCs and macrophages have distinct roles to play during the early innate response to M. tuberculosis. Cytokines and chemokines are key participants in the early innate response, and so, the goal of the present study was to use a global expression analysis and determine if besides IL-12, other chemokines and cytokines are regulated differentially in DCs and macrophages following M. tuberculosis infection.
Studies examining the gene activation program in macrophages induced by M. tuberculosis have been reported [7]. However, only one study has compared the degree to which DC and macrophage responses to M. tuberculosis are distinct or similar at the gene transcriptional level [8]. A key difference between the previously published work and our current study is the length of host cell exposure to M. tuberculosis. In our study, gene expression was analyzed after a 4-h cell contact with M. tuberculosis. This short exposure time most likely is indicative of early gene expression occurring in response to M. tuberculosis interaction with TLRs and other innate receptors. The published work examined gene modulation following a 16-h exposure to M. tuberculosis and thus, reflects reprogramming that may have taken place as a result of secondary influences from cytokines and chemokines that are secreted after infection. Therefore, in this study, we have been able to identify genes that were not recognized previously to be differentially regulated in DCs and macrophages in response to M. tuberculosis infection in vitro. In addition, the differential expression pattern of some genes was extended to DCs and macrophages isolated from lungs of M. tuberculosis-infected mice.
MATERIALS AND METHODS
Mice and mycobacteria
BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). M. tuberculosis Erdman strain (Trudeau Institute, Saranac Lake, NY, USA), obtained after mouse passage, was grown in culture, titrated and stored at −70ºC. Before infection, aliquots were thawed, briefly sonicated, and then added to cultures at a multiplicity of infection of three.
Macrophages and DC preparation
Bone marrow-derived DCs were prepared as described previously [4]. Briefly, bone marrow was flushed out from the femur and tibia, and 2 × 106 bone marrow cells were seeded into 10 cm Petri dishes in 10 ml RPMI containing 10% FBS (HyClone Laboratories, Logan, UT, USA) and supplemented with penicillin (100 U/ml), streptomycin (100 μg/ml), glutamine (2 mM), 2-ME (50 μM), and 20 ng/ml murine recombinant (r)GM-CSF; (Peprotech, Rocky Hill, NJ, USA). On Day 3, an additional 10-ml complete medium containing rGM-CSF was added to the cultures. On Day 7, the cultures were fed by changing 50% of medium. Day 9 cultures were used in all experiments, and we routinely obtained ∼75% DC purity (CD11b+c+Gr-1−). A representative FACS analysis, presented in Supplemental Figure 1, shows that 72% of cells in bone marrow cultures supplemented with GM-CSF were CD11b+c+, and the rest were CD11b+c−. Of the CD11b+c+ cells, only 4% were Gr-1-positive, and of the CD11b+c–cells, ∼50% were Gr-1-positive. Consistent with previous reports [9, 10], the FACS analysis indicates that the cultures contain predominantly DCs and a small percentage of granulocytes and macrophages.
For microarray studies, on Day 9, nonadherent cells were purified further using the MACS anti-CD11c magnetic beads and positive selection columns (Miltenyi Biotec Inc., Auburn, CA, USA), according to the manufacturer’s instruction. The enriched populations were subjected to FACS analysis for surface expression of CD11c+CD11b+, and purity was >95%.
For obtaining bone marrow-derived macrophages, cells were grown in D10 medium (DMEM; Mediatech, Herdon, VA, USA), containing 10% FBS (HyClone Laboratories), penicillin (100 U/ml), streptomycin (100 μg/ml), and sodium pyruvate (1 mM) and supplemented with 20% L929 cell-conditioned medium on Days 0 and 4. On Day 7, cells were harvested and rested overnight in media lacking L cell-conditioned medium prior to infection with M. tuberculosis. The purity of the macrophage population (CD11b+F-480+Gr-1−) was ∼95%.
Cytokine assays
The presence of cytokines in the supernatants was determined by sandwich ELISA using the following antibody pairs from BD PharMingen (San Diego, CA, USA): C15.6 and C17.8 (biotinylated) for IL-12p40; 9A5 and C17.8 (biotinylated) for IL-12p70; and JES5-2A5 and JES5-16E3 (biotinylated) for IL-10. Detection of CXCL10 and IL-23 was performed with ELISA kits from R&D Systems (Minneapolis, MN, USA). CCL2 and CCL5 were evaluated with ELISA kits purchased from Peprotech.
Microarray
Microarray experiments and basic analysis were performed by the Neuroscience Gene Expression Laboratory (Rutgers University, New Brunswick, NJ, USA) with bioinformatics provided on the website (http://www.ngelab.org). Mouse probes (21,997; of which 21,578 were unique oligonucleotides), from Compugen Sigma Genosys (Rockville, MD, USA), were printed on custom microarrays at the Neuroscience Gene Expression Laboratory (Rutgers University). Total RNA was isolated from cells using TRIzol reagent (Invitrogen, Calsbad, CA, USA) and RNeasy columns (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. RNA samples were quantified by spectrophotometry. All samples had a A260/A280 ratio of between 1.7 and 2.1.
The three-dimensional nucleic acid (3DNA) dendrimer labeling technology was used to prepare the hybridization target (Array 350HS dendrimer system, Genisphere, Hatfield, PA, USA), as it provides a more predictable and consistent signal than direct or indirect dye incorporation. 3DNA is a highly branched molecule assembled from individual strands of DNA that are covalently linked and thus, resistant to denaturation. Oligos and fluoroprobes can be readily incorporated into the layered structure of the dendrimer, providing a labeling technology for microarray experiments, where dyes do not have to be incorporated during the cDNA preparation. The underlying principle of the technology includes reverse-transcribing RNA using deoxynucleotide triphosphate mix and special RT primer oligo that contains a capture sequence on the 5′ end. The resulting cDNA thus has a tag on its 5′ end. Next, a two-step hybridization is performed, where the cDNA is first hybridized to the microarray, and the binding of cDNA is quantitated by a second hybridization with the fluorescent-labeled 3DNA reagent, as it contains the complementary capture sequences.
cDNA was prepared from 5 μg total cellular RNA, extracted from each of the uninfected and infected macrophage and DC cultures as described. RNA from uninfected cells was combined with 2 pmol Genisphere oligo-dT18 primer with attached “capture” sequence for Cy3, and RNA from infected cells was combined with 2 pmol Genisphere oligo-dT18 primer with an attached capture sequence for Cy5. The RNA was reverse-transcribed with SuperScript II RT (Invitrogen), 10 mM unlabeled dNTP mix, and RNase inhibitor. Reactions were terminated by the addition of 3.5 μl 0.5 M NaOH/50 mM EDTA for 10 min at 65°C to denature the DNA/RNA hybrids and degrade the RNA. After a pulse spin, 5 μl 1 M Tris-HCl, pH 7.5, was added for neutralization. The cDNA was concentrated and purified using YM30 columns (Millipore, Beford, MA, USA).
Next, two-step, automated hybridization was performed using the Discovery workstation (Ventana Medical Systems, Tuscon, AZ, USA). Purified cDNAs (prepared from uninfected cells and its infected counterpart) were added to 200 μl ChipHyb solution (Ventana Medical Systems) and hybridized at 58°C for 12 h. Microarrays were washed on the instrument, twice in 2× SSC for 10 min at 55°C and once in 2× SSC for 2 min at 42°C to wash away unbound cDNA. The second hybridization was carried out with Cy3- and Cy5-labeled dendrimers containing the appropriate complement of the capture sequence. The microarrays were washed on the Discovery workstation (Ventana Medical Systems) to remove unbound dendrimers. Arrays were spin-dried in a centrifuge and scanned on an Axon GenePix 4000B (Axon Instruments, Union City, CA, USA). The gain for each laser was adjusted so that ratios of positive control spots were equal to 1. Image files were processed using Axon GenePix 4.0 software. Normalization and data analysis were conducted in Gene-Spring (Silicon Genetics, Redwood City, CA, USA), using the Lowess method of normalization.
Mouse infection and isolation of CD11b+ cells and CD11c+ cells from infected lung
The Erdman strain of M. tuberculosis was used to infect mice via aerosol (∼100 CFU), and lungs were removed at 2 and 3 weeks postinfection. Lungs were perfused with PBS containing 50 U/ml heparin and digested with collagenase D at 2 mg/ml (Roche, Indianapolis, IN, USA) at 37°C for 20 min to obtain single-cell suspensions. RBC were lysed with RBC lysis buffer. The lung CD11c+ cell population was purified using the MACS anti-CD11c-positive selection column (Miltenyi Biotec Inc.). For the CD11b+ cells, the flow-through cells from the anti-CD11c column were admixed with anti-CD11b magnetic beads, and a positive selection column was used (Miltenyi Biotec, Auburn CA, USA). The cells were spun down and lysed in TRIzol, and the RNA was isolated as described in the microarray section.
Real-time PCR
Total RNA was reverse-transcribed using Superscript II enzyme (Invitrogen), as directed by the manufacturer. The PCR reactions were performed in an Mx3000P machine (Stratagene, La Jolla, CA, USA) to generate cycles [comparative threshold cycle (Ct)]. CCL1 and CXCL10 primers were purchased from Superarray (Frederick, MD, USA). Other primer sequences are as follows: p19 (forward, 5′-GGGAACAAGATGCTGGATT-3′, p19 reverse, 5′-CTTCACACTGGATACGGGG-3′), IL-6 (forward, 5′-TTC CAT CCA GTT GCC TTC TTG-3′, reverse, 5′-CTA CCT GGA GTA CAT GAA G-3′), CCL2 (forward, 5′-TCA CCT GCT GCT ACT CAT TCA-3′, reverse, 5′-GGA GTG ACC AGT GTG ACA GTG-3′), CCL5 (forward, 5′-AGT CGA TCT CCC ACA GCC-3′, reverse, 5′-GGGT TTC TTG ATT CTG ACC CTG-3′), CCL17 (forward, 5′-CAG GAA GTT GGT GAG CTG GTA T-3′, reverse, 5′-TAT GCA CTA CAG GCG AAC ACA A-3′), NO synthase 2 (NOS2; forward, 5′-TGC CCC TTC AAT GGT TGG TA-3′, reverse, 5′-ATT TGG CTG GTC CCT CCA GT-3′), IL-12p40 (forward, 5′-CCA GAG ACA TGG AGT CAT AG-3′, reverse, 5′-AGA TGT GAG TGG CTC AGA GT-3′), IL-10 (forward, 5′-TCCAAGACCAAGGTGTCTAC-3′, reverse, 5′-TATTGAGTCTGCTGGACTCC-3′), TNF-α (forward, 5′-GACGTGGAACTGGCAGAAGA-3′, reverse, 5′-CTCATTCCTGCTTGTGGCAG-3′), IFN-β forward, 5′-CTCCAGCTCCAAGAAAGGGACG-3′, reverse, 5′-CGAAGACTTACCAGAAACTTC-3′), IL-17 (forward, 5′-GCTCCAGAAGGCCCT-3′, reverse, 5′-ATGCGGAGGGAAAGCT-3′), and β-actin (forward, 5′-CCG TGA AAA GAT GAC CCA GAT C-3′, reverse, 5′-ACG TAC CCA TCC AGG CTG TG-3′).
Relative gene expression was calculated as 2(–ΔΔCt), where ΔCt = Ct (gene of interest) – Ct (normalizer=β-actin). Using the relative gene expression values, the fold change (ΔΔCt) was calculated as ΔCt (sample)/ΔCt (calibrator). The calibrator was pooled uninfected macrophages (for in vitro experiments) or pooled CD11b+c− cells from several uninfected mice (for in vivo experiments).
RESULTS
Cytokine and chemokine gene expression of M. tuberculosis-infected DCs and macrophages
The microarray data (Supplemental Tables 1 and 2) were first examined to establish a list of all cytokine and chemokine genes whose mRNA expression was elevated greater than twofold in M. tuberculosis-infected bone marrow-derived DCs or macrophages. As shown in Tables 1 and 2, expression of several cytokines and chemokines was up-regulated greater than twofold in macrophages or DCs in at least two of the three replicate samples. However, for some genes, we observed a differential expression pattern between the two APC types. The fold-increase in mRNA expression for IL-6, IL-10, IL-12p40, and p19 subunits of IL-23 was higher in M. tuberculosis-infected DCs compared with macrophages, and that of IL-1α, TNF, and MIF was higher in infected macrophages compared with DCs (Table 1). M. tuberculosis infection up-regulated transcript levels for NOS2 similarly in both cell types (Table 1). A consistent gene expression pattern in the two cell types was not observed for IL-1β and IFN-β. With regards to chemokine gene expression, transcript levels for CCL3, CCL6, CCL9, CCL22, CXCL1, CXCL2, CXCL5, and CXCL11 were up-regulated similarly in infected DCs and macrophages (Table 2). CCL4 expression was inconsistent in macrophage replicate samples. However, the mRNA expression levels for CCL1 (I-309) and CCL17 [thymus and activation-regulated chemokine (TARC)] were up-regulated several-fold higher in infected DCs than macrophages, while CCL2 (MCP-1), CCL5, CCL12, CCL24, CXCL4, and CXCL10 (IFN-γ-inducible protein-10) were up-regulated several-fold higher in infected macrophages compared with DCs (Table 2). Together, these data indicate that the cytokine and chemokine gene induction program differs between DCs and macrophages following infection with M. tuberculosis.
TABLE 1.
Differential Cytokine Gene Expression in Macrophages and DCs
| Gene access # | Common name | DC1 | DC2 | DC3 | Mac1 | Mac2 | Mac3 |
|---|---|---|---|---|---|---|---|
| NM_010554 | IL-1α | 37.0 | 25.6 | 42.3 | 54.4 | 122.7 | 139.8 |
| NM_008361 | IL-1β | 40.8 | 30.3 | 11.9 | – | 53.0 | – |
| J03783 | IL-6 | 81.0 | 38.0 | 27.3 | 9.4 | 5.8 | 26.4 |
| NM_010548 | IL-10 | 8.5 | 58.3 | 101.2 | 22.8 | 22.9 | 16.1 |
| M86671 | IL-12b | 7.0 | 10.2 | 11.6 | 2.8 | 2.7 | – |
| NM_008357 | IL-15 | 6.2 | 5.8 | 7.0 | 3.6 | 3.3 | 5.3 |
| NM_008360 | IL-18 | 1.9 | 5.7 | – | 3.9 | 19.6 | 9.2 |
| AF301619 | IL-23, α subunit p19 | 15.4 | 4.3 | 10.3 | 10.7 | 1.8 | 1.6 |
| NM_013693 | TNF | 41.0 | 24.6 | 19.7 | 64.6 | 74.1 | 46.5 |
| NM_010798 | macrophage migration inhibitory factor (MIF) | 2.0 | 1.0 | 2.3 | 4.8 | 3.8 | 5.3 |
| NM_010510 | IFN-β | 24.0 | 4.9 | 4.9 | 9.8 | 2.2 | 4.2 |
| NM_010927 | NOS2 | 21.11 | 22.28 | 34.01 | 14.07 | 14.61 | 13.52 |
Total RNA was isolated from uninfected DCs and macrophages and from both cell types after 4 h of M. tuberculosis infection. The RNA was converted to cDNA and subjected to a microarray analysis as described in Materials and Methods. Changes in gene expression are represented as the ratio of signal intensities of infected cells to uninfected cells. Values represent gene expression changes from three separate experiments. – indicates that gene expression of infected and uninfected samples was below the background signal. Mac, Macrophages.
TABLE 2.
Differential Chemokine Gene Expression in Macrophages and DCs
| Gene access # | Common name | DC1 | DC2 | DC3 | Mac1 | Mac2 | Mac3 |
|---|---|---|---|---|---|---|---|
| NM_011329 | chemokine (C-C motif) ligand 1 | 59.9 | 17.2 | 9.9 | 0.8 | – | – |
| NM_011333 | chemokine (C-C motif) ligand 2 | 15.8 | 17.1 | 9.1 | 108.0 | 117.1 | 57.0 |
| NM_011337 | chemokine (C-C motif) ligand 3 | 37.3 | 18.8 | 22.7 | 16.9 | 13.8 | 19.8 |
| NM_013652 | chemokine (C-C motif) ligand 4 | 25.3 | 23.4 | 47.4 | 117.2 | 15.0 | 32.3 |
| NM_013653 | chemokine (C-C motif) ligand 5 | 6.7 | 7.1 | 7.4 | 38.9 | 49.4 | 49.5 |
| NM_009139 | chemokine (C-C motif) ligand 6 | 2.1 | 2.2 | 2.1 | 1.6 | 0.8 | 1.3 |
| NM_011338 | chemokine (C-C motif) ligand 9 | 1.5 | 2.5 | 2.1 | 4.1 | 2.2 | 11.3 |
| NM_011331 | chemokine (C-C motif) ligand 12 | 2.0 | 4.8 | 2.6 | 8.8 | 6.9 | 6.3 |
| NM_011332 | chemokine (C-C motif) ligand 17 | 23.4 | 6.7 | 9.8 | – | – | – |
| NM_009137 | chemokine (C-C motif) ligand 22 | 8.5 | 11.8 | 18.3 | 14.3 | 12.0 | 29.5 |
| NM_019577 | chemokine (C-C motif) ligand 24 | 1.2 | 1.3 | 1.1 | 22.1 | 14.0 | 18.3 |
| NM_008176 | chemokine (C-X-C motif) ligand 1 | 83.5 | 68.8 | 103.0 | 93.2 | 135.0 | 144.2 |
| NM_009140 | chemokine (C-X-C motif) ligand 2 | 66.6 | 19.9 | 22.8 | 85.0 | 54.5 | 9.1 |
| NM_019932 | chemokine (C-X-C motif) ligand 4 | 3.2 | 1.3 | 1.1 | 6.0 | 10.4 | 5.3 |
| NM_009141 | chemokine (C-X-C motif) ligand 5 | 5.2 | 6.5 | 4.0 | 4.3 | 2.3 | 4.7 |
| NM_021274 | chemokine (C-X-C motif) ligand 10 | 3.9 | 3.6 | 3.0 | 56.1 | 13.9 | 12.5 |
| NM_019494 | chemokine (C-X-C motif) ligand 11 | 10.1 | 7.8 | 8.3 | – | 7.9 | 7.6 |
Total RNA was collected from uninfected DCs and macrophages and from both cell types after 4 h of M. tuberculosis infection. The RNA was converted to cDNA and subjected to microarray analysis as described in Materials and Methods. Changes in gene expression are represented as the ratio of signal intensities of infected cells to uninfected cells. Values represent gene expression changes from three separate experiments. – indicates that gene expression of infected and uninfected samples was below the background signal.
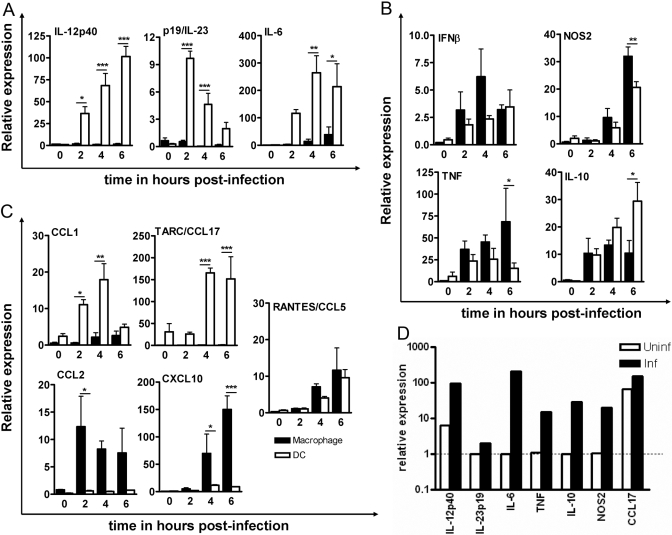
A real-time PCR assay was performed as a function of time to further validate the differential expression of some of the cytokine and chemokine genes detected by microarray analysis. Microarray data represent the fold-change between the uninfected and infected DCs or macrophages and not between DCs and macrophages. To compare target gene expression between DCs and macrophages, gene expression of uninfected macrophages was used as a calibrator (relative value=1).
Overall, the real-time PCR results were in agreement with the microarray findings, albeit the magnitude of change in the expression of the genes varied. Akin to microarray findings, M. tuberculosis infection significantly up-regulated expression of IL-12p40, IL-23p19, and IL-6 in DCs but not in macrophages (Fig. 1A). IL-12p40 and IL-6 mRNA transcripts were present in infected DCs as early as 2 h following infection, and their expression continued to increase over the 6-h experimental period with significantly higher expression in DCs compared with macrophages at all time-points. Interestingly, p19 expression in DCs peaked at 2 h following infection, and by 6 h, expression declined to baseline, and no expression was detectable in macrophages. Also, consistent with the microarray data, IFN-β, NOS2, and TNF expression was similar in the two cell types by real-time PCR (Fig. 1B), although expression of NOS2 was considerably delayed in both cell types. At 6 h postinfection, significantly higher expression of NOS2 and TNF was detected in macrophages in comparison with DCs. There was an equivalent expression of IL-10 in DCs and macrophages shortly following infection, but as infection progressed, expression was significantly higher in DCs compared with macrophages (Fig. 1B).
Fig. 1.
Confirmation of microarray data by real-time PCR. Total RNA from DCs and macrophages was assayed for gene expression at 0, 2, 4, and 6 h after M. tuberculosis infection by real-time PCR. The following genes were examined: IL-12p40, IL-23p19, and IL-6 in infected macrophages and DCs (A), IFN-β, NOS2, TNF, and IL-10 in infected macrophages and DCs (B), CCL1, CCL2, CCL17, CCL5, and CXCL10 in infected macrophages and DCs (C), and IL-12p40, IL-23p19, IL-6, TNF, NOS2, IL-10, and CCL17 in DCs cultured in vitro for 6 h in the presence of M. tuberculosis infection (D). Gene expression was normalized to β-actin, and the fold-change was calculated with respect to gene expression in pooled, uninfected macrophages (universal calibrator). Data are from three individual experiments and are expressed as the mean ± sd. The presence of significant differences in gene expression between DCs and macrophages was calculated by a two-way ANOVA. *, **, ***, P < 0.05, 0.01, and 0.001, respectively. In panels A, B, and C, open bars represent macrophage and closed bars represent DC. In panel D, open bars represent uninfected culture and closed bars represent infected culture.
To ensure that in vitro culturing of DCs did not in itself up-regulate gene expression, we studied mRNA expression for several cytokines and chemokines in DCs that had been cultured for 6 h in the absence of M. tuberculosis infection. As shown in Figure 1D, DCs cultured for 6 h did not exhibit up-regulation of IL-23p19, IL-6, TNF, IL-10, or NOS2 but showed a low-level induction of the IL-12p40 gene. Nevertheless, in the presence of M. tuberculosis, the induction of the IL-12p40 gene was significantly higher, indicating infection-specific induction.
The restricted expression of CCL1 and CCL17 to DCs was also validated by real-time PCR analysis. As shown in Figure 1C, CCL1 and CCL17 expression was up-regulated significantly only in infected DCs (Fig. 1C). However, a significant CCL17 expression was seen in DCs cultured for 6 h in vitro. Despite the high basal level expression in uninfected cells, CCL17 expresssion was up-regulated further following M. tuberculosis infection in DCs (Fig. 1D). Again, consistent with the microarray data, macrophages expressed significantly higher CCL2 and CXCL10 compared with DCs. The higher CCL5 expression found in macrophages by microarray could not be confirmed by RT-PCR, as in the latter analysis, CCL5 expression was found to be similar in the two cell types (Fig. 1C). The differential expression pattern of some select cytokine and chemokine genes was further confirmed by examining their protein levels by ELISA.
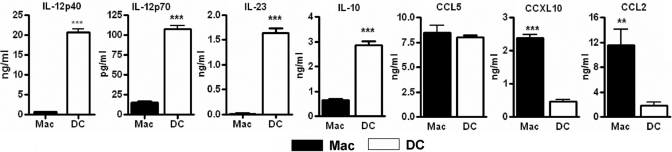
Consistent with the gene expression pattern, comparative analysis of supernatants from infected DCs and macrophages showed that CCL2 and CXCL10 release was significantly higher in macrophages, and release of IL-12p40, IL-12p70, IL-23, and IL-10 was enhanced significantly in DCs (Fig. 2). CCL5 release was similar in infected DCs and macrophages (Fig. 2). Expression of uninfected supernatants was below detection limits for all of the tested cytokines and chemokines (data not shown).
Fig. 2.
Differential cytokine and chemokine production from macrophages and DCs in response to M. tuberculosis. Cells were infected with M. tuberculosis. After 20 h, supernatants were harvested and analyzed for the presence of IL-12p40, IL-12p70, IL-23, IL-10, CCL2, CCL5, and CXCL10 by sandwich ELISA. **, P < 0.01; ***, P < 0.001.
In vivo expression of CXCL10, IL-12p40, and CCL17 in lung DCs and macrophages
To validate the relevance of the in vitro findings, expression of CXCL10, IL-12p40, and CCL17 was analyzed further in the lungs of mice that were infected by aerosol with ∼100 CFU of the virulent Erdman strain of M. tuberculosis. CD11b+CD11c− cells and CD11c+ cells were isolated from the lungs of infected mice at 2 and 3 weeks postinfection. Total RNA was isolated from the CD11b+CD11c− and CD11c+ population, and real-time RT-PCR was performed to evaluate the expression level of CXCL10, CCL17, and IL-12p40 in the two-cell populations. Relative gene expression in the two isolated cell populations from infected lungs was calculated by using gene-expression level in the CD11b+CD11c− population isolated from the lungs of uninfected mice as the calibrator. IL12p40 (Fig. 3A) and CCL17 (Fig. 3B) were highly expressed in lung CD11c+ cells compared with CD11b+CD11c− cells at Week 2 of infection, whereas enhanced CXCL10 expression was observed in lung CD11b+CD11c− cells compared with CD11c+ cells (Fig. 3C). A similar enhanced CXCL10 expression was observed in CD11b+CD11c− lung cells compared with CD11c+ cells at Week 3 of infection (Fig. 3C). Interestingly, a small increase in IL-12p40 gene expression was also observed in the CD11b+CD11c− lung cells isolated at 3 weeks following infection (Fig. 3A). It is possible that as infection progresses, Th1 cells are recruited to the lung, and the IFN-γ released from these cells up-regulates IL-12p40 expression in macrophages. This possibility is supported by in vitro studies, where IL-12p40 expression was enhanced following M. tuberculosis infection of IFN-γ-primed macrophages [4]. Together with the in vitro findings, these in vivo data strongly support that DCs and macrophages respond differently upon infection with M. tuberculosis, suggesting their distinct roles in the early host innate immune response to M. tuberculosis infection.
Fig. 3.
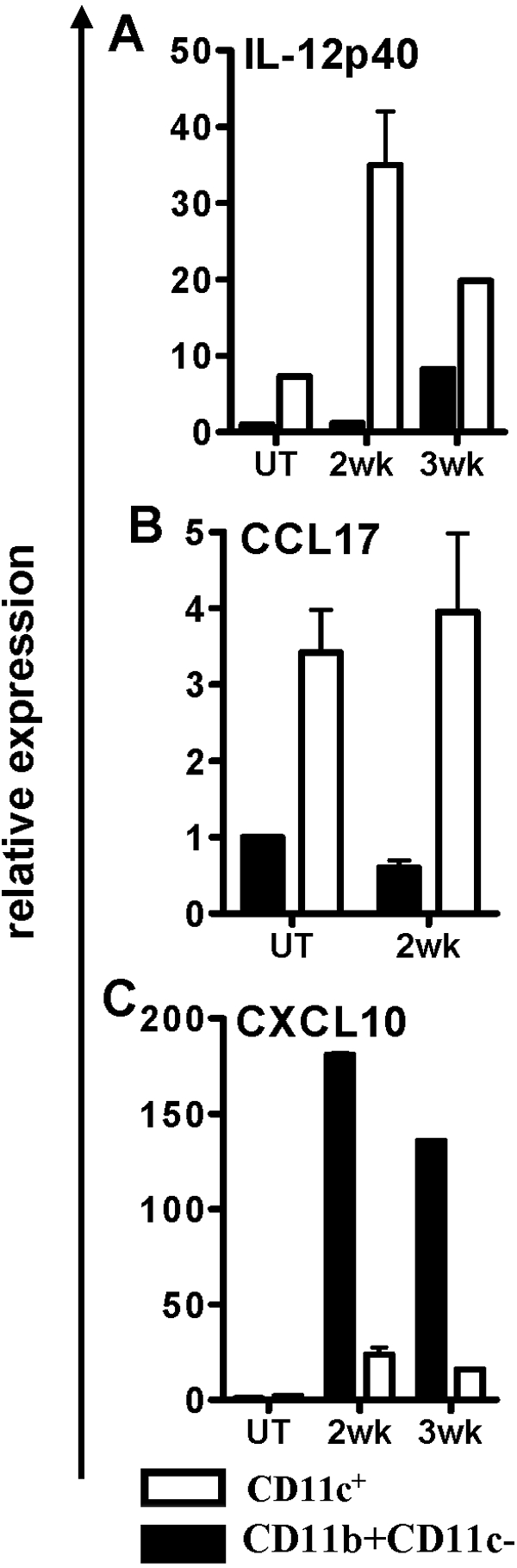
In vivo expression of IL-12p40, CCL17, and CXCL10. Lung CD11c+ cells or CD11b+ cells were isolated 2 and 3 weeks following aerosol infection of mice with M. tuberculosis and assayed for expression of IL-12p40 (A), CCL17 (B), and CXCL10 (C) by real-time PCR. Lung CD11b+ cells from uninfected mice (UT) were used as the calibrator. Lungs from three mice were pooled for each experiment and assayed in triplicate. Two separate experiments were performed for the 2-week time-point and one experiment for the 3-week time-point.
DISCUSSION
Previously, we had shown that following M. tuberculosis infection, DCs release a significantly high amount of IL-12, while secretion is highly limited in macrophages [4]. The present study confirms and extends our previous finding and demonstrates that in addition to IL-12, there are several other cytokines and chemokines that are differentially modulated in DCs and macrophages upon M. tuberculosis infection. Based on these findings, we posit that macrophages and DCs have distinct roles to play in host immune response to M. tuberculosis.
The bioactive IL-12p70 is a heterodimeric protein consisting of covalently linked p40 and p35 subunits, both of which are regulated independently [11]. The production of IL-12 is essential for the development of host Th1 immunity and resistance against intracellular pathogens [12]. IL-23 is another proinflammatory type-1 cytokine, consisting of IL-12p40 heterodimerized to the IL-12p35 homologue p19 and participates in promoting type-1 immunity (reviewed in ref. [13]) and is a proximal trigger for IL-17 release from T cells [14]. IL-23 was also shown to be required in the establishment of IL-17-producing T cells in the lungs of M. tuberculosis-infected mice [15, 16]. Our data show that DCs, and not macrophages, up-regulate IL-12 and IL-23 in response to M. tuberculosis infection, suggesting that the early innate response of DCs is perhaps important for promoting the Th1 and Th17 lineages.
Interestingly, DCs also show enhanced expression of CCL1 and CCL17, chemokines that have been implicated in the recruitment of cells that can dampen Th1 responses. Chemokine receptor CCR8 is constitutively expressed on CD4+CD25+forkhead box p3+ T regulatory cells (Tregs), and its ligand CCL1 has been shown to selectively attract Tregs [17]. The IL-10-secreting, CD4+CD25+ Treg population [18] and skin-homing Tregs [19] have also been reported to express CCR8. Blood-borne CD4+CD25+ Tregs also express CCR8 and strongly respond to CCL1. The specific, functional, high-affinity receptor for TARC/CCL17 is CCR4, and this chemokine receptor is expressed by CD4+CD25+ Tregs [20]. In studies addressing the mechanism of how Tregs are recruited to tolerized cardiac allografts, it was noted that Tregs depended on CCR4 expression for directed migration [21]. In addition, DCs released significantly more IL-10, which can potentially temper the innate immune response. Thus, the cytokine and chemokine response of M. tuberculosis-infected DCs positions it to function as an initiator and a moderator of Th1 cell induction in draining lymph nodes.
M. tuberculosis-infected DCs have been shown to up-regulate their expression of CXCL10 via a IFNR-αβ-dependent manner [22]. We also find a small increase in CXCL10 in infected DCs, but the new finding from the present study is the significantly enhanced gene expression and release of CXCL10 in infected macrophages. The early IFN-β secretion from macrophages may explain the enhanced CXCL10 release from this cell type.
CXCL10 binds to CXCR3-A, which is expressed on Th1 cells and NK cells [23, 24]. Thus, the elevated secretion of CXCL10 by M. tuberculosis-infected macrophages may serve to recruit Th1 cells from the draining lymph nodes to the lung and NK cells from the periphery to initiate the lung granuloma response. That the macrophage-derived CXCL10 has a role in regulating the granulomatous response in the lung is supported by a previous detailed study of chemokine expression in vivo during M. tuberculosis infection in TNF-neutralized mice [25]. In that study, the authors have shown that CD11b+ cells isolated from the disorganized lung granulomas of TNF-neutralized animals have reduced CXCL10 expression compared with cells isolated from wild-type mice. CXCL10 also promotes Th1 dominance at sites of inflammation [26], and binding to CXCR3-A has been shown to up-regulate Th1-specific T box transcription factor (T-bet) expression and concomitantly down-regulate GATA binding protein 3 (GATA3) expression in T cells [27]. Thus, in addition to recruiting T cells to the granuloma, CXCL10 from M. tuberculosis-infected macrophages could selectively promote and enhance the Th1 response in the lung. In contrast, a recent study reported that the expression of CXCL10 is down-regulated in mycobacteria-infected alveolar macrophages [28]. A reason for lack of up-regulation of CXCL10 gene expression could be a result of the fact that the animals in this study were infected with bacillus Calmette-Guerin, and additionally, the infection was carried out intratracheally at a high inoculum of 2 × 105 bacteria.
Flow cytometric characterization of lung phagocytic cells has shown that several different cell types including CD11b−/CD11c+/high alveolar macrophages, CD11b+/CD11c+ myeloid DCs, and CD11b+/CD11c–/low-recruited macrophages take up M. tuberculosis following an aerosol infection [29, 30]. As alveolar macrophages express CD11c, our cell purification protocol for DCs could have included this subset. However, a recent study using GFP-labeled M. tuberculosis for tracking infected cells has shown that the cell types predominantly containing the pathogen, as infection progresses in the lung, are the myeloid DCs and recruited macrophages. This supports our reasoning that the enhanced IL-12p40 and CCL17 gene expression in the purified CD11c+ population from the 2- and 3-week-infected lungs is attributable to myeloid DCs.
Overall, findings from this study provide insight into the early events in gene expression in murine macrophages and DCs in response to M. tuberculosis infection and for the first time, provide definitive evidence that the two cell types have distinct roles to play following a pulmonary infection with M. tuberculosis. We propose a model wherein immature DCs in the lung are induced to differentiate and mature in response to infection. The mature DCs migrate to the draining lymph nodes, where they initiate Th1 differentiation. The DCs at the same time also secrete chemokines that selectively recruit Tregs to the draining lymph nodes to curtail the developing Th1 response. In contrast, infected macrophages do not migrate to the draining lymph nodes but play a prominent role in initiating the granulomatous response by secreting chemokines that recruit and expand Th1 cells in the lung. The IFN-γ from activated Th1 cells enhances the mycobactericidal activity of macrophages to control M. tuberculosis replication. The M. tuberculosis-infected DCs also attract Tregs to the inflamed lung and attenuate immunopathology. The gene expression analysis has thus revealed several paradigms that can be experimentally tested in future studies.
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grant AI055377 to P. S. We thank James Burns (Drexel University, Philadelphia, PA, USA) for initial help with the microarray work and Donna Wilson (University of Pennsylvania, Philadelphia, PA, USA) for analysis of the microarray data.
References
- Bodnar K A, Serbina N V, Flynn J L. Fate of Mycobacterium tuberculosis within murine dendritic cells. Infect Immun. 2001;69:800–809. doi: 10.1128/IAI.69.2.800-809.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Orme I M. Characterization of murine lung dendritic cells infected with Mycobacterium tuberculosis. Infect Immun. 2001;69:1127–1133. doi: 10.1128/IAI.69.2.1127-1133.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tascon R E, Soares C S, Ragno S, Stavropoulos E, Hirst E M, Colston M J. Mycobacterium tuberculosis-activated dendritic cells induce protective immunity in mice. Immunology. 2000;99:473–480. doi: 10.1046/j.1365-2567.2000.00963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickman S P, Chan J, Salgame P. Mycobacterium tuberculosis induces differential cytokine production from dendritic cells and macrophages with divergent effects on naive T cell polarization. J Immunol. 2002;168:4636–4642. doi: 10.4049/jimmunol.168.9.4636. [DOI] [PubMed] [Google Scholar]
- Bhatt K, Hickman S P, Salgame P. Cutting edge: a new approach to modeling early lung immunity in murine tuberculosis. J Immunol. 2004;172:2748–2751. doi: 10.4049/jimmunol.172.5.2748. [DOI] [PubMed] [Google Scholar]
- Tian T, Woodworth J, Skold M, Behar S M. In vivo depletion of CD11c+ cells delays the CD4+ T cell response to Mycobacterium tuberculosis and exacerbates the outcome of infection. J Immunol. 2005;175:3268–3272. doi: 10.4049/jimmunol.175.5.3268. [DOI] [PubMed] [Google Scholar]
- Ehrt S, Schnappinger D, Bekiranov S, Drenkow J, Shi S, Gingeras T R, Gaasterland T, Schoolnik G, Nathan C. Reprogramming of the macrophage transcriptome in response to interferon-γ and Mycobacterium tuberculosis: signaling roles of nitric oxide synthase-2 and phagocyte oxidase. J Exp Med. 2001;194:1123–1140. doi: 10.1084/jem.194.8.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaussabel D, Semnani R T, McDowell M A, Sacks D, Sher A, Nutman T B. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- Talmor M, Mirza A, Turley S, Mellman I, Hoffman L A, Steinman R M. Generation or large numbers of immature and mature dendritic cells from rat bone marrow cultures. Eur J Immunol. 1998;28:811–817. doi: 10.1002/(SICI)1521-4141(199803)28:03<811::AID-IMMU811>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Inaba K, Inaba M, Romani N, Aya H, Deguchi M, Ikehara S, Muramatsu S, Steinman R M. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176:1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- Watford W T, Moriguchi M, Morinobu A, O'Shea J J. The biology of IL-12: coordinating innate and adaptive immune responses. Cytokine Growth Factor Rev. 2003;14:361–368. doi: 10.1016/s1359-6101(03)00043-1. [DOI] [PubMed] [Google Scholar]
- Hunter C A. New IL-12-family members: IL-23 and IL-27, cytokines with divergent functions. Nat Rev Immunol. 2005;5:521–531. doi: 10.1038/nri1648. [DOI] [PubMed] [Google Scholar]
- Kolls J K, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- Khader S A, Pearl J E, Sakamoto K, Gilmartin L, Bell G K, Jelley-Gibbs D M, Ghilardi N, deSauvage F, Cooper A M. IL-23 compensates for the absence of IL-12p70 and is essential for the IL-17 response during tuberculosis but is dispensable for protection and antigen-specific IFN-{γ} responses if IL-12p70 is available. J Immunol. 2005;175:788–795. doi: 10.4049/jimmunol.175.2.788. [DOI] [PubMed] [Google Scholar]
- Lockhart E, Green A M, Flynn J L. IL-17 production is dominated by γδ T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- Annunziato F, Cosmi L, Liotta F, Lazzeri E, Manetti R, Vanini V, Romagnani P, Maggi E, Romagnani S. Phenotype, localization, and mechanism of suppression of CD4(+)CD25(+) human thymocytes. J Exp Med. 2002;196:379–387. doi: 10.1084/jem.20020110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman C M, Chiu B C, Stolberg V R, Hu J, Zeibecoglou K, Lukacs N W, Lira S A, Kunkel S L, Chensue S W. CCR8 is expressed by antigen-elicited, IL-10-producing CD4+CD25+ T cells, which regulate Th2-mediated granuloma formation in mice. J Immunol. 2005;174:1962–1970. doi: 10.4049/jimmunol.174.4.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colantonio L, Iellem A, Sinigaglia F, D'Ambrosio D. Skin-homing CLA+ T cells and regulatory CD25+ T cells represent major subsets of human peripheral blood memory T cells migrating in response to CCL1/I-309. Eur J Immunol. 2002;32:3506–3514. doi: 10.1002/1521-4141(200212)32:12<3506::AID-IMMU3506>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Iellem A, Colantonio L, D'Ambrosio D. Skin-versus gut-skewed homing receptor expression and intrinsic CCR4 expression on human peripheral blood CD4+CD25+ suppressor T cells. Eur J Immunol. 2003;33:1488–1496. doi: 10.1002/eji.200323658. [DOI] [PubMed] [Google Scholar]
- Lee I, Wang L, Wells A D, Dorf M E, Ozkaynak E, Hancock W W. Recruitment of Foxp3+ T regulatory cells mediating allograft tolerance depends on the CCR4 chemokine receptor. J Exp Med. 2005;201:1037–1044. doi: 10.1084/jem.20041709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lande R, Giacomini E, Grassi T, Remoli M E, Iona E, Miettinen M, Julkunen I, Coccia E M. IFN-α β released by Mycobacterium tuberculosis-infected human dendritic cells induces the expression of CXCL10: selective recruitment of NK and activated T cells. J Immunol. 2003;170:1174–1182. doi: 10.4049/jimmunol.170.3.1174. [DOI] [PubMed] [Google Scholar]
- Bonecchi R, Bianchi G, Bordignon P P, D'Ambrosio D, Lang R, Borsatti A, Sozzani S, Allavena P, Gray P A, Mantovani A, Sinigaglia F. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1) and Th2s. J Exp Med. 1998;187:129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inngjerdingen M, Damaj B, Maghazachi A A. Expression and regulation of chemokine receptors in human natural killer cells. Blood. 2001;97:367–375. doi: 10.1182/blood.v97.2.367. [DOI] [PubMed] [Google Scholar]
- Algood H M, Lin P L, Yankura D, Jones A, Chan J, Flynn J L. TNF influences chemokine expression of macrophages in vitro and that of CD11b+ cells in vivo during Mycobacterium tuberculosis infection. J Immunol. 2004;172:6846–6857. doi: 10.4049/jimmunol.172.11.6846. [DOI] [PubMed] [Google Scholar]
- Gangur V, Simons F E, Hayglass K T. Human IP-10 selectively promotes dominance of polyclonally activated and environmental antigen-driven IFN-γ over IL-4 responses. FASEB J. 1998;12:705–713. doi: 10.1096/fasebj.12.9.705. [DOI] [PubMed] [Google Scholar]
- Romagnani P, Maggi L, Mazzinghi B, Cosmi L, Lasagni L, Liotta F, Lazzeri E, Angeli R, Rotondi M, Filì L, Parronchi P, Serio M, Maggi E, Romagnani S, Annunziato F. CXCR3-mediated opposite effects of CXCL10 and CXCL4 on TH1 or TH2 cytokine production. J Allergy Clin Immunol. 2005;116:1372–1379. doi: 10.1016/j.jaci.2005.09.035. [DOI] [PubMed] [Google Scholar]
- Srivastava M, Meinders A, Steinwede K, Maus R, Lucke N, Bühling F, Ehlers S, Welte T, Maus U A. Mediator responses of alveolar macrophages and kinetics of mononuclear phagocyte subset recruitment during acute primary and secondary mycobacterial infections in the lungs of mice. Cell Microbiol. 2007;9:738–752. doi: 10.1111/j.1462-5822.2006.00824.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Juarrero M, Shim T S, Kipnis A, Junqueira-Kipnis A P, Orme I M. Dynamics of macrophage cell populations during murine pulmonary tuberculosis. J Immunol. 2003;171:3128–3135. doi: 10.4049/jimmunol.171.6.3128. [DOI] [PubMed] [Google Scholar]
- Pedroza-Gonzalez A, Garcia-Romo G S, Aguilar-Leon D, Calderon-Amador J, Hurtado-Ortiz R, Orozco-Estevez H, Lambrecht B N, Estrada-García I, Hernández-Pando R, Flores-Romo L. In situ analysis of lung antigen-presenting cells during murine pulmonary infection with virulent Mycobacterium tuberculosis. Int J Exp Pathol. 2004;85:135–145. doi: 10.1111/j.0959-9673.2004.00381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.