Abstract
Because medication adherence is critical to improving the virologic and immunologic response to therapy and reducing the risk of drug resistance, it is important that we understand the predictors of nonadherence. The goal of the current study is to examine demographic, health behavior and psychosocial correlates (e.g., stressful life events, depressive symptoms) of nonadherence among a sample of HIV infected men and women from one south Florida metropolitan area. We collected questionnaire data from on 105 HIV infected men and women who were taking antiretroviral medication during the years 2004 to 2007. In this sample, 44.8% had missed a medication dose in the past 2 weeks, and 22.1% had missed their medication during the previous weekend. Those with three or more stressful life events in the previous 6 months were 2.5 to more than 3 times as likely to be nonadherent (in the past 2 weeks and previous weekend, respectively) compared to those without such events. Fully 86.7% of those with six or more stresses were nonadherent during the prior 2 weeks compared to 22.2% of those with no stressors. Although alcohol consumption, drug use, and symptoms of depression were related to nonadherence in the bivariate analyses, the effects of these predictors were reduced to nonsignificance by the stressful event measure. These findings underscore the importance of addressing the often chaotic and stressful lives of HIV infected persons within medical settings.
Introduction
Although highly active antiretroviral therapy (HAART) has reduced morbidity and mortality in HIV infection,1 adherence to medication regimens is critical to improving the virologic and immunologic response to therapy.2,3 Medication nonadherence increases the risk of developing multiple drug resistant strains of HIV, faster disease progression, and worse survival.2,4 Despite the importance of adherence to drug regimens, studies consistently show poor adherence among HIV infected persons.5,6
Because medication adherence is critical in the treatment of HIV, there is a growing body of research examining predictors of adherence, such as, socioeconomic status,7–9 health behaviors (alcohol and drug use),5,8–13 and psychosocial factors (stress, depression).2,5,6,8,9,14–19 Although there is extensive research on the association of depression with nonadherence, only three studies have examined the role of recent stressful life events.18–20 All showed that stressful events were associated with nonadherence. Two studies18,19 measured adherence using short (and perhaps less valid21) time frames (2–4 days); one study examined the number of reasons for missing medication doses.20 Furthermore, stressful events and lifetime trauma have been shown in some studies to be stronger correlates of nonadherence compared to depression.8,18 The association of stressful events with nonadherence may imply a different treatment strategy (e.g., stress management) than the association of depression with nonadherence. Clearly, we need more studies examining the relative importance of stressful events and depression on nonadherence over longer and varying recall time frames.
The goal of the current study is to examine demographic, health behavior and psychosocial correlates of nonadherence, using several recall time frames, among a sample of HIV infected men and women. We hypothesized that nonadherence would be related to more alcohol and illicit drug use, more depressive symptoms, and more stressful life events.
Methods
Sample and procedure
We collected data from February 2004 to February 2007 on 158 HIV-infected men and women at study entry before starting a randomized stress or trauma treatment intervention. Of the 158 participants, 105 were taking antiretroviral medications and thus comprise the sample for the current paper. Almost all of those on antiretroviral medications (96%) were on HAART.
All subjects were recruited from South Florida (Miami-Dade and Broward Counties) using newspaper and newsletter advertisements, and by making contact with community organizations, doctor's offices, sexually transmitted disease clinics, and word of mouth. Although subjects were part of a stress and/or trauma treatment study (writing about stress/trauma), we assumed the stress of HIV was sufficient to enter the study and did not require the presence of additional trauma. The treatment study did require excluding those: less than 18 and greater than 68 years of age, who were illiterate or could not speak, read and write in English, with active systemic diseases or disorders that would interfere with participation or confound prediction of disease progression (e.g., heart, lung, kidney, liver, cancer, stroke), with current alcohol or substance dependence, planning to change HIV medication in the next 6 months or recently changed HIV medications in the past three months, and initiating use of antidepressant medication during the 1 month before the study. The Institutional Review Board at the University of Miami approved the study, and all patients signed an informed consent.
Information from participants was obtained from questionnaires completed in a private room and taking approximately 45 minutes to complete. We also performed a blood draw to assay baseline CD4 T lymphocyte number and HIV RNA viral load.
Measures
To measure nonadherence to antiretroviral medication, we administered the questionnaire developed by the Patient Care Committee and the Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trial Group.22 The following question in reference to their HIV treatment was asked, “When was the last time you missed any of your medications?” We defined nonadherence in several ways; first it was defined as missing medications in the past 2 weeks (0 = none missing, 1 = nonadherent). We used this recall time frame because a recent meta analysis of adherence interventions showed that longer recall periods (2 weeks and 1 month) may be more sensitive,23 particularly as medication regimens have simplified. As in other studies,24 there was a trend for this 2-week recall time point to be associated with undetectable HIV RNA viral load (< 400 copies per milliliter) (e.g., 74% of the adherent had undetectable viral load compared to 58.7% of the nonadherent (χ2 = 2.53, p = 0.11, n = 96). To ensure that the longer recall time period did not make a difference in the results, we also examined whether subjects missed any of their HIV medications in the previous weekend (Saturday or Sunday), because adherence tends to be more problematic on weekends. Furthermore, to establish consistency in the findings regardless of recall time frame, we also report nonadherence (missing any medication) during the past week, past month, and past 3 months.
Recent stressful life events were measured using a modified version of the Life Events Survey (LES)25,26 that assessed the presence of stressful events during the 6 months before the baseline assessment. This measure was shown to correlate with poor health related functioning in a large sample of HIV infected persons in the rural southeast.27 The list of events was modified from the LES to include only those events that were considered moderate to severely stressful based on our previous studies with interviewer based objectively rated stresses.28–30 This objective rating was shown to be consistently related to immune decline and HIV disease progression.28–30 For the current analysis, we examined number of stresses out of 43 including: change in relationships (e.g., marriage, divorce, estrangement from family), death or serious illness of family members or close friends, work/financial problems (e.g., unemployment, worked long hours, large drop in income), illness (non-HIV), accidents or safety issues (e.g., physical or sexual assault), crime or legal problems (e.g., subject or close relative arrested, burglarized), other life changes (e.g., you or partner became pregnant, moved residence more than once). To avoid outliers, we truncated the number of stresses at 10; all but two subjects had 10 or fewer stresses. To avoid confounding patient's mood with ratings of adherence, we count the number of events, ignoring patient ratings of distress.
The Beck Depression Inventory (BDI) is a 21-item instrument that assesses cognitive, affective, and somatic symptoms of depression.31 The BDI has acceptable Cronbach α = 0.81 for nonpsychiatric subjects, and is highly correlated with other depression measures.
We examined several health behaviors, including smoking, drinking alcohol, and drug use. Cigarette smoking was coded as 0 = no, 1 = yes. We computed the number of alcoholic drinks (glass of wine, can of beer, shot of liquor) per month by multiplying the number of days drinking by usual number of drinks consumed. We truncated 8 responses at 25, due to outliers. Illicit drug use (0 = no, 1 = yes) included using any of the following substances in the past month: cocaine, heroin, other injecting drugs (e.g., ketamine), opiates, amphetamines, hallucinogens, and sedatives/tranquilizers.
The questionnaire also included demographic and background data on age, gender, education (coded 1 [less than eighth grade] to 6 [graduate school]), household income (coded 1 [$5000 or less] to 9 [over $70,000]), race/ethnicity (coded as two dummy variables, African American and Hispanic, with Caucasian as the comparison), and gay or bisexual orientation.
Statistical analyses
Descriptive data were run on all variables including means, standard deviations, and medians on continuous variables, and percentages on all categorical variables. We ran separate bivariate logistic regression models with each demographic, background, health behavior, and psychosocial variable predicting nonadherence. African American and Hispanic dummy variables were entered into models together (with Caucasian as the comparison group) to test the effect of race/ethnicity. To test the primary hypotheses of this study, we used logistic regression with nonadherence measures as our outcomes. We used a stepwise analysis strategy, entering variables into equations in the following steps: (1) demographic and background variables including age, education, household income, gender, gay and race/ethnicity (two dummy variables, African American and Hispanic) and (2) number of total recent stresses in the previous 6 months, depression symptoms, and health behaviors (smoking, alcohol consumption, drug use). We allowed variables to stay in the models if p < 0.10 at the step it went in. We show the results of these stepwise logistic regression analyses as well as all variables in the model that had a significant bivariate relationship with each nonadherence measure.
Results
Description of the sample
Descriptive information about the sample is shown in Table 1. The sample of 105 HIV-infected persons had an average age of 44, 61.0% were male, approximately half had a high school degree or less, and most had household incomes at or below $20,000 per year (74.0%). Most were minorities (81.9%) including African Americans (56.2%) and Hispanics (25.7%); 47.1% were gay or bisexual.
Table 1.
Descriptive Information About the Samplea
| Characteristic | Mean (SD) | % |
|---|---|---|
| Age in years (range, 24–68) | 43.95 ± 8.99 | |
| Education | ||
| Less than high school degree | 28.6 | |
| High school degree | 21.9 | |
| Some college/trade school | 27.6 | |
| College graduate or beyond | 21.9 | |
| Household income | ||
| $5000 or less | 39.4 | |
| $5001–$10,000 | 23.1 | |
| $10,001–$20,000 | 11.5 | |
| $20,000–$40,000 | 15.4 | |
| >$40,000 | 10.6 | |
| Gender (male) | 61.0 | |
| Race/ethnicity | ||
| African American | 56.2 | |
| Caucasian | 18.1 | |
| Hispanic | 25.7 | |
| Gay/bisexual | 47.1 | |
| Cigarette smoking | 51.0 | |
| Alcohol (drinks per month) (range, 0–25) | 4.64 ± 8.00 | |
| Drug use (past month) | 34.3 | |
| Number of stressful events (range 0–10) | 3.15 ± 2.53 | |
| Depressive symptoms (0–36) | 11.17 ± 9.16 | |
| Nonadherence (skipped within past two-weeks) | 44.8 | |
| Nonadherence (skipped past weekend) | 22.1 |
Continuous variables are reported as means ± standard deviations; categorical variables are listed as percentages. n = 105, except gay sexual orientation, household income and nonadherence in the past weekend was based on 104, smoking was based on 102, and drinking was based on 101, due to missing data.
In terms of health habits, approximately half (51.0%) smoked cigarettes, approximately a third (34.3%) used illicit drugs (e.g., cocaine, opiates, amphetamines) in the past month, but only 37.6% drank alcoholic beverages in the past month. The median number of stressful events in the past 6 months was 3. The most common stressors included: worsening financial status or chronic financial stress (41.4%), serious arguments or separation from partner (23.8%), estrangement from family (23.8%), major illness or injury (not HIV related; 19.1%), serious illness or injury of close family member(s) (18.1%), trouble finding employment (17.3%), death of close friend (16.4%), death of close family member(s) (15.2%), and marriage or engagement (15.2%). Average depressive symptoms was 11.2, with 21.0 % meeting criteria for depression using a cutoff of 19 for moderate depression.31 In all, 44.8% had missed a medication dose in the past 2 weeks, and 22.1% missed at least one dose during the previous weekend.
Bivariate relationship of nonadherence to demographic, behavior, and psychosocial variables
Table 2 shows bivariate logistic regression odds ratios and confidence intervals (CI) of each demographic, behavioral, and psychological variable with each nonadherence measure. Regardless of nonadherence definition, drinking more alcohol, more stressful events and greater depressive symptoms were associated with nonadherence. Gay men had greater likelihood of reporting nonadherence during the past 2 weeks, and those who smoked cigarettes or used drugs had greater odds for skipped medication during the previous weekend. Age, education, household income, gender, race, and ethnicity were not significantly related to either measure of nonadherence, although there was a trend for younger persons to miss their medication during the past weekend (p = 0.054).
Table 2.
Bivariate Logistic Regression Odds Ratios and Confidence Intervals (CI) of Demographic, Health Behaviors and Psychosocial Variables with Adherence Measuresa
| |
Nonadherence |
|||
|---|---|---|---|---|
| |
Skipped in past 2 weeks |
Skipped in past weekend |
||
| Predictor variables | Odds ratio | 95% CI | Odds ratio | 95% CI |
| Age | 0.97 | 0.93–1.02 | 0.94b | 0.89–1.00 |
| Education | 1.20 | 0.88–1.65 | 0.99 | 0.68–1.44 |
| Household income | 1.12 | 0.93–1.35 | 1.04 | 0.84–1.29 |
| Male gender | 0.58 | 0.26–1.29 | 0.46 | 0.17–1.30 |
| Race/ethnicity | ||||
| African American | 0.58 | 0.20–1.63 | 1.41 | 0.35–5.64 |
| Hispanic | 0.97 | 0.30–3.14 | 1.75 | 0.39–7.91 |
| Gay | 2.74c | 1.23–6.09 | 2.10 | 0.82–5.43 |
| Cigarette smoking | 1.01 | 0.46–2.21 | 2.90c | 1.02–8.25 |
| Alcohol drinks/month | 1.08d | 1.02–1.15 | 1.09d | 1.03–1.15 |
| Drug use (past month) | 1.64 | 0.73–3.68 | 2.59c | 1.00–6.68 |
| # of stressful events | 1.36d | 1.13–1.63 | 1.54d | 1.25–1.92 |
| Depressive symptoms | 1.05c | 1.00–1.09 | 1.07d | 1.02–1.12 |
Odds ratios are from separate logistic regression models that each include a single independent variable, except African-American and Hispanic are in the model together because they are two dummy variables of race/ethnicity; Caucasian is the comparison category.
p < 0.10.
p < 0.05.
p < 0.01.
Multivariate relationship of nonadherence to demographic, behavior, and psychosocial variables
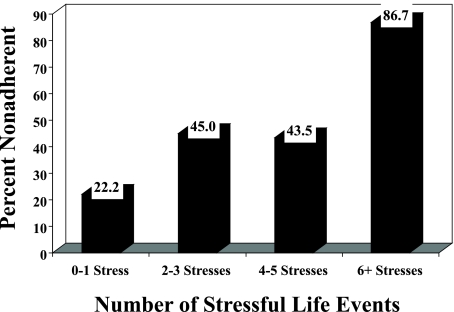
Logistic regression analyses in Table 3 shows that having more stressful events in the previous six months was associated with both measures of adherence. For each increase in one stressor, the odds of reporting missed medication in the past two weeks increased by 36%. For every 3-point (median) increase in stressful events, there was a 2.5 times greater odds of nonadherence in the past 2 weeks. Figure 1 shows this stressful events and nonadherence relationship graphically; among those with no or one stressful event, 22.2% were nonadherent compared to 86.7% of those with six or more stresses. On the short-term nonadherence measure, persons with three stressful events had 3.2 times the likelihood of missing medication during the past weekend compared with those having no stressors.
Table 3.
Nonadherence with Demographic, Background, Health Behaviors, and Psychosocial Variablesa
|
Nonadherence: skipped in past 2 weeks | ||||||
|---|---|---|---|---|---|---|
| |
Model 1 |
Model 2 |
||||
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Gay | 2.55 | 1.04–6.28 | 0.041 | 2.73 | 1.17–6.39 | 0.020 |
| Alcohol drinks/month | 1.03 | 0.97–1.10 | 0.344 | |||
| # of stressful events | 1.26 | 1.02–1.58 | 0.037 | 1.36 | 1.12–1.64 | 0.002 |
| Depressive symptoms | 1.02 | 0.97–1.08 | 0.365 | |||
|
Nonadherence: skipped past weekend | ||||||
|---|---|---|---|---|---|---|
| Odds ratio | 95% CI | p value | Odds ratio | 95% CI | p value | |
| Age | 0.99 | 0.92–1.06 | 0.737 | 0.99 | 0.92–1.06 | 0.822 |
| Cigarette smoking | 3.06 | 0.89–10.54 | 0.076 | 2.98 | 0.92–9.58 | 0.068 |
| Alcohol drinks/month | 1.04 | 0.97–1.11 | 0.290 | |||
| Drug use (past month) | 1.60 | 0.48–5.37 | 0.446 | |||
| # of stressful events | 1.33 | 1.03–1.71 | 0.030 | 1.47 | 1.17–1.86 | 0.0007 |
| Depressive symptoms | 1.04 | 0.98–1.11 | 0.171 | |||
Table shows two models. Model 1 shows the logistic regression including all predictor variables that had p < 0.10 bivariate relationship with each adherence measure. Model 2 shows the results from multivariable stepwise logistic regression for each of the adherence measures with predictor variables stepped into the model in two stages: (1) demographic and background variables (e.g., age, education, racial/ethnic, household income, gender, gay) and (2) psychosocial and health behaviors (e.g., smoking, drug use, alcohol use, number of stresses, depression). Variables with p ≤ 0.10 at the step that they were entered, were included in the final model 2.
FIG. 1.
The association of antiretroviral nonadherence (past 2 weeks) by the number of stressful life events (χ2 = 16.22, p = 0.001).
In addition to stressful events, those who were gay reported about 2.7 times greater odds of missed medication during the past 2 weeks compared to heterosexuals, but were not different on nonadherence reported during the past weekend. There was a nonsignificant trend for cigarette smoking to be related to nonadherence during the previous weekend, but no relationship of smoking to nonadherence measured for the past 2 weeks. Although in bivariate analyses, depressive symptoms and drinking more alcohol were related to both measures of adherence, and drug use was related to missing medication during the previous weekend, the effects of these variables were diminished by the inclusion of stressful events in the equations. Stressful life events was moderately correlated with depressive symptoms (r = 0.31, p = 0.001), greater alcohol consumption (r = 0.48, p < 0.0001), and drug use (r = 0.23, p = 0.02).
To ensure that our findings relative to stressful life events were not due to choosing particular nonadherence recall time periods, we also tested models using different cutoffs. Using the same analysis strategy, we found that stressful events were related to increased odds of nonadherence reported during the past week (odds ratio = 1.42, CI = 1.16–1.75, p = 0.0009), month (odds ratio = 1.33, CI = 1.10–1.61, p = 0.003), and 3 months (odds ratio = 1.34, CI = 1.08–1.66, p = 0.007). In this sample, nonadherence was reported by 37.1% during the past week, 48.6% during the past month, and 64.8% during the past 3 months.
Discussion
We found high levels of nonadherence (44%) during the past 2 weeks in this sample of patients from a large metropolitan city. Our high levels of nonadherence were similar to other studies.5,6,18 Stressful life events was consistently associated with missing HIV medication, regardless of the recall time period used in assessing adherence (from preceding weekend to three months). For every increase of three stressful events, the odds of nonadherence were increased 2.5 to over 3 times, depending on the recall time period. Stressful life events have also been linked to faster HIV disease progression.29,32–34 The current findings showing that stress is associated with medication nonadherence may be a possible mediator of this stress/health relationship.
Although symptoms of depression were related to nonadherence in the bivariate analysis, depression decreased to nonsignificance when the stressful event measure was in the equation. Other studies have also shown that depression was unrelated to nonadherence when trauma or life events were considered.8,18 Therefore, having many stressful events may be a more robust correlate of nonadherence than depression. Persons who report more stressful events may have more chaotic lifestyles that may account for their missed medications. O'Cleirigh and colleagues20 found that those with many life events and poor distress tolerance endorsed a greater number of reasons for missing their medication. These findings suggest that while interventions for depression may be useful, cognitive behavioral interventions that address stress and coping may have a greater impact on adherence to HIV medications.23
Gay men had more nonadherence when measuring missed medication during the previous 2 weeks, but not when examining the previous weekend. We could find no other studies that reported a similar finding and therefore we need further research to determine if this is unique to our sample. There was a nonsignificant trend for smoking cigarettes to be related to nonadherence during the past weekend but no relationship with missed medication during the previous 2 weeks. Smoking has been shown to be positively related to nonadherence in several studies done in France,35–37 where there has been an emphasis on smoking cessation in HIV.38 These differences on adherence measures may reflect measurement error when using varying length recall periods,23 lending support for the multiple measures approach used here.
Many studies have shown that drinking alcohol and drug use are related to nonadherence.5,8–13,35 The current study showed that these health behaviors were related to nonadherence in the bivariate analyses, but that life stress reduced the effects of these variables to nonsignificance. Because our sample omitted those with substance dependence, the relationship of these health behaviors with adherence is likely to be reduced. However, it is also likely that persons with many life stressors tend to use more drugs and alcohol and that those who use more drugs and alcohol tend to engage in behaviors that lead to more life stress (e.g., conflicts with families, financial and employment problems). While life stress may be a more potent correlate of nonadherence than poor health behaviors, effective adherence treatment must still address issues of substance abuse and dependence.
Interpretation of our study findings must be balanced against a number of limitations in the current study design. First, our sample was drawn from persons volunteering for a psychological treatment study in one south Florida metropolitan area and may not be representative of HIV infected persons. Comparing our sample on stress and trauma to a large population based clinic study in the rural southeast33 showed similar levels of recent stress with somewhat more trauma (e.g., sexual and physical abuse) in the current urban sample. It is certainly possible that more trauma history might affect subject's ability to manage current life stress and thus affect the generalizability of this study.
Second, without longitudinal data it is difficult to determine the causal nature of the relationships, although it does seem unlikely that poor adherence could lead to having more stressful events. Using discrete events rather than patient's ratings of their stress, and measuring stress during the 6 months before baseline, also helps to establish the causal direction of this relationship. However, it is always possible that some third variable might account for the relationship between stressful events and nonadherence, despite controlling for many demographic and background characteristics. Finally, our study is limited by the use of self-report measures of adherence that may not accurately reflect actual practice. The consistency of the stress findings, regardless of measurement time frame, lends support to the validity of our results.
This is among the few studies that have examined the role of stressful life events on medication adherence. Previous studies of stressful events18,19 have focused on short recall time frames of adherence. By showing that more stressful life events correlates with nonadherence using multiple recall time frames (from short to long), our study demonstrates the robust nature of these findings. The role of stressful events in medication nonadherence lends credence to considering cognitive behavioral interventions that address issues of stress, coping and chaotic lifestyles for treating inconsistent conformance with medication guidelines. These findings underscore the importance of addressing stressful events within the medical setting, and providing referrals to psychologists, social workers or case managers for patients having many such stressors.
Acknowledgments
This study was supported in part by the National Center for Complementary and Alternative Medicine/National Institutes of Health (NCCAM/NIH): R01 AT002035 (G. Ironson, PI), and 5T32MH18917.
References
- 1.Lima VD. Hogg RS. Harrigan PR, et al. Continued improvement in survival among HIV-infected individuals with newer forms of highly active antiretroviral therapy. AIDS. 2007;21:685–692. doi: 10.1097/QAD.0b013e32802ef30c. [DOI] [PubMed] [Google Scholar]
- 2.Paterson DL. Swindells S. Mohr J, et al. Adherence to protease inhibitor therapy and outcomes in patients with HIV infection. Ann Intern Med. 2000;133:21–30. doi: 10.7326/0003-4819-133-1-200007040-00004. [DOI] [PubMed] [Google Scholar]
- 3.Wood E. Hogg RS. Yip B. Harrigan PR. O'Shaughnessy MV. Montaner JS. The impact of adherence on CD4 cell count responses among HIV-infected patients. J Acquir Immune Defic Syndr. 2004;35:261–268. doi: 10.1097/00126334-200403010-00006. [DOI] [PubMed] [Google Scholar]
- 4.Harrigan PR. Hogg RS. Dong WW, et al. Predictors of HIV drug-resistance mutations in a large antiretroviral-naive cohort initiating triple antiretroviral therapy. J Infect Dis. 2005;191:339–347. doi: 10.1086/427192. [DOI] [PubMed] [Google Scholar]
- 5.Tucker JS. Burnam MA. Sherbourne CD. Kung FY. Gifford AL. Substance use and mental health correlates of nonadherence to antiretroviral medications in a sample of patients with human immunodeficiency virus infection. Am J Med. 2003;114:573–580. doi: 10.1016/s0002-9343(03)00093-7. [DOI] [PubMed] [Google Scholar]
- 6.Carrieri MP. Leport C. Protopopescu C, et al. Factors associated with nonadherence to highly active antiretroviral therapy: A 5-year follow-up analysis with correction for the bias induced by missing data in the treatment maintenance phase. J Acquir Immune Defic Syndr. 2006;41:477–485. doi: 10.1097/01.qai.0000186364.27587.0e. [DOI] [PubMed] [Google Scholar]
- 7.Bouhnik AD. Chesney M. Carrieri P, et al. Nonadherence among HIV-infected injecting drug users: the impact of social instability. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S149–S153. doi: 10.1097/00126334-200212153-00013. [DOI] [PubMed] [Google Scholar]
- 8.Mugavero M. Ostermann J. Whetten K, et al. Barriers to antiretroviral adherence: The importance of depression, abuse, and other traumatic events. AIDS Patient Care STDs. 2006;20:418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- 9.Spire B. Duran S. Souville M. Leport C. Raffi F. Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: From a predictive to a dynamic approach. Soc Sci Med. 2002;54:1481–1496. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 10.Mohammed H. Kieltyka L. Richardson-Alston G, et al. Adherence to HAART among HIV-infected persons in rural Louisiana. AIDS Patient Care STDs. 2004;18:289–296. doi: 10.1089/108729104323076025. [DOI] [PubMed] [Google Scholar]
- 11.Liu H. Longshore D. Williams JK, et al. Substance abuse and medication adherence among HIV-positive women with histories of child sexual abuse. AIDS Behav. 2006;10:279–286. doi: 10.1007/s10461-005-9041-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Arnsten JH. Demas PA. Grant RW, et al. Impact of active drug use on antiretroviral therapy adherence and viral suppression in HIV-infected drug users. J Gen Intern Med. 2002;17:377–381. doi: 10.1046/j.1525-1497.2002.10644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Golin CE. Liu H. Hays RD, et al. A prospective study of predictors of adherence to combination antiretroviral medication. J Gen Intern Med. 2002;17:756–765. doi: 10.1046/j.1525-1497.2002.11214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Starace F. Ammassari A. Trotta MP, et al. Depression is a risk factor for suboptimal adherence to highly active antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S136–S139. doi: 10.1097/00126334-200212153-00010. [DOI] [PubMed] [Google Scholar]
- 15.Ammassari A. Antinori A. Aloisi MS, et al. Depressive symptoms, neurocognitive impairment, and adherence to highly active antiretroviral therapy among HIV-infected persons. Psychosomatics. 2004;45:394–402. doi: 10.1176/appi.psy.45.5.394. [DOI] [PubMed] [Google Scholar]
- 16.Phillips KD. Moneyham L. Murdaugh C, et al. Sleep disturbance and depression as barriers to adherence. Clin Nurs Res. 2005;14:273–293. doi: 10.1177/1054773805275122. [DOI] [PubMed] [Google Scholar]
- 17.Boarts JM. Sledjeski EM. Bogart LM. Delahanty DL. The differential impact of PTSD and depression on HIV disease markers and adherence to HAART in people living with HIV. AIDS Behav. 2006;10:253–261. doi: 10.1007/s10461-006-9069-7. [DOI] [PubMed] [Google Scholar]
- 18.Nilsson Schonnesson L. Diamond PM. Ross MW. Williams M. Bratt G. Baseline predictors of three types of antiretroviral therapy (ART) adherence: A 2-year follow-up. AIDS Care. 2006;18:407–414. doi: 10.1080/09540120500456631a. [DOI] [PubMed] [Google Scholar]
- 19.Mellins CA. Kang E. Leu CS. Havens JF. Chesney MA. Longitudinal study of mental health and psychosocial predictors of medical treatment adherence in mothers living with HIV disease. AIDS Patient Care STDs. 2003;17:407–416. doi: 10.1089/108729103322277420. [DOI] [PubMed] [Google Scholar]
- 20.O'Cleirigh C. Ironson G. Smits JA. Does distress tolerance moderate the impact of major life events on psychosocial variables and behaviors important in the management of HIV? Behav Ther. 2007;38:314–323. doi: 10.1016/j.beth.2006.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simoni JM. Kurth AE. Pearson CR. Pantalone DW. Merrill JO. Frick PA. Self-report measures of antiretroviral therapy adherence: A review with recommendations for HIV research and clinical management. AIDS Behav. 2006;10:227–245. doi: 10.1007/s10461-006-9078-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chesney MA. Ickovics JR. Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. Patient Care Committee & Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 23.Simoni JM. Pearson CR. Pantalone DW. Marks G. Crepaz N. Efficacy of interventions in improving highly active antiretroviral therapy adherence and HIV-1 RNA viral load. A meta-analytic review of randomized controlled trials. J Acquir Immune Defic Syndr. 2006;43(Suppl 1):S23–S35. doi: 10.1097/01.qai.0000248342.05438.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nieuwkerk PT. Oort FJ. Self-reported adherence to antiretroviral therapy for HIV-1 infection and virologic treatment response: A meta-analysis. J Acquir Immune Defic Syndr. 2005;38:445–448. doi: 10.1097/01.qai.0000147522.34369.12. [DOI] [PubMed] [Google Scholar]
- 25.Sarason IG. Johnson JH. Siegel JM. Assessing the impact of life changes: Development of the Life Experiences Survey. J Consult Clin Psychol. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- 26.Sarason IG. Johnson JH. The Life Experiences Survey: Preliminary findings. Technical Report SCS-LS-001. Office of Naval Research. 1976.
- 27.Leserman J. Whetten K. Lowe K. Stangl D. Swartz MS. Thielman NM. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the south. Psychosom Med. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- 28.Leserman J. Jackson ED. Petitto JM, et al. Progression to AIDS: The effects of stress, depressive symptoms, and social support. Psychosom Med. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- 29.Leserman J. Petitto JM. Gu H, et al. Progression to AIDS, a clinical AIDS condition, and mortality: Psychosocial and physiological predictors. Psychol Med. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- 30.Leserman J. Petitto JM. Perkins DO. Folds JD. Golden RN. Evans DL. Severe stress, depressive symptoms, and changes in lymphocyte subsets in human immunodeficiency virus-Infected men. Arch Gen Psychiatry. 1997;54:279–285. doi: 10.1001/archpsyc.1997.01830150105015. [DOI] [PubMed] [Google Scholar]
- 31.Beck AT. Steer RA. Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 32.Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biol Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 33.Leserman J. Pence BW. Whetten K, et al. Lifetime trauma and depressive symptoms predict mortality in HIV. Am J Psychiatry. (in press). [DOI] [PubMed]
- 34.Ironson G. O'Cleirigh C. Fletcher MA, et al. Psychosocial factors predict CD4 and viral load change in men and women with human immunodeficiency virus in the era of highly active antiretroviral treatment. Psychosom Med. 2005;67:1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peretti-Watel P. Spire B. Lert F. Obadia Y. Drug use patterns and adherence to treatment among HIV-positive patients: Evidence from a large sample of French outpatients (ANRS-EN12-VESPA 2003) Drug Alcohol Depend. 2006;82(Suppl 1):S71–S79. doi: 10.1016/s0376-8716(06)80012-8. [DOI] [PubMed] [Google Scholar]
- 36.Spire B. Duran S. Souville M. Leport C. Raffi F. Moatti JP. Adherence to highly active antiretroviral therapies (HAART) in HIV-infected patients: From a predictive to a dynamic approach. Soc Sci Med. 2002;54:1481–1496. doi: 10.1016/s0277-9536(01)00125-3. [DOI] [PubMed] [Google Scholar]
- 37.Goujard C. Bernard N. Sohier N, et al. Impact of a patient education program on adherence to HIV medication: A randomized clinical trial. J Acquir Immune Defic Syndr. 2003;34:191–194. doi: 10.1097/00126334-200310010-00009. [DOI] [PubMed] [Google Scholar]
- 38.Benard A. Bonnet F. Tessier JF, et al. Tobacco addiction and HIV infection: toward the implementation of cessation programs. ANRS CO3 Aquitaine Cohort. AIDS Patient Care STDs. 2007;21:458–468. doi: 10.1089/apc.2006.0142. [DOI] [PubMed] [Google Scholar]