Abstract
Living with HIV involves management of multiple stressful disease-related and other life events. Distress tolerance may provide a functional, individual-based context for qualifying the established relationships between major life events and psychosocial variables important in the management of HIV. The present study provided a preliminary test of the hypothesis that distress tolerance moderates the impact of major life events on these predictors of disease progression. HIV-positive patients (n= 116) completed psychosocial and medical questionnaires. Results indicated that major life events interacted with distress tolerance such that lower distress tolerance and higher life events were associated with significantly higher levels of depressive symptoms, substance use coping, alcohol and cocaine use, and medication adherence. In addition, distress tolerance was directly related to self-reported HIV-related symptoms. These results suggest that low distress tolerance, particularly in the face of major life events, may present significant challenges to adaptive management of HIV. Distress tolerance assessment may help to specify targets for cognitive-behavioral and stress management treatments for people living with HIV.
Life stress is ubiquitous among persons suffering from HIV. Not only do people with HIV experience the distress from living with a chronic illness, they also report high incidence of other life stressors. For example, Leserman et al. (2005) found that approximately half (53%) of a large cohort (n=611) of HIV-positive adults reported a history of either sexual or physical abuse. Moreover, more than 70% of the sample had experienced at least two lifetime traumas. Other common life stressors among HIV-positive adults include job loss, interpersonal rejection, bereavement, and symptom onset (Leserman, 2003; O’Cleirigh et al., 2003).
Evidence suggests that life stress may operate prominently in the adaptive management of HIV through its impact on established psychosocial and behavioral predictors of poorer HIV disease course (see Leserman, 2003). Specifically, life event stress has been associated with higher levels of depressed symptoms in HIV-positive women (Catz, Gore-Felton & McClure, 2002; Siegel, Schrimshaw & Pretter, 2005) and among bereaved HIV-positive men and women (Sikkema, Kochman, DiFranceisco, Kelly, & Hoffman, 2003). Similarly, live event stress has been related to denial coping strategies in HIV-positive gay and bisexual men (Penedo et al., 2003) and less use of active coping strategies in women (Weaver et al., 2005). Likewise, life event stress has been linked to increased substance use in HIV (Ibanez, Purcell, Stall, Parsons, & Gomez, 2005; Pence, Miller, Whetten, Eron, & Gaynes, 2006), and to poorer adherence to antiretroviral medication (Chesney et al., 2000; Mugavero et al., 2006; Vanable, Carey, Blair, & Littlewood, 2006). Lastly, life event stress and PTSD symptom severity are associated with more sexual risk taking (Roberts, Wechsberg, Zule, & Burroughs, 2003; Stein, Rotheram-Borus, Swendeman, & Milburn, 2005) and with increased HIV symptom presentation and symptom burden (Leserman et al., 2005; Smith, Egert, Winkel, & Jacobson, 2002).
Tests of the hypothesis that life event stress is predictive of poorer disease course have yielded mixed findings. A series of longitudinal studies has shown a link between life stress and accelerated HIV disease progression (Ironson et al., 2005; Kemeny & Dean, 1995; Patterson et al., 1995), speed of AIDS onset (Leserman et al., 1999, 2002), and survival time (Patterson et al., 1996). However, several studies have failed to find a relationship between adverse life events and biological markers of HIV disease progression (Kessler et al., 1991) or with progression to AIDS over 5 years (Patterson et al., 1996). To account for these discrepant findings, Leserman (2003) has suggested that the stress from a life event needs to be evaluated with reference to the context within which it occurs.
One context that moderates the impact of life stress may be the characteristics of an individual’s response to stress. More specifically, an individual’s ability to approach the stress of a major life event and to endure the negative emotional experiences associated with the event may help to provide an “individual-based” context for assessing the impact of the life event. Support for considering the effect of the individual’s response to stress comes from longitudinal studies indicating that avoidant coping predicts greater HIV disease progression over 2 years (Ironson et al., 2005) and that denial coping predicts more rapid progression to AIDS (Ironson et al., 1994; Leserman et al., 2002; Solano et al., 1993) and mortality (Ironson et al., 1994). Similarly, recent research in the psychopathology literature suggests that the impact of stress on clinical outcomes may depend on the individual’s level of distress tolerance. Distress tolerance is an individual difference variable that refers to the capacity to experience and withstand emotional discomfort (Simons & Gaher, 2005). Simons and Gaher define distress tolerance as a higher-order emotional construct; individuals with low distress tolerance tend to (a) describe the experience of emotional distress as unbearable, (b) appraise emotional distress as unacceptable, (c) engage in efforts to alleviate the negative emotional state; and (d) be unable to focus their attention away from their feelings of distress. Brown and colleagues (Brown et al., 2002) reported that the interaction between distress tolerance and nicotine withdrawal stress predicted smoking relapse. Likewise, Schmidt and colleagues (1997, 1999) reported that anxiety sensitivity, or the fear of anxiety-related sensations, predicted the development of panic among cadets experiencing the stress of basic training. Most recently, Brozina and Abela (2006) reported that behavioral inhibition predicted the onset of anxiety symptoms at 6-week follow-up for those high in stress (hassles) in a cohort of over 1,300 elementary school children.
If anxiety sensitivity and behavioral inhibition can be interpreted as measures of distress tolerance (broadly conceived), then collectively, these results provide good initial evidence that the impact of stress on important clinical outcomes may depend on the extent to which the individual can tolerate distress. In the context of HIV, it is plausible that low distress tolerance may increase the negative appraisal of major life events or interfere with an adaptive response to disease-related stressors. For example, new symptom onset may require treatment planning with a health care provider, accommodating to a new treatment regimen or adapting to increasing levels of impairment. In the presence of low distress tolerance the patient may experience difficulty enduring the negative emotions associated with new symptom onset when trying to implement these approach-oriented strategies. The patient may also be predisposed to use avoidant strategies that may delay or even preclude an adaptive response to the new symptom. In this way, low distress tolerance may negatively affect a range of health and health risk behaviors. Thus, specifying the individual’s level of distress tolerance may well help provide a more functional context for assessing the impact of major life events on disease-related outcomes and health behaviors in HIV and, as such, may be particularly relevant for the development of psychosocial treatment strategies.
The purpose of this study was to provide a preliminary test of the hypothesis that distress tolerance moderates the impact of major life events on variables important in the management of HIV, including depressive symptoms, substance use coping, alcohol use, cocaine use, medication adherence, risky sexual behavior, and self-reported HIV-related symptoms. To this end, we administered a battery of psychosocial assessments, symptom checklists, and medication and adherence questionnaires to a diverse, HIV-positive volunteer sample.
Method
PARTICIPANTS
Participants were a paid volunteer sample recruited through physician offices, specialty clinics, service organizations, and hospitals as part of a larger longitudinal study (Ironson et al., 2005). Entry into the longitudinal study required participants to have CD4 cell counts between 150 and 500 (mid-range of disease) at study entry. Participants were excluded if they had ever had CD4 cell counts below 75 or experienced an AIDS-defining symptom (e.g., Kaposi’s sarcoma, HIV wasting syndrome, lymphoma). These are examples of medical conditions that, when they present in people with HIV, represent progression to AIDS and are characteristic of advanced HIV disease. Participants were also excluded if they were under age 18, had other life-threatening illnesses (e.g., cancer), were actively psychotic or suicidal, or had dementia or current substance dependence. Participants were included in these cross-sectional analyses if they had completed the 6-month assessment time point at which distress tolerance was assessed (n=116). Data were collected from this sample between January 2005 and October 2005.
The average age of the sample was 44 (SD=9.40) and ranged from 24 to 73. Participants were predominantly male (60%). Just over 40% of the sample was African-American; 25% of the sample identified as Hispanic and 20% as Caucasian. This was a well-educated sample, with 80% graduating high school and approximately 30% with college degrees. However, only one-quarter of the sample was employed at study entry, 40% reported being on disability, and two-thirds reported an annual income of $10,000 or less. Forty-seven percent of the participants identified themselves as gay or bisexual, and just over a 25% identified themselves as married or in a committed relationship.
The mean CD4 cell number for the sample was 399 (SD=196) and HIV-1 viral load was 27,000 (SD=84,000) identifying the average participant in the mid-range of HIV disease progression, although there was broad variability around these means. Two-thirds of the sample were taking highly active antiretroviral therapy, and 25% were not taking any anti-HIV medication.
PROCEDURE
As part of the parent longitudinal study (Ironson et al., 2005), participants completed written informed consent, psychosocial questionnaires, a clinical assessment interview, and a blood draw for CD4 and viral load assay. Follow-up visits, repeated every 6 months, included the questionnaire battery, brief interview, and blood draw. Study procedures, including informed consent, were approved by the Institutional Review Board.
MEASURES
Demographics (e.g., age, gender, ethnicity, sexual orientation, education level) and background medical information (e.g., date of HIV diagnosis, symptoms) were assessed by self-report. CD4 lymphocyte count (CD3+CD4+) (T-helper cell number) was determined by whole-blood 4-color direct immunofluorescence using a Coulter XL-MCL flow cytometer. Viral load was estimated using the Roche Amplicor RT/PCR assay sensitive to 400 copies of plasma RNA.
Distress Tolerance Scale (DTS; Simons & Gaher, 2005)
The DTS is a 14-item measure that evaluates the participant’s ability to experience and endure negative emotional states by answering on a 5-point Likert-type scale (5=strongly disagree, 1=strongly agree). This scale has good psychometric properties, including high internal consistency (α=.89) and appropriate convergence with other self-report ratings of affective distress and regulation (Simons & Gaher, 2005). In addition, the DTS has demonstrated adequate 6-month test-retest retest reliability (r=.61; Simons & Gaher, 2005). The scale incorporates items that assess appraisal (e.g., “Being distressed or upset is always a major ordeal for me”), tolerance (e.g., “I can’t handle feeling distressed or upset”), absorption (e.g., “When I feel distressed or upset, all I can think about is how bad I feel”), and regulation (e.g., “I’ll do anything to avoid feeling distressed or upset”). These four second-order factors are comprehensively assessed with the inclusion of an additional item that was not administered. The 14-item scale administered in the present study yields only a score for the single higher-order distress tolerance factor (see Simon & Gaher).
Life Events Scale (LES; Sarason, Johnson & Siegel 1978)
Life events were assessed using the LES, in which participants report the occurrence of major life events in the previous 6 months. Participants also identify how stressful these events were on a 5-point scale (1=extremely stressful to 5=not at all stressful). Because this appraisal of a life event may be influenced by a host of factors (e.g., neuroticism, depression, or possibly distress tolerance), our current analysis separated out the occurrence of the negative event itself from the intensity of the stress appraisal. The measure used in this analysis was the unweighted sum of life events rated negatively, in the past 6 months. This sum was modified to exclude health-related events when examining the relationship with HIV-related symptoms to reduce the possibility of construct overlap.
Beck Depression Inventory (BDI; Beck, Rush, Shaw, & Emery, 1979)
The BDI is a widely used 21-item self-report measure that assesses severity of depressive symptoms. The scale has good psychometric properties, including high internal consistency (α=.87), adequate 1-month test-retest reliability of .60, and appropriate convergence with clinical and other self-report ratings of depressive symptoms (Beck, Steer, & Garbin, 1988).
COPE (Carver, Scheier & Weintraub, 1989)
Coping strategies were assessed using the COPE, a 24-item scale, modified for HIV populations, that assesses the endorsement of each of 12 cognitive and behavioral coping strategies. Each coping strategy is assessed by two items which participants rate on a 4-point scale (1=I haven’t been doing this at all, 4=I’ve been doing this a great deal). The substance use coping subscale (e.g., “I’ve been using alcohol or other drugs to help me get through it”) was used because of its reported relationship with distress tolerance (Simons & Gaher, 2005).
AIDS Clinical Trials Group Adherence Measure (ACTG; Chesney et al., 2000)
Prescribed antiretroviral medications, medication adherence and symptoms associated with HIV were assessed with the interviewer-administered ACTG. The adherence measure used was the sum of the number of reasons endorsed for missed doses (e.g., “because of a change in your daily routine,” “because you felt sick or ill from side effects,” “because you didn’t want other people to notice you taking your medications”).
AIDS Clinical Trials Group HIV Symptom Checklist (ACTG; Chesney et al., 2000)
The ACTG questionnaire includes a list of 20 symptoms associated with HIV infection. Eight of these symptoms that could overlap with the emotional (e.g., “felt sad, down or depressed”) or physical (e.g., “fatigue or loss of energy”) symptoms of depression or anxiety were excluded from this analysis to avoid the possibility of construct overlap between symptoms of HIV and symptoms of distress associated with either depression and anxiety and to increase the confidence that relationships assessed between distress tolerance and symptoms of HIV were not confounded with measures of emotional distress. These 12 remaining items together had good internal consistency (α=.84). The total number of symptoms reported in the past 4 weeks was the measure used.
SUBSTANCE USE
The frequency of alcohol and cocaine use in the past month was assessed though self-report on an interviewer-administered 5-point Likert-type scale ranging from “no use” (0) to “about every day” (4) for each substance.
RISKY SEXUAL BEHAVIOR
Risky sexual behavior was assessed though self-report whereby participants were asked to estimate on a 7-point Likert-type scale the frequency with which they had engaged in unprotected receptive or insertive (vaginal or anal) intercourse in the past 6 months. This information was assessed for male and female sexual partners. The sum of unprotected sexual intercourse events in the past 6 months was the measure used.
Statistical Analyses
In line with previous studies examining psychosocial relationships in HIV (e.g., Ironson et al., 2005), age, gender, ethnicity, SES, and disease stage were considered as covariates. CD4 cell count was employed to control for possible variation in outcomes as a function of disease stage. Education level was used as an unbiased indicator of SES (as both income and employment may be affected by advancing HIV disease) and was controlled using one categorical variable to identify three levels (less than high school graduate, high school graduate, and college graduate and above). Pearson correlations revealed that neither of the continuous covariates (age, CD4 cell count) was significantly associated with any of the outcomes (all ps>.15). Similarly, no significant relationships emerged for education level, gender or ethnicity with any of the outcome measures (all ps>.2).
The main analyses were conducted using separate hierarchical multiple regression analyses for each of the dependent variables. The regression models were constructed by first entering distress tolerance and life events (which had been centered) into the model, followed by the interaction of distress tolerance and life events. Consistent with established procedures for testing moderation (Baron & Kenny, 1986), significant interaction terms in these regression models provide evidence of the moderated effects of distress tolerance on the relationship between major life events and the specified outcomes. That is, the relationship between life events and the dependent variable (e.g., depression) varies as a function of distress tolerance.
Results
DESCRIPTIVE ANALYSES
Participants reported a mean of 1.50 (SD=1.41) major life events in the past 6 months, with a range from 0 to 7. The average score on the BDI was 10.29 (SD=9.29), showing some elevation in depressed symptoms but placing the majority of the sample below the clinical cutoff of 18, indicating moderate levels of depression. The mean coping scores on the substance use coping subscale of the COPE suggest that our participants were, on average, employing substance use coping between “a little bit” and “a medium amount.” The scores on the alcohol and cocaine use variables suggest that, on average, our participants reported alcohol use about once a month and that on average the group reported cocaine use less than once a month. On average, participants endorsed multiple (>6) reasons for missing their medications.
The mean number of reported unprotected sexual intercourse acts in the past 6 months was 0.63 (SD=1.61). There was no significant difference in the number of unprotected sexual intercourse acts based upon sexual orientation, t(83) = −1.10, p=.28.
The mean number of HIV-related symptoms reported in the past month on the modified ACTG symptom checklist was 5.00 (SD=3.60, maximum=12). The means and standard deviations for these variables are presented in Table 1.
Table 1.
Means, standard deviations for major study variables
| M | SD | |
|---|---|---|
| Distress tolerance (DTS) | 3.21 | 0.91 |
| Life events (LES) | 1.74 | 1.60 |
| Depressive symptoms (BDI) | 10.29 | 9.29 |
| Substance use coping (COPE) | 2.89 | 1.44 |
| Substance use past month | ||
| Alcohol use | 0.67 | 1.05 |
| Cocaine use | 0.16 | 0.44 |
| Reasons for missed medication doses (ACTG) | 6.06 | 7.20 |
| Unprotected intercourse in past 6 months | 0.63 | 1.61 |
| HIV-related symptoms in past month (ACTG) | 4.98 | 3.61 |
Note. DTS=Distress Tolerance Scale; LES=Life Event Scale; BDI=Beck Depression Inventory; ACTG=AIDS Clinical Trials Group Adherence Measure; Frequency of substance use was assessed using a 5-point Likert scale; Risky sexual behavior was assessed using a 7-point Likert-type scale.
MAIN EFFECTS FOR DISTRESS TOLERANCE AND MAJOR LIFE EVENTS
The main effects of distress tolerance and major live events on the psychosocial variables are presented in Table 2. They are expressed as partial correlations. Significant main effects were observed for distress tolerance on depressive symptoms, substance use coping, medication adherence, and HIV-related symptoms in the past month. However, only the significant relationship between distress tolerance and HIV-related symptoms, pr=−.35, t(113)=−3.97, p<.01, was observed in the absence of a significant interaction effect. This result suggests lower distress tolerance is associated with more reported HIV-related symptoms in the past month and that distress tolerance accounts for approximately 11% of the variance in HIV-related symptoms. The other significant relationships with distress tolerance are interpreted with reference to the significant interaction effect. No significant relationships were observed between distress tolerance and alcohol use in the past month or unprotected sexual intercourse in the past 6 months.
Table 2.
Main effects for distress tolerance and life events and their interaction effects
| Distress Tolerance (DTS)
|
Life Events (LES)
|
Interaction (DTS ×LES)
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dependent variable | pr | t | df | p | pr | t | df | p | pr | r2Δ | FΔ | df | p |
| Depressive Symptoms (BDI) | −.53 | −6.56 | 113 | <.01 | .38 | 4.32 | 113 | <.01 | −.21 | .03 | 4.02 | 112 | .02 |
| Substance Use Coping (COPE) | −.20 | −2.11 | 113 | .04 | .15 | 1.56 | 113 | .12 | −.19 | .03 | 4.02 | 112 | .05 |
| Alcohol use | −.08 | −.86 | 113 | .39 | .24 | 2.61 | 113 | .01 | −.24 | .05 | 6.49 | 112 | .01 |
| Cocaine use | −.18 | −1.95 | 113 | .05 | .25 | 2.77 | 113 | <.01 | −.19 | .03 | 4.09 | 112 | .05 |
| Medication adherence (ACTG) | −.35 | −3.33 | 81 | <.01 | .40 | 4.16 | 81 | <.01 | −.21 | .03 | 3.84 | 80 | .05 |
| Unprotected sexual Intercourse | −.10 | −1.07 | 113 | .29 | .14 | 1.45 | 113 | .15 | −.12 | .02 | 1.71 | 112 | .19 |
| HIV-Related symptoms (ACGT) | −.35 | −3.97 | 113 | <.01 | .36 | 4.05 | 113 | <.01 | .02 | .00 | .00 | 112 | .80 |
Note. DTS=Distress Tolerance Scale; LES=Life Event Scale; BDI=Beck Depression Inventory; ACTG=AIDS Clinical Trials Group Adherence Measure; Substance use frequency and risky sexual behavior were assessed on 5-and 7-point Likert-type scales, respectively.
Significant main effects for major life events were observed for depressive symptoms, alcohol use, cocaine use, medication adherence, and HIV-related symptoms. However, only the significant relationship between life events and HIV-related symptoms, pr=.36, t(113) =4.05, p <.01, was observed in the absence of a significant interaction effect. This result suggests that more negative life events in the past 6 months is associated with more reported HIV-related symptoms in the past month and that negative life events accounts for approximately 10% of the variance in HIV-related symptoms. It should be recalled that, in this particular analysis, health-related life events were not included in the life event total. The other significant relationships with life events are interpreted below with reference to the significant interaction effect.
DISTRESS TOLERANCE × LIFE EVENTS INTERACTION EFFECTS
The interaction effects are presented in Table 2 as partial correlations in the full model that also includes the main effects for distress tolerance and major life events. In line with our hypotheses, the interaction of distress tolerance and major life events was significantly and negatively related to depression, pr=-.21, r2 =.03, p<.05, substance use coping, pr=−.19, r2 =.03, p=.05, the frequency of alcohol use in the past month, pr=−.24, r2 =.05, p=.01, the use of cocaine in the past month, pr=−.19, r2 =.03, p=.05, and to the number of reported reasons for missed medication doses, pr=−.21, r2 =.03, p=.05. The proportion of variance in psychosocial variables explained by the interaction of distress tolerance and life events controlling for the main effects ranged from 3% (i.e., depressive symptoms, coping, cocaine use adherence) to 5% (i.e., alcohol use). The interaction of distress tolerance and major life events was not significantly related to risky sexual behavior or to reported HIV symptoms (all ps>.1).
Although the most precise test of the interaction effects was presented above, an examination of the mean differences for those above and below the mean in distress tolerance and life events illustrates the effect of these interactions. These four groups (presented in Fig. 1) did not differ significantly on demographic characteristics (i.e., age, gender, education level) or on disease stage (as measured by CD4 cell count), all ps>.1. The mean depression score for those high in life events and low in distress tolerance was 18.90 (SD=9.50), which was significantly higher than that of the other three groups [HighDT/HighLE: t(43)=5.07, p<.001; LowDT/LowLE: t(58)=3.76, p<.001; and HighDT/LowLE: t(62)=6.30, p<.001]. Interestingly, those low in both distress tolerance and major life events reported significantly higher depression than those high in distress tolerance. This suggests that the presence or absence of life events does not affect depression in the presence of high distress tolerance. A similar interaction pattern emerged for the effects on coping and substance use: those low in distress tolerance and high in life events were significantly more likely to endorse the use of substance use coping, F(3, 112)=2.70, p<.05, have a higher frequency of alcohol use in the past month, F(3, 112)= 5.32, p<.01, were more likely to use cocaine, F(3, 112)=5.66, p<.01, and endorsed a greater number of reasons for missing medication doses, F(3, 80)= 4.19, p<.01.
FIGURE 1.
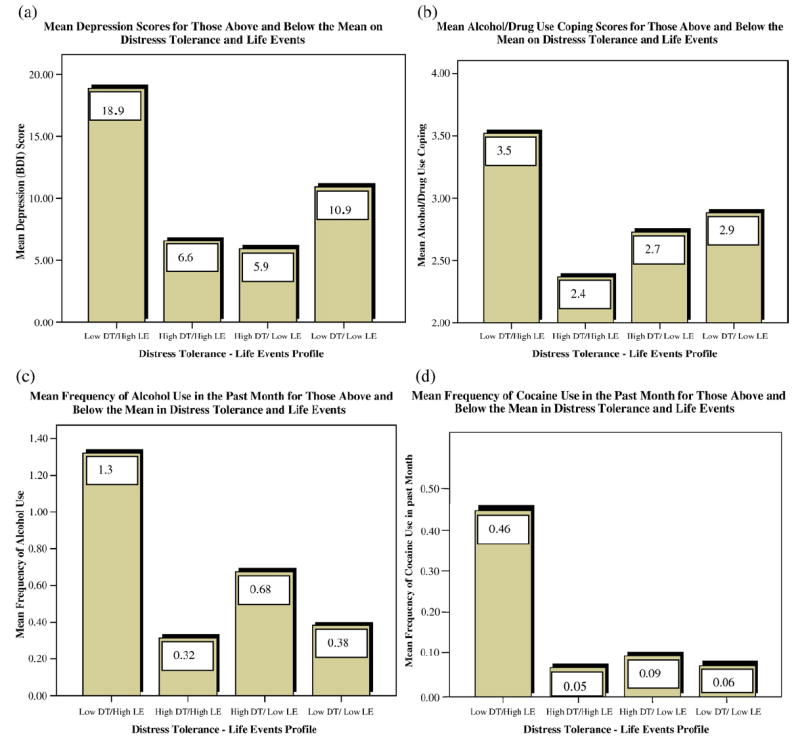
Distress Tolerance/Life Events Interaction Effects for Depression (a), Substance Use Coping (b), Alcohol Use (c), and Cocaine Use (d) (DT=Distress Tolerance; LE=Life Events).
Discussion
In line with our hypotheses, distress tolerance significantly moderated the relationship between major life events in the previous 6 months and relevant outcomes in HIV populations, namely depressive symptoms, substance use coping, alcohol and cocaine use in the past month, and number of reported reasons for missed medication doses. That is, low distress tolerance appeared to combine with a high frequency of major life events to significantly increase the strength of these relationships. These results are consistent with and extend previous findings indicating that levels of specific stress interacted with distress tolerance to predict subsequent onset of panic (Schmidt et al., 1997, 1999), anxiety symptoms (Brozina & Abela, 2006), and smoking relapse (Brown et al., 2002).
This interaction may suggest a vulnerability for depression in HIV-positive populations. Specifically, the mean depression score for those low in distress tolerance and high in major life events was 18.90, which is above the clinical cutoff of 18 on the BDI for moderate depression, suggesting that this interaction may contribute to clinically significant elevations in depression. The presence of the moderated relationship with depression has particular clinical relevance in HIV because depression has been shown to predict accelerated disease progression (Ironson et al., 2005) and death (Ickovics et al., 2001). In addition, depression in people living with HIV interferes with the adaptive management of the disease through its association with higher levels of drug use (Dixit & Crum, 2000; Kalichman et al., 1997), poorer adherence to antiretroviral medication (Safren et al., 2001), and with more risky sex (Kennedy et al., 1993; Lehman et al., 1998). As the rates of depressive disorders in people with HIV have been estimated at up to 37% (e.g., Atkinson & Grant, 1994; Dew et al., 1997; Rabkin, 1996), effective treatment for depression in HIV is critical.
The measure of coping used in this study asked participants to identify the coping strategies used to deal with HIV-specific stressors. The moderated relationship with substance use coping suggests that low distress tolerance may predispose patients with HIV to utilize substance use coping when confronting disease-related stressors. Similarly, our data suggest that the effect of life stress on alcohol and cocaine use may depend on the individual’s level of distress tolerance. These findings have important implications, because drug use in this patient population has been associated with both poorer adherence and accelerated disease progression (Arnsten et al., 2001; Lucas, Gebo, Chaisson, & Moore, 2002). It should be noted that that the low reported rates of alcohol and cocaine use in this sample are likely an artifact of the study design as current alcohol or substance dependence at study entry were exclusion criteria. As a result, the restricted range of substance users in the sample may well limit the external validity of these specific findings.
Those with low distress tolerance and high life events endorsed more reasons for missing their antiretroviral medications. Poorer adherence has been previously related to stressful life events, depression, hopelessness and anxiety, lower social support, and to lower levels of patient knowledge about HIV (Chesney et al., 2000; O’Cleirigh & Ironson, 2005; Sorensen et al., 1998), although these results may be the first to establish a relationship between adherence and distress tolerance in HIV. Adherence to antiretroviral medications is critical for the effective management of HIV (Carpenter et al., 2000; Powderly, Landry, & Lederman, 1998), and poorer adherence has been repeatedly associated with more rapid HIV disease progression (Bangsberg et al., 2000; Castillo et al., 2004; Catz, Kelly, Bogart, Benotsch, & McAuliffe, 2000; Kitahata et al., 2004).
The observed relationship between distress tolerance and reported HIV symptoms in the past month raises the possibility that those with low distress tolerance are more sensitive to the onset of symptoms and therefore more likely to endorse more physical symptoms associated with HIV disease. Although it is feasible that the onset on symptoms in the past month may well incline our participants to lower their estimates of their own distress tolerance, it is also possible that low distress tolerance may cause increased symptoms to occur, through either poor disease management or increased physiological arousal. Future research on this relationship could usefully examine the possibility that lower distress tolerance may well be accompanied by underlying physiological profiles (e.g., dysregulation of the hypothalamic-adrenal cortical axis or the sympathetic-adrenal-medullary axis) to the extent that immune function and responsiveness is compromised, thus contributing to the increased symptom burden reported by our participants with lower distress tolerance. Recently, Leserman et al. (2005) reported that trauma, stressful events, and posttraumatic stress disorder accounted for up to 27% of the variance in health-related functioning in a large sample of HIV-seropositive patients, suggesting that life stress and the emotional distress associated with it are strongly associated with physical functioning. Whatever the mechanism underlying this relationship, our results suggest that an evaluation of distress tolerance may well be indicated, not just to support the adaptive management of HIV but also to inform the treatment of the disease.
Contrary to our hypothesis, we did not observe main effects or moderated effects of distress tolerance and major life events for risky sexual behavior. These results are in contrast to previous work that reported significant relationships between higher levels of psychological distress and depression and risky sexual behavior in homosexual (Beck, McNally & Petrak, 2003) and African-American men (Myers, Javanbakht, Martinez, & Obediah, 2003). The sexual behavior measure required participants to recall their behavior over the past 6 months, which may weaken the reliability of the measure.
The principal limitation of this study is the cross-sectional design, which prohibits the assertion of causal relationships or the specification of the directionality of the effects. Thus, it is possible that higher levels of depressive symptoms or poor health habits are not consequences, but instead cause decreases in distress tolerance. In this case, our data suggest that distress tolerance may be a legitimate target for treatment, particularly in patients with depression that is persistent or not responsive to treatments. A second limitation is that the measures used in this study were based upon self-report and are therefore vulnerable to the biases of that methodology. Specifically, the use of self-reported HIV-related physical symptoms should not be confused with physician-diagnosed symptoms. The measures of substance use were single-item self-report measures that have not been formally validated. Although the sample was diverse with respect to age, gender, ethnicity, and sexual orientation, this was a paid volunteer sample, and these results may not generalize to the broader population of patients with HIV.
Despite these limitations, our results provide preliminary evidence suggesting that the treatment of depression, substance use coping, substance use, and medication adherence in HIV may well benefit from an assessment of the patient’s distress-tolerance profile. Specifically, the direct relationship between distress tolerance and reported HIV symptoms suggests that an assessment of the patient’s distress-tolerance profile may inform medical care by helping to interpret symptom reports. Another clinical implication of our findings is that the efficacy of traditional behavioral medicine components of relaxation/meditation training and problem solving, which already provide strategies for approach-oriented coping and management of negative affect (Antoni et al., 2006; Safren et al., 2004), may be enhanced by the inclusion of emotional acceptance strategies (e.g., mindfulness training, exposure-based treatments).
The moderated relationships described here raise some interesting questions for future research. These findings would be strengthened by replication using a longitudinal design. The associations between distress tolerance and the established psychosocial predictors of HIV disease progression (i.e., depression, avoidant coping, substance use) suggest the possibility that distress tolerance may be an underlying process that itself may account for significant variation in disease progression over time. Perhaps the most important avenue for future research would be the examination of therapeutic strategies designed to increase distress tolerance in patients living with HIV. These initiatives could usefully examine the relationship between intervention related changes in distress tolerance to changes in health behaviors, disease progression, and survival.
Acknowledgments
This research was funded by NIH (R01MH53791 and R01MH 066697; Principal Investigator: Gail Ironson) and T32MH18917. Dr. O’Cleirigh is now at the Department of Psychiatry, Massachusetts General Hospital, Harvard Medical School and The Fenway Institute, Boston, MA.
Contributor Information
Conall O’Cleirigh, University of Miami.
Gail Ironson, University of Miami.
Jasper A. J. Smits, Southern Methodist University
References
- Antoni MH, Carrico AW, Duran RE, Spitzer S, Penedo F, Ironson G, Fletcher MA, Klimas N, Schneiderman N. Randomized clinical trial of cognitive behavioral stress management on human immunodeficiency virus viral load in gay men treated with highly active antiretroviral therapy. Psychosomatic Medicine. 2006;68:143–151. doi: 10.1097/01.psy.0000195749.60049.63. [DOI] [PubMed] [Google Scholar]
- Arnsten JH, Demas PA, Farzadegan H, Grant RW, Gourevitch MN, Chang CJ, Buono D, Eckholt H, Howard AA, Schoenbaum EE. Antiretroviral therapy adherence and viral suppression in HIV-infected drug users: Comparison of self-report and electronic monitoring. Clinical Infectious Diseases. 2001;33:1417–1423. doi: 10.1086/323201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson JH, Grant I. Natural history of neuropsychiatric manifestations of HIV disease. Psychiatric Clinics of North America. 1994;17:17–33. [PubMed] [Google Scholar]
- Bangsberg DR, Hecht FM, Charlebois ED, Zolopa AR, Holodniy MG, Sheiner L, Bamberger JD, Chesney MA, Moss A. Adherence to protease inhibitors, HIV-1 viral load, and development of drug resistance in an indigent population. AIDS. 2000;14:357–366. doi: 10.1097/00002030-200003100-00008. [DOI] [PubMed] [Google Scholar]
- Baron R, Kenny D. The moderator-mediator distinction in social psychological research: Conceptual strategic and statistical considerations. Journal of Personality and Social Psychology. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- Beck A, McNally I, Petrak J. Psychosocial predictors of HIV/STI risk behaviors in a sample of homosexual men. Sexually Transmitted Infections. 2003;79:142–146. doi: 10.1136/sti.79.2.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Rush A, Shaw B, Emery G. Cognitive therapy of depression. New York: The Guilford Press; 1979. [Google Scholar]
- Beck AT, Steer RA, Garbin MG. Psychometric properties of the Beck Depression Inventory: Twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR. Distress tolerance and duration of past smoking cessation attempts. Journal of Abnormal Psychology. 2002;111:180–185. [PubMed] [Google Scholar]
- Brozina K, Abela JR. Behavioral inhibition, anxious symptoms, and depressive symptoms: A short-term prospective examination of a diathesis-stress model. Behaviour Research and Therapy. 2006;44:1337–1346. doi: 10.1016/j.brat.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Carpenter CC, Cooper DA, Fischl MA, Gatell JM, Gazzard BG, Hammer SM, et al. Antiretroviral therapy in adults: Updated recommendations of the International AIDS Society-USA Panel. Journal of the American Medical Association. 2000;283:381–390. doi: 10.1001/jama.283.3.381. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF, Weintraub JK. Assessing coping strategies: A theoretically based approach. Journal of Personality and Social Psychology. 1989;56:267–283. doi: 10.1037//0022-3514.56.2.267. [DOI] [PubMed] [Google Scholar]
- Castillo E, Palepu A, Beardsell A, Akagi L, Yip B, Montaner JS, Hogg RS. Outpatient pharmacy care and HIV viral load response among patients on HAART. AIDS Care. 2004;16:446–457. doi: 10.1080/09540120410001683385. [DOI] [PubMed] [Google Scholar]
- Catz S, Gore-Felton C, McClure J. Psychological distress among minority and low-income women living with HIV. Behavioral Medicine. 2002;28:53–60. doi: 10.1080/08964280209596398. [DOI] [PubMed] [Google Scholar]
- Catz SL, Kelly JA, Bogart LM, Benotsch EG, McAuliffe TL. Patterns, correlates, and barriers to medication adherence among persons prescribed new treatments for HIV disease. Health Psychology. 2000;19:124–133. [PubMed] [Google Scholar]
- Chesney MA, Ickovics JR, Chambers DB, Gifford AL, Neidig J, Zwickl B, Wu AW. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: The AACTG adherence instruments. AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- Dew MA, Becker JT, Sanchez J, Cladararo R, Lopez OL, Wess J, et al. Prevalence and predictors of depressive, anxiety, and substance use disorders in HIV-infected and uninfected men: A longitudinal evaluation. Psychological Medicine. 1997;27:395–409. doi: 10.1017/s0033291796004552. [DOI] [PubMed] [Google Scholar]
- Dixit AR, Crum RM. Prospective study of depression and the risk of heavy alcohol use in women. American Journal of Psychiatry. 2000;157:751–758. doi: 10.1176/appi.ajp.157.5.751. [DOI] [PubMed] [Google Scholar]
- Ibanez GE, Purcell DW, Stall R, Parsons JT, Gomez CA. Sexual risk, substance use, and psychological distress in HIV-positive gay and bisexual men who also inject drugs. AIDS. 2005;19:S49–S55. doi: 10.1097/01.aids.0000167351.00503.92. [DOI] [PubMed] [Google Scholar]
- Ickovics JR, Hamburger ME, Vlahov D, Schoenbaum EE, Schuman P, Boland RJ, et al. Mortality, CD4 cell count decline, and depressive symptoms among HIV-seropositive women: Longitudinal analysis from the HIV Epidemiology Research Study. Journal of the American Medical Association. 2001;285:1460–1465. doi: 10.1001/jama.285.11.1466. [DOI] [PubMed] [Google Scholar]
- Ironson G, O’Cleirigh C, Fletcher MA, Laurenceau JP, Balbin E, Klimas N, et al. Psychosocial factors predict CD4 and viral load change in men and women with HIV in the era of HAART. Psychosomatic Medicine. 2005;67:1013–1021. doi: 10.1097/01.psy.0000188569.58998.c8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ironson G, Friedman A, Klimas N, Antoni M, Fletcher M, LaPerriere A, et al. Distress, denial, and low adherence to behavioral interventions predict faster disease progression in gay men infected with human immunodeficiency virus. International Journal of Behavioral Medicine. 1994;1:90–105. doi: 10.1207/s15327558ijbm0101_6. [DOI] [PubMed] [Google Scholar]
- Kalichman SC, Kelly JA, Rompa D. Continued high-risk sex among HIV seropositive gay and bisexual men seeking HIV prevention services. Health Psychology. 1997;16:369–375. doi: 10.1037//0278-6133.16.4.369. [DOI] [PubMed] [Google Scholar]
- Kemeny ME, Dean L. Effects of AIDS-related bereavement on HIV progression among New York City gay men. AIDS Education and Prevention. 1995;7:36–47. [PubMed] [Google Scholar]
- Kennedy CA, Skurnick J, Wan JY, Quattrone G, Sheffet A, Quionenes M, et al. Psychological distress, drug and alcohol use as correlates of condom use in HIV-serodiscordant heterosexual couples. AIDS. 1993;7:1493–1499. doi: 10.1097/00002030-199311000-00014. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Foster C, Joseph J, Ostrow D, Wortman C, Phair J, et al. Stressful life events and symptom onset in HIV infection. American Journal of Psychiatry. 1991;148:733–738. doi: 10.1176/ajp.148.6.733. [DOI] [PubMed] [Google Scholar]
- Kitahata MM, Reed SD, Dillingham PW, Van Rompaey SE, Young AA, Harrington RD, et al. Pharmacy-based assessment of adherence to HAART predicts virologic and immunologic treatment response and clinical progression to AIDS and death. International Journal of STD and AIDS. 2004;15:803–810. doi: 10.1258/0956462042563666. [DOI] [PubMed] [Google Scholar]
- Lehman JS, Hecht FM, Fleming PL, Coleman S, Chesney M, Bindman A, et al. HIV testing behavior among at-risk populations: Why do persons seek, defer, or avoid getting tested in the United States? International Conference on AIDS. 1998;12:867. [Google Scholar]
- Leserman J. HIV disease progression: Depression, stress, and possible mechanisms. Biological Psychiatry. 2003;54:295–306. doi: 10.1016/s0006-3223(03)00323-8. [DOI] [PubMed] [Google Scholar]
- Leserman J, Jackson ED, Petitto JM, Golden RN, Silva SG, Perkins DO, et al. Progression to AIDS. The effects of stress, depressive symptoms, and social support. Psychosomatic Medicine. 1999;61:397–406. doi: 10.1097/00006842-199905000-00021. [DOI] [PubMed] [Google Scholar]
- Leserman J, Petitto JM, Gu H, Gaynes BN, Barroso J, Golden RN, et al. Progression to AIDS, a clinical AIDS condition and mortality: Psychosocial and physiological predictors. Psychosomatic Medicine. 2002;32:1059–1073. doi: 10.1017/s0033291702005949. [DOI] [PubMed] [Google Scholar]
- Leserman J, Whetten K, Lowe K, Strangl D, Schwartz M, Thielman N. How trauma, recent stressful events, and PTSD affect functional health status and health utilization in HIV-infected patients in the South. Psychosomatic Medicine. 2005;67:500–507. doi: 10.1097/01.psy.0000160459.78182.d9. [DOI] [PubMed] [Google Scholar]
- Lucas GM, Gebo KA, Chaisson RE, Moore RD. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- Mugavero M, Ostermann J, Whetten K, Leserman J, Swartz M, Stangl D, Thielman N. Barriers to antiretroviral adherence: The importance of depression, abuse, and other traumatic events. AIDS Patient Care STDS. 2006;20:418–428. doi: 10.1089/apc.2006.20.418. [DOI] [PubMed] [Google Scholar]
- Myers HF, Javanbakht M, Martinez M, Obediah S. Psychosocial predictors of risky sexual behavior in African American men. AIDS Education and Prevention. 2003;15:66–79. doi: 10.1521/aeap.15.1.5.66.23615. [DOI] [PubMed] [Google Scholar]
- O’Cleirigh C, Ironson G. The relationship between emotional processing of traumatic life experiences, distress and antiretroviral medication adherence in gay and bisexual HIV positive men. Poster presented to the 23rd Annual Conference of the Society for Behavioral Medicine; Boston, MA. 2005. [Google Scholar]
- O’Cleirigh C, Ironson G, Antoni M, Fletcher MA, McGuffey L, Balbin E, et al. Emotional expression and depth processing: Their relation to long-term survival patients living with HIV/AIDS. The Journal of Psychosomatic Research. 2003;54:225–235. doi: 10.1016/s0022-3999(02)00524-x. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Semple SJ, Temoshok LR, Atkinson JH, McCutchan JA, Straits-Troster K, et al. Stress and depressive symptoms prospectively predict immune change among HIV-seropositive men. Psychiatry. 1995;58:299–312. doi: 10.1080/00332747.1995.11024735. [DOI] [PubMed] [Google Scholar]
- Patterson TL, Shaw WS, Semple SJ, Cherner M, McCutchan A, Atkinson H, et al. Relationship of psychosocial factors to HIV disease progression. Annals of Behavioral Medicine. 1996;18:30–39. doi: 10.1007/BF02903937. [DOI] [PubMed] [Google Scholar]
- Penedo FJ, Gonzalez JS, Davis C, Dahn J, Antoni M, Ironson G, et al. Coping and psychological distress among symptomatic HIV+men who have sex with men. Annals of Behavioral Medicine. 2003;25:203–213. doi: 10.1207/S15324796ABM2503_06. [DOI] [PubMed] [Google Scholar]
- Pence BW, Miller WC, Whetten K, Eron JJ, Gaynes BN. Prevalence of DSM-IV-defined mood, anxiety, and substance use disorders in an HIV clinic in the Southeastern United States. Journal of Acquired Immune Deficiency Syndrome. 2006;42:298–306. doi: 10.1097/01.qai.0000219773.82055.aa. [DOI] [PubMed] [Google Scholar]
- Powderly WG, Landry A, Lederman M. Recovery of the immune system with antiretroviral therapy: The end of opportunism? Journal of the American Medical Association. 1998;280:72–77. doi: 10.1001/jama.280.1.72. [DOI] [PubMed] [Google Scholar]
- Rabkin JG. Prevalence of psychiatric disorders in HIV illness. International Review of Psychiatry. 1996;8:157–166. [Google Scholar]
- Roberts AC, Wechsberg WM, Zule W, Burroughs AR. Contextual factors and other correlates of sexual risk of HIV among African-American crack-abusing women. Addictive Behaviors. 2003;28:523–536. doi: 10.1016/s0306-4603(01)00255-6. [DOI] [PubMed] [Google Scholar]
- Safren S, Hendriksen E, Mayer K, Pickard R, Otto M. Cognitive behavioral therapy for HIV medication adherence and depression. Cognitive and Behavioral Practice. 2004;11:415–424. [Google Scholar]
- Safren SA, Otto MW, Worth J, Salomon E, Johnson W, Mayer K, et al. Two strategies to increase adherence to HIV antiretroviral medication: Life-Steps and medication monitoring. Behaviour Research and Therapy. 2001;39:1151–1162. doi: 10.1016/s0005-7967(00)00091-7. [DOI] [PubMed] [Google Scholar]
- Sarason IG, Johnson J, Siegel J. Assessing the impact of life changes: Development of the Life Experiences Survey. Journal of Consulting and Clinical Psychology. 1978;46:932–946. doi: 10.1037//0022-006x.46.5.932. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. The role of anxiety sensitivity in the pathogenesis of panic: Prospective evaluation of spontaneous panic attacks during acute stress. Journal of Abnormal Psychology. 1997;106:355–364. doi: 10.1037//0021-843x.106.3.355. [DOI] [PubMed] [Google Scholar]
- Schmidt NB, Lerew DR, Jackson RJ. Prospective evaluation of anxiety sensitivity in the pathogenesis of panic: Replication and extension. Journal of Abnormal Psychology. 1999;108:532–537. doi: 10.1037//0021-843x.108.3.532. [DOI] [PubMed] [Google Scholar]
- Siegel K, Schrimshaw EW, Pretter S. Stress-related growth among women living with HIV/AIDS: Examination of an explanatory model. Journal of Behavioral Medicine. 2005;28:403–414. doi: 10.1007/s10865-005-9015-6. [DOI] [PubMed] [Google Scholar]
- Sikkema K, Kochman A, DiFranceisco W, Kelly J, Hoffman R. AIDS-related grief and coping with loss among HIV-positive men and women. Journal of Behavioral Medicine. 2003;26:165–181. doi: 10.1023/a:1023086723137. [DOI] [PubMed] [Google Scholar]
- Simons JS, Gaher RM. The Distress Tolerance Scale: Development and validation of a self-report measure. Motivation and Emotion. 2005;29:83–102. [Google Scholar]
- Solano L, Costa M, Salvati S, Coda R, Aiuti F, Mezzaroma I, et al. Psychosocial factors and clinical evolution in HIV-1 infection: A longitudinal study. Journal of Psychosomatic Research. 1993;37:39–51. doi: 10.1016/0022-3999(93)90122-v. [DOI] [PubMed] [Google Scholar]
- Sorensen JL, Mascovich A, Wall TL, DePhilippis D, Batki SL, Chesney M. Medication adherence strategies for drug abusers with HIV/AIDS. AIDS Care. 1998;10:297–312. doi: 10.1080/713612419. [DOI] [PubMed] [Google Scholar]
- Smith MY, Egert J, Winkel G, Jacobson J. The impact of PTSD on pain experience in persons with HIV/ AIDS. Pain. 2002;98:9–17. doi: 10.1016/s0304-3959(01)00431-6. [DOI] [PubMed] [Google Scholar]
- Stein J, Rotheram-Borus MJ, Swendeman D, Milburn NG. Predictors of sexual transmission risk behaviors among HIV-positive young men. AIDS Care. 2005;17:433–442. doi: 10.1080/09540120412331291724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanable PA, Carey MP, Blair DC, Littlewood RA. Impact of HIV-related stigma on health behaviors and psychological adjustment among HIV-positive men and women. AIDS and Behaivor. 2006;10:473–482. doi: 10.1007/s10461-006-9099-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver KE, Antoni MH, Lechner S, Duran R, Penedo F, Fernandez MI, et al. Perceived stress mediates the effects of coping on the quality of life of HIV-positive women on highly active antiretroviral therapy. AIDS and Behavior. 2005;8:175–183. doi: 10.1023/B:AIBE.0000030248.52063.11. [DOI] [PubMed] [Google Scholar]


