Abstract
Purpose
The aims of this study were to determine whether IL-17+ T cells were present in CD4 and CD8 interphotoreceptor retinoid-binding protein (IRBP)-specific T cells and to determine the role of antigen-specific and nonspecific IL-17+ T cells in the pathogenesis of experimental autoimmune uveitis (EAU).
Methods
B6 mice were immunized with uveitogenic peptide IRBP1–20. In vivo-primed T cells were separated and stimulated with the immunizing peptide. Intracellular expression of IFN-γ and IL-17 by the T cells was assessed, and the pathogenic activity of the activated T cells was determined.
Results
A subset of autoreactive IRBP-specific CD8 T cells expressed IL-17. IRBP-specific T cells preferentially expressed IL-17 when expanded by IL-23, whereas IFN-γ–expressing cells were dominant when the T cells were cultured with IL-2. Importantly, both expanded T-cell populations were uveitogenic. In addition, IL-23 promoted the expansion of antigen-specific and non–antigen-specific IL-17+ T cells, whereas TGF-β and IL-6 acted only on non–antigen-specific IL-17+ T cells. Only the antigen-specific IL-17+ T cells were uveitogenic. The activation of autoreactive IL-17+ T cells was markedly increased in vivo by the mycobacterial component of CFA and pertussis toxin (PTX) and in vitro by the ligation of Toll-like receptors.
Conclusions
IL-17+ T cells can be readily detected among activated autoreactive and bystander T cells and may play a major role in the pathogenesis of EAU.
Experimental autoimmune uveitis (EAU) is a T cell-mediated autoimmune disease that serves as a model for several posterior uveitides, such as Behçet disease, Vogt-Koyanagi-Harada syndrome, birdshot retinochoroidopathy, and sympathetic ophthalmia.1,2 The histopathology of EAU is characterized by posterior retinal and choroidal inflammation, granuloma formation, vasculitis, photoreceptor damage, vitreitis, and varying degrees of anterior uveitis.2 EAU is induced in animals by immunization with retinal antigens or by the adoptive transfer of retinal antigen-specific T lymphocytes.3,4 Among the ocular antigens known to induce EAU in rodent models are interphotoreceptor retinoid-binding protein (IRBP)5 and the soluble retinal antigen (S-antigen).6,7
Thus far, it is thought that the major subsets of pathogenic autoreactive T cells produce proinflammatory cytokines, including IFN-γ and IL-2, and belong to the Th1-type of CD4 T cells.8 Recent studies have shown that a specific autoreactive T-cell subset that produces IL-17, but not IFN-γ or IL-4, is crucially involved in the pathogenesis of autoimmune diseases, such as rheumatoid arthritis, experimental autoimmune encephalomyelitis (EAE),9–11 and other allergic diseases,12–14 especially during the chronic phase. IL-17–deficient mice are resistant to an arthritislike disease15,16 and have impaired host defense against microbial infection and an increased incidence of acquired delayed-type hypersensitivity.15 In addition, autoimmune-prone mice become disease resistant after they are treated with an IL-17R antagonist.17
To determine the role of IL-17+ T cells in the pathogenesis of EAU and the interrelationship between previously characterized uveitogenic T-cell subsets and IL-17+ T cells, we studied the induction and pathologic function of IL-17+ T cells. We were particularly interested in determining whether IL-17 was expressed only by CD4 autoreactive T cells or also by CD8 autoreactive T cells and in comparing the pathogenic effect of IL-17+ T cells specific for the uveitogenic antigen with that of nonspecific IL-17+ T cells. We showed that CD4 and CD8 autoreactive T cells in EAU were equally capable of expressing IL-17 and that IRBP-specific T cells preferentially expressed IL-17 when they were expanded by IL-23, whereas IFN-γ– expressing cells were dominant when the T cells were cultured with IL-2. We also showed that both sets of expanded T cells were uveitogenic. Comparison of IL-17+ T cells elicited by antigen-specific stimulation in vitro with those elicited by antigen-nonspecific activation (anti-CD3 antibody) demonstrated that only antigen-specific IL-17+ T cells were uveitogenic. Finally, we showed that the ligation of Toll-like receptor (TLR) greatly enhanced the activation of IL-17–producing T cells in vivo and in vitro. Our results provide direct evidence that IL-17+ autoreactive T cells elicited by uveitogenic antigen are pathogenic, that the number and activation of these T cells is regulated by available cytokines, that the activation of such T-cell subsets is regulated by factors other than autoantigen, such as mycobacterial components in complete Freund adjuvant (CFA), and that pertussis toxin (PTX), which is essential for the induction of EAU in animal models, promotes the activation of IL-17+ autoreactive T cells.
METHODS
Animals and Reagents
Pathogen-free female C57BL/6 (B6) and B10RIII mice (12–14 weeks old) were purchased from the Jackson Laboratory (Bar Harbor, ME) and were housed and maintained in the animal facilities of the University of Louisville. All animal studies conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Institutional approval was obtained, and institutional guidelines regarding animal experimentation were followed. Recombinant murine IL-2, IL-4, IL-6, IL-23, mouse GM-CSF, and TGF-β were purchased from R&D Systems (Minneapolis, MN). FITC-conjugated anti–IL-17 antibody was purchased from Biolegend (San Diego, CA); all other antibodies were from BD Biosciences (La Jolla, CA). Poly IC, CpG1826, and LPS were purchased from Sigma (St. Louis, MO), and BLP (Pam3CSK), a synthetic tripalmitoylated lipopeptide that mimics the acylated amino terminus of bacterial lipopeptide, was purchased from EMC Microcollection (Tübingen, Germany). IRBP1–20 and IRBP161–180 peptides and incomplete Freund adjuvant (IFA) or complete Freund adjuvant (CFA) were obtained from Sigma.
Preparation of IRBP1–20–Specific T Cells
Briefly, B6 mice were immunized subcutaneously with 200 μL emulsion containing 200 μg IRBP1–20 in either IFA or CFA, distributed over six spots at the tail base and on the flank. At postimmunization (p.i.) day 13, T cells were isolated from lymph node cells and spleen cells by passage through a nylon wool column. Then 1 × 107 cells in 2 mL RPMI medium in a six-well plate (Costar; Corning Life Sciences, Corning, NY) were stimulated for 48 hours with 10 μg/mL IRBP1–20 in the presence of 1 × 107 irradiated syngeneic antigen-presenting cells (APCs; irradiated spleen cells) in the presence of IL-2 or IL-23 (10 ng/mL). Activated T-cell blasts were separated by Ficoll gradient centrifugation and cultured for another 72 hours in the same medium used for stimulation minus the peptide. B10RIII mice were immunized in the same way with the uveitogenic peptide IRBP161–180.
Purification of CD4 and CD8 T Cells Using Auto-MACS Columns
Purified CD4 and CD8 T cells were prepared from the spleens and draining lymph nodes of immunized B6 mice (CD4 and CD8 Isolation Kit; Miltenyi Biotec GmbH) as we previously reported.18 Lymph node and spleen cells were first incubated for 10 minutes at 4°C with a cocktail of biotin-conjugated antibodies against CD8 (CD8a, Ly-2) or CD4 (CD4, L3T4) T cells (H57–597), B cells (CD45R, B220), NK cells (CD49b, DX5), hematopoietic cells (CD11b, Mac-1), and erythroid cells (Ter119) and then were incubated for 15 minutes at 4°C with antibiotin microbeads. The cells were separated into bound and non-bound groups on a separator column (AutoMACS; Miltenyi Biotec GmbH) and were washed with 15 mL medium according to the manufacturer’s protocol. The flow-through fraction containing CD4- or CD8-enriched cells was collected. The purity of the isolated cell fraction was in the range of more than 95%, as determined by flow cytometric analysis using FITC-conjugated anti-TCR antibodies and PE-conjugated antibodies directed against CD8 or CD4 (BD Biosciences). Data collection and analysis were performed on a flow cytometer (FACSCalibur; BD Biosciences) with acquisition and analysis software (CellQuest; Becton Dickinson, Franklin Lakes, NJ).
Expansion of IRBP-Specific T Cells In Vitro
MACS column-purified CD4 or CD8 T cells or unfractionated T cells prepared from IRBP1–20 immunized or naive B6 mice were seeded at 1 × 106 cells/well in 24-well plates and stimulated with immunizing antigen (10 μg/mL) or anti-CD3 antibodies (1 μg/mL) in the presence of syngeneic APCs (irradiated spleen cells). To obtain T cells preferentially expressing IFN-γ or IL-17, the stimulation medium was supplemented with 10 ng/mL recombinant IL-2 or IL-23, respectively.
Scoring of EAU
Mice were examined by indirect funduscopy three times a week for clinical signs of EAU. Pupils were dilated with 0.5% tropicamide and 1.25% phenylephrine hydrochloride ophthalmic solutions. Grading of disease was performed according to the scoring systems described previously.19 For histopathologic evaluation, whole eyes were collected at the end of the experiment and were immersed for 1 hour in 4% glutaraldehyde in phosphate buffer, pH 7.4, and were transferred to 10% formaldehyde in phosphate buffer until processed. Fixed and dehydrated tissues were embedded in methacrylate, and 5-μm sections were cut through the pupillary–optic nerve plane and stained with hematoxylin and eosin. The presence or absence of disease was evaluated in a masked fashion through examination of six sections cut at different levels for each eye. Disease was graded depending on cellular infiltration and structural changes.20
Bone Marrow–Derived Dendritic Cells and Testing the Effects of TLR Ligation
Bone marrow was collected from the femurs of naive B6 mice and cultured for 6 days in medium containing mouse GM-CSF (10 ng/ mL) and IL-4 (10 ng/mL). On day 4, the nonadherent granulocytes were removed, and the medium was replaced with fresh GM-CSF–and IL-4 – containing medium. On day 6, the loosely adherent cells were transferred to a fresh culture dish and were used as bone marrow– derived dendritic (BMDCs). FACS analysis showed that more than 95% of the cells were CD11c+ major histocompatibility complex (MHC) class II antigen+. To test the effect of TLR ligation on the activation of IL-17+ T cells in vitro, BMDCs on day 6 were left untreated or were pretreated with different TLR ligands for 18 hours. After washings, they were cocultured with IRBP-specific T cells for 3 to 4 days, when the production of IL-17 and IFN-γ was assessed by ELISA. The TLR ligands used were BLP (TLR2, 5 μg/mL), LPS (TLR4, 1 μg/mL), poly(I:C) (TLR3, 50 μg/mL), and CpG1826 (TLR9, 10 μg/mL).
Immunofluorescence Flow Cytometry
Aliquots of 2 × 105 cells were double-stained with combinations of FITC- or PE-conjugated monoclonal antibodies against mouse αβTCR, CD4, or CD8. Data collection and analysis were performed on a flow cytometer (FACSCalibur; BD Biosciences) using acquisition and analysis software (CellQuest; Becton Dickinson).
Intracellular Cytokine Flow Cytometry
Unfractionated or purified CD4 or CD8 IRBP1–20–specific T cells were stimulated in vitro with 50 ng/mL PMA, 1 μg/mL ionomycin, and 1 μg/mL antibiotic (Brefeldin A; Sigma) for 4 hours and then were washed, fixed, permeabilized overnight with buffer (Cytofix/Cyto-perm; eBioscience, San Diego, CA), intracellularly stained with antibodies against IFN-γ and IL-17, and analyzed on a flow cytometer (FACSCalibur; BD Biosciences).
ELISA
IL-17 and IFN-γ were measured using commercially available ELISA kits (R&D Systems).
Statistical Analysis
Data are expressed as the mean ± SD of the results for at least three separate experiments.
RESULTS
Evidence of IL-17+ T Cells in IRBP-Reactive CD8 T Cells
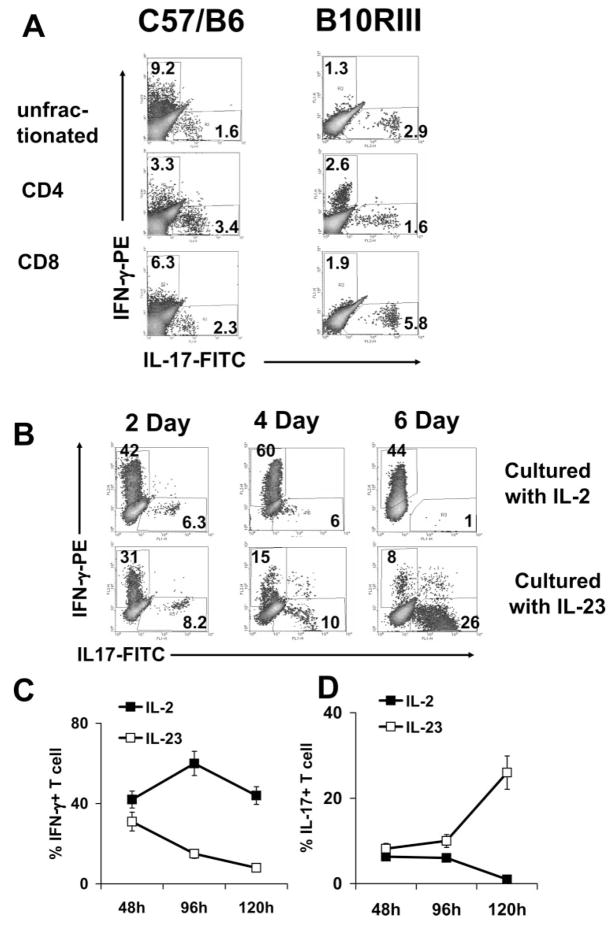
To determine whether autoreactive CD8 T cells of EAU mice expressed IL-17, we used a magnetic column (MACS) to separate CD4 and CD8 T cells from the spleens and the draining lymph nodes of IRBP-immunized B6 mice at p.i. day 13. Separated CD4 and CD8 T cells were then exposed to in vitro stimulation with immunizing antigen (10 μg/mL) and APCs (irradiated spleen cells) for 4 days, when activated T-cell blasts were separated by Ficoll gradient centrifugation and stained intracellularly with FITC-anti–IL-17 and PE-anti–IFN-γ antibodies. As shown in the left panels of Figure 1A, a significant proportion of the IRBP-specific CD8 T-cell blasts expressed IL-17; the percentage of IL-17+ T cells induced in the CD8 and CD4 IRBP-specific T cells was comparable to and higher than that induced in unfractionated T cells. Kinetic studies on unfractionated T cells from B6 mice showed that, unlike the IFN-γ–expressing T cells in the IL-2–stimulated cultures, which were activated within 24 hours (a percentage of which remained high for the next 3 to 5 days; Fig. 1B, upper panels), the percentage of IL-17+ cells in IL-23–stimulated cultures increased gradually with time (Fig. 1B, lower panels). The percentage of activated IL-17+ T cells in the cultured autoreactive T cells was strictly dependent on the supplemental cytokine used in expanding the antigen-stimulated T cells. As shown in Figures 1C and 1D, unfractionated expanding T cells preferentially expressed IFN-γ when cultured with antigen and 10 ng/mL IL-2; IL-17+ cells were seen transiently. However, when the same T-cell cultures were stimulated with antigen in the presence of 10 ng/mL IL-23, an increased percentage of the activated T cells persistently expressed IL-17, and only transient expression of IFN-γ was seen.
Figure 1.
IL-17 expression by IRBP-specific T-cell subsets. (A) IL-17–expressing IRBP-specific unfractionated T cells and CD4 and CD8 T cells in C57BL/6 (left) and B10RIII (right) mice. T cells were prepared from the spleens, and the draining lymph nodes of IRBP1–20–immunized B6 mice or IRBP161–180–immunized B10RIII mice at p.i. day 13 were fractionated into CD4 or CD8 cells using antibodies and a magnetic column (MACS). Unfractionated or purified CD4 or CD8 T cells were exposed to in vitro stimulation with immunizing antigen (10 μg/mL) and APCs (irradiated spleen cells) for 4 days, and the activated T-cell blasts were separated by Ficoll gradient centrifugation and stained intracellularly with FITC-anti–IL-17 and PE-anti–IFN-γ antibodies. (B) Kinetic study of IL-17 expression by unfractionated IRBP-specific T cells in the presence of IL-2 or IL-23. Responder T cells were enriched from IRBP1–20–immunized B6 mice at p.i. day 13 and were incubated with the immunizing peptide for 2 days in the presence of syngeneic APCs. Then the activated T cells were separated by Ficoll gradient centrifugation and continuously cultured in IL-2– or IL-23–supplemented medium for 4 more days. Intracellular expression of IFN-γ and IL-17 was assessed by FACS analysis. (C and D) As in (B), results show the statistical data for 40 mice.
To confirm that the expression of IL-17 by autoreactive T cells was not restricted to the B6 EAU model, we examined the B10RIII EAU model in which autoreactive CD4 and CD8 T cells can be induced by the uveitogenic peptide IRBP161–180. As shown in Figure 1A, IL-17+ CD8 autoreactive T cells were again seen.
Adoptively Transferred Disease by IRBP-Specific T Cells Expanded by IL-23 or IL-2
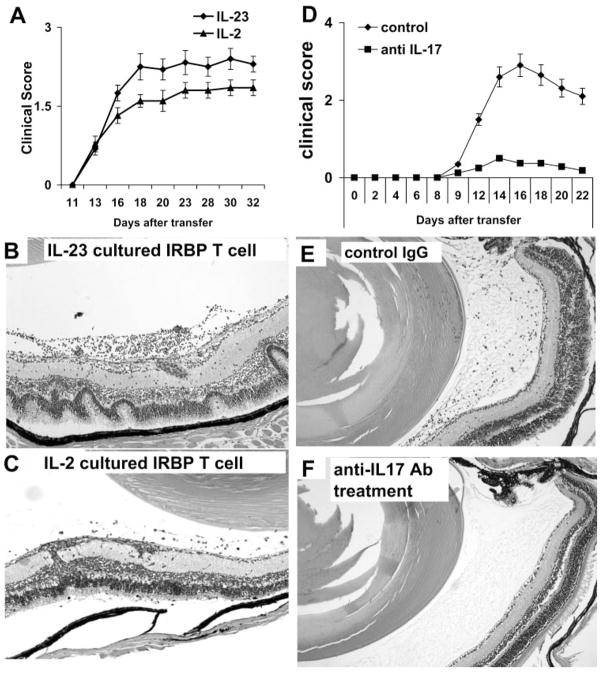
We then examined whether IRBP-specific T cells enriched for IL-17+ cells as a result of expansion in IL-23–containing medium were pathogenic. To test this, T cells from IRBP1–20–immunized B6 mice prepared at p.i. day 13 were exposed to immunizing peptide in the presence of IL-2 or IL-23 (10 ng/ mL)-containing medium for 3 days, activated T-cell blasts were separated by Ficoll gradient centrifugation, and 5 × 106 activated T cells were adoptively transferred to each naive B6 mouse. As shown in Figure 2, the IRBP-specific T cells expanded by IL-2 preferentially expressing IFN-γ and those expanded by IL-23 preferentially expressing IL-17 induced severe EAU in recipient mice.
Figure 2.
Determination of the uveitogenic activity of IRBP-specific T cells expanded by IL-23. Unfractionated T cells from IRBP1–20–immunized B6 mice at p.i. day 13 were exposed to immunizing peptide in the presence of IL-2 or IL-23 (10 ng/ mL)-containing medium for 3 days. Activated T-cell blasts were separated by Ficoll gradient centrifugation, and 5 × 106 activated T cells were adoptively transferred into a naive B6 mouse. (A) Clinical score by funduscopy with time (six mice per group) and (B, C) pathologic examination of the retinas of mice receiving IL-23–cultured (Th17) (B) or IL-2-cultured (C) IRBP-specific T cells. (D, E) Recipient B6 mice were injected with four doses (100 μg) of anti–IL-17 antibodies twice a week, and control mice were treated with isotype-matched rat antibody. All animals were induced for EAU by injection of 5 × 106 IL-17+ IRBP-specific T cells. Animals were monitored by funduscope examination (D). Pathologic examinations were conducted 15 days after the disease induction (E, F). Mice treated with anti–IL-17 antibodies had significantly milder disease (F).
We also tested whether the injection of anti–mouse IL-17 antibodies could ameliorate the EAU induced by adoptive transfer of IL-17+ IRBP-specific T cells. Thus, recipient B6 mice were randomly separated into two groups: treated mice were injected with four doses (100 μg) of anti–IL-17 antibodies twice a week, and control mice received similar doses of isotype-matched rat antibody. All the animals were monitored by funduscope examination, and pathologic examination was conducted 15 days after disease induction (7 days after disease onset). Our results showed that injection of recipient mice with anti–IL-17 antibodies significantly inhibited EAU development (Figs. 2D–F).
Expansion of Antigen-Specific and Nonantigen-Specific IL-17+ T Cells
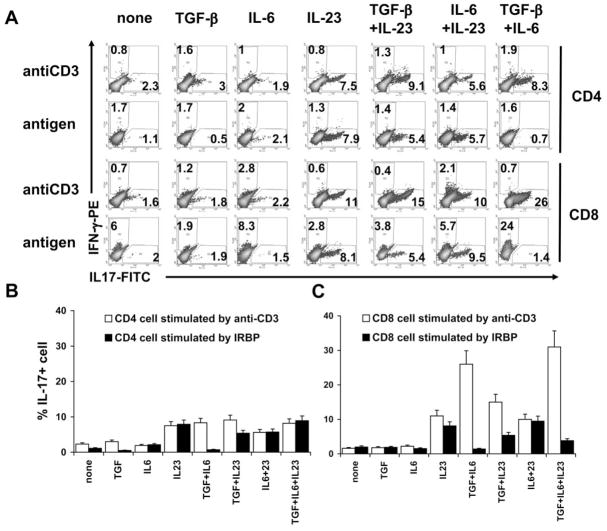
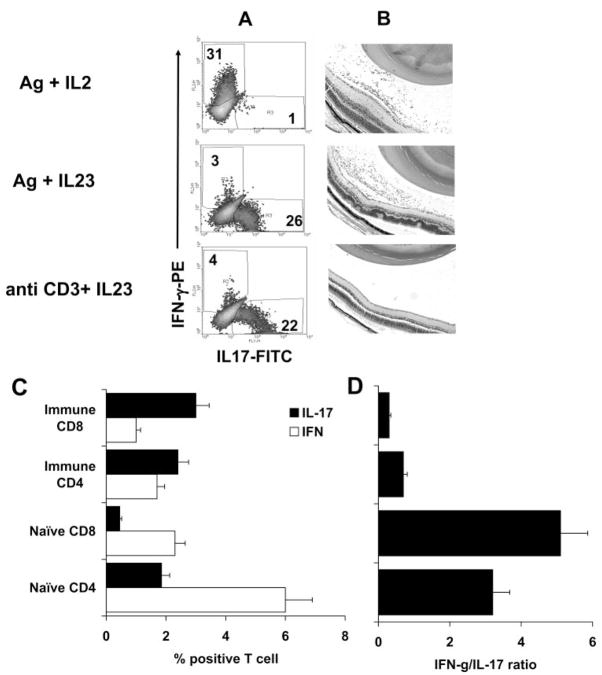
Previous studies have shown that several cytokines, including TGF-β,21,22 IL-6, and IL-23,12,14 can promote the activation or expansion of IL-17+ T cells. To determine whether antigen-specific and nonspecific IL-17+ T cells and CD4 and CD8 IL-17+ T cells are regulated by distinct cytokines, we incubated in vivo-primed CD4 or CD8 T cells from IRBP1–20–immunized mice with the immunizing antigen or anti-CD3 antibody in the presence or absence of various cytokines for 3 to 4 days, then separated the activated T cells by Ficoll gradient centrifugation and stained them intracellularly with anti–IFN-γ and anti–IL-17 antibodies before FACS analysis. As shown in Figure 3, a sig-nificant increase in IL-17+ T cells (CD4 and CD8 cells) was seen when the cultures where supplemented with IL-23 (10 ng/mL), regardless of whether the T cells were stimulated with immunizing antigen or anti-CD3 antibody. Addition of the combination of IL-6 and TGF-β1 significantly increased the number of activated IL-17+ T cells stimulated by anti-CD3 antibodies but did not significantly increase the activation of IL-17+ T cells stimulated by immunizing antigen. Adoptive transfer of antigen-specific IFN-γ+ T cells generated by stimulation with antigen and expanded by IL-2, antigen-specific IL-17+ T cells generated by stimulation with antigen and expanded with IL-23, or nonantigen-specific IL-17+ T cells generated using anti-CD3 antibody and expanded by IL-23 (Fig. 4A) into naive B6 mice showed that only the antigen-stimulated T cells were uveito-genic, as shown by Figure 4B. The same T cells did not acquire pathogenic activity after in vitro stimulation with anti-CD3 antibody, even when cultured in IL-23–containing medium and containing a high percentage of IL-17+ T cells and few IFN-γ+ T cells (Fig. 4A).
Figure 3.
IL-23 promotes the expansion of antigen-specific and antigen-nonspecific IL-17+ T cells, whereas TGF-β and IL-6 act primarily on antigen-nonspecific IL-17+ T cells. (A) CD4 or CD8 T cells prepared from IRBP1–20–immunized mice (1 × 106 cells/well on a 24-well plate) were exposed for 4 days to immunizing antigen (10 μg/mL) or anti-CD3 antibody (1 μg/mL) in the presence or absence of the indicated cytokines. Activated T cells were separated by Ficoll gradient centrifugation, stained intracellularly with anti–IFN-γ and anti–IL-17 antibodies, and subjected to FACS analysis. (B, C) Statistical data for 30 mice taken from (A).
Figure 4.
Comparison of the uveitogenic activity of antigen-specific and nonspecific IL-17+ T cells. (A) Phenotype of the transferred T cells. T cells from IRBP1–20–immunized B6 mice were exposed to the immunizing antigen (10 μg/mL) or to anti-CD3 antibody (1 μg/mL) in the presence of IL-2 or IL-23 (10 ng/mL)-containing medium for 3 days. Activated T-cell blasts were separated by Ficoll gradient centrifugation before adoptive transfer, and the disease-inducing T cells were assessed for expression of IFN-γ and IL-17 by intracellular staining. (B) Disease induction. Cells shown in (A) were adoptively transferred (5 × 106 activated T cells/mouse) to naive B6 mice, and the retinas were examined at p.i. day 15. Antigen/IL-2–expanded cells (top) and antigen/IL-23–expanded cells (center) were pathogenic (infiltration of immune cells and retinal tissues disarranged), whereas anti-CD3 antibody/IL-23–expanded cells were not. (C, D) Expression of IFN-γ and IL-17 by CD4 and CD8 T cells from naive and antigen-immunized B6 mice. Nylon wool–enriched T cells prepared from naive or IRBP–immunized B6 mice and stimulated in vitro with anti-CD3 for 3 days. Separated T-cell blasts were intracellularly stained with anti–IFN-γ and anti–IL-17. Each group consisted of 10 mice. (D) IFN-γ /IL-17 ratio.
We also compared the induction of IL-17+ T cells by anti-CD3 antibody treatment of T cells from antigen-immunized or unimmunized (naive) B6 mice. As shown in Figure 4C, a greater proportion of CD4 and CD8 T cells from naive B6 mice expressed IFN-γ rather than IL-17 when stimulated with anti-CD3 antibodies in vitro (IFN-γ+/IL-17+ ratio of 3.2:5.1), whereas responder T cells from antigen-immunized mice stimulated in the same way predominantly expressed IL-17+ (IFN-γ+/IL-17+ ratio of 0.3–0.4; P < 0.01), suggesting that antigen immunization promoted the expansion of IL-17+ T cells.
Effect of TLR Ligands on In Vivo–Primed IRBP-Specific T Cells
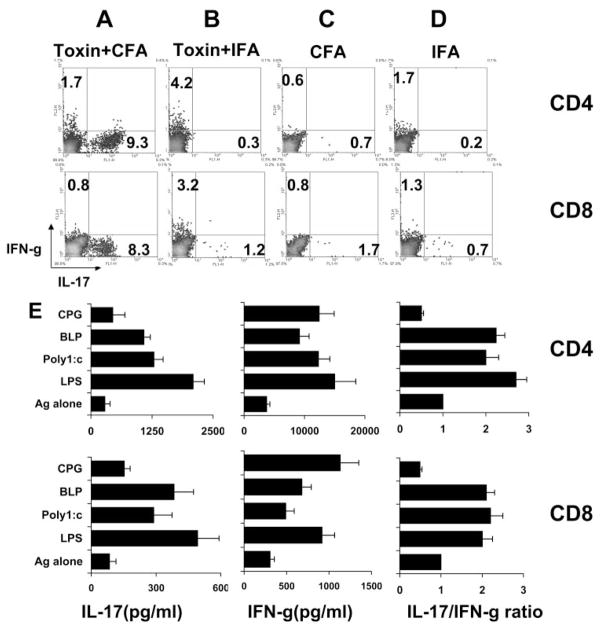
It has been repeatedly shown that mycobacteria in CFA and in pertussis toxin (PTX) are necessary for the induction of many autoimmune diseases, especially in mouse models. To determine whether bacterial products increased the activation of IL-17+ T cells and thus resulted in a broader spectrum of pathogenic T cells, we immunized B6 mice with IRBP1–20 emulsified in CFA or IFA with or without PTX pretreatment. At p.i. day 13, we prepared and stimulated T cells for 3 days with IRBP1–20, as described in Methods. As shown in Figures 5A to 5D, the in vivo-primed CD4 or CD8 T cells from PTX-pretreated B6 mice immunized with antigen in CFA generated more IL- 17+ T cells than PTX-pretreated B6 mice immunized with antigen in IFA or non–PTX-treated mice immunized with antigen in IFA or CFA, suggesting that the mycobacteria in CFA and PTX pretreatment play a synergistic role in the activation of IL-17+ autoreactive T cells.
Figure 5.
The role of CFA, pertussis toxin, and TLR ligands in the induction of IL-17+ IRBP-specific T cells. Synergistic effect of CFA and PTX on the induction of IL-17+ IRBP-specific T cells in vivo. B6 mice were immunized with IRBP1–20 emulsified in CFA (A, C) with (A) or without (C) PTX pretreatment or in IFA (B, D). CD4 and CD8 T cells were prepared at p.i. day 13 and were stimulated with immunizing peptide for 4 days. Activated T cells were separated by Ficoll, intracellularly stained with the indicated antibodies, and analyzed by FACS analysis. (E) In vitro effect of TLR ligation of BMDCs on IL-17 and IFN-γ production. In vivo-primed unfractionated IRBP-specific T cells were incubated for 3 days with immunizing peptide and APCs (BMDCs), which had been left untreated or were treated for 18 hours with the TLR ligands, BLP (TLR2, 5 μg/mL), LPS (TLR4, 1 μg/ mL), poly(I:C) (TLR3, 50 μg/mL), or CpG1826 (TLR9, 10 μg/mL). IL-17 and IFN-γ production was measured by ELISA (left and center) and the IL-17/IFN-γ ratio was calculated.
To determine the mechanism by which mycobacterial components in CFA promote IL-17+ activation, we tested the in vitro effect of TLR ligation on APCs in the activation of IL-17+ T cells using BMDCs (see Methods). As shown in Figure 5E, exposure of BMDCs to ligands of TLR2 (BLP), TLR4 (LPS), or TLR3 (poly(I:C)) markedly enhanced the production of IFN-γ and IL-17, whereas the TLR9 ligand, CpG1826, primarily enhanced IFN-γ production. BLP, LPS, and poly(I:C) caused a greater increase in IL-17 than IFN-γ production, resulting in an increase in the production of the IL-17-/IFN-γ ratio.
DISCUSSION
Recent studies have identified a unique CD4 T-cell subset that expresses IL-17, but not IFN-γ or IL-4,22–24 and have demonstrated that such T cells play a major role in the pathogenesis of autoimmune diseases.14,25,26 Given our recent observation that the interaction of CD4 and CD8 autoreactive T cells plays a major role in the pathogenesis of EAU and EAE,27–29 we wanted to determine whether CD8 autoreactive T cells in IRBP-induced EAU also express IL-17, whether IL-17–producing CD4 and CD8 autoreactive T cells are driven by different immune factors, and whether antigen-specific and nonspecific IL-17–producing T cells have common immune functions. In this report, we showed that the subset of CD8 autoreactive T cells in B6 mice with IRBP-induced EAU expressed IL-17. We also showed that antigen-specific and nonspecific IL-17+ T cells were induced by different growth factors and that only the antigen-specific IL-17+ autoreactive T cells were uveitogenic. Further characterization should allow us to gain much needed insight into the pathogenesis of the disease and to define the interactive effects of different autoreactive T-cell subsets.
Before the realization that different culture conditions could fundamentally affect the expansion of antigen-specific T-cell subsets in vitro, antigen-primed T cells were usually propagated using a standard protocol that included 2-day stimulation of in vivo- primed T cells with immunizing antigen and APCs in vitro, followed by culture of these T cells in IL-2–containing medium.27,28,30–32 Comparing the antigen-specific T cells expanded in IL-2- or IL-23–containing medium, we showed that, though IL-2 preferentially promoted the expansion of IFN-γ–producing T cells, IL-23 promoted the expansion of IL-17–producing T cells. We showed that though transient expansion of IL-17–producing T cells in the cultured IRBP-specific T cells occurred on culture in IL-2–containing medium, the persistent growth and expansion of these T cells required IL-23. This suggested that the expansion of antigen-specific T cells in vitro is heavily influenced by the culture conditions and determined by specific cytokine(s). Importantly, the IL-23–expanded IRBP-specific T cells had a strong pathogenic effect in EAU. Thus, our results provided direct evidence that IL-17+ autoreactive T cells are pathogenic in autoimmune uveitis.
Many previous reports have evaluated IL-17–expressing cells using in vitro stimulation with anti-CD3 antibody,23,33,34 but we considered it necessary to distinguish between antigen-specific and nonantigen-specific IL-17 cell expansion. Given that an overwhelming majority of the IL-17–producing cells expanded from naive mice using anti-CD3 antibody stimulation are T cells with unknown antigen specificity and anti-CD3–stimulated IL-17–producing cells from antigen-immunized mice contain antigen-specific and nonspecific T cells, we made a number of comparisons between IL-17+ T cells triggered by stimulation with immunizing autoantigen (IRBP peptide) and those stimulated in vitro with nonspecific anti-CD3 antibodies and between T cells from unimmunized mice and those from immunized mice. Our results demonstrated that antigen-specific IL-17+ T cells rely on IL-23 for expansion, whereas non-specific IL-17+ T cells can exploit a number of cytokines, such as TGF-β and IL-6, for expansion. Anti-CD3 stimulation resulted in preferential expansion of IFN-γ+ T cells from naive mice, but preferential expansion of IL-17+ T cells from immunized mice suggested that immunization favored T-cell subset(s) producing IL-17+ T cells or Th17 differentiation of antigen-specific T cells. It is possible that the mycobacteria in CFA direct T-cell activation toward Th17-type responder T cells.
The expression of IL-17 by IRBP-specific autoreactive T cells differed from their expression of Th1-type cytokines, such as IFN-γ. For example, IFN-γ expression peaked within 2 days of in vitro antigen stimulation, whereas IL-17 expression was maximal at 3 to 5 days after antigen stimulation. FACS analysis of the activated T cells demonstrated that IL-17 was expressed by medium-sized activated T cells, whereas IL-2 and IFN-γ were associated with fully activated T-cell blasts (not shown). It is our hypothesis that the functional activity of IL-17+ T cells is mediated by their ability to interact with other immune cells or the parenchymal of the autoimmune organ, probably through the cytokines they produce, whereas Th1-type autoreactive T cells are direct effectors, the activity of which is strictly dependent on the activation status of the T cell.
Autoimmune disease is frequently associated with various infections, but the mechanisms by which infection affects disease development are largely unknown. Previous studies have shown that TLR–ligand interaction on DCs directly affects the process of antigen presentation35 and that treatment of highly purified activated CD4+ T cells with the dsRNA synthetic analog, poly(I:C), and CpG oligodeoxynucleotides (CpG DNA)—respective ligands for TLR-3 and TLR-9—directly enhance their survival without augmenting proliferation.36 In addition, in the absence of MyD88 signaling or of certain TLRs, DCs show impaired uptake of bacteria or LPS-coated beads and fail to stimulate CD4+ T cells.35 In this study, we showed that exposure to TLR ligands greatly enhanced the induction of IL-17+ T cells. It is likely that, after exposure to microbial agents, APCs such as DCs respond by producing immunostimulatory cytokines, including IL-12, and further upregulating their expression of MHC and costimulatory molecules and that the microbial-induced DC maturation provides crucial elements required for selective activation of pathogenic T-cell subsets, including those that are IL-17+. These observations agree with the previous observation that the ligation of TLR237 or TLR438 plays a vital role in IL-23 expression. It should be noted that recent studies have also shown that TLRs can be activated by endogenous ligands, such as heat shock protein, oligosaccha-rides,39 and the contents of necrotic cells.40–42 Conceivably, endogenous TLR ligands may also play a role in inflammatory reactions and the activation of pathogenic autoreactive T cells.
Although previous studies have repeatedly proved that pathogenic T cells producing Th1-like cytokines are essential for the development of autoimmune diseases,43–48 the interrelationship between Th1- and IL-17–producing T cells is largely unknown. It is likely that the two pathogenic T-cell subsets have a synergistic effect, leading to disease progression. This appears to be supported by recent observations that, during the chronic phase of autoimmune disease, IL-17–producing cells accumulate more prominently in the autoimmune organs.49,50
In summary, our study demonstrates that CD4 and CD8 T cells can express and produce IL-17. IRBP-specific T cells that express IL-17 are strong effector cells for EAU. We show that antigen-specific, but not nonantigen-specific, IL-17+ T cells are uveitogenic and that in vitro TLR ligation greatly enhances the activation of IL-17+ autoreactive T cells.
Acknowledgments
The authors thank Tom Barkas for his editorial assistance.
Supported in part by National Institutes of Health/National Eye Institute Grants NEI-EY014366 and EY017373, Vision Research Infrastructure Development Grant R24 EY015636, National Multiple Sclerosis Society Grant RG3413A4, and the Commonwealth of Kentucky Research Challenge Trust Fund.
Footnotes
Disclosure: Y. Peng, None; G. Han, None; H. Shao, None; Y. Wang, None; H.J. Kaplan, None; D. Sun, None
References
- 1.Faure JP. Autoimmunity and the retina. Curr Top Eye Res. 1980;2:215–302. [PubMed] [Google Scholar]
- 2.Wacker WB, Donoso LA, Kalsow CM, et al. Experimental allergic uveitis: isolation, characterization, and localization of a soluble uveitopathogenic antigen from bovine retina. J Immunol. 1977;119:1949–1958. [PubMed] [Google Scholar]
- 3.Caspi RR, Roberge FG, McAllister CG, et al. T cell lines mediating experimental autoimmune uveoretinitis (EAU) in the rat. J Immunol. 1986;136:928–933. [PubMed] [Google Scholar]
- 4.Fox GM, Redmond TM, Wiggert B, et al. Dissociation between lymphocyte activation for proliferation and for the capacity to adoptively transfer uveoretinitis. J Immunol. 1987;138:3242–3246. [PubMed] [Google Scholar]
- 5.Chan CC, Nussenblatt RB, Wiggert B, et al. Immunohistochemical analysis of experimental autoimmune uveoretinitis (EAU) induced by interphotoreceptor retinoid-binding protein (IRBP) in the rat. Immunol Invest. 1987;16:63–74. doi: 10.3109/08820138709055713. [DOI] [PubMed] [Google Scholar]
- 6.Gregerson DS, Fling SP, Obritsch WF, et al. Identification of T cell recognition sites in S-antigen: dissociation of proliferative and pathogenic sites. Cell Immunol. 1989;123:427–440. doi: 10.1016/0008-8749(89)90302-x. [DOI] [PubMed] [Google Scholar]
- 7.Rozenszajn LA, Muellenberg-Coulombre C, Gery I, et al. Induction of experimental autoimmune uveoretinitis by T-cell lines. Immunology. 1986;57:559–565. [PMC free article] [PubMed] [Google Scholar]
- 8.Avichezer D, Silver PB, Chan CC, et al. Identification of a new epitope of human IRBP that induces autoimmune uveoretinitis in mice of the H-2b haplotype. Invest Ophthalmol Vis Sci. 2000;41:127–131. [PubMed] [Google Scholar]
- 9.Chen Y, Langrish CL, McKenzie B, et al. Anti-IL-23 therapy inhibits multiple inflammatory pathways and ameliorates autoimmune encephalomyelitis. J Clin Invest. 2006;116:1317–1326. doi: 10.1172/JCI25308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Axtell RC, Xu L, Barnum SR, Raman C. CD5-CK2 binding/activation-deficient mice are resistant to experimental autoimmune encephalomyelitis: protection is associated with diminished populations of IL-17-expressing T cells in the central nervous system. J Immunol. 2006;177:8542–8549. doi: 10.4049/jimmunol.177.12.8542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides “preferentially” polarize CD4+ Th-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–180. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 12.Cua DJ, Sherlock J, Chen Y, et al. Interleukin-23 rather than interleukin-12 is the critical cytokine for autoimmune inflammation of the brain. Nature. 2003;421:744–748. doi: 10.1038/nature01355. [DOI] [PubMed] [Google Scholar]
- 13.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 14.Langrish CL, Chen Y, Blumenschein WM, et al. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J Exp Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nakae S, Komiyama Y, Nambu A, et al. Antigen-specific T cell sensitization is impaired in IL-17-deficient mice, causing suppression of allergic cellular and humoral responses. Immunity. 2002;17:375–387. doi: 10.1016/s1074-7613(02)00391-6. [DOI] [PubMed] [Google Scholar]
- 16.Nakae S, Saijo S, Horai R, et al. IL-17 production from activated T cells is required for the spontaneous development of destructive arthritis in mice deficient in IL-1 receptor antagonist. Proc Natl Acad Sci USA. 2003;100:5986–5990. doi: 10.1073/pnas.1035999100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li J, Simeoni E, Fleury S, et al. Gene transfer of soluble interleukin-17 receptor prolongs cardiac allograft survival in a rat model. Eur J Cardiothorac Surg. 2006;29:779–783. doi: 10.1016/j.ejcts.2006.01.052. [DOI] [PubMed] [Google Scholar]
- 18.Peng Y, Shao H, Ke Y, et al. In vitro activation of CD8 interphotoreceptor retinoid-binding protein-specific T cells requires not only antigenic stimulation but also exogenous growth factors. J Immunol. 2006;176:5006–5014. doi: 10.4049/jimmunol.176.8.5006. [DOI] [PubMed] [Google Scholar]
- 19.Thurau SR, Chan CC, Nussenblatt RB, Caspi RR. Oral tolerance in a murine model of relapsing experimental autoimmune uveoretinitis (EAU): induction of protective tolerance in primed animals. Clin Exp Immunol. 1997;109:370–376. doi: 10.1046/j.1365-2249.1997.4571356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao H, Liao T, Ke Y, et al. Severe chronic experimental autoimmune uveitis (EAU) of the C57BL/6 mouse induced by adoptive transfer of IRBP1–20-specific T cells. Exp Eye Res. 2006;82:323–331. doi: 10.1016/j.exer.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Harrington LE, Hatton RD, Mangan PR, et al. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 22.Park H, Li Z, Yang XO, et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mangan PR, Harrington LE, O’Quinn DB, et al. Transforming growth factor-β induces development of the Th17 lineage. Nature. 2006;441:231–234. doi: 10.1038/nature04754. [DOI] [PubMed] [Google Scholar]
- 24.Liang SC, Tan XY, Luxenberg DP, et al. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J Exp Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Komiyama Y, Nakae S, Matsuki T, et al. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 26.Lubberts E, Joosten LAB, Oppers B, et al. IL-1–independent role of IL-17 in synovial inflammation and joint destruction during collagen-induced arthritis. J Immunol. 2001;167:1004–1013. doi: 10.4049/jimmunol.167.2.1004. [DOI] [PubMed] [Google Scholar]
- 27.Shao H, Peng Y, Liao T, et al. A shared epitope of the interphoto-receptor retinoid-binding protein (IRBP) recognized by the CD4+ and CD8+ autoreactive T cells. J Immunol. 2005;175:1851–1857. doi: 10.4049/jimmunol.175.3.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shao H, Sun SL, Kaplan HJ, Sun D. Characterization of rat CD8+ uveitogenic T cells specific for interphotoreceptor retinal-binding protein 1177–1191. J Immunol. 2004;173:2849–2854. doi: 10.4049/jimmunol.173.4.2849. [DOI] [PubMed] [Google Scholar]
- 29.Sun D, Zhang Y, Wei B, et al. Encephalitogenic activity of truncated myelin oligodendrocyte glycoprotein (MOG) peptides and their recognition by CD8+ MOG-specific T cells on oligomeric MHC class I molecules. Int Immunol. 2003;15:261–268. doi: 10.1093/intimm/dxg023. [DOI] [PubMed] [Google Scholar]
- 30.Sun D, Wekerle H. Ia-restricted encephalitogenic T lymphocytes mediating EAE lyse autoantigen-presenting astrocytes. Nature. 1986;320:70–72. doi: 10.1038/320070a0. [DOI] [PubMed] [Google Scholar]
- 31.Linington C, Izumo S, Suzuki M, et al. A permanent rat T cell line that mediate experimental allergic neuritis in the Lewis rat in vivo. J Immunol. 1984;133:1946. [PubMed] [Google Scholar]
- 32.Sun D, Whitaker JN, Huang Z, et al. Myelin antigen-specific CD8+ T cells are encephalitogenic and produce severe disease in C57BL/6 mice. J Immunol. 2001;166:7579–7587. doi: 10.4049/jimmunol.166.12.7579. [DOI] [PubMed] [Google Scholar]
- 33.Bettelli E, Carrier Y, Gao W, et al. Reciprocal developmental pathways for the generation of pathogenic effector Th17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 34.Veldhoen M, Hocking RJ, Atkins CJ, et al. TGFβ in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 35.Blander JM, Medzhitov R. Regulation of phagosome maturation by signals from Toll-like receptors. Science. 2004;304:1014–1018. doi: 10.1126/science.1096158. [DOI] [PubMed] [Google Scholar]
- 36.Gelman AE, Zhang J, Choi Y, Turka LA. Toll-like receptor ligands directly promote activated CD4+ T cell survival. J Immunol. 2004;172:6065–6073. doi: 10.4049/jimmunol.172.10.6065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Re F, Strominger JL. Toll-like receptor 2 (TLR2) and TLR4 differentially activate human dendritic cells. J Biol Chem. 2001;276:37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 38.Happel KI, Zheng M, Young E, et al. Cutting edge: roles of Toll-like receptor 4 and IL-23 in IL-17 expression in response to Klebsiella pneumoniae infection. J Immunol. 2003;170:4432–4436. doi: 10.4049/jimmunol.170.9.4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Termeer C, Benedix F, Sleeman J, et al. Oligosaccharides of hyaluronan activate dendritic cells via Toll-like receptor 4. J Exp Med. 2002;195:99–111. doi: 10.1084/jem.20001858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Johnson GB, Brunn GJ, Kodaira Y, Platt JL. Receptor-mediated monitoring of tissue well-being via detection of soluble heparan sulfate by Toll-like receptor 4. J Immunol. 2002;168:5233. doi: 10.4049/jimmunol.168.10.5233. [DOI] [PubMed] [Google Scholar]
- 41.Li M, Carpio DF, Zheng Y, et al. An essential role of the NFκB/ Toll-like receptor pathway in induction of inflammatory and tissue-repair gene expression by necrotic cells. J Immunol. 2001;166:7128. doi: 10.4049/jimmunol.166.12.7128. [DOI] [PubMed] [Google Scholar]
- 42.Ohashi K, Burkart V, Flohe S, Kolb H. Cutting edge: heat shock protein 60 is a putative endogenous ligand of the Toll-like receptor-4 complex. J Immunol. 2000;164:558. doi: 10.4049/jimmunol.164.2.558. [DOI] [PubMed] [Google Scholar]
- 43.Ando DG, Clayton J, Kono D, et al. Encephalitogenic T cells in the B10. PL model of experimental allergic encephalomyelitis (EAE) are of the Th-1 lymphokine subtype. Cell Immunol. 1989;124:132–143. doi: 10.1016/0008-8749(89)90117-2. [DOI] [PubMed] [Google Scholar]
- 44.Caspi RR, Silver PB, Chan CC, et al. Genetic susceptibility to experimental autoimmune uveoretinitis in the rat is associated with an elevated Th1 response. J Immunol. 1996;157:2668–2675. [PubMed] [Google Scholar]
- 45.Finotto S, Neurath MF, Glickman JN, et al. Development of spontaneous airway changes consistent with human asthma in mice lacking T-bet. Science. 2002;295:336–338. doi: 10.1126/science.1065544. [DOI] [PubMed] [Google Scholar]
- 46.Liblau RS, Singer SM, McDevitt HO. Th1 and Th2 CD4+ T cells in the pathogenesis of organ- specific autoimmune diseases. Immunol Today. 1995;16:34–38. doi: 10.1016/0167-5699(95)80068-9. [DOI] [PubMed] [Google Scholar]
- 47.Mauri C, Williams RO, Walmsley M, Feldmann M. Relationship between Th1/Th2 cytokine patterns and the arthritogenic response in collagen-induced arthritis. Eur J Immunol. 1996;26:1511–1518. doi: 10.1002/eji.1830260716. [DOI] [PubMed] [Google Scholar]
- 48.Nicholson LB, Greer JM, Sobel RA, et al. An altered peptide ligand mediates immune deviation and prevents autoimmune encephalomyelitis. Immunity. 1995;3:397–405. doi: 10.1016/1074-7613(95)90169-8. [DOI] [PubMed] [Google Scholar]
- 49.Kotake S, Udagawa N, Takahashi N, et al. IL-17 in synovial fluids from patients with rheumatoid arthritis is a potent stimulator of osteoclastogenesis. J Clin Invest. 1999;103:1345–1352. doi: 10.1172/JCI5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matusevicius D, Kivisakk P, He B, et al. Interleukin-17 mRNA expression in blood and CSF mononuclear cells is augmented in multiple sclerosis. Mult Scler. 1999;5:101–104. doi: 10.1177/135245859900500206. [DOI] [PubMed] [Google Scholar]