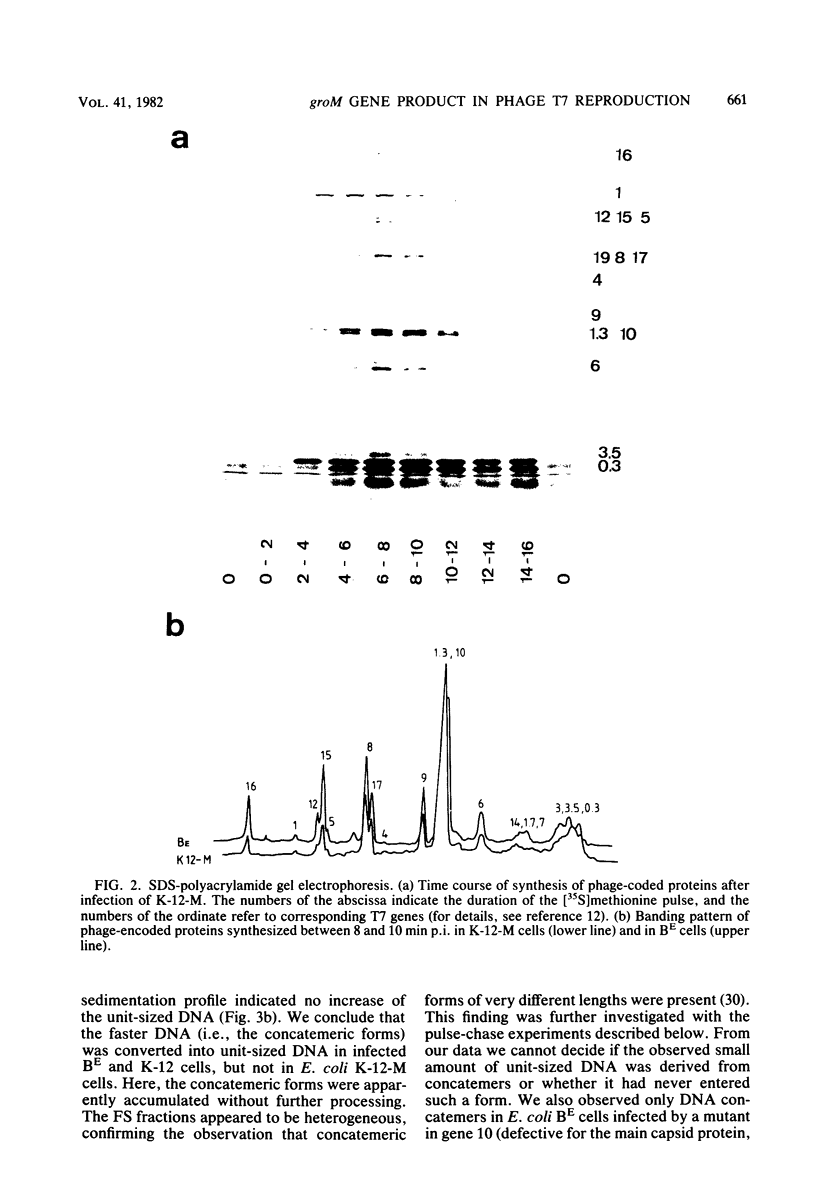
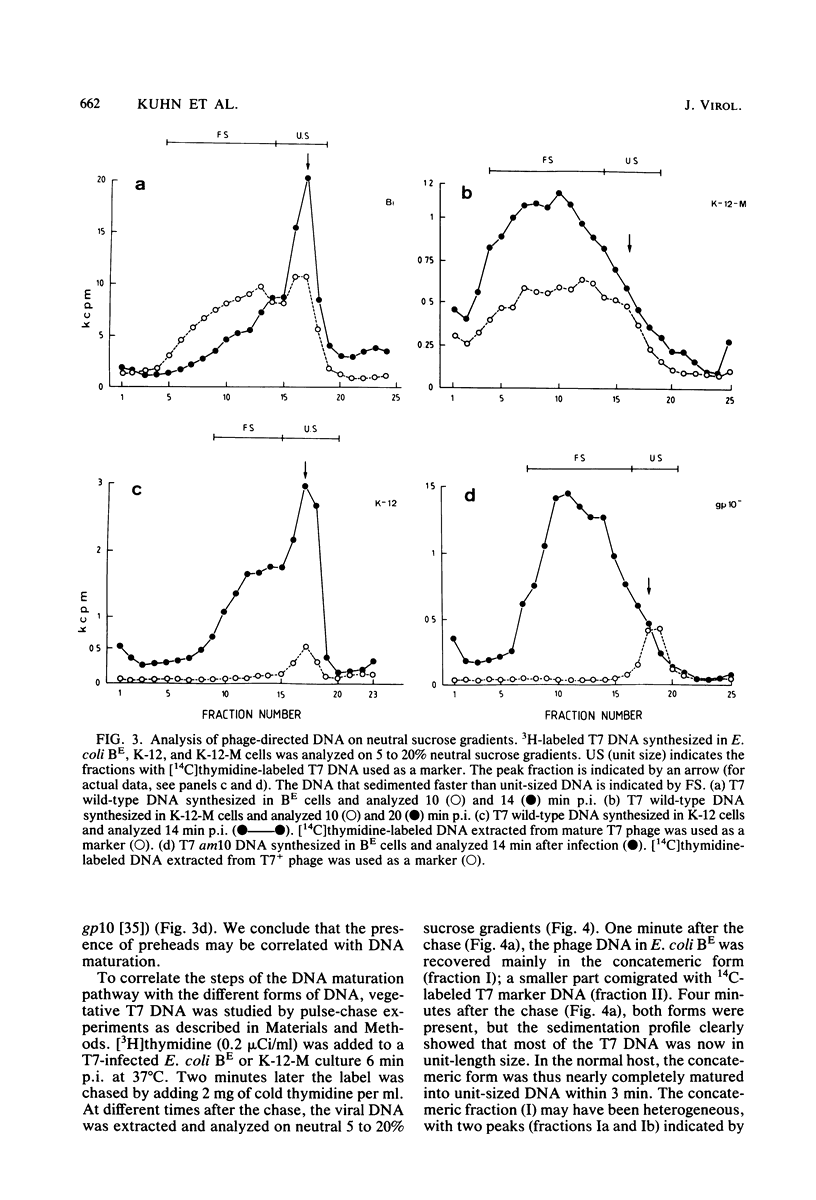
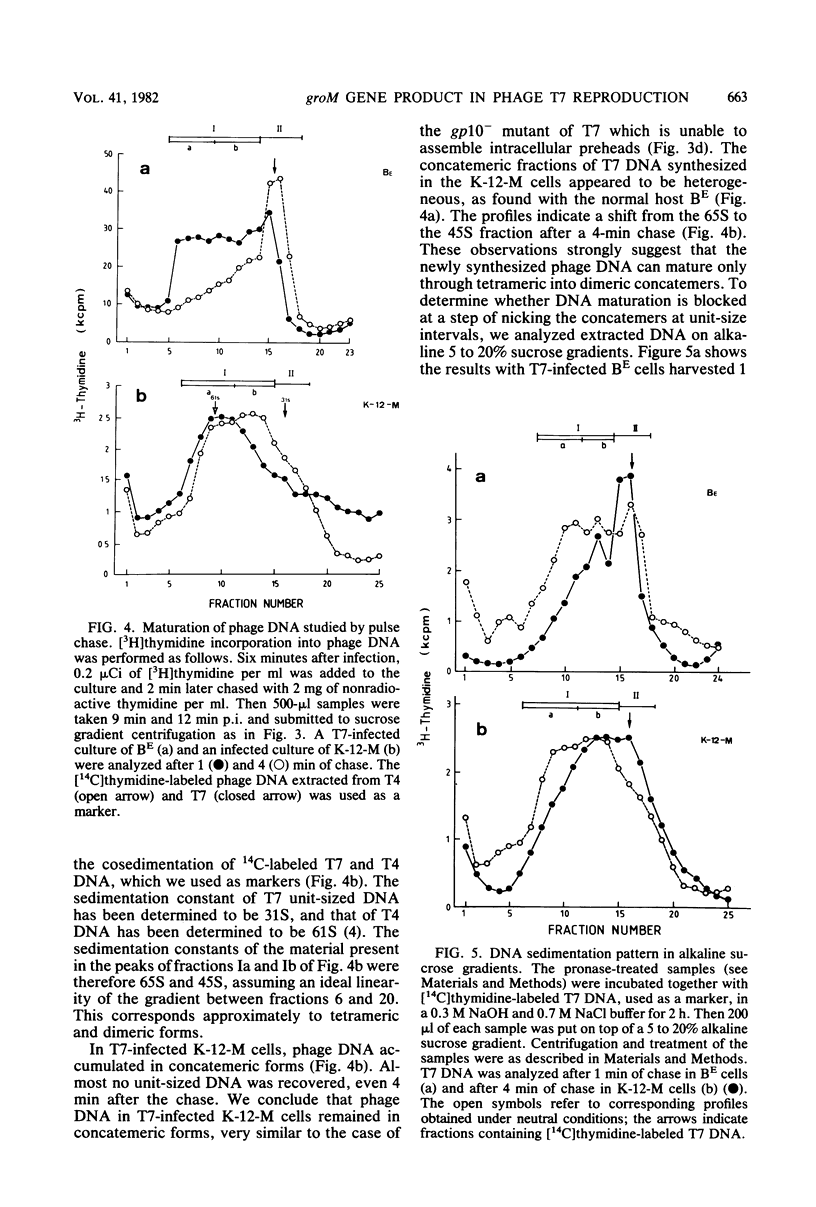
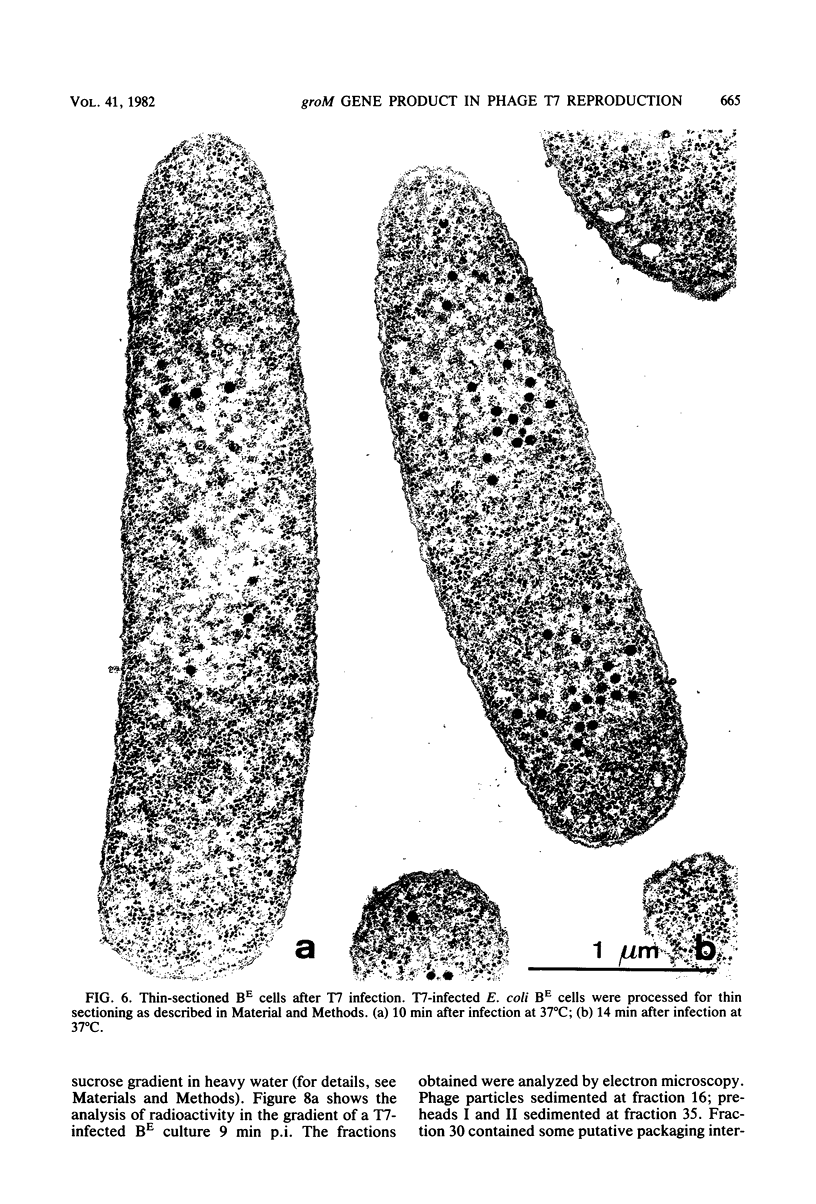
Abstract
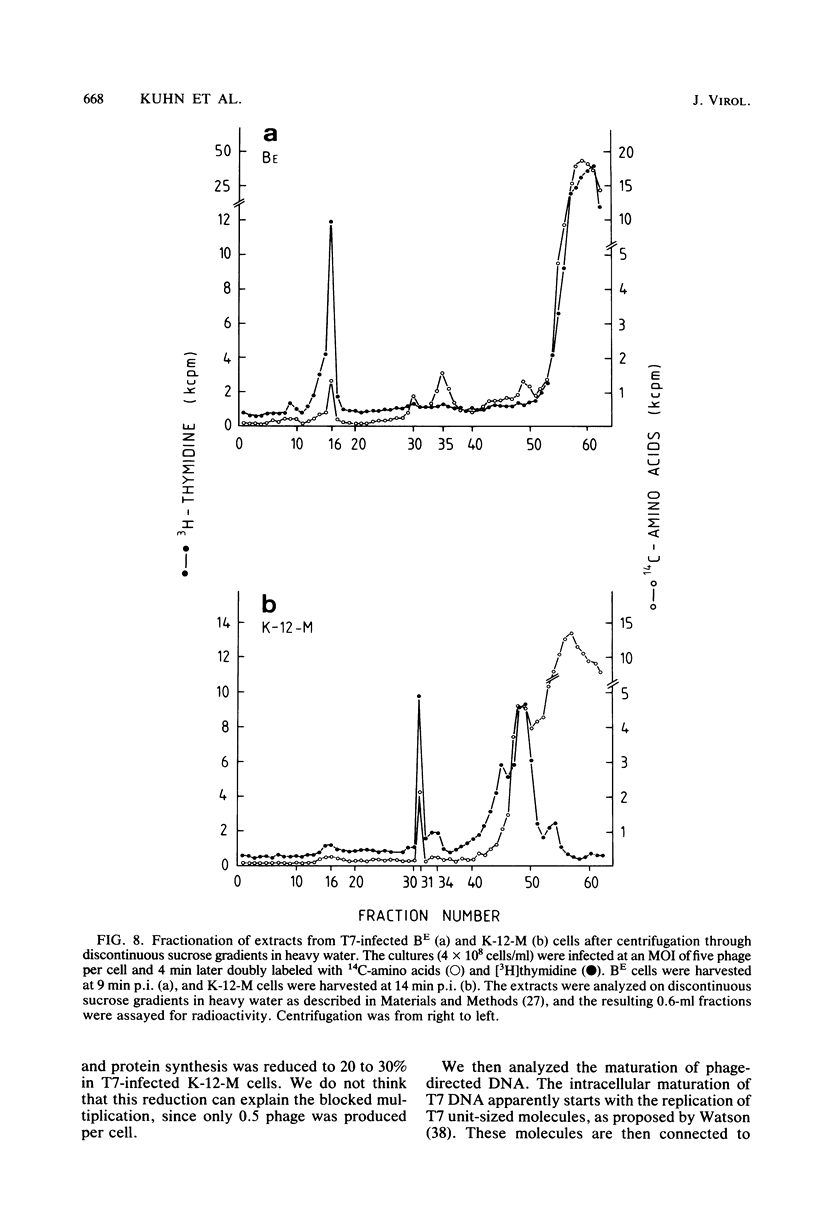
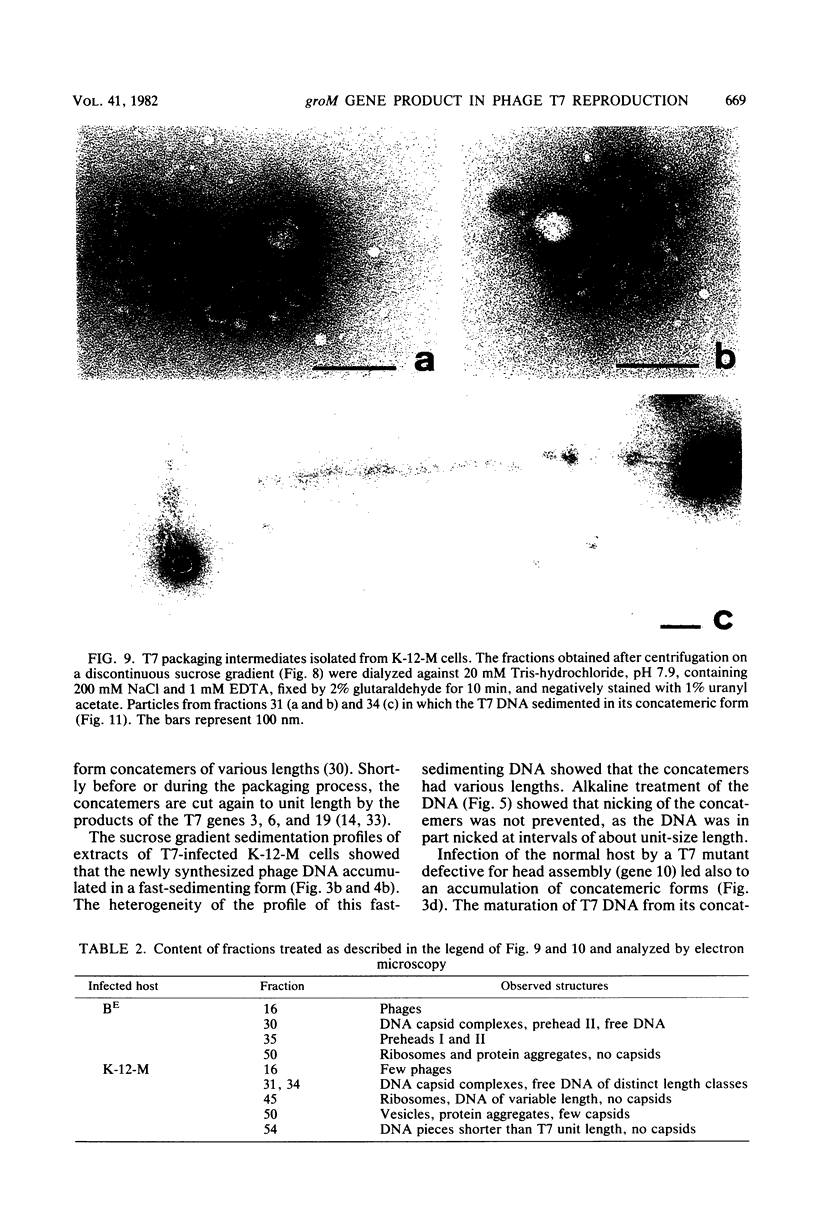
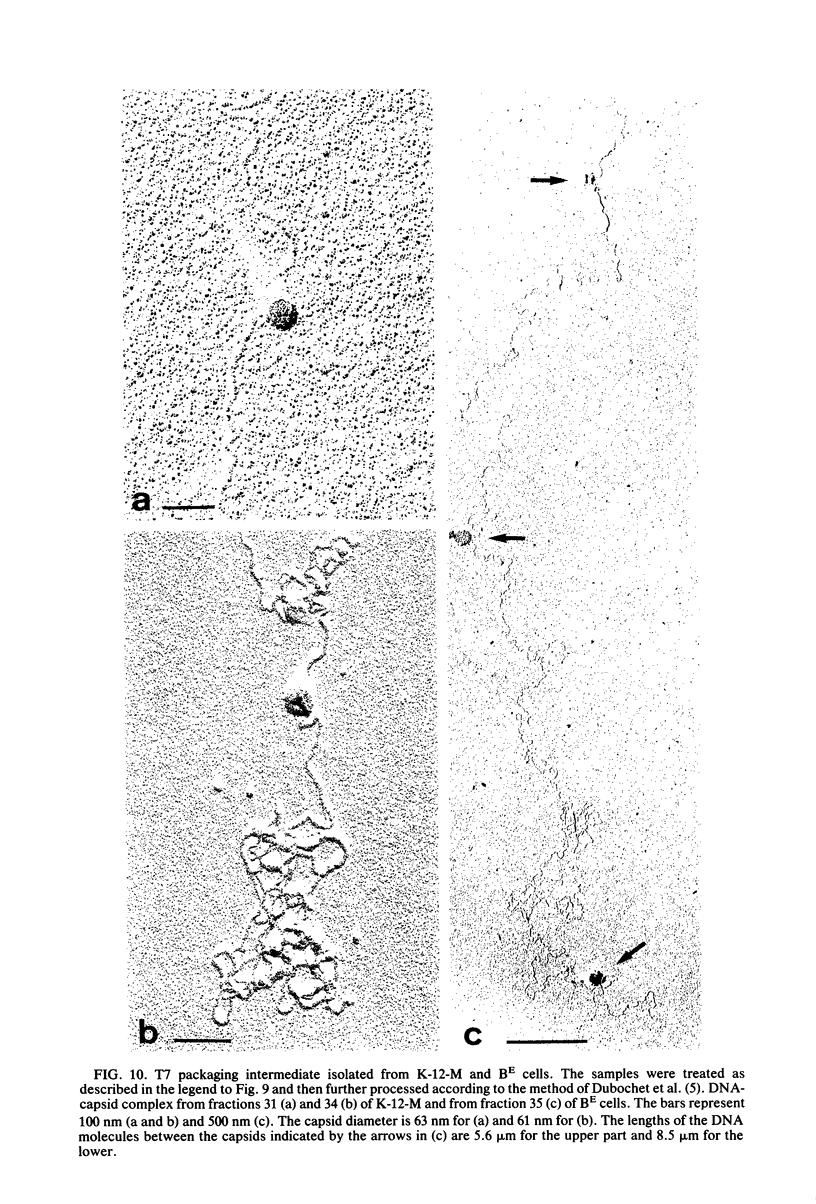
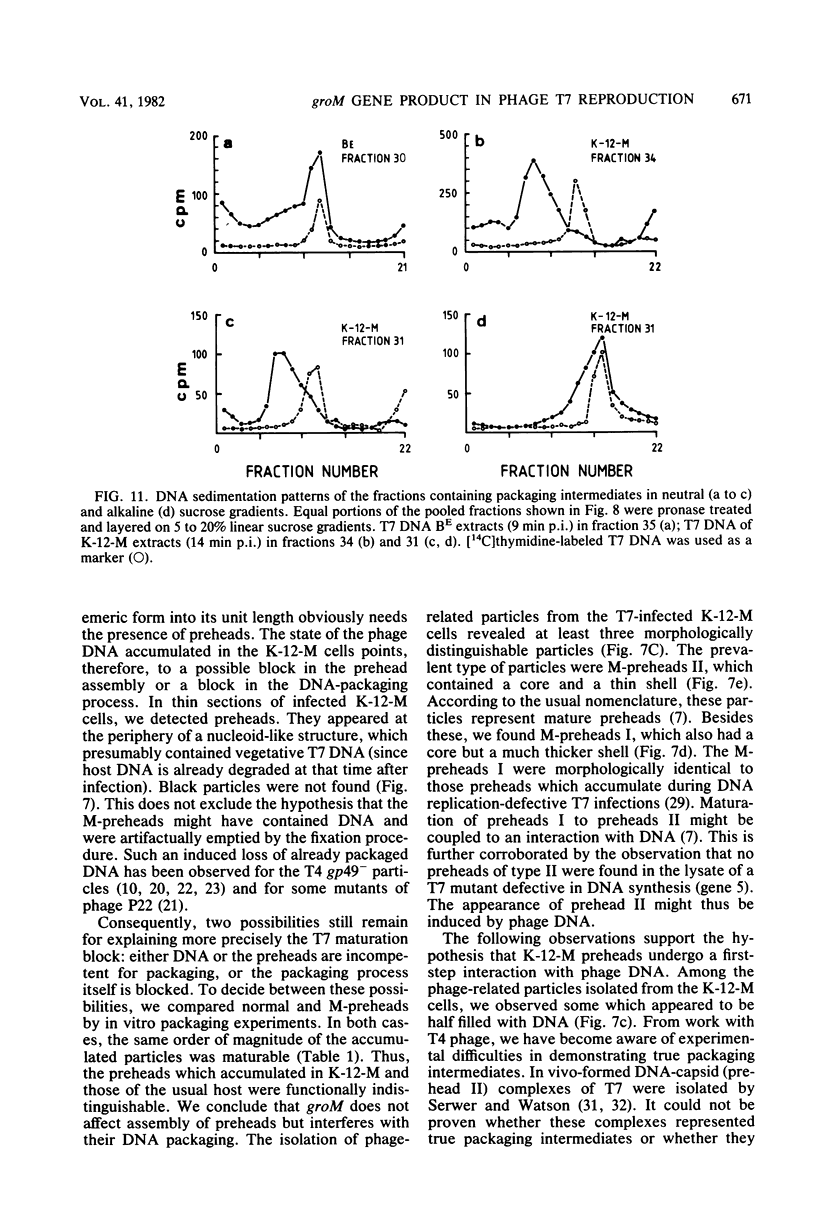
The multiplication of bacteriophage T7 is blocked in Escherichia coli M. The genetic determinant of this ability (groM) to inhibit T7 growth was transferred to an E. coli K-12 recipient by means of conjugation. We determined at which precise step T7 maturation is blocked. Phage-directed protein and DNA synthesis as well as degradation of host DNA were not qualitatively affected. Instead of infective phages, only preheads were produced. These, however, were maturable in vitro. The newly synthesized phage DNA accumulated in a concatemeric form and matured from its tetrameric or longer forms (very fast sedimenting DNA) only into its dimeric form (fast-sedimenting DNA) or longer forms. The following step, i.e., the maturation of the dimeric to unit-length DNA, was not observed. Since the concatemeric form of T7 DNA accumulated in spite of the presence of maturable preheads, it is likely that the maturation process was blocked at the level of DNA packaging. As intermediates in the packaging process, we found some prehead-DNA complexes. We interpreted these as true assembly intermediates (or breakdown products thereof), since the attached DNA was still in its concatemeric form. This shows that the very first DNA packaging step, the binding of the progeny DNA to the preheads, was obviously not blocked. Rather, a later step, such as the filling of the preheads with T7 DNA or the stabilization of completely packaged particles (i.e., the final cutting of the concatemers into unit-size length), was inhibited.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ARBER W., DUSSOIX D. Host specificity of DNA produced by Escherichia coli. I. Host controlled modification of bacteriophage lambda. J Mol Biol. 1962 Jul;5:18–36. doi: 10.1016/s0022-2836(62)80058-8. [DOI] [PubMed] [Google Scholar]
- Center M. S. Role of gene 2 in bacteriophage T7 DNA synthesis. J Virol. 1975 Jul;16(1):94–100. doi: 10.1128/jvi.16.1.94-100.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. W., Wever G. H., Wiberg J. S. High-molecular-weight DNA and the sedimentation coefficient: a new perspective based on DNA from T7 bacteriophage and two novel forms of T4 bacteriophage. J Virol. 1980 Jan;33(1):438–448. doi: 10.1128/jvi.33.1.438-448.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Duckworth D. H., Glenn J., McCorquodale D. J. Inhibition of bacteriophage replication by extrachromosomal genetic elements. Microbiol Rev. 1981 Mar;45(1):52–71. doi: 10.1128/mr.45.1.52-71.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Bacterial mutants in which the gene N function of bacteriophage lambda is blocked have an altered RNA polymerase. Proc Natl Acad Sci U S A. 1971 Dec;68(12):2977–2981. doi: 10.1073/pnas.68.12.2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Hamilton D. L., Luftig R. B. Bacteriophage T4 head morphogenesis. VII. Terminal stages of head maturation. J Virol. 1976 Feb;17(2):550–567. doi: 10.1128/jvi.17.2.550-567.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R. Bacteriophage T7 genetics. Curr Top Microbiol Immunol. 1976;75:77–110. doi: 10.1007/978-3-642-66530-1_3. [DOI] [PubMed] [Google Scholar]
- Hausmann R., Gomez B. Amber mutants of bacteriophages T3 and T7 defective in phage-directed deoxyribonucleic acid synthesis. J Virol. 1967 Aug;1(4):779–792. doi: 10.1128/jvi.1.4.779-792.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann R., LaRue K. Variations in sedimentation patterns among deoxyribonucleic acids synthesized after infection of Escherichia coli by different amber mutants of bacteriophage T7. J Virol. 1969 Feb;3(2):278–281. doi: 10.1128/jvi.3.2.278-281.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrix R. W., Tsui L. Role of the host in virus assembly: cloning of the Escherichia coli groE gene and identification of its protein product. Proc Natl Acad Sci U S A. 1978 Jan;75(1):136–139. doi: 10.1073/pnas.75.1.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Thomas C. A., Jr An intermediate in the replication of bacteriophage T7 DNA molecules. J Mol Biol. 1969 Sep 28;44(3):459–475. doi: 10.1016/0022-2836(69)90373-8. [DOI] [PubMed] [Google Scholar]
- Kerr C., Sadowski P. D. Packaging and maturation of DNA of bacteriophage T7 in vitro. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3545–3549. doi: 10.1073/pnas.71.9.3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsten K. H., Tomkiewicz C., Hausmann R. The strategy of infection as a criterion for phylogenetic relationships of non-coli phages morphologically similar to phage T7. J Gen Virol. 1979 Apr;43(1):57–73. doi: 10.1099/0022-1317-43-1-57. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol Rev. 1981 Mar;45(1):9–51. doi: 10.1128/mr.45.1.9-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Teaff N., D'Ambrosia J. Maturation of the head of bacteriophage T4. III. DNA packaging into preformed heads. J Mol Biol. 1974 Oct 5;88(4):749–765. doi: 10.1016/0022-2836(74)90397-0. [DOI] [PubMed] [Google Scholar]
- Lenk E., Casjens S., Weeks J., King J. Intracellular visualization of precursor capsids in phage P22 mutant infected cells. Virology. 1975 Nov;68(1):182–199. doi: 10.1016/0042-6822(75)90160-9. [DOI] [PubMed] [Google Scholar]
- Luftig R. B., Ganz C. Bacteriophage T4 head morphogenesis. II. Studies on the maturation of gene 49-defective head intermediates. J Virol. 1972 Feb;9(2):377–389. doi: 10.1128/jvi.9.2.377-389.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luftig R. B., Wood W. B., Okinaka R. Bacteriophage T4 head morphogenesis. On the nature of gene 49-defective heads and their role as intermediates. J Mol Biol. 1971 May 14;57(3):555–573. doi: 10.1016/0022-2836(71)90109-4. [DOI] [PubMed] [Google Scholar]
- Mark D. F., Richardson C. C. Escherichia coli thioredoxin: a subunit of bacteriophage T7 DNA polymerase. Proc Natl Acad Sci U S A. 1976 Mar;73(3):780–784. doi: 10.1073/pnas.73.3.780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masker W. E., Kuemmerle N. B., Allison D. P. In vitro packaging of bacteriophate T7 DNA synthesized in vitro. J Virol. 1978 Jul;27(1):149–163. doi: 10.1128/jvi.27.1.149-163.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki J. I., Fujisawa H., Minagawa T. Biological activity of purified bacteriophage T3 prohead and proheadlike structures as precursors for in vitro head assembly. Virology. 1978 Dec;91(2):283–290. doi: 10.1016/0042-6822(78)90376-8. [DOI] [PubMed] [Google Scholar]
- Moncany M. L., Révet B., Girard M. Characterization of a new adenovirus type 5 assembly intermediate. J Gen Virol. 1980 Sep;50(1):33–47. doi: 10.1099/0022-1317-50-1-33. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Schlegel R. A., Thomas C. A., Jr Some special structural features of intracellular bacteriophage T7 concatemers. J Mol Biol. 1972 Jul 21;68(2):319–345. doi: 10.1016/0022-2836(72)90216-1. [DOI] [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Capsid-DNA complexes in the DNA packaging pathway of bacteriophage T7: characterization of the capsids bound to monomeric and concatemeric DNA. Virology. 1981 Jan 15;108(1):164–176. doi: 10.1016/0042-6822(81)90536-5. [DOI] [PubMed] [Google Scholar]
- Strätling W., Krause E., Knippers R. Fast sedimenting deoxyribonucleic acid in bacteriophage T7-infected cells. Virology. 1973 Jan;51(1):109–119. doi: 10.1016/0042-6822(73)90371-1. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Relationships among different strains of T7 and among T7-related bacteriophages. Virology. 1979 May;95(1):70–84. doi: 10.1016/0042-6822(79)90402-1. [DOI] [PubMed] [Google Scholar]
- Watson J. D. Origin of concatemeric T7 DNA. Nat New Biol. 1972 Oct 18;239(94):197–201. doi: 10.1038/newbio239197a0. [DOI] [PubMed] [Google Scholar]
- Wunderli H., vd Broek J., Kellenberger E. Studies related to the head-maturation pathway of bacteriophages T4 and T2:I. morphology and kinetics of intracellular particles produced by mutants in the maturation genes. J Supramol Struct. 1977;7(2):135–161. doi: 10.1002/jss.400070202. [DOI] [PubMed] [Google Scholar]
- Yamada Y., Silnutzer J., Nakada D. Accumulation of bacteriophage T7 head-related particles in an Escherichia coli mutant. J Virol. 1979 Jul;31(1):209–219. doi: 10.1128/jvi.31.1.209-219.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi H., Okamoto M. Visualization of the intracellular development of bacteriophage lambda, with special reference to DNA packaging. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3206–3210. doi: 10.1073/pnas.75.7.3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Driel R., Traub F., Showe M. K. Probable localization of the bacteriophage T4 prehead proteinase zymogen in the center of the prehead core. J Virol. 1980 Oct;36(1):220–223. doi: 10.1128/jvi.36.1.220-223.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]







