Abstract
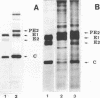
Brief treatment of Sindbis virus-infected BHK-21 or Vero cells with low concentrations of trypsin irreversibly blocked further production of progeny virions after removal of the enzyme. The inhibitory effects of the trypsin treatment could only be demonstrated in cells in which virus infection was established; optimal inhibition occurred at ca. 3 h postinfection. Production of virus structural proteins PE2, E1, and C occurred at normal levels in inhibited cells. PE2 and E1 were also transported to the cell plasma membrane during inhibition; however, PE2 was not cleaved to E2, and little capsid protein became membrane associated relative to control cells. Although trypsin treatment had no effect on Sindbis protein synthesis, the production of both 26S and 42S RNA was greatly reduced. Similar trypsin treatment of BHK cells infected with vesicular stomatitis virus had no detectable effect on the course of virus infection.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Bracha M., Schlesinger M. J. Defects in RNA+ temperature-sensitive mutants of Sindbis virus and evidence for a complex of PE2-E1 viral glycoproteins. Virology. 1976 Oct 15;74(2):441–449. doi: 10.1016/0042-6822(76)90350-0. [DOI] [PubMed] [Google Scholar]
- Brown D. T., Smith J. F. Morphology of BHK-21 Cells Infected with Sindbis Virus Temperature-Sensitive Mutants in Complementation Groups D and E. J Virol. 1975 May;15(5):1262–1266. doi: 10.1128/jvi.15.5.1262-1266.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. T., Waite M. R., Pfefferkorn E. R. Morphology and morphogenesis of Sindbis virus as seen with freeze-etching techniques. J Virol. 1972 Sep;10(3):524–536. doi: 10.1128/jvi.10.3.524-536.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton C. J., Kennedy S. I. Semliki Forest virus intracellular RNA: properties of the multi-stranded RNA species and kinetics of positive and negative strand synthesis. J Gen Virol. 1975 Jul;28(1):111–127. doi: 10.1099/0022-1317-28-1-111. [DOI] [PubMed] [Google Scholar]
- Burke D., Keegstra K. Carbohydrate structure of Sindbis virus glycoprotein E2 from virus grown in hamster and chicken cells. J Virol. 1979 Feb;29(2):546–554. doi: 10.1128/jvi.29.2.546-554.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- Erwin C., Brown D. T. Intracellular distribution of Sindbis virus membrane proteins in BHK-21 cells infected with wild-type virus and maturation-defective mutants. J Virol. 1980 Dec;36(3):775–786. doi: 10.1128/jvi.36.3.775-786.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frattali V. Soybean inhibitors. 3. Properties of a low molecular weight soybean proteinase inhibitor. J Biol Chem. 1969 Jan 25;244(2):274–280. [PubMed] [Google Scholar]
- Fujinami R. S., Oldstone M. B. Antiviral antibody reacting on the plasma membrane alters measles virus expression inside the cell. Nature. 1979 Jun 7;279(5713):529–530. doi: 10.1038/279529a0. [DOI] [PubMed] [Google Scholar]
- Hirschberg C. B., Robbins P. W. The glycolipids and phospholipids of Sindbis virus and their relation to the lipids of the host cell plasma membrane. Virology. 1974 Oct;61(2):602–608. doi: 10.1016/0042-6822(74)90295-5. [DOI] [PubMed] [Google Scholar]
- Igarashi A., Koo R., Stollar V. Evolution and properties of Aedes albopictus cell cultures persistently infected with sindbis virus. Virology. 1977 Oct 1;82(1):69–83. doi: 10.1016/0042-6822(77)90033-2. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J., Elson E. L. Fluorescence photobleaching recovery measurements reveal differences in envelopment of Sindbis and vesicular stomatitis viruses. Cell. 1981 Feb;23(2):423–431. doi: 10.1016/0092-8674(81)90137-9. [DOI] [PubMed] [Google Scholar]
- Johnson D. C., Schlesinger M. J. Vesicular stomatitis virus and sindbis virus glycoprotein transport to the cell surface is inhibited by ionophores. Virology. 1980 Jun;103(2):407–424. doi: 10.1016/0042-6822(80)90200-7. [DOI] [PubMed] [Google Scholar]
- Kos K. A., Osborne B. A., Goldsby R. A. Inhibition of group B arbovirus antigen production and replication in cells enucleated with cytochalasin B. J Virol. 1975 Apr;15(4):913–917. doi: 10.1128/jvi.15.4.913-917.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Ortin J., Vińuela E. Requirement of cell nucleus for African swine fever virus replication in Vero cells. J Virol. 1977 Mar;21(3):902–905. doi: 10.1128/jvi.21.3.902-905.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PFEFFERKORN E. R., CLIFFORD R. L. THE ORIGIN OF THE PROTEIN OF SINDBIS VIRUS. Virology. 1964 Jun;23:217–223. doi: 10.1016/0042-6822(64)90285-5. [DOI] [PubMed] [Google Scholar]
- Penman S. RNA metabolism in the HeLa cell nucleus. J Mol Biol. 1966 May;17(1):117–130. doi: 10.1016/s0022-2836(66)80098-0. [DOI] [PubMed] [Google Scholar]
- Pesonen M., Renkonen O. Serum glycoprotein-type sequence of monosaccharides in membrane glycoproteins of Semliki Forest virus. Biochim Biophys Acta. 1976 Dec 2;455(2):510–525. doi: 10.1016/0005-2736(76)90321-7. [DOI] [PubMed] [Google Scholar]
- Renz D., Brown D. T. Characteristics of Sindbis virus temperature-sensitive mutants in cultured BHK-21 and Aedes albopictus (Mosquito) cells. J Virol. 1976 Sep;19(3):775–781. doi: 10.1128/jvi.19.3.775-781.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riedel B., Brown D. T. Role of extracellular virus on the maintenance of the persistent infection induced in Aedes albopictus (mosquito) cells by Sindbis virus. J Virol. 1977 Sep;23(3):554–561. doi: 10.1128/jvi.23.3.554-561.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheefers-Borchel U., Scheefers H., Edwards J., Brown D. T. Sindbis virus maturation in cultured mosquito cells is sensitive to actinomycin D. Virology. 1981 Apr 30;110(2):292–301. doi: 10.1016/0042-6822(81)90061-1. [DOI] [PubMed] [Google Scholar]
- Scheid A., Choppin P. W. Identification of biological activities of paramyxovirus glycoproteins. Activation of cell fusion, hemolysis, and infectivity of proteolytic cleavage of an inactive precursor protein of Sendai virus. Virology. 1974 Feb;57(2):475–490. doi: 10.1016/0042-6822(74)90187-1. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S., Burge B. W. Identification of a second glycoprotein in Sindbis virus. Virology. 1972 Feb;47(2):539–541. doi: 10.1016/0042-6822(72)90298-x. [DOI] [PubMed] [Google Scholar]
- Schlesinger M. J., Schlesinger S. Large-molecular-weight precursors of sindbis virus proteins. J Virol. 1973 Jun;11(6):1013–1016. doi: 10.1128/jvi.11.6.1013-1016.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. Formation of Sindbis virus proteins: identification of a precursor for one of the envelope proteins. J Virol. 1972 Nov;10(5):925–932. doi: 10.1128/jvi.10.5.925-932.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Fatty acid binding to vesicular stomatitis virus glycoprotein: a new type of post-translational modification of the viral glycoprotein. Cell. 1979 Aug;17(4):813–819. doi: 10.1016/0092-8674(79)90321-0. [DOI] [PubMed] [Google Scholar]
- Schmidt M. F., Schlesinger M. J. Relation of fatty acid attachment to the translation and maturation of vesicular stomatitis and Sindbis virus membrane glycoproteins. J Biol Chem. 1980 Apr 25;255(8):3334–3339. [PubMed] [Google Scholar]
- Sefton B. M., Burge B. W. Biosynthesis of the Sindbis virus carbohydrates. J Virol. 1973 Dec;12(6):1366–1374. doi: 10.1128/jvi.12.6.1366-1374.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. F., Brown D. T. Envelopments of Sindbis virus: synthesis and organization of proteins in cells infected with wild type and maturation-defective mutants. J Virol. 1977 Jun;22(3):662–678. doi: 10.1128/jvi.22.3.662-678.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Wirth D. F., Lodish H. F., Robbins P. W. Requirements for the insertion of the Sindbis envelope glycoproteins into the endoplasmic reticulum membrane. J Cell Biol. 1979 Apr;81(1):154–162. doi: 10.1083/jcb.81.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Bonsdorff C. H., Harrison S. C. Hexagonal glycoprotein arrays from Sindbis virus membranes. J Virol. 1978 Nov;28(2):578–583. doi: 10.1128/jvi.28.2.578-583.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]