Abstract
The biological effects of type I serine/threonine kinase receptors and Smad proteins were examined using an adenovirus-based vector system. Constitutively active forms of bone morphogenetic protein (BMP) type I receptors (BMPR-IA and BMPR-IB; BMPR-I group) and those of activin receptor–like kinase (ALK)-1 and ALK-2 (ALK-1 group) induced alkaline phosphatase activity in C2C12 cells. Receptor-regulated Smads (R-Smads) that act in the BMP pathways, such as Smad1 and Smad5, also induced the alkaline phosphatase activity in C2C12 cells. BMP-6 dramatically enhanced alkaline phosphatase activity induced by Smad1 or Smad5, probably because of the nuclear translocation of R-Smads triggered by the ligand. Inhibitory Smads, i.e., Smad6 and Smad7, repressed the alkaline phosphatase activity induced by BMP-6 or the type I receptors. Chondrogenic differentiation of ATDC5 cells was induced by the receptors of the BMPR-I group but not by those of the ALK-1 group. However, kinase-inactive forms of the receptors of the ALK-1 and BMPR-I groups blocked chondrogenic differentiation. Although R-Smads failed to induce cartilage nodule formation, inhibitory Smads blocked it. Osteoblast differentiation induced by BMPs is thus mediated mainly via the Smad-signaling pathway, whereas chondrogenic differentiation may be transmitted by Smad-dependent and independent pathways.
INTRODUCTION
Bone morphogenetic proteins (BMPs) are multifunctional regulators of cell growth, differentiation, and apoptosis (Reddi, 1994; Hogan, 1996). BMPs were originally isolated as proteins that induce bone and cartilage formation in vivo (Wozney et al., 1988), but it is now known that BMPs play critical roles in morphogenesis during development in vertebrates and invertebrates. BMPs have been shown to induce differentiation of mesenchymal cells into osteoblast and chondroblast lineages and to inhibit their differentiation into myocytes in vitro (Katagiri et al., 1994; Luyten et al., 1994; Shukunami et al., 1996). More than a dozen BMP proteins have been identified in mammals, which can be subclassified into several groups depending on their structures. BMP-2 and BMP-4 are highly similar to each other and most similar to Decapentaplegic in Drosophila. BMP-5, BMP-6, osteogenic protein (OP)-1 (also called BMP-7), and OP-2/BMP-8 are structurally similar to each other. Growth-differentiation factor (GDF)-5 (also termed cartilage-derived morphogenetic protein-1), GDF-6/cartilage-derived morphogenetic protein-2, and GDF-7 form another group (Kawabata et al., 1998a). In contrast to BMP-2, BMP-4, BMP-6, and OP-1/BMP-7, which induce bone and cartilage formation in vivo (Wozney et al., 1988; Sampath et al., 1992; Gitelman et al., 1994), GDF-5, GDF-6, and GDF-7 more efficiently induce cartilage and tendon-like structures in vivo (Hötten et al., 1996; Wolfman et al., 1997).
BMPs belong to the transforming growth factor (TGF)-β superfamily, which includes TGF-βs, activins, and Müllerian-inhibiting substance. Members of the TGF-β superfamily exert their effects via binding to two types of serine/threonine kinase receptors, both of which are essential for signal transduction (Kawabata et al., 1998a; Massagué, 1998). The type II receptors are constitutively active kinases, which transphosphorylate type I receptors upon ligand binding. The type I receptors activate intracellular substrates such as Smad proteins and thus determine the specificity of intracellular signals. Seven different type I receptors have been isolated in mammals, which were originally termed activin receptor–like kinase (ALK)-1–ALK7 (ten Dijke et al., 1994a,b). BMP type IA receptor (BMPR-IA or ALK-3) and BMPR-IB (ALK-6) are structurally highly similar to each other and specifically bind BMPs together with type II receptors. ALK-2 has been shown to bind activin, but recent data revealed that it is a type I receptor for certain BMPs, e.g., OP-1/BMP-7 (ten Dijke et al., 1994b; Macías-Silva et al., 1998). ALK-1 is structurally highly similar to ALK-2, but its physiological ligand is still unknown. ALK-5 and ALK-4 are type I receptors for TGF-β (TβR-I) and activin (ActR-IB), respectively. ALK-7 is structurally similar to ALK-4 and ALK-5, but its ligand has not been determined yet.
Type I receptors function as downstream components of type II receptors. Mutation of Thr-204 in TβR-I to acidic amino acids such as aspartic acid [TβR-I(TD)] leads to constitutive activation of the type I receptor kinase (Wieser et al., 1995). Thus, TβR-I(TD) induces signals in the absence of ligands or type II receptors. Similarly, mutations of corresponding threonine or glutamine residues in the other type I receptors to aspartic acid or glutamic acid lead to constitutive activation of the type I receptor kinases. The specificity of the intracellular signals by type I receptors is determined by a specific region in the serine/threonine kinase domain, termed the L45 loop (Feng and Derynck, 1997). Thus, the structures of the L45 loop of BMPR-IA/ALK-3 and BMPR-IB/ALK-6 (BMPR-I group) are identical to each other, and they may transduce similar signals in cells. Similarly, the L45 loops of TβR-I/ALK-5, ActR-IB/ALK-4, and ALK-7 (TβR-I group) are identical to each other, and they activate similar substrates (Y.G. Chen et al., 1998; Persson et al., 1998). The L45 loops of ALK-1 and ALK-2 (ALK-1 group) are most divergent from the other type I receptors, but they activate substrates similar to that of the type I receptors of the BMPR-I group (Armes and Smith, 1997; Armes et al., 1999; Chen and Massagué, 1999).
Signals from the serine/threonine kinase receptors may be transduced by various proteins. Among them, the best-studied molecules are proteins of the Smad family (Heldin et al., 1997; Attisano and Wrana, 1998; Derynck et al., 1998; Massagué, 1998). Eight different Smad proteins have been identified in mammals, and these proteins are classified into three subgroups, i.e., receptor-regulated Smads (R-Smads), common partner Smads (Co-Smads), and inhibitory Smads. R-Smads are directly activated by type I receptors, form complexes with Co-Smads, and translocate into the nucleus. The Smad heteromers bind to DNA directly and indirectly via other DNA-binding proteins and thus regulate the transcription of target genes. Smad1, Smad5, and Smad8 are activated by BMPs, whereas Smad2 and Smad3 are activated by TGF-β and activin. Smad4 functions as a Co-Smad. Smad6 and Smad7 are structurally distantly related to the other Smads and act as inhibitory Smads.
BMP type I receptors have been shown to exhibit various biological effects, including osteoblast differentiation (Akiyama et al., 1997; Namiki et al., 1997; D. Chen et al., 1998). However, biological effects of the type I receptors of the ALK-1 group have not been determined. Smad1 and Smad5 have also been shown to elicit various effects of BMPs, e.g., ventral mesoderm formation in Xenopus embryos (Graff et al., 1996; Thomsen, 1996; Suzuki et al., 1997). They were also shown to induce differentiation of C2C12 cells into osteoblast cells (Yamamoto et al., 1997), although this differentiation was not efficient compared with that stimulated by ligands or constitutively active BMP receptors. It is not known whether Smads are sufficient for the induction of osteoblast differentiation or whether other signaling molecules activated by BMP receptors are required for this induction. Smad6 and Smad7 were shown to inhibit the transcriptional activity induced by TGF-β (Hayashi et al., 1997; Imamura et al., 1997; Nakao et al., 1997), but recent data revealed that Smad7 is more potent than Smad6 in inhibiting TGF-β–induced growth inhibitory activity (Itoh et al., 1998). Inhibitory Smads were also shown to inhibit the effects of BMPs as well as those of activins in Xenopus embryos (Bhushan et al., 1998; Hata et al., 1998; Nakayama et al., 1998a,b); however, the effects of inhibitory Smads on osteoblast and chondroblast differentiation have not been determined. An adenovirus-based vector system allows high efficiencies of transfection of DNAs in many cell types. In this study, we examined the effects of various type I receptors and Smads in the regulation of differentiation into osteoblast and chondroblast cells using an adenovirus vector system.
MATERIALS AND METHODS
Constructions of Recombinant Adenoviruses
Recombinant adenoviruses were constructed as described previously (Saito et al., 1985; Miyake et al., 1996; Saitoh et al., 1998). Briefly, various hemagglutinin (HA)-tagged type I receptor, FLAG-tagged Smad, and β-galactosidase cDNAs were subcloned into the SwaI site of the pAxCAwt cassette cosmid. Each cosmid carrying the expression unit and adenovirus DNA–terminal protein complex were cotransfected into E1 transcomplemental cell line 293. The recombinant adenoviruses generated by homologous recombination were isolated, and the insertion of Smad or type I receptor cDNAs was confirmed by digestion using restriction endonucleases. High-titered stocks of recombinant viruses were grown in 293 cells and purified. Infection of recombinant adenoviruses was performed at a multiplicity of infection (m.o.i.) of <8 × 102 pfu/cell.
Cell Culture
The mouse muscle myoblast cell line C2C12 was obtained from American Type Culture Collection (Rockville, MD). The cells were maintained in DMEM containing 15% fetal bovine serum (FBS) and 100 U/ml penicillin. The mouse clonal teratocarcinoma cell line ATDC5 was obtained from Riken Cell Bank (Saitama, Japan). The cells were grown in a medium consisting of a 1:1 mixture of DMEM and Ham’s F-12 medium containing 5% FBS, 10 μg/ml bovine insulin, 10 μg/ml transferrin, 3 × 10−8 M sodium selenite (Boehringer Mannheim, Indianapolis, IN), and antibiotics as described (Shukunami et al., 1996).
Immunoblotting
Cells infected with adenoviruses were washed with phosphate-buffered saline (PBS) and solubilized in a buffer containing 20 mM Tris-HCl, pH 7.5, 150 mM NaCl, 1% Triton X-100, 1% aprotinin, and 1 mM phenylmethylsulfonyl fluoride. Lysates were cleared by centrifugation and subjected to immunoprecipitation by anti-FLAG antibody or directly subjected to SDS-gel electrophoresis (Kawabata et al., 1998b; Yagi et al., 1999). Proteins were then electrotransferred to polyvinylidene difluoride membranes, immunoblotted with anti-FLAG M2 antibody (Sigma, St. Louis, MO), anti-HA 3F10 antibody (Boehringer Mannheim), or anti-phosphoserine antibody (Zymed Labs, San Francisco, CA), and visualized using an enhanced chemiluminescence detection system (Pharmacia, Piscataway, NJ).
Assay for Alkaline Phosphatase Activity
Histochemical analysis of alkaline phosphatase activity was performed as described (Katagiri et al., 1994). Briefly, cells were fixed for 10 min with 3.7% formaldehyde at room temperature. After washing with PBS, the cells were incubated for 20 min with a mixture of 0.1 mg/ml naphthol AS-MX phosphate (Sigma), 0.5% N,N-dimethylformamide, 2 mM MgCl2, and 0.6 mg/ml fast blue BB salt (Sigma) in 0.1 M Tris-HCl, pH 8.5, at room temperature, followed by histochemical analysis using phase-contrast microscopy. For quantitative analysis of alkaline phosphatase activity, cells were washed and extracted with a lysis buffer as described (Asahina et al., 1993; Nishitoh et al., 1996). Alkaline phosphatase activity was determined using p-nitrophenyl phosphate (Sigma) as a substrate.
Measurements of Chondrogenic Differentiation
Chondrogenic differentiation of ATDC5 cells was determined by staining of sulfated glycosaminoglycans of the cells with Alcian Blue as described (Asahina et al., 1996). Cells were washed with PBS, fixed with 4% paraformaldehyde for 10 min, and stained with 0.5% Alcian Blue 8GX (Wako, Osaka, Japan) in 0.1N HCl overnight. After washing with distilled water, the cells were examined by histochemical analysis. Synthesis of sulfated glycosaminoglycans was measured by incorporation of [35S]sulfate as described (Lietman et al., 1997). Briefly, cells were labeled with Na235SO4 (10 μCi; Pharmacia) for 4 h. The radioactive medium was removed, and cells were washed with ice-cold buffer (10 mM EDTA and 0.1 M sodium phosphate, pH 6.5) and digested in 12-well plates with 200 μl of proteinase K (1 mg/ml) for 24 h at 37°C. One hundred microliters of the samples were subjected to chromatography on Microspin G-25 columns (Pharmacia) in 4 M guanidine hydrochloride to remove unincorporated 35SO4. Radioactivity in the macromolecular fraction was determined by a scintillation counter.
Immunofluorescence and Confocal Microscopy
Cells were grown in LAB-TEK chambers (Nalge, Rochester, NY), infected with adenoviruses carrying different Smad and receptor cDNAs, washed with PBS, and fixed with acetone. Cells were then incubated with 10% normal goat serum, washed with PBS, and incubated with an anti-Smad5 antiserum (provided by P. ten Dijke and C.-H. Heldin). The cells were then washed again with PBS, followed by incubation with fluorescein isothiocyanate–conjugated antibody against mouse immunoglobulin (Cappel, West Chester, PA). After a final wash, cells were covered with glycerine and examined by confocal laser-scanning microscopy (Olympus, Lake Success, NY).
RESULTS
Differentiation Induction of C2C12 Cells by Type I Receptors
To obtain a high-transfection efficiency, we infected DNAs in C2C12 cells using a recombinant adenovirus system. More than 80% of the cells were infected as determined by staining of the cells for β-galactosidase. Similar results were obtained for ATDC5 cells.
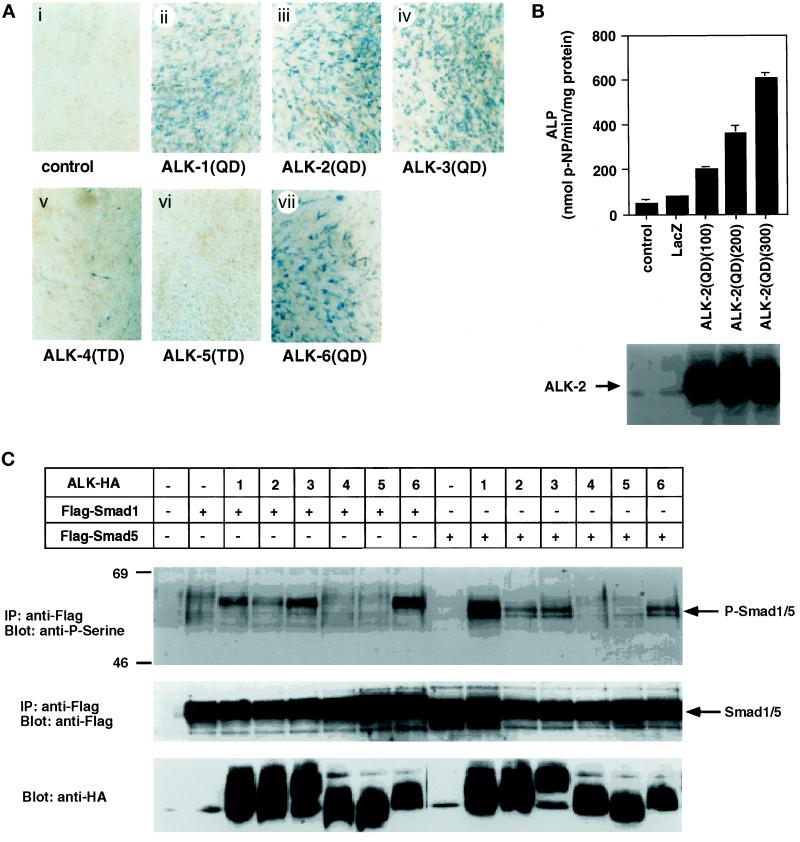
C2C12 undifferentiated mesenchymal cells differentiate into osteoblast-like cells after treatment with BMP-2 and OP-1/BMP-7 (Katagiri et al., 1994; Takeda et al., 1998). Osteoblast differentiation by constitutively active forms of type I receptors was examined by the induction of alkaline phosphatase activity by staining the cells 4 d after infection. Similar expression levels of HA-tagged type I receptors were observed when determined by immunoblotting using anti-HA antibody (see Figure 1C). Histochemical analysis revealed that the cells infected with adenoviruses carrying BMPR-IA/ALK-3(QD) and BMPR-IB/ALK-6(QD) were positively stained (Figure 1A). In addition, constitutively active forms of type I receptors of the ALK-1 group (ALK-1 and ALK-2), but not those of the TβR-I group (TβR-I/ALK-5 and ActR-IB/ALK-4), induced alkaline phosphatase activity (Figure 1A). Quantitative analysis of alkaline phosphatase activity also revealed that the constitutively active ALK-2 induced alkaline phosphatase activity (Figure 1B). Differences in the alkaline phosphatase activity were caused by the difference in the numbers of the alkaline phosphatase–positive cells in certain experiments (see below). Therefore, further studies on osteoblast differentiation were performed by histochemical analysis.
Figure 1.
Histochemical analysis of alkaline phosphatase activity and phosphorylation of Smad1 and Smad5 by constitutively active forms of type I receptors in the adenovirus vector. (A) Alkaline phosphatase production in C2C12 cells by constitutively active forms of type I receptors is shown. C2C12 cells were infected with adenoviruses carrying constitutively active forms of type I receptor cDNA at an m.o.i. of 300 (ii–vii). Four days after infection, the cells were observed by phase-contrast microscopy. (B) Quantification of alkaline phosphatase activity in C2C12 cells by the constitutively active form of ALK-2 [ALK-2(QD)] is shown. Top, alkaline phosphatase activity was determined as described in MATERIALS AND METHODS; m.o.i. is shown in parentheses. Bottom, expression of ALK-2(QD) was determined by anti-HA immunoblotting. (C) Phosphorylation of Smad1 and Smad5 by the constitutively active forms of type I receptors is shown. C2C12 cells were infected with adenoviruses with HA-tagged type I receptor (m.o.i. of 200) and FLAG-tagged Smad1 or Smad5 cDNAs (ALK-HA; m.o.i. of 200). Top, cell lysates were immunoprecipitated with anti-FLAG antibody followed by immunoblotting with anti-phosphoserine antibody. Middle, expression of Smad1 and Smad5 was confirmed by reblotting the membrane with anti-FLAG antibody. Bottom, expression of type I receptors was detected by immunoblotting using anti-HA antibody of the cell lysates. ALP, alkaline phosphatase; IP, immunoprecipitation; p-NP, p-nitrophenyl phosphate.
Activation of Smad1 and Smad5 by type I receptors was examined by immunoblotting using anti-phosphoserine antibody. Constitutively active forms of ALK-1, ALK-2, BMPR-IA/ALK-3, and BMPR-IB/ALK-6 induced the phosphorylation of Smad1 and Smad5 (Figure 1C). In contrast, neither ActR-IB/ALK-4 nor TβR-I/ALK-5 efficiently phosphorylated Smad1 or Smad5. Thus, the ability of type I receptors to induce osteoblast differentiation of C2C12 cells was correlated with their ability to activate Smad1 and Smad5.
Induction of Alkaline Phosphatase Activity in C2C12 Cells by Smad1 and Smad5
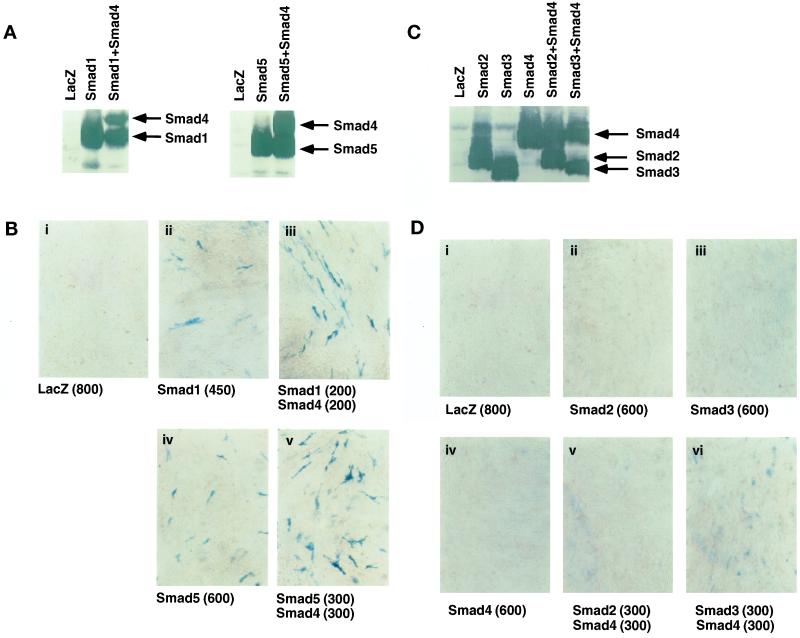
Osteoblast differentiation by Smads was then examined by induction of alkaline phosphatase activity by staining the cells infected with adenoviruses carrying Smad cDNAs. Expression of FLAG-tagged Smads was confirmed by anti-FLAG immunoblotting (Figure 2, A and C). Small fractions of the cells expressing Smad1 or Smad5 were positively stained at an m.o.i. of >450 (Figure 2B). In contrast, neither Smad2 nor Smad3, which are activated by TGF-β and activin pathways, induced alkaline phosphatase activity even at an m.o.i. of 600 (Figure 2D), although Smad proteins were expressed in the infected cells. Smad4, the common partner Smad in mammals, did not induce alkaline phosphatase activity when infected alone (Figure 2D); however, coinfection of Smad4 potentiated the effect of Smad1 and Smad5 (Figure 2B). Cells positively stained for alkaline phosphatase were only sparsely observed in the presence of Smad1 or Smad5 and Smad4, whereas most of the cells infected with the adenoviruses containing type I receptors were positively stained (see Figure 1A). Interestingly, Smad4 weakly induced alkaline phosphatase activity in the C2C12 cells infected with the Smad3 adenovirus but not in the cells infected with the Smad2 adenovirus (Figure 2D), suggesting that Smad3 weakly, but significantly, activates the transcription of the alkaline phosphatase gene in the presence of Smad4.
Figure 2.
Induction of alkaline phosphatase activity by Smads in the adenovirus vector. (A and B) Effects of Smad1 and Smad5 in the presence and absence of Smad4 are shown. C2C12 cells were infected with adenoviruses carrying Smad1, Smad5, and Smad4 at an m.o.i. of 200–600 (shown in parentheses in B). Adenovirus carrying β-galactosidase (m.o.i. of 800) was used as a control (i). (A) Expression of Smads was confirmed by anti-FLAG immunoblotting. (B) C2C12 cells were stained for alkaline phosphatase activity. (C and D) Effects of Smad2, Smad3, and Smad4 on C2C12 cells are shown. C2C12 cells were infected with adenoviruses with Smad2, Smad3, or Smad4 alone (m.o.i. of 600; ii–iv) or with combinations of Smad2 or Smad3 and Smad4 (m.o.i. of 300 for each; v and vi). (C) Expression of Smads was confirmed by anti-FLAG immunoblotting. (D) C2C12 cells were stained for alkaline phosphatase activity.
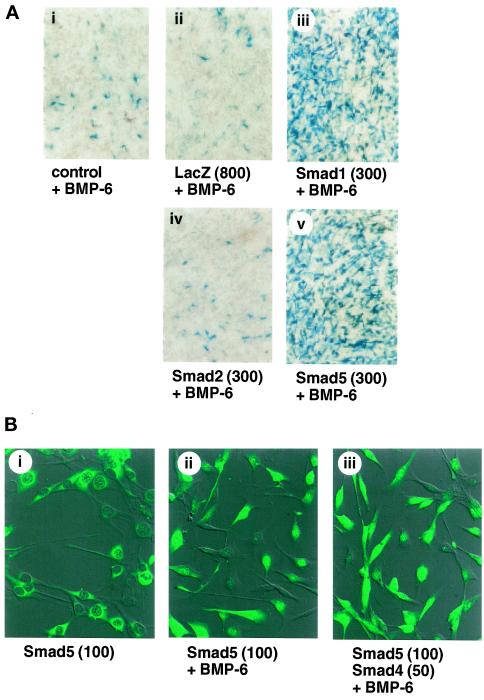
BMP-6 is structurally most similar to OP-1/BMP-7. BMP-6 (200 ng/ml) induced the differentiation of C2C12 cells into osteoblast cells (Figure 3A). When the C2C12 cells were infected with Smad1 or Smad5 and treated with 200 ng/ml BMP-6, induction of alkaline phosphatase activity was dramatically enhanced, and most of the infected cells were positively stained for alkaline phosphatase activity (Figure 3A).
Figure 3.
Increase in alkaline phosphatase activity by Smad1 and Smad5 in the presence of BMP-6. (A) Effects of Smads in the presence of BMP-6. C2C12 cells were infected with adenoviruses carrying Smad1, Smad2, and Smad5 at an m.o.i of 300 and treated with 200 ng/ml BMP-6 (iii–v). Adenovirus carrying β-galactosidase (m.o.i. of 800; ii) was used as a control. Cells were stained for alkaline phosphatase activity 4 d after infection. (B) Nuclear translocation of Smad5 by BMP-6. The cells infected with Smad5 (m.o.i. of 100) and Smad4 (m.o.i. of 50) and treated with BMP-6 (200 ng/ml) were stained by indirect immunofluorescence using an anti-Smad5 antibody.
To study the mechanism of efficient induction of alkaline phosphatase activity of R-Smads by BMP-6, we examined subcellular localization of Smad5 by indirect immunofluorescence staining of cells using an anti-Smad5 antiserum (Figure 3B). Smad5 was mainly observed in the cytoplasm in the absence of BMP-6, whereas addition of BMP-6 induced the nuclear accumulation of Smad5. Constitutively active forms of ALK-1, ALK-2, BMPR-IA/ALK-3, and BMPR-IB/ALK-6 also induced the nuclear translocation of Smad5 (our unpublished results; see Figure 4). Overexpression of Smad5 does not lead to its nuclear accumulation, although small fractions of Smad5 may spontaneously translocate into the nucleus. Acceleration of Smad5-induced alkaline phosphatase induction by BMP-6 may thus be induced by nuclear translocation of Smad5. Similar results were obtained for Smad1.
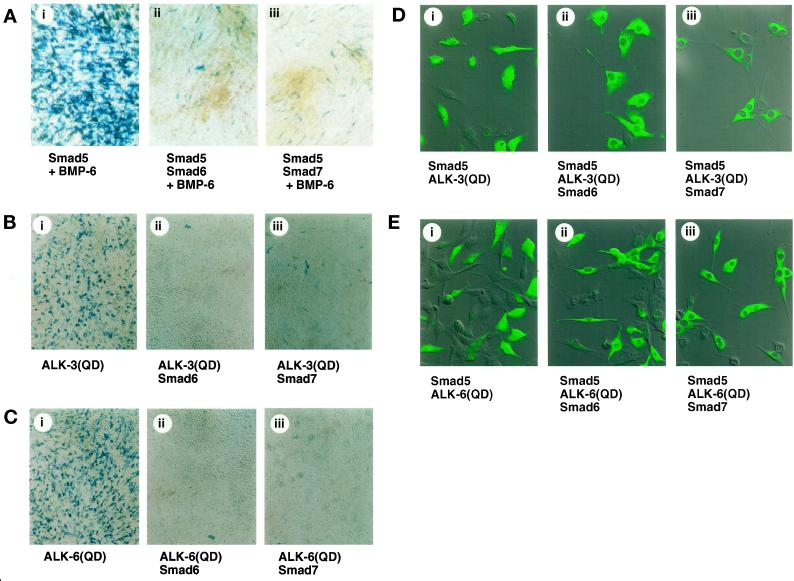
Figure 4.
Smad6 and Smad7 inhibit the osteoblast differentiation of C2C12 cells. (A) C2C12 cells infected with adenoviruses carrying Smad5, Smad6, and Smad7 (m.o.i. of 150 for each) were treated with 200 ng/ml BMP-6 and stained for alkaline phosphatase activity 4 d after infection. (B and C) C2C12 cells infected with adenoviruses with the constitutively active forms of BMPR-IA/ALK-3 [ALK-3(QD); B] or BMPR-IB/ALK-6 [ALK-6(QD); C] (m.o.i. of 300) were coinfected with Smad6 (ii) or Smad7 (iii) (m.o.i. of 100). Cells were stained for alkaline phosphatase activity 4 d after infection. (D and E) Subcellular localization of Smad5 coinfected with the adenoviruses carrying BMPR-IA/ALK-3(QD) (D) or BMPR-IB/ALK-6(QD) (E) and Smad6 (ii) or Smad7 (iii) is shown. Each adenovirus was infected at an m.o.i. of 100. Cells were stained by indirect immunofluorescence using an anti-Smad5 antibody 4 d after infection.
Inhibitory Smads Block the Differentiation of C2C12 Cells into Osteoblasts Induced by BMPs
Next, the effects of inhibitory Smads on differentiation of osteoblasts were determined. C2C12 cells were infected with the adenoviruses with Smad6 or Smad7 together with Smad5 and treated with BMP-6. Alkaline phosphatase activity was induced by BMP-6 and Smad5 but was inhibited by Smad6 or Smad7 (Figure 4A). Coinfection of Smad6 or Smad7 adenoviruses in the cells expressing BMPR-IA/ALK-3(QD) or BMPR-IB/ALK-6(QD) also prevented differentiation into osteoblast cells (Figure 4, B and C). Similar data were obtained when adenovirus carrying ALK-2(QD) was used (our unpublished results).
Smad5 was observed in the nucleus after stimulation of BMPR-IA/ALK-3(QD) or BMPR-IB/ALK-6(QD), when the cells were stained with anti-Smad5 antibody. However, coinfection of the Smad6 or Smad7 adenovirus clearly blocked the nuclear translocation of Smad5, and the Smad5 protein was observed predominantly in the cytoplasm (Figure 4, D and E). These findings indicate that Smad6 and Smad7 prevent the nuclear translocation of Smad5 and thus inhibit the differentiation of C2C12 cells into osteoblast-like cells.
Chondrogenic Differentiation of ATDC5 Cells by Type I Receptors
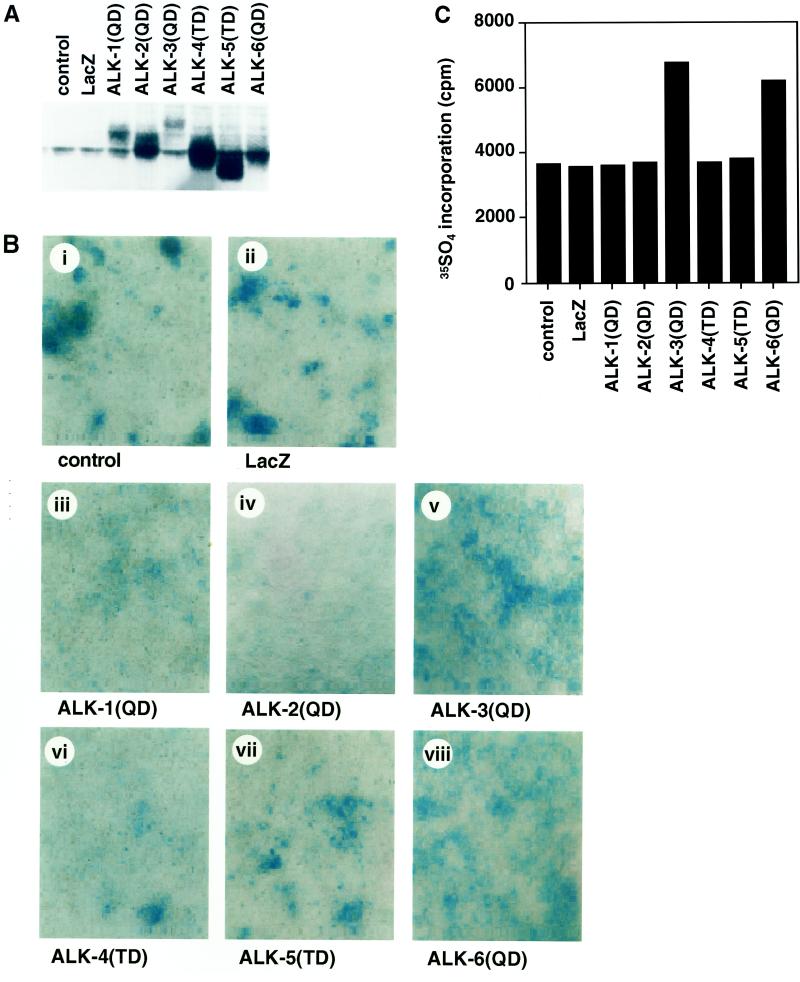
We next examined the effects of type I receptors on differentiation into chondrogenic cells of a mouse teratocarcinoma cell line, ATDC5. Comparable expression levels of the type I receptors were obtained when the immunoblotting was performed using anti-HA antibody (Figure 5A). By 8 d after infection, a certain number of the cells spontaneously differentiated into chondrogenic cells, and formation of Alcian Blue–positive cartilage nodules was observed, probably because of endogenously produced BMPs (Figure 5B) (Shukunami et al., 1996, 1998). The constitutively active type I receptors of the BMPR-I group (BMPR-IA/ALK-3 and BMPR-IB/ALK-6) increased cartilage nodules, whereas those of the ALK-1 group (ALK-1 and ALK-2) did not. Chondrogenic differentiation was also examined by the in corporation of [35S]sulfate into glycosaminoglycans 10 d after adenovirus infection. In agreement with the results obtained by Alcian Blue staining, BMPR-IA/ALK-3(QD) and BMPR-IB/ALK-6(QD), but not the other type I receptors, induced incorporation of [35S]sulfate (Figure 5C).
Figure 5.
Chondrogenic differentiation of ATDC5 cells by constitutively active forms of type I receptors. (A and B) ATDC5 cells were infected with adenoviruses carrying the constitutively active forms of type I receptors at an m.o.i. of 300 (iii–viii). (A) Expression of each receptor was determined by immunoblotting using anti-HA antibody. (B) Cells were stained by Alcian Blue 8 d after infection. Adenovirus carrying β-galactosidase (m.o.i. of 300) was used as a control (ii). (C) Incorporation of [35S]sulfate into ATDC5 cells by the constitutively active forms of type I receptors was determined 10 d after infection.
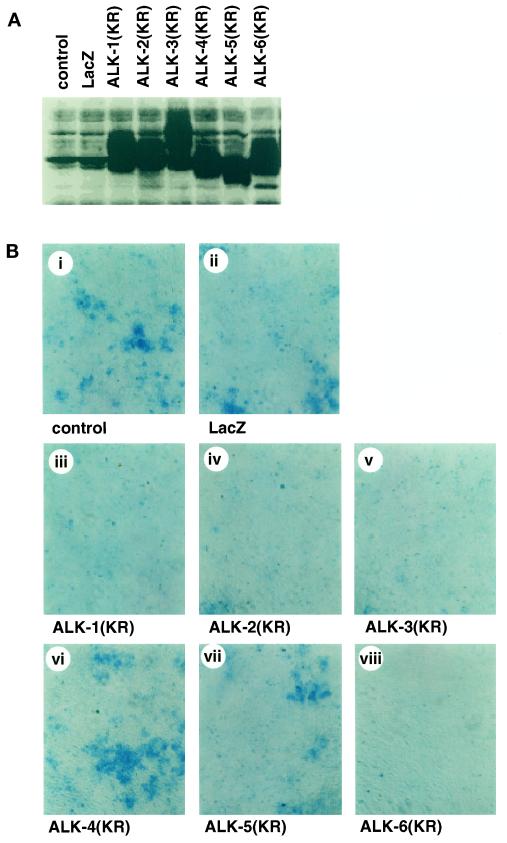
Because chondrogenic differentiation is spontaneously induced in ATDC5 cells, we tested the effects of kinase-inactive forms of type I receptors, which act as dominant-negative receptors (Imamura et al., 1997). BMPR-IA/ALK-3(KR) and BMPR-IB/ALK-6(KR) prevented the cartilage nodule formation of ATDC5 cells (Figure 6). In addition, ALK-1(KR) and ALK-2(KR), but not TβR-I/ALK-5(KR) or ActR-IB/ALK-4(KR), blocked chondrogenic differentiation. Thus, the constitutively active type I receptors of the ALK-1 group failed to induce chondrogenic differentiation, whereas the kinase-inactive receptors of the ALK-1 group blocked this differentiation.
Figure 6.
Prevention of chondrogenic differentiation of ATDC5 cells by kinase-inactive forms of type I receptors. ATDC5 cells were infected with adenoviruses carrying the kinase-inactive forms of type I receptors at an m.o.i. of 300 (iii–viii). (A) Expression of each receptor was determined by immunoblotting using anti-HA antibody. (B) Cells were stained by Alcian Blue 8 d after infection. Adenovirus carrying β-galactosidase (m.o.i. of 300) was used as a control (ii).
Effects of R-Smads and Inhibitory Smads on Chondrogenic Differentiation
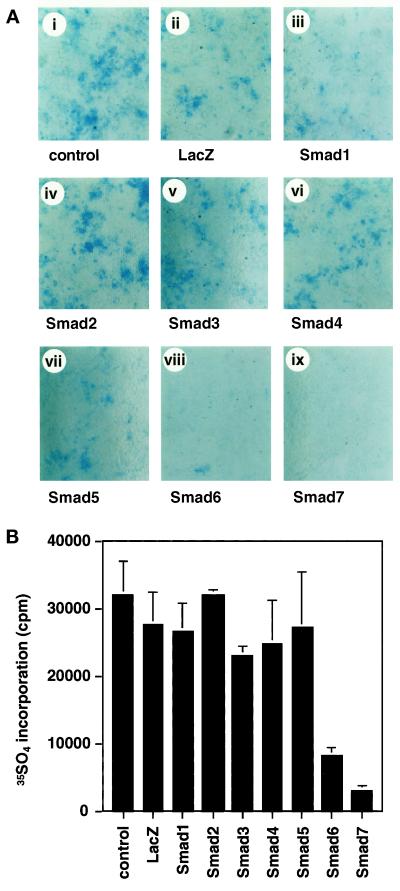
To determine whether Smads are involved in chondrogenic differentiation of ATDC5 cells, adenoviruses carrying Smad1–Smad7 were infected into these cells. None of the Smads efficiently induced Alcian Blue–positive cartilage nodule formation in the absence (Figure 7A) or presence (our unpublished results) of BMPR-IB/ALK-6(QD). In the presence of BMPR-IB/ALK-6(KR), BMP-specific R-Smad did not induce cartilage nodule formation in the presence or absence of Smad4 (our unpublished results). However, Smad6 and Smad7 strongly inhibited the formation of cartilage nodules, indicating that R-Smads and Co-Smads do not induce chondrogenic differentiation but that inhibitory Smads can block it. Chondrogenic differentiation was also studied by the incorporation of [35S]sulfate (Figure 7B). In agreement with the results of histochemical analysis, Smad6 and Smad7 inhibited the chondrogenic differentiation of ATDC5 cells.
Figure 7.
Chondrogenic differentiation of ATDC5 cells by Smads. (A) ATDC5 cells were infected with adenoviruses carrying Smad1–Smad7 at an m.o.i. of 300 (iii–ix). Cells were stained by Alcian Blue 8 d after infection. Adenovirus with β-galactosidase (m.o.i. of 300) was used as a control (ii). (B) Incorporation of [35S]sulfate into ATDC5 cells by Smad1–Smad7 was determined 10 d after infection.
DISCUSSION
Induction of Osteoblast Differentiation by Type I Receptors of BMPR-I and ALK-1 Groups
BMP-2, BMP-4, BMP-6, and OP-1/BMP-7 have been shown to induce osteoblast and chondroblast differentiation both in vitro and in vivo (reviewed in Kawabata et al., 1998a). In addition, GDF-5 and its related proteins play important roles in chondrocyte differentiation (Hötten et al., 1996; Wolfman et al., 1997; Francis-West et al., 1999; Tsumaki et al., 1999). BMPR-IA/ALK-3 and BMPR-IB/ALK-6 are type I receptors that specifically bind BMPs. GDF-5 was shown to bind predominantly to BMPR-IB/ALK-6, compared with other type I receptors (Nishitoh et al., 1996). In contrast, ALK-2 was shown to be a type I receptor for OP-1/BMP-7, BMP-6, and possibly other BMPs (ten Dijke et al., 1994b; Macías-Silva et al., 1998; Ebisawa et al., 1999) (our unpublished results). Thus, the type I receptors of the BMPR-I group as well as certain members of the ALK-1 group act as type I receptors for BMPs. A difference in the biological effects of BMPR-IA/ALK-3 and BMPR-IB/ALK-6 has been reported (Zou et al., 1997; D. Chen et al., 1998); D. Chen et al. showed that BMPR-IA/ALK-3 is important for adipocyte differentiation, whereas BMPR-IB/ALK-6 is critical for osteoblast differentiation and apoptosis (D. Chen et al., 1998). No functional difference was found between the two receptors in our assays, probably because of the difference in cell types used in the present study.
The specificity of the intracellular signals is determined by the L45 loops of type I receptors, which are composed of nine amino acid residues (Feng and Derynck, 1997). The L45 loop of type I receptors interacts with specific sequences in the C-terminal Mad homology 2 domain of R-Smads, including the L3 loop and α-helix 1 (Lo et al., 1998; Y.G. Chen et al., 1998; Chen and Massagué, 1999). The L45 loops of BMPR-IA/ALK-3 and BMPR-IB/ALK-6 are identical to each other, and those of the TβR-I group are also identical to each other. However, the L45 loops of BMPR-IA and BMPR-IB and those of the TβR-I group differ at four amino acid residues; this appears to be most important for the specific interaction with different R-Smads. Thus, the BMPR-I group activates Smad1, Smad5, and presumably Smad8, whereas the TβR-I group phosphorylates Smad2 and Smad3. Although the L45 loop of the ALK-1 group diverges from those of the other type I receptors, it was shown to interact with Smad1 and Smad5, similar to that of the BMPR-I group (Chen and Massagué, 1999) (our unpublished results). Thus, the ability of type I receptors to induce alkaline phosphatase activity correlated with their ability to activate Smad1 and Smad5.
Smad1 and Smad5 Induce Alkaline Phosphatase Activity in C2C12 Cells
An important question with regard to signal transduction by serine/threonine kinase receptors is whether Smads alone are sufficient to induce osteoblast differentiation. As shown in Figure 2, Smad1 and Smad5 could induce osteoblast differentiation, which was enhanced by the presence of Co-Smad, Smad4. However, the differentiation-inducing effects of Smad1 and Smad5 in the presence of Smad4 were less potent than were those of the constitutively active type I receptors. When the cells were stimulated with BMP-6 at a concentration that did not fully induce osteoblast differentiation in culture, Smad1 and Smad5 dramatically induced alkaline phosphatase activity. This may have been because R-Smads efficiently translocated into the nucleus upon ligand stimulation; R-Smads were otherwise predominantly localized in the cytoplasm. Nuclear localization of R-Smads is thus an important event in their biological activity, because they act as transcription factors together with other DNA-binding proteins. Our present findings suggest that Smads are major signaling molecules for the differentiation of osteoblasts, but it is still possible that other signaling pathways independent of that of Smads, which act cooperatively with Smad pathways, may be required for efficient osteoblast differentiation induction.
Inhibitory Smads Inhibit Osteoblast Differentiation
Smad6 and Smad7 have been shown to inhibit the effects of R-Smads by competing for binding to activated type I receptors (Hayashi et al., 1997; Imamura et al., 1997; Nakao et al., 1997). It was also shown that Smad6 inhibits the activity of Smad1 by competing for complex formation with Smad4 (Hata et al., 1998). Inhibition of BMP signals by inhibitory Smads has been reported using Xenopus assays (Bhushan et al., 1998; Hata et al., 1998; Nakayama et al., 1998a,b), but their effects on osteoblast differentiation have not been reported. In the present study, we showed that both Smad6 and Smad7 inhibited the osteoblast differentiation induced by ligand or receptor stimulation. In the presence of inhibitory Smads, Smad5 (Figure 4, D and E) and Smad1 (our unpublished results) were detected in the cytoplasm, supporting the notion that inhibitory Smads exhibit their effects by inhibiting the activation of R-Smads. These findings again indicate that Smad pathways are essential for the induction of osteoblast differentiation.
We have shown previously that Smad6 inhibits the activation of R-Smad by BMPR-IB/ALK-6 but not efficiently that by BMPR-IA/ALK-3 (Imamura et al., 1997). However, using constitutively active forms of type I receptors in the adenovirus vector, which allowed us to obtain sufficient protein expression levels, we found that both Smad6 and Smad7 inhibit the activation of Smad1 and Smad5 by BMPR-IA/ALK-3 and BMPR-IB/ALK-6, as well as that by ALK-2 (Figure 4; our unpublished results).
Induction of Chondrogenesis by Different Type I Receptors
Formation of cartilaginous bone rudiments is a critical step in the initiation of endochondral bone formation. BMPs, including BMP-2, BMP-4, and OP-1/BMP-7, have been shown to regulate the growth and maturation of chondrocytes in vitro (Luyten et al., 1994; Rosen et al., 1994; Asahina et al., 1996; Shukunami et al., 1998). Stimulation of chondrogenesis by BMPR-IA/ALK-3 and BMPR-IB/ALK-6 was also demonstrated in vivo (Zou et al., 1997). We therefore studied the chondrogenic differentiation induced by type I receptors using ATDC5 cells. The receptors of the BMPR-I group, but not those of the ALK-1 group, induced chondrogenic differentiation (Figure 5). The lack of ability of ALK-1 and ALK-2 to induce differentiation of ATDC5 cells suggests that Smads may not be sufficient for chondrogenic differentiation. In agreement with this notion, none of the Smads efficiently induced cartilage formation in vitro (Figure 7).
Prevention of Chondrogenic Differentiation by Kinase-inactive Type I Receptors and Inhibitory Smads
ATDC5 cells spontaneously form cartilage nodules in the absence of exogenously added BMPs, possibly because of BMPs endogenously produced by these cells. Spontaneous cartilage nodule formation was blocked by the kinase-inactive forms of type I receptors of the BMPR-I group as well as those of the ALK-1 group (Figure 6). Moreover, inhibitory Smads, Smad6 and Smad7, efficiently blocked ATDC5 differentiation. These findings suggest that the type I receptors of the BMPR-I group activate the Smad pathway as well as Smad-independent signaling pathways and that the latter may not be efficiently activated by ALK-1 or ALK-2. Smad-dependent and -independent pathways may be required to act in concert for chondrogenic differentiation. Type I receptors have been shown to activate various Smad-independent signaling pathways, including ERK, JNK, and p38 MAP kinase pathways (Hartsough and Mulder, 1995; Atfi et al., 1997; Hannigan et al., 1998; Liberati et al., 1999). Production of fibronectin has been shown recently to be induced by the JNK pathway (Hocevar et al., 1999). It may thus be important to determine the Smad-independent signaling pathways that are involved in chondrogenic differentiation.
Our present data revealed that osteoblast differentiation of C2C12 cells is induced by the type I receptors of the BMPR-I and ALK-1 groups, the signaling from which appears to be mainly transmitted by the Smad pathways. In contrast, chondrogenic differentiation of ATDC5 cells is induced by BMPR-IA/ALK-3 and BMPR-IB/ALK-6, and Smads may be required, but not sufficient, for this differentiation. Further studies will be needed to identify the signaling pathways responsible for these biological effects and to elucidate whether there are cooperative or antagonistic effects between Smad-dependent and -independent signaling pathways.
ACKNOWLEDGMENTS
We thank Drs. Chisa Shukunami and Yuji Hiraki for valuable comments, Peter ten Dijke and Carl-Henrik Heldin for the Smad5 antibody, and Yasufumi Yuuki, Yuri Inada, and Aki Hanyu for technical help. This study was supported by grants-in-aid for scientific research from the Ministry of Education, Science, Sports and Culture of Japan and by Special Coordination Funds for Promoting Science and Technology from the Science and Technology Agency.
Abbreviations used:
- ActR
activin receptor
- ALK
activin receptor–like kinase
- BMP
bone morphogenetic protein
- BMPR
BMP receptor
- Co-Smad
common partner Smad
- FBS
fetal bovine serum
- GDF
growth-differentiation factor
- HA
hemagglutinin
- m.o.i.
multiplicity of infection
- OP
osteogenic protein
- PBS
phosphate-buffered saline
- R-Smad
receptor-regulated Smad
- TGF-β
transforming growth factor-β
- TβR
TGF-β receptor
REFERENCES
- Akiyama S, Katagiri T, Namiki M, Yamaji N, Yamamoto N, Miyama K, Shibuya H, Ueno N, Wozney JM, Suda T. Constitutively active BMP type I receptors transduce BMP-2 signals without the ligand in C2C12 myoblasts. Exp Cell Res. 1997;235:362–369. doi: 10.1006/excr.1997.3680. [DOI] [PubMed] [Google Scholar]
- Armes NA, Neal KA, Smith JC. A short loop on the ALK-2 and ALK-4 activin receptors regulates signaling specificity but cannot account for all their effects on early Xenopus development. J Biol Chem. 1999;274:7929–7935. doi: 10.1074/jbc.274.12.7929. [DOI] [PubMed] [Google Scholar]
- Armes NA, Smith JC. The ALK-2 and ALK-4 activin receptors transduce distinct mesoderm-inducing signals during early Xenopus development but do not cooperate to establish thresholds. Development. 1997;124:3797–3804. doi: 10.1242/dev.124.19.3797. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Hauschka PV. Human osteogenic protein-1 induces chondroblastic, osteoblastic, and/or adipocytic differentiation of clonal murine target cells. Exp Cell Res. 1996;222:38–47. doi: 10.1006/excr.1996.0005. [DOI] [PubMed] [Google Scholar]
- Asahina I, Sampath TK, Nishimura I, Hauschka PV. Human osteogenic protein-1 induces both chondroblastic and osteoblastic differentiation of osteoprogenitor cells derived from newborn rat calvaria. J Cell Biol. 1993;123:921–933. doi: 10.1083/jcb.123.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atfi A, Djelloul S, Chastre E, Davis R, Gespach C. Evidence for a role of Rho-like GTPases and stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) in transforming growth factor β-mediated signaling. J Biol Chem. 1997;272:1429–1432. doi: 10.1074/jbc.272.3.1429. [DOI] [PubMed] [Google Scholar]
- Attisano L, Wrana JL. Mads and Smads in TGFβ signaling. Curr Opin Cell Biol. 1998;10:188–194. doi: 10.1016/s0955-0674(98)80141-5. [DOI] [PubMed] [Google Scholar]
- Bhushan A, Chen Y, Vale W. Smad7 inhibits mesoderm formation and promotes neural cell fate in Xenopus embryos. Dev Biol. 1998;200:260–268. doi: 10.1006/dbio.1998.8965. [DOI] [PubMed] [Google Scholar]
- Chen D, Ji X, Harris MA, Feng JQ, Karsenty G, Celeste AJ, Rosen V, Mundy GR, Harris SE. Differential roles for bone morphogenetic protein (BMP) receptor type IB and IA in differentiation and specification of mesenchymal precursor cells to osteoblast and adipocyte lineages. J Cell Biol. 1998;142:295–305. doi: 10.1083/jcb.142.1.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Hata A, Lo RS, Wotton D, Shi Y, Pavletich N, Massagué J. Determinants of specificity in TGF-β signal transduction. Genes Dev. 1998;12:2144–2152. doi: 10.1101/gad.12.14.2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YG, Massagué J. Smad1 recognition and activation by the ALK1 group of transforming growth factor-β family receptors. J Biol Chem. 1999;274:3672–3677. doi: 10.1074/jbc.274.6.3672. [DOI] [PubMed] [Google Scholar]
- Derynck R, Zhang Y, Feng X-H. Smads: transcriptional activators of TGF-β responses. Cell. 1998;95:737–740. doi: 10.1016/s0092-8674(00)81696-7. [DOI] [PubMed] [Google Scholar]
- Ebisawa, T., Tada, K., Kitajima, I., Tojo, A., Sampath, T.K., Kawabata, M., Miyazono, K., and Imamura, T. (1999). Characterization of bone morphogenetic protein-6 signaling pathways in osteoblast differentiation. J. Cell Sci. (in press). [DOI] [PubMed]
- Feng X-H, Derynck R. A kinase subdomain of transforming growth factor-β (TGF-β) type I receptor determines the TGF-β intracellular signaling specificity. EMBO J. 1997;16:3912–3923. doi: 10.1093/emboj/16.13.3912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francis-West PH, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten FP, Archer CW. Mechanisms of GDF-5 action during skeletal development. Development. 1999;126:1305–1315. doi: 10.1242/dev.126.6.1305. [DOI] [PubMed] [Google Scholar]
- Gitelman SE, Kobrin MS, Ye JQ, Lopez AR, Lee A, Derynck R. Recombinant Vgr-1/BMP-6-expressing tumors induce fibrosis and endochondral bone formation in vivo. J Cell Biol. 1994;126:1595–1609. doi: 10.1083/jcb.126.6.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff JM, Bansal A, Melton DA. Xenopus Mad proteins transduce distinct subsets of signals for the TGFβ superfamily. Cell. 1996;85:479–487. doi: 10.1016/s0092-8674(00)81249-0. [DOI] [PubMed] [Google Scholar]
- Hannigan M, Zhan L, Ai Y, Huang C-K. The role of p38 MAP kinase in TGF-β1-induced signal transduction in human neutrophils. Biochem Biophys Res Commun. 1998;246:55–58. doi: 10.1006/bbrc.1998.8570. [DOI] [PubMed] [Google Scholar]
- Hartsough MT, Mulder KM. Transforming growth factor β activation of p44mapk in proliferating cultures of epithelial cells. J Biol Chem. 1995;270:7117–7124. doi: 10.1074/jbc.270.13.7117. [DOI] [PubMed] [Google Scholar]
- Hata A, Lagna G, Massagué J, Hemmati-Brivanlou A. Smad6 inhibits BMP/Smad1 signaling by specifically competing with the Smad4 tumor suppressor. Genes Dev. 1998;12:186–197. doi: 10.1101/gad.12.2.186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H, et al. The MAD-related protein Smad7 associates with the TGFβ receptor and functions as an antagonist of TGFβ signaling. Cell. 1997;89:1165–1173. doi: 10.1016/s0092-8674(00)80303-7. [DOI] [PubMed] [Google Scholar]
- Heldin C-H, Miyazono K, ten Dijke P. TGF-β signaling from cell membrane to nucleus through SMAD proteins. Nature. 1997;390:465–471. doi: 10.1038/37284. [DOI] [PubMed] [Google Scholar]
- Hocevar BA, Brown TL, Howe PH. TGF-β induces fibronectin synthesis through a c-Jun N-terminal kinase-dependent, Smad4-independent pathway. EMBO J. 1999;18:1345–1356. doi: 10.1093/emboj/18.5.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan BL. Bone morphogenetic proteins: multifunctional regulators of vertebrate development. Genes Dev. 1996;10:1580–1594. doi: 10.1101/gad.10.13.1580. [DOI] [PubMed] [Google Scholar]
- Hötten GC, et al. Recombinant human growth/differentiation factor 5 stimulates mesenchyme aggregation and chondrogenesis responsible for the skeletal development of limbs. Growth Factors. 1996;13:65–74. doi: 10.3109/08977199609034567. [DOI] [PubMed] [Google Scholar]
- Imamura T, Takase M, Nishihara A, Oeda E, Hanai J, Kawabata M, Miyazono K. Smad6 inhibits signaling by the TGF-β superfamily. Nature. 1997;389:622–626. doi: 10.1038/39355. [DOI] [PubMed] [Google Scholar]
- Itoh S, Landström M, Hermansson A, Itoh F, Heldin C-H, Heldin N-E, ten Dijke P. Transforming growth factor β1 induces nuclear export of inhibitory Smad7. J Biol Chem. 1998;273:29195–29201. doi: 10.1074/jbc.273.44.29195. [DOI] [PubMed] [Google Scholar]
- Katagiri T, Yamaguchi A, Komaki M, Abe E, Takahashi N, Ikeda T, Rosen V, Wozney JM, Fujisawa-Sehara A, Suda T. Bone morphogenetic protein-2 converts the differentiation pathway of C2C12 myoblasts into the osteoblast lineage. J Cell Biol. 1994;127:1755–1766. doi: 10.1083/jcb.127.6.1755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawabata M, Imamura T, Miyazono K. Signal transduction by bone morphogenetic proteins. Cytokine Growth Factor Rev. 1998a;9:49–61. doi: 10.1016/s1359-6101(97)00036-1. [DOI] [PubMed] [Google Scholar]
- Kawabata M, Inoue H, Hanyu A, Imamura T, Miyazono K. Smad proteins exist as monomers in vivo and undergo homo- and hetero-oligomerization upon activation by serine/threonine kinase receptors. EMBO J. 1998b;17:4056–4065. doi: 10.1093/emboj/17.14.4056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberati NT, Datto MB, Frederick JP, Shen X, Wong C, Rougier-Chapman EM, Wang X-F. Smads bind directly to the Jun family of AP-1 transcription factors. Proc Natl Acad Sci USA. 1999;96:4844–4849. doi: 10.1073/pnas.96.9.4844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lietman SA, Yanagishita M, Sampath TK, Reddi AH. Stimulation of proteoglycan synthesis in explants of porcine articular cartilage by recombinant osteogenic protein-1 (bone morphogenetic protein-7) J Bone Joint Surg Am. 1997;79:1132–1137. doi: 10.2106/00004623-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Lo RS, Chen YG, Shi Y, Pavletich NP, Massagué J. The L3 loop: a structural motif determining specific interactions between SMAD proteins and TGF-β receptors. EMBO J. 1998;17:996–1005. doi: 10.1093/emboj/17.4.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luyten FP, Chen P, Paralkar V, Reddi AH. Recombinant bone morphogenetic protein-4, transforming growth factor-β1, and activin A enhance the cartilage phenotype of articular chondrocytes in vitro. Exp Cell Res. 1994;210:224–229. doi: 10.1006/excr.1994.1033. [DOI] [PubMed] [Google Scholar]
- Macías-Silva M, Hoodless PA, Tang SJ, Buchwald M, Wrana JL. Specific activation of Smad1 signaling pathways by the BMP7 type I receptor, ALK2. J Biol Chem. 1998;273:25628–25636. doi: 10.1074/jbc.273.40.25628. [DOI] [PubMed] [Google Scholar]
- Massagué J. TGF-β signal transduction. Annu Rev Biochem. 1998;67:753–791. doi: 10.1146/annurev.biochem.67.1.753. [DOI] [PubMed] [Google Scholar]
- Miyake S, Makimura M, Kanegae Y, Harada S, Sato Y, Takamori K, Tokuda C, Saito I. Efficient generation of recombinant adenoviruses using adenovirus DNA-terminal protein complex and a cosmid bearing the full-length virus genome. Proc Natl Acad Sci USA. 1996;93:1320–1324. doi: 10.1073/pnas.93.3.1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, et al. Identification of Smad7, a TGFβ-inducible antagonist of TGF-β signaling. Nature. 1997;389:631–635. doi: 10.1038/39369. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Gardner H, Berg LK, Christian JL. Smad6 functions as an intracellular antagonist of some TGF-β family members during Xenopus embryogenesis. Genes Cells. 1998a;3:387–394. doi: 10.1046/j.1365-2443.1998.00196.x. [DOI] [PubMed] [Google Scholar]
- Nakayama T, Snyder MA, Grewal SS, Tsuneizumi K, Tabata T, Christian JL. Xenopus Smad8 acts downstream of BMP-4 to modulate its activity during vertebrate embryonic patterning. Development. 1998b;125:857–867. doi: 10.1242/dev.125.5.857. [DOI] [PubMed] [Google Scholar]
- Namiki M, Akiyama S, Katagiri T, Suzuki A, Ueno N, Yamaji N, Rosen V, Wozney JM, Suda T. A kinase domain-truncated type I receptor blocks bone morphogenetic protein-2-induced signal transduction in C2C12 myoblasts. J Biol Chem. 1997;272:22046–22052. doi: 10.1074/jbc.272.35.22046. [DOI] [PubMed] [Google Scholar]
- Nishitoh H, Ichijo H, Kimura M, Matsumoto T, Makishima F, Yamaguchi A, Yamashita H, Enomoto S, Miyazono K. Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5. J Biol Chem. 1996;271:21345–21352. doi: 10.1074/jbc.271.35.21345. [DOI] [PubMed] [Google Scholar]
- Persson U, Izumi H, Souchelnytskyi S, Itoh S, Grimsby S, Engström U, Heldin C-H, Funa K, ten Dijke P. The L45 loop in type I receptors for TGF-β family members is a critical determinant in specifying Smad isoform activation. FEBS Lett. 1998;434:83–87. doi: 10.1016/s0014-5793(98)00954-5. [DOI] [PubMed] [Google Scholar]
- Reddi AH. Bone and cartilage differentiation. Curr Opin Genet Dev. 1994;4:737–744. doi: 10.1016/0959-437x(94)90141-o. [DOI] [PubMed] [Google Scholar]
- Rosen V, Nove J, Song JJ, Thies RS, Cox K, Wozney JM. Responsiveness of clonal limb bud cell lines to bone morphogenetic protein 2 reveals a sequential relationship between cartilage and bone cell phenotypes. J Bone Miner Res. 1994;9:1759–1768. doi: 10.1002/jbmr.5650091113. [DOI] [PubMed] [Google Scholar]
- Saito I, Oya Y, Yamamoto K, Yuasa T, Shimojo H. Construction of nondefective adenovirus type 5 bearing a 2.8-kb hepatitis B virus DNA near the right end of its genome. J Virol. 1985;54:711–719. doi: 10.1128/jvi.54.3.711-719.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saitoh M, Nishitoh H, Fujii M, Takeda K, Tobiume K, Sawada Y, Kawabata M, Miyazono K, Ichijo H. Mammalian thioredoxin is a direct inhibitor of apoptosis signal-regulating kinase (ASK) 1. EMBO J. 1998;17:2596–2606. doi: 10.1093/emboj/17.9.2596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampath TK, et al. Recombinant human osteogenic protein-1 (hOP-1) induces new bone formation in vivo with a specific activity comparable with natural bovine osteogenic protein and stimulates osteoblast proliferation and differentiation in vitro. J Biol Chem. 1992;267:20352–20362. [PubMed] [Google Scholar]
- Shukunami C, Ohta Y, Sakuda M, Hiraki Y. Sequential progression of the differentiation program by bone morphogenetic protein-2 in chondrogenic cell line ATDC5. Exp Cell Res. 1998;241:1–11. doi: 10.1006/excr.1998.4045. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, Hiraki Y. Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J Cell Biol. 1996;133:457–468. doi: 10.1083/jcb.133.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A, Chang C, Yingling JM, Wang X-F, Hemmati-Brivanlou A. Smad5 induces ventral fates in Xenopus embryo. Dev Biol. 1997;184:402–405. doi: 10.1006/dbio.1997.8548. [DOI] [PubMed] [Google Scholar]
- Takeda K, Ichijo H, Fujii M, Mochida Y, Saitoh M, Nishitoh H, Sampath TK, Miyazono K. Identification of a novel bone morphogenetic protein-responsive gene that may function as a noncoding RNA. J Biol Chem. 1998;273:17079–17085. doi: 10.1074/jbc.273.27.17079. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Ichijo H, Franzén P, Laiho M, Miyazono K, Heldin C-H. Characterization of type I receptors for transforming growth factor-β and activin. Science. 1994a;264:101–104. doi: 10.1126/science.8140412. [DOI] [PubMed] [Google Scholar]
- ten Dijke P, Yamashita H, Sampath TK, Reddi AH, Estevez M, Riddle DL, Ichijo H, Heldin C-H, Miyazono K. Identification of type I receptors for osteogenic protein-1 and bone morphogenetic protein-4. J Biol Chem. 1994b;269:16985–16988. [PubMed] [Google Scholar]
- Thomsen GH. Xenopus mothers against decapentaplegic is an embryonic ventralizing agent that acts downstream of the BMP-2/4 receptor. Development. 1996;122:2359–2366. doi: 10.1242/dev.122.8.2359. [DOI] [PubMed] [Google Scholar]
- Tsumaki N, Tanaka K, Arikawa-Hirasawa E, Nakase T, Kimura T, Thomas JT, Ochi T, Luyten FP, Yamada Y. Role of CDMP-1 in skeletal morphogenesis: promotion of mesenchymal cell recruitment and chondrocyte differentiation. J Cell Biol. 1999;144:161–173. doi: 10.1083/jcb.144.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieser R, Wrana JL, Massagué J. GS domain mutations that constitutively activate TβR-I, the downstream signaling component in the TGF-β receptor complex. EMBO J. 1995;14:2199–2208. doi: 10.1002/j.1460-2075.1995.tb07214.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfman NM, et al. Ectopic induction of tendon and ligament in rats by growth and differentiation factors 5, 6, and 7, members of the TGF-β gene family. J Clin Invest. 1997;100:321–330. doi: 10.1172/JCI119537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney JM, Rosen V, Celeste AJ, Mitsock LM, Whitters MJ, Kriz RW, Hewick RM, Wang EA. Novel regulators of bone formation: molecular clones and activities. Science. 1988;242:1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Yagi K, Goto D, Hamamoto T, Takenoshita S, Kato M, Miyazono K. Alternatively-spliced variant of Smad2 lacking exon 3: comparison with wild-type Smad2 and Smad3. J Biol Chem. 1999;274:703–709. doi: 10.1074/jbc.274.2.703. [DOI] [PubMed] [Google Scholar]
- Yamamoto N, Akiyama S, Katagiri T, Namiki M, Kurokawa T, Suda T. Smad1 and Smad5 act downstream of intracellular signalings of BMP-2 that inhibits myogenic differentiation and induces osteoblast differentiation in C2C12 myoblasts. Biochem Biophys Res Commun. 1997;238:574–580. doi: 10.1006/bbrc.1997.7325. [DOI] [PubMed] [Google Scholar]
- Zou H, Wieser R, Massague J, Niswander L. Distinct roles of type I bone morphogenetic protein receptors in the formation and differentiation of cartilage. Genes Dev. 1997;11:2191–2203. doi: 10.1101/gad.11.17.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]