Abstract
Purpose
The goal of this project is to develop a model of retinal pigment epithelium (RPE) transplantation that permits extensive and reliable analysis of the transplants.
Methods
Cultures of newborn rabbit RPE were evaluated by morphology, electrophysiology and the expression of zonula occludens-1, cytokeratin and a melanocyte marker (S-100). Cells labeled with 5,6-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE) were transplanted into the subretinal space of rabbits using a 30 gauge needle without making a conjunctival flap or sclerotomy. The transplants were examined by fundus photography, confocal scanning laser ophthalmoscopy (cSLO), optical coherence tomography (OCT) and angiography. At two months the retina was examined histochemically.
Results
A one minute incubation at 37°C with 20μM CFDA-SE did not affect morphology or the expression of marker proteins. In co-culture, the labeled cells integrated into monolayers that developed a normal transepithelial electrical resistance of 400-450 Ωcm2. Dye was not transferred from labeled to non-labeled RPE cells. Transplanted RPE was detectable for at least 2 months. Angiography demonstrated an intact blood retinal barrier. The normal morphology of the retina and lack of debris in the subretinal space, suggested the transplanted RPE was functional.
Conclusions
Primary cultures of newborn rabbit RPE were highly differentiated even when labeled with CFDA-SE. Labeled cells could be followed long-term in vitro and in vivo. This model can examine how culture and transplantation protocols affect the reformation of a functional RPE monolayer. The similar size of rabbit and human eyes will facilitate the translation of these protocols to the bedside.
Introduction
Age-related macular degeneration is the leading cause of legal blindness in the elderly populations of the Western world.1 Despite elegant descriptions of the alterations in Bruch's membrane and associated degeneration of the retinal pigment epithelium (RPE), there is no successful treatment for the retinal disorders caused by RPE degeneration. In recent decades, procedures to replace aged or diseased RPE with a healthy RPE allograft or xenograft have been extensively investigated in well-known models.2-12 Encouraging results suggest that some blinding disorders may be amenable to treatment by RPE transplantation.12-14 Nonetheless, RPE engineering and transplantation remain a difficult strategy. The ideal experimental model would be economical for thorough experimentation, homologous to avoid immunorejection as a complication and similar to human to facilitate translation from the laboratory to the bedside. Rabbit eyes are closer in size and structure to human than rodent eyes, which means techniques developed in rabbit can be more readily adapted to human surgery. Further, a rabbit model would allow extensive experimentation and analysis of the essential retinal and choroidal interactions that would re-establish the blood-retinal barrier needed for a successful transplantation. These data would inform the experimental design for more expensive, primate models, and ultimately, clinical trials.
Two important criteria for any experimental model are the quality of the RPE used for transplantation and the marker used to identify and localize the grafted RPE cells in the host. Different donor cells and labeling techniques have been used for RPE transplantation with variable results.2-12, 15-19 Some donor RPE cells are limited by loss of differentiated properties and also by rejection.20-24 Many reported markers were toxic, leaked from cells or labeled cells with low efficiency.2, 15-19, 25-27 To overcome these limitations, we developed an effective method to isolate and grow newborn rabbit RPE cells and adapted a reliable label, 5,6-carboxyfluorescein diacetate succinimidyl ester (CFDA-SE).
CFDA-SE is a lipophilic molecule that is minimally fluorescent until it enters cells by passive diffusion and esterases cleave the acetyl groups to form carboxyfluorescein succinimidyl ester (CFSE). With the acetyl groups removed, CFSE becomes positively charged, which greatly reduces transmembrane diffusion, and binds the amino groups of proteins. With time, CFSE remains bound to stable macromolecular complexes of the nucleus.28 CFSE gives a green fluorescence under the proper illumination. CFDA-SE does not affect cell viability or function and has been successfully used to track neuronal cells and to monitor lymphocytes proliferation and migration.28-30 It has also been used to label hepatocytes, leukocytes and erythrocytes.31, 32 In these applications, CFSE was stable and non-diffusible both in vitro and in vivo.30, 33-35
To our knowledge, the use of CFDA-SE for labeling RPE cells has not been reported. The current study describes the conditions for isolating and labeling newborn rabbit RPE cells with CFDA-SE, and an improved method to transplant the RPE. In co-culture, we found the label was not transferred to neighboring cells, and in vivo, labeled RPE cells were found in the subretinal space two months post-transplantation.
Materials and Methods
Preparation of purified newborn rabbit RPE cells
The animals were obtained from the Animal Center of Harbin Medical University. All animals were used after receiving institutional approval and were handled in a humane manner. The procedure complied strictly with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Newborn pigmented rabbits were anesthetized 5-8 days after birth by intravenous injection of sodium pentobarbital (50mg/kg). Their eyes were enucleated and incubated in a solution of calcium-magnesium-free Dulbecco's phosphate buffered saline (DPBS; Hyclone, Logan, UT, USA) supplemented with Gentamicin (400U/ml) for 15 minutes at 4°C. The eyeballs were immersed in 2.4U/ml dispase-II solution (Roche, Mannheim, Germany) for 1 hour at 37°C. Under the dissecting microscope a circular incision was made through the choroid, RPE, and neural retina following the circumference of the scleral rim, and the anterior segment with the lens and vitreous was removed. Then the posterior segment was incubated in Dulbecco's modified Eagle's medium: Nutrient Mixture F12, 1:1 mixture (DMEM-F12 medium; Hyclone, Logan, UT, USA) without fetal bovine serum for 20 minutes at 37°C. A distinct cleavage plane is identifiable between the taut monolayer of RPE and the adjacent choroid so that an isolated sheet of RPE can be dissected. The RPE patches were separated gently from Bruch's membrane and choroid with fine forceps. The isolated cells were transferred to a conical centrifuge tube containing growth medium. This medium consisted of DMEM-F12 medium supplemented with 20% fetal bovine serum (FBS; Gibco BRL, Gland Island NY, USA), 2mM L-glutamine and penicillin (100U/ml) /streptomycin (100U/ml).19 To obtain cell suspensions, the RPE patches were triturated into single cells or small clusters by repeated pipetting. The suspensions were centrifuged for 5 minutes at 1500U/min. Cells were resuspended with the growth medium. The concentration of cells in suspension was determined with a hemocytometer. 12-well polycarbonate filter (0.4-μm pore size) Transwell (Corning-Costar, Corning, NY, USA) were coated with 5μg laminin (Roche, Mannheim, Germany) per filter according to the supplier's instructions, and used the same day. The primary isolated newborn rabbit RPE cells were plated on the filter at the density of 4×105 cells/cm2. The medium in the apical chamber and the basolateral chamber were both DMEM-F12 medium supplemented with 20% FBS. The cultures on microporous filter-supports were maintained at 37°C in a humidified atmosphere of 95% air/5% CO2. After cell plating, the media in the apical and basolateral chambers were replaced by DMEM-F12 medium supplemented with 5% FBS. The cells were fed every 3 days, and examined daily. The cells reached confluence in 1-2 weeks. For some experiments, the cells were passaged 2-4 times, as indicated
Immunocytochemistry
RPE was plated on glass slides or Transwell filters. At the indicated time for each experiment, cultures were fixed in 2% paraformaldehyde and blocked with 3% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, Missouri, USA). To detect cytokeratins or S-100, cultures grown on slides were stained with monoclonal mouse anti-Human Cytokeratin (Clone MNF116; Dako, Wiesentheid, Germany) or monoclonal anti-S-100 (Clone SH-B1; Sigma-Aldrich, St. Louis, Missouri, USA). After washing with PBS, the cells were incubated with biotinylated IgG, followed by an avidin-biotin peroxidase complex.36 Immunoreactivity was visualized by using diaminobenzidine and the nuclei were counterstained with hematoxylin. The sites of antibody deposition were visible as brownish granular spots. In control experiments, the primary antibody was omitted.
To detect ZO-1, cultures grown on filters were stained with rat-anti-ZO-1 polyclonal antibody (Santa Cruz, CA, USA).37, 38 After washing with PBS, the cultures were incubated with goat-anti-rat IgG conjugated with Texas Red (Santa Cruz, CA, USA). The cells were viewed with a fluorescence microscope. Images were captured and prepared with image-analysis software (Photoshop; Adobe Systems, Inc., San Jose, CA, USA).
Measurement of the transepithelial electrical resistance (TER)
The TER of newborn rabbit RPE monolayers cultured on Transwell filters was measured using endohm electrodes (World Precision Instruments, Sarasota, FL, USA) according to the manufacturer's instructions at 33°C after the cells were cultured for 5 days.38 This temperature was easier to maintain than 37°C and avoided the phase transition that occurs in tight junctions at ambient temperatures.39 Measurements were made in a modified DMEM-F12 medium that was supplemented with 20mM HEPES (pH 7.2). TER was recorded at several time-points from day 5 until the TER reached a plateau after approximately 4-5 weeks. The experiments were performed three times using three filters per experiment. Net TER measurements were calculated by subtracting the value of a blank filter coated with laminin (12 Ω) from the value recorded from each culture. The measurements were reported as Ω×cm2.
Preparation of CFDA-SE
Since CFDA-SE is relatively insoluble in aqueous solutions,35 the dispersing agent dimethylsulfoxide (DMSO, Amresco, Solon, Ohio, USA) was used to facilitate cell loading. Then, a 10mM CFDA-SE stock solution was prepared by dissolving 2.5mg CFDA-SE (Molecular Probes Inc., Eugene, OR, USA) into 500μl of this solution and stored at −20°C. The CFDA-SE solution was used immediately upon thawing due to its instability.
Determination of optimal labeling conditions for CFDA-SE
10mM CFDA-SE stock solution was diluted to concentrations of 5, 10, 20, 40 and 80μM with PBS. Secondary cultures of newborn rabbit RPE were harvested and washed three times with PBS. After measuring the viability by trypan blue, 1.0 ml of 5×104 cells/ml PBS was added to 16 10ml glass centrifuge tubes. Immediately, each tube received 1.0 ml of a CFDA-SE dilution to make the final concentrations of 2.5, 5, 10, 20 and 40 μM. There were 3 tubes for each concentration and one tube, without CFDA-SE, served as a control. Tubes from each concentration were incubated at 37°C with shaking for 1, 5 or 10 minutes. The RPE cells were washed three times with PBS following incubation. The viability of RPE cells in each of the 16 tubes was tested by trypan blue staining and the fluorescence was observed by fluorescence microscopy. Then the cells were plated on laminin-coated tissue culture plates (Corning-Costar, Corning, NY, USA) with DMEM-F12 medium supplemented with 20% FBS. To determine the plating efficiency of labeled RPE cells, the number of cells attached to the plate 24 hours post-plating was divided by the number of the RPE cells added to the plate. Each experiment was performed in triplicate. Based on the cell viability, plating efficiency, and fluorescence intensity, an optimal labeling condition was selected for the following in vitro experiments.
Leakage and reuptake test
Secondary cultures of newborn rabbit RPE were labeled and co-cultured with human RPE cells, as described in other tests of CFDA-SE labeling.35 Human eyeballs were obtained from the Heilongjiang Eye Bank after the corneas were harvested for keratoplasty, and the RPE was isolated, as described.40 The rabbit RPE was labeled, washed and incubated in PBS for 4 hours. Then 1×104 cells/cm2 of human and rabbit RPE were mixed and plated on laminin-coated slides. After 48 hours, human and rabbit RPE were distinguished by fixing the cultures and incubating them with purified rat anti-human TNF receptor type-II (CD120b) monoclonal antibody (hTNFR-M1; BD PharMingen, San Diego, CA, USA), which will not bind rabbit RPE. The human cells were then revealed using goat-anti-rat IgG-Texas Red (Santa Cruz, CA, USA) to impart a red immunofluorescence signal. The CFDA-SE-labeled rabbit RPE cells would appear green. CFDA-SE fluorescence observed in any human RPE cells would suggest CFDA-SE leakage and transfer from rabbit to human RPE cells. The test was repeated 3 times.
Flow cytometric analysis
Cells harvested from secondary cultures of newborn rabbit RPE were labeled with CFDA-SE. Four hours after labeling, 1×105 cells in 1.0 ml PBS were analyzed by fluorescence-activated flow cytometry (Elite, Culter, Hialeah, FL, USA). The same density of non-labeled RPE cells was analyzed as a control. The remaining control and labeled cells were plated at a density of 2×104 cells/cm2 onto 25cm2 tissue culture flasks (Corning Costar Corp., Cambridge, MA, USA). To maintain the cells in log phase growth, the cells were passaged in duplicate every 3-4 days. At 2, 3 and 4 weeks a flask was selected to measure the mean and geometric fluorescence intensity and fraction of labeled cells by fluorescence-activated flow cytometry. For analysis, the cell samples were gated on forward scatter (FS) vs. side scatter (SS) to exclude debris and clumps. These experiments were repeated 4 times.
In vitro co-culture model
Freshly isolated newborn rabbit RPE cells were labeled with CFDA-SE. After washing and incubation for 4 hours in PBS, the labeled cells were mixed with an equal number of unlabeled newborn rabbit RPE to imitate the transplantation of RPE into the sub-retinal space. The mixed cells were plated on laminin-coated filters at a density of 4×105 cells/cm2 on 12mm filter. For control filters, unlabeled RPE was plated at the same density. The laminin-coated filters were prepared according to the supplier's instructions, with 5μg laminin per filter, and used the same day. The medium in the apical chamber and the basolateral chamber were both DMEM-F12 medium supplemented with 20% FBS. The co-cultures were maintained at 37°C in a humidified atmosphere of 95% air/5% CO2 .After 1 day in culture, the media in the apical and basolateral chambers were replaced by DMEM-F12 medium supplemented with 5% FBS. The cells were fed every 3 days.
In vivo rabbit RPE cells transplantation
For subretinal transplantation, each animal was anesthetized with sodium pentobarbital (25mg/kg, intramuscularly) and xylazine (10mg/kg, intramuscularly). The pupil was dilated and a lid speculum was used to keep the eye lids open. Without preparing a conjunctival flap, the sclera was punctured 1.5mm posterior to the limbus. A 30 gauge needle with a 1-ml syringe was introduced into the vitreous cavity with the aid of a 90D preset lens (Ocular Instruments, Bellevue, WA, USA) in the surgeon's left hand under a surgical microscope. The needle was directed toward the infero-nasal quadrant, and a stream of RPE cell suspension (∼25 μl, containing ∼105 cells) was slowly injected under the neural retina, which produced a small bleb. To prevent reflux of the transplanted cells into the vitreous, a small amount of sterile air followed the suspension. The RPE suspension had been prepared from secondary cultures of newborn rabbit RPE cells that were harvested and labeled with CFDA-SE, washed and incubated in PBS for 4 hours, and concentrated by centrifugation. Because the injection site was self-sealing, there was no need to suture the sclera and conjunctiva after the procedure. For each rabbit, the left eye had RPE transplantation. As a control, the right eye had a mock transplantation with BSS (Alcon, Fort Worth, TX) in place of the RPE suspension.
Rabbits were examined 1, 3 and 5 days after surgery and then weekly for 2 months by confocal scanning laser ophthalmoscope (cSLO, Heidelberg Engineering, Heidelberg, Germany) and optical coherence tomography (OCT, Zeiss, Germany). The cSLO provided 820nm, 790nm, and 488 nm illumination. We examined the fluorescence of CFSE with 488nm illumination without injecting other dyes under cSLO. Fundus fluorescein angiography (FFA) and indocyanine green angiography (ICGA) were performed simultaneously with a cSLO double-detection system. The dyes were injected into an ear vein in one bolus containing 0.2ml fluorescein (100mg/ml) and 0.7ml ICG (4.2mg/ml). The timer was started immediately when the dyes were injected, and sequential photographs (30 images per second) were recorded in computer for approximately 30 min. We monitored the fluorescein and indocyanine green with 488nm and 790nm illumination. Angiography was performed at 2 weeks and monthly thereafter.
The rabbit eyes were enucleated after the rabbit was anesthetized and euthanatized. The eyes were immersed in liquid nitrogen. Sections were cut every 100μm serially and every section was 2μm thick. All the sections were examined by fluorescence microscopy.
Results
General properties of the newborn rabbit RPE cells
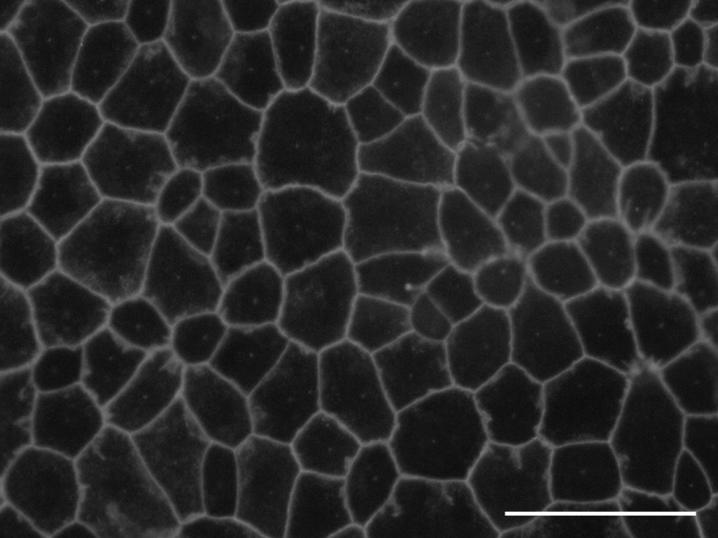
After 1-2 weeks, primary cultures of rabbit RPE cells formed a confluent, pigmented monolayer with the characteristic ‘cobblestone’ appearance typical of a simple epithelium (Fig. 1). The nuclei and cell borders were prominent, because of the density of melanin granules.
Figure 1.
Primary cultures of newborn rabbit RPE exhibited an epitheloid morphology. Freshly isolated RPE was plated and examined by phase contrast microscopy after 10 days of culture, when the cultures were confluent. The RPE exhibited defined cell borders, because of the density of melanin granules, and an overall ‘cobblestone’ appearance. Bar, 50μm.
The purity of the cultures was established by examining the expression of epithelial cytokeratins and the melanocyte maker S-100.41 Normally, RPE expresses cytokeratins-5, 6, 7, 8, 14, 15, 16, 17, 18, and 19. The antibody, MNF116, binds a subset of these, cytokeratins 5, 6, 8, 17 and 19, but none of this subset are expressed in iris pigment epithelium (IPE) cells.41-44 Indirect immunocytochemistry revealed that one or more of these cytokeratins were expressed by virtually all the cells (Fig. 2 A). S-100 is normally expressed by melanocytes, but not fibroblasts. All cells in the culture were positive for S-100, as determined by the antibody SH-B1 (Fig. 2 B). There was no evidence of contamination by fibroblasts or IPE.
Figure 2.
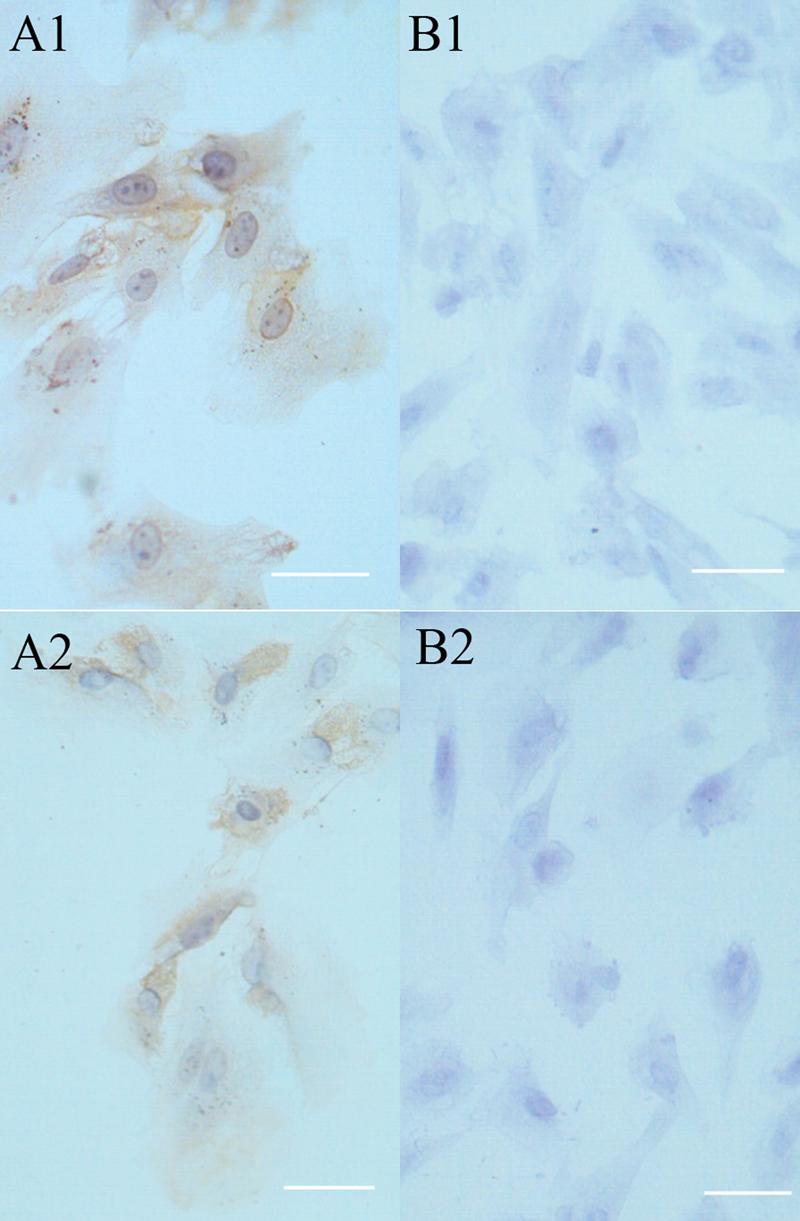
Secondary cultures of newborn rabbit RPE cells expressed cytokeratin and S-100. RPE cells (passage 4) were plated on slides at a density of 5-10×104 cells per 1ml and cultured for 2 days. The cells were prepared for immunocytochemistry to determine whether the cells expressed RPE-specific cytokeratins using the monoclonal antibody MNF116 (A1) or S-100 using the monoclonal antibody of SH-B1 (B1). In panels (A2) and (B2), the primary antibody was omitted. The cells were counterstained with hematoxylin (blue) and observed by bright field. In each case, the brown immunostain was observed in virtually all of the cells. Bar, 25 μm.
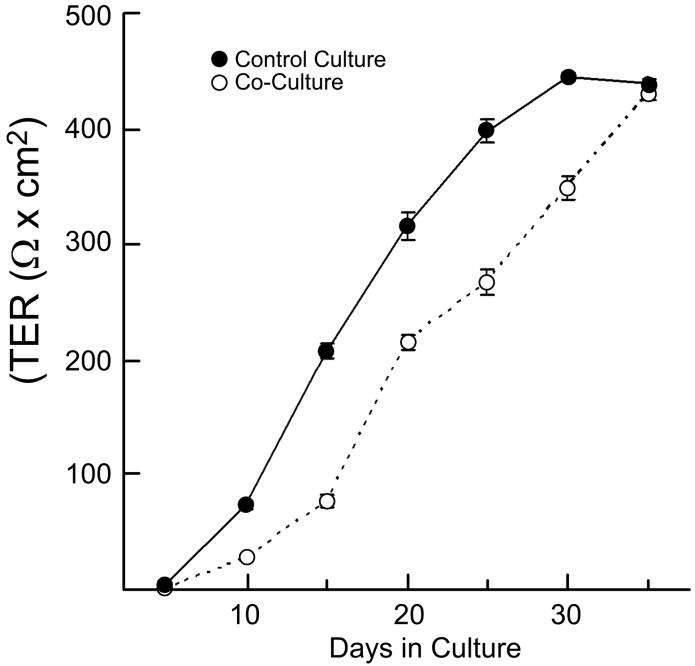
The formation of tight junctions is a highly differentiated property that is difficult to achieve in culture.45 The localization of ZO-1 and the TER were used to assess the formation of tight junctions. Primary cultures of RPE were established on polycarbonate filters. In quiescent monolayers, ZO-1 localized to continuous, circumferential bands along cell-cell contacts (Fig. 3). Although commonly used to establish the presence of tight junctions, this distribution of ZO-1 is a necessary, but insufficient, criterion.37, 46, 47 The presence of functional tight junctions was evident by the high TER. The TER of the primary cultures was recorded periodically for 5 weeks post-plating. The TER began to rise on day 5 and reached a plateau of 400-450 Ω-cm2 after 4 weeks in culture (unlabeled, control culture, Fig. 4). With cell passage, the ability of the RPE to establish a high TER diminished. Secondary cultures (passages 2-4) established a TER of only 150 Ω-cm2. Further, microscopy revealed that spindle shaped cells were intermixed with clusters of polygonal cells (data not shown). Cultures of freshly isolated adult RPE contained a large percentage of spindle shaped cells and only occasionally attained a TER as high as 50 Ω-cm2 (data not shown). These data indicate that functional tight junctions had formed in primary cultures of newborn rabbit RPE, but that properties of highly differentiated RPE diminished with cell passage and were never attained by adult RPE.
Figure 3.
ZO-1 distributed to circumferential bands where neighboring cells abutted in confluent cultures. Cells, freshly isolated from newborn rabbits, were cultured for 30 days on Transwell filters and stained for ZO-1 by indirect immunofluorescence. The ‘cobblestone’ appearance was typical of an epithelial monolayer. Bar, 50μm
Figure 4.
In vivo-like levels of the transepithelial electrical resistance (TER) were attained by primary cultures of newborn rabbit RPE. The TER was monitored at the indicated times for cultures of unlabeled RPE (filled symbols) or co-cultures of CFSE-labeled and unlabeled rabbit RPE (open symbols). Each culture achieved a high TER, indicating the formation of tight junctions. Each data point is the average of 3 cultures. Data are plotted as mean ± sd. Some error bars are smaller than the symbol.
Optimal conditions for newborn rabbit RPE cells labeling
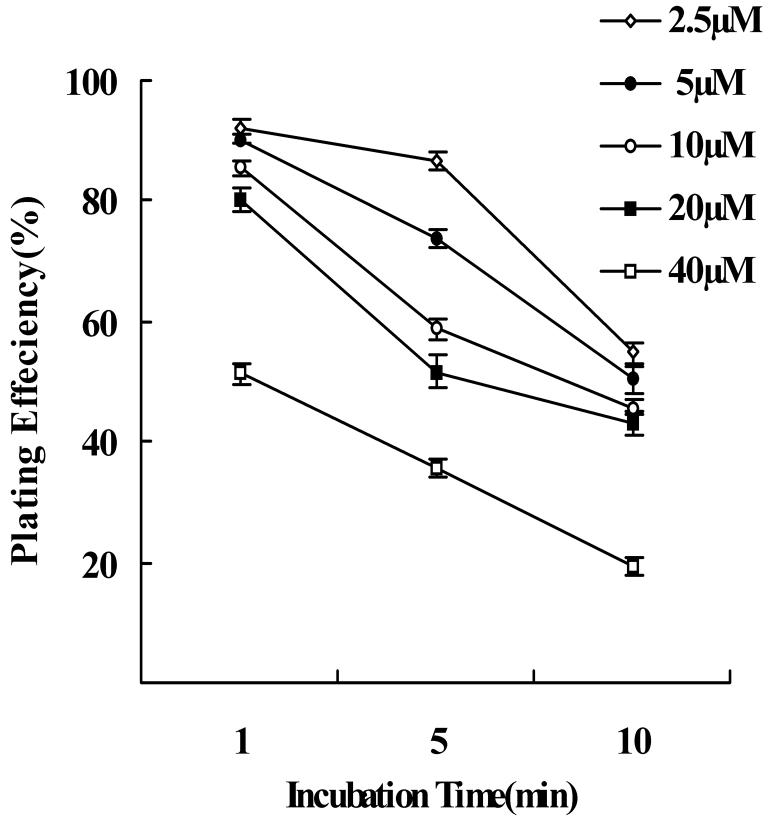
The optimal conditions for labeling RPE with CFDA-SE were determined using fluorescence microscopy before and after cell plating. To balance strong labeling with cell viability, we sought the lowest concentration of CFDA-SE and the shortest incubation time that labeled the cells well. The experiments were repeated 4 times, using secondary cultures of newborn rabbit RPE. Cell labeling occurred rapidly with an intense green fluorescence signal that was evenly distributed within the cytoplasm and the nucleus. Some differences were observed among the different CFDA-SE concentrations and different incubation periods. We graded and tabulated the fluorescence signal using a scale of 0 to 4 (0, no fluorescence; 1, just detectable; 2, distinct; 3, strong; 4, very strong) (Table 1). RPE was optimally labeled with 20 or 40 μM CFDA-SE, as compared to 2.5, 5 or 10μM. Although cell viability, by trypan blue, was unaffected by 20 or 40 μM CFDA-SE for all incubation times (P<0.05), the efficiency of cell-plating decreased with increasing CFDA-SE concentration and incubation time (Fig. 5). The decrease was statistically significant for both increasing concentration (P<0.01) and increasing incubation time (P<0.01). As an optimal protocol, 1 minute incubation at 37°C with a concentration of 20μM of CFDA-SE was selected for further in vitro and in vivo experiments (Fig. 6 A,B).
TABLE 1.
Fluorescence intensity of CFSE labeled cells, as a function of labeling conditions.
| CFDA-SE Concentration (μM) |
Incubation time (minute) |
||
|---|---|---|---|
| 1 | 5 | 10 | |
| control | 0 | 0 | 0 |
| 2.5 | 1 | 1 | 1 |
| 5 | 1 | 2 | 2 |
| 10 | 2 | 2 | 3 |
| 20 | 3 | 3 | 4 |
| 40 | 3 | 4 | 4 |
Grading scale: 0, no fluorescence; 1, just detectable; 2, distinct; 3, strong; 4, very strong. The experiment was performed in triplicate.
Figure 5.
Plating efficiency of newborn rabbit RPE cells following CFDA-SE labeling. The plating efficiency of RPE cells decreased with increasing incubation time in all concentrations of CFDA-SE. Following 10 minutes of incubation the plating efficiency decreased significantly at all dye concentrations. There was also significant decreased plating efficiency at concentrations 40μM compared to 2.5, 5, 10 and 20μM following 1 minute incubation (one-way ANOVA, P>0.05). Each point in the graph represents the mean percentage of plated RPE cells, after 24 hours. Error bars indicate the standard error.
Figure 6.

CFSE was not transferred between labeled and unlabeled cells. (A) The suspended newborn rabbit RPE cells were labeled by CFDA-SE for 1 minute at 37°C with 20 μM of dye. The cells expressed bright and uniform green fluorescence 4 hours after labeling. (B) The labeled newborn rabbit RPE cells were plated and attached to the plate by 24 hours. (C) Leakage of CFSE and reuptake by neighboring cells was tested using co-cultures of CFDA-SE labeled newborn rabbit RPE cells and human RPE cells. After 48 hours in culture, human RPE cells were labeled with a human specific hTNFR-M1 antibody (red) and did not take up any CFSE fluorescence (green) in their cytoplasm. Bars, 50μm.
Characterization of labeled RPE in vitro
To determine if the label from cells that did not survive replating could be phagocytised or if label could be transferred from cell to cell by some other mechanism, we adapted the procedure of Li et. al.35 RPE was isolated from human and newborn rabbit eyes. The rabbit RPE was labeled with CFDA-SE and co-cultured with the human RPE. The human RPE was identified using an antibody that was specific for the human TNF receptor, hTNFR-M1. After 24 hours, the cultures were fixed and labeled for hTNFR-M1. All of the cells in the culture exhibited either green or red fluorescence (Fig. 6 C). None of the cells were double-labeled, even when rabbit and human RPE cells were in direct contact. There was no evidence of CFSE leaking from labeled newborn rabbit RPE with subsequent uptake by adult human RPE.
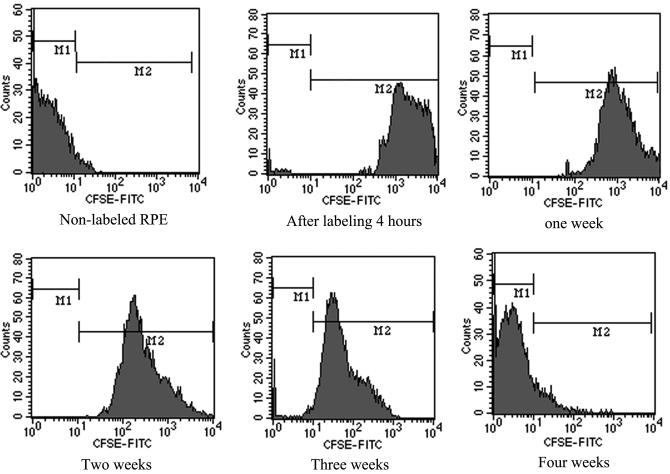
In quiescent cultures, the CFSE label was stable but appeared to associate with the nucleus and perhaps other stable structures, as has been described for other cells28 (for example, see Fig. 8 below). To determine how long cell labeling could be detected in a proliferating culture, cells were plated at low density and at the appropriate time, analyzed by fluorescence-activated flow cytometry. With each cell division, the label was diluted, as it was divided between daughter cells. To maintain the cultures in log phase growth, the cells were passaged before they reached confluence. At the indicated time points, a fraction of the cells was analyzed by flow cytometry (Fig. 7). Initially, 99.05% of the cells were labeled. The intensity of CFDA-SE fluorescence was high in each cell during the first week, and gradually decreased thereafter. By 4 weeks, after approximately 20 cell doublings, the label was not detected in most cells.
Figure 8.

The CFSE signal persisted in quiescent cultures of RPE. Labeled and unlabeled newborn rabbit RPE were mixed 50:50 and cultured for 5 weeks. The cells were fixed and ZO-1 revealed by indirect immunofluorescence (red). The CFSE-labeled cells survived and integrated into a ‘cobblestone’ monolayer with the unlabeled cells. The fluorescence due to CFSE appeared to associate with the nucleus and perhaps other stable structures. ZO-1 expression demonstrated that the labeled RPE cells formed junctions with neighboring cells, regardless of whether they were labeled. Bar, 50μm.
Figure 7.
The CFSE signal was detected for up to four weeks in rapidly dividing cells. Labeled cells were repeatedly subcultured to maintain the cells in log-phase growth. At the indicated a portion of the cultures were analyzed using a fluorescence-activated cell sorter. The M1 pool identifies unlabeled cells. The M2 pool identifies labeled cells of varying intensity. The ratio of fluorescence cells in M2 gradually decreased to almost 10.11% and the average fluorescence intensity decreased to almost 0.02% during the course of the experiment.
To imitate transplantation in vitro, freshly isolated newborn rabbit RPE was split into two pools. One pool was labeled with CFDA-SE and then mixed 50:50 with the unlabeled pool. The TER of the co-cultured cells was recorded at several time-points over the next 5 weeks. The co-culture cells developed a resistance by day 14 that increased to more than 400 Ω×cm2 after 5 weeks (Fig. 4). By contrast, control cultures of pure unlabeled cells developed a TER by day 7 and attained a TER over 400 Ω×cm2 by 4 weeks.
Fluorescence microscopy demonstrated that the labeled RPE cells survived, but the fraction of fluorescing cells decreased to about 29.69% after 5 weeks of co-culture (Fig. 8). Although the fluorescence in cytoplasm of the labeled RPE cells became faint, the fluorescence in nuclear-like structures was bright. Some cells also exhibited bright spherical bodies in the cytoplasm. ZO-1 distributed to continuous junctions between each cell, indicating that labeled and unlabeled RPE cells formed an integrated monolayer, as confirmed by the high TER. The decrease in the percentage of labeled cells by roughly 40% and the lag in the formation of a confluent monolayer, as assessed by the TER, suggest that the plating efficiency or rate of propagation for the labeled cells was lower than the control cells. Nonetheless, the labeled cells were able to incorporate into a highly functional monolayer, as evidenced by the high TER. The labeling protocol had no obvious effects on the morphology of the RPE, nor the expression of the MNF116 and SH-B1 antigens, as determined by immunocytochemistry (data not shown).
Retinal examination after transplantation
The labeled newborn rabbit RPE cells were transplanted into the subretinal space of pigmented rabbit eyes. The transplantation was successful in 12 of 14 attempts. Color fundus photography showed the transplantation entrance and the round elevated region clearly (Fig. 9). One day after transplantation the transplant site could be detected by cSLO (Fig. 10 B1). By OCT imaging, the transplanted suspension appears as the dark region under the neural retina (Fig. 10 B2). The retinal elevation was evident for nearly a week. Thereafter, the fluorescence due to CFSE became bright and distinct (Fig. 10 C1), likely because the detached retina reattached to the retinal pigment epithelium (Fig. 10 C2). At 1 month, the donor RPE cells appear to be distributed evenly in the host subretinal space (Fig. 10 D1). OCT at 1 month demonstrated that the donor RPE cells had already formed a layer on the host RPE layer (Fig. 10 D2). After 2 months, the fluorescence due to CFSE diminished, but was still detectable. Nonetheless, the distribution of labeled cells appeared to be unchanged between months one and two (Fig. 10 E).
Figure 9.
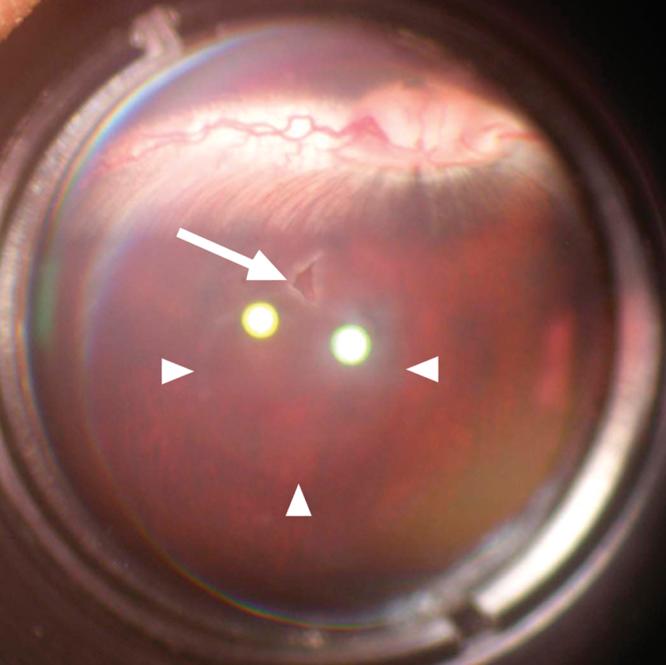
A transplant site in rabbit retina seen by fundus photography through a hand-held 90D preset lens 1 day after surgery. The transplant site (arrow) and the extent of the retinal elevation (arrowheads) are evident.
Figure 10.

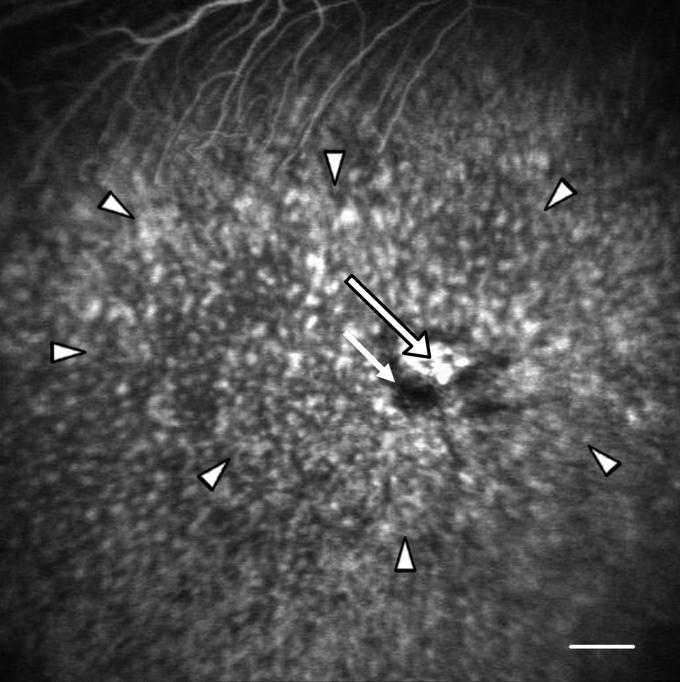
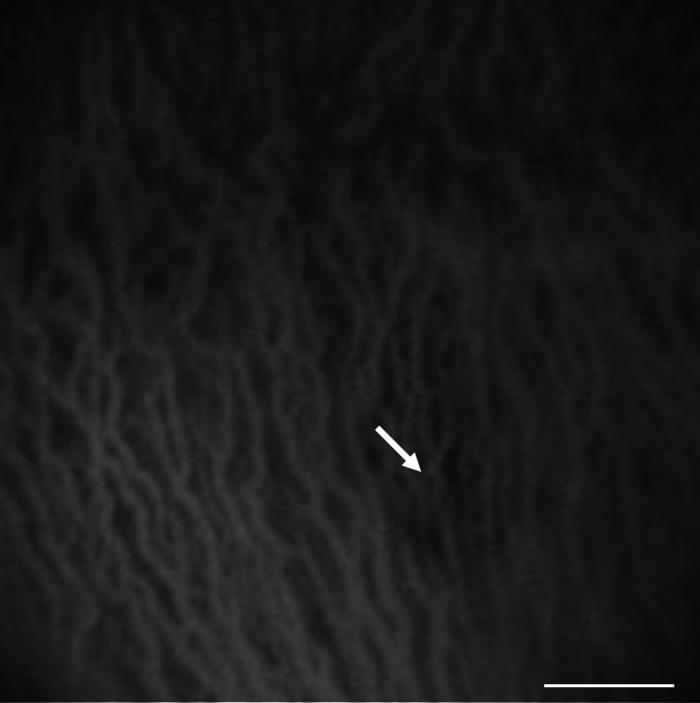
The labeled RPE could be observed and the overlying retina assessed by cSLO and OCT photography. The data presented were collected from the same rabbit. The apparent variability in the thickness of the retina was due to the difficulty in precisely repositioning the instrument from time point to time point. Retinas were examined pre-transplantation (A) or 1 day (B), 1 week (C), 1 month (D) and 2 months (E) post-transplantation by cSLO (1) or OCT (2). (A1) No fluorescent signal can be observed by cSLO. (A2) The normal RPE (white arrow) and neural retina (red arrow) can be distinguished in the OCT image. (B1) White arrow shows the injection site; the region of the transplant was vague in the cSLO image. (B2) The OCT image shows the elevation of the neural retina (red arrow) and the transplanted RPE (white arrow). The host RPE layer was unbroken (arrowhead). (C1) At 1 week, the labeled RPE cells become evident (white arrow), as the retina reattached. (C2). The retina elevation is no longer evident and the transplanted cells could be detected (white arrow). (D1) After 1 month the fluorescence (white arrow) could be easily detected by cSLO. (D2) From the OCT image, the RPE cells of the host (arrowhead) and the transplanted RPE cells (white arrow) could be distinguished. In the region of the transplant, the thickness of the RPE layer obscured the resolution of the RPE layer and the underlying choroid that was evident near the host RPE (arrowhead). (E1) At 2 months after surgery, the region of the transplantation reduced in size and the intensity of fluorescence (white arrow) decreased. (E2) The retina regained its normal morphology (see panel A2) as the transplanted RPE integrated into the RPE monolayer (white arrow). Distinct neural retina, RPE and choroid layers were observed. Bar (A1-E1), 500 μm; Bar (A2-E2) 100 μm
The RPE and the choroidal and retinal vascular beds appeared to be intact, as assessed by FFA and ICGA. Fig. 11 shows the transplant site 1 month after surgery by FFA. The large vessels near the optic disc were filled with fluorescein. There was no tortuosity of vessels or leakage of the dye. A fluorescence defect could be detected around the transplantation entrance with hyperfluorescence in the center. The fluorescence defect may indicate scar formation at the transplantation entrance, whereas the hyperfluorescence in the center may indicate a high-density of CFSE-labeled RPE cells. The even distribution of punctate hyperfluorescence within the arrowheads of the image indicates the distribution of the transplanted cells and was evident prior to the intravenous injection of fluorescein. There was no evidence of a leak in the RPE monolayer. The choroidal vasculature was also intact, as seen by ICGA (Fig. 12). Under this illumination, CFSE would not be evident. The center of the transplantation site was a little darker than the other regions, which may indicate multilayering of the RPE.
Figure 11.
The retinal vascular bed was intact 1 month after RPE transplantation, as assessed by FFA. The region of hyperfluorescence (long arrow) with a fluorescence defect (short arrow) around it shows the transplantation entrance. The arrowheads indicate the extent of the transplant. The bright punctate signal within this region is due to the CFSE signal from the transplanted cells. The normal filling of the vessels (top of image) by the intravenous fluorescein indicates there was no leakage from the retinal vascular bed. There was no evidence of a fluorescein leak across the RPE layer inside or outside the region of the transplant. Bar, 500 μm
Figure 12.
The choroidal vascular bed was intact 1 month after RPE transplantation, as assessed by ICGA. There was no disturbance of the choroidal vessels, because there was no evidence of leakage of ICG. The slightly darker center of the transplantation site (white arrow) may indicate a multilayer of transplanted RPE. Bar, 500 μm
Histopathologic examination of the graft site was performed after hematoxylin and eosin staining (Fig. 13). The retina appeared to be intact. No accumulated debris was evident in the subretinal space. A layer of pigmented cells was evident in the site of the RPE. When viewed by fluorescence microscopy, this layer corresponded to cells labeled by CFSE (Fig. 14). The layer of labeled cells appeared to be multilayer or composed of enlarged cells. The label was punctate, similar to the spherical bodies exhibited in some cultured cells. This layer was co-linear with native RPE, which appeared black because of the quenching of background fluorescence.
Figure 13.
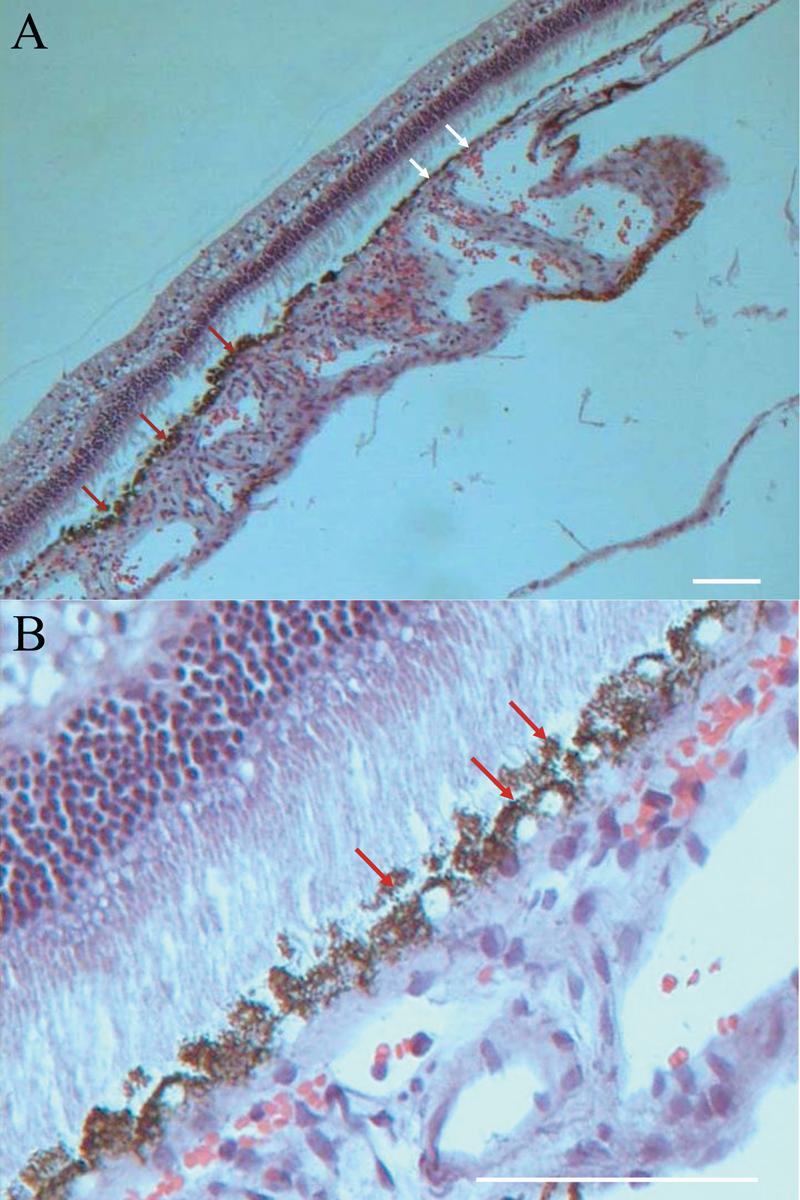
The neural retina that overlay the transplant had a normal morphology. Red arrows show several layers of the RPE clearly under the light microscope. (A) The grafted cells survived with numerous pigment granules in the cytoplasm 2 months after transplantation. White arrows show the normal monolayer of the host RPE. The apparent retinal detachment was an artifact of processing. (B) A higher magnification of the transplantation site shows a bilayer of RPE. The outer nuclear layer was intact and no debris accumulated in the subretinal space. Bars, 100 μm
Figure 14.
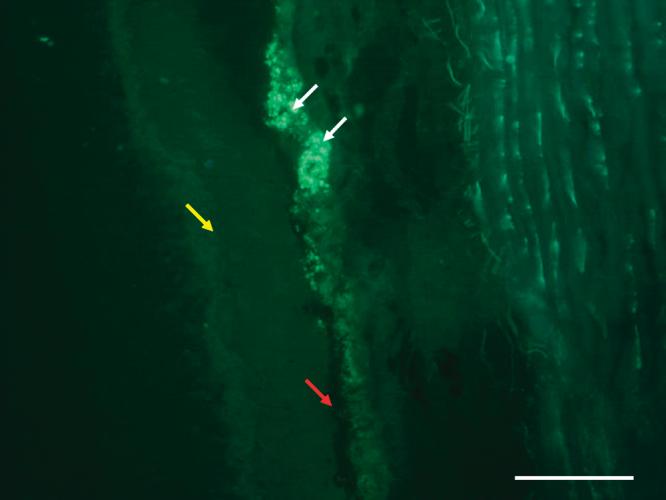
The green fluorescence of CFSE could be seen clearly under the fluorescence microscope on frozen sections. White arrows show the fluorescence of CFDA-SE- labeled RPE cells in subretinal space. The black retinal pigment epithelium of the host (red arrow) and the neural retina (yellow arrow) could be observed clearly. Bar, 100 μm
Discussion
RPE transplantation is a promising therapeutic strategy for the treatment of retinal degenerations and dystrophies that affect RPE function. To build on previous advances, an animal model is needed that is reliable, easy to work with, and employs techniques that can be readily translated to the bedside. Rodent eyes are too small to model human surgery and primates are too expensive to rigorously evaluate the cell biology and physiology of the transplanted RPE. Without an appropriate experimental model, evaluation of human transplantation is limited by the inability to understand what factors make transplantation succeed and what factors limit success. The rabbit model and labeling protocol that we propose addresses these issues. Specifically, rabbit eyes are large enough to develop surgical techniques that can be translated to human eyes. The labeling protocol makes it easy to evaluate the viability and functionality of the transplanted cells.
It is well known that RPE often dedifferentiates in culture, but it is unclear if cultured cells fully redifferentiate when returned to their native environment. Obviously, cell culture is required to expand the number of cells available for transplantation. To explore the effects of differentiation in culture, we sought protocols that result in highly differentiated cells. The most successful reports in the literature rely on RPE isolated from developing eyes and cultured in highly specialized media.47-51 Although these primary cultures are useful for experimentation, secondary cultures derived from them lose various properties of differentiated RPE, and therefore, might be suboptimal for transplantation. In the current study, primary cultures of the newborn rabbit RPE exhibited a cobblestone appearance and circumferential bands of ZO-1. The TER is a more stringent criterion for RPE differentiation, because it determines whether ZO-1 and other junctional proteins have been assembled into a functional tight junction. With the appearance of a high TER, the monolayer becomes an integrated unit that can form a blood-retinal barrier. Simple expression of tight junctional proteins does not guarantee that this transition has occurred.37 Indeed, it is common for cultured RPE to incorporate ZO-1 in a junctional complex that lacks tight junctions.45 Like young human RPE,52 we found that primary cultures of newborn rabbit RPE exhibited an in vivo like TER.53 Remarkably, serum-supplemented DMEM-F12 medium was sufficient for the RPE to differentiate. Like other animal models, the TER diminished with cell passage, and adult rabbit RPE never attained a high TER, even in primary culture (data not shown). By using cultures with different levels of differentiation, the rabbit model would test how differentiation in culture affects transplantation.
Various labeling and tracing techniques have been tried to evaluate the fate of transplanted RPE. Although DNA can be labeled with 3H-thymidine this label cannot be observed directly in vivo. Further, the label was not permanent and was unfortunately toxic to nearby cells.25 Bromodeoxyuridine also labels the nucleus and is also diluted with cell division. However, it is toxic and requires immunohistochemical methods for visualization.27 Carbon particles and natural pigment granules are readily observed in vivo, but they proved to be unreliable markers. Stressed RPE cells can release these markers and surrounding RPE cells may engulf them.2, 15-17, 19 Green fluorescent protein (GFP), introduced by retroviral-mediated gene transfer, can be seen in vivo. However, the fraction of cells expressing detectable GFP was low and variable.26 The cat Y chromosome probe identified transplanted RPE cells with high precision. However, the Y chromosome probe labeled only a fraction of the donor cells.54
The current study demonstrates that RPE cells can be easily and efficiently labeled with CFDA-SE without compromising function. Consistent with studies of other cell types.28, 55-57 CFDA-SE was not toxic at the concentrations used here. The labeled cells maintained their typical ‘cobblestone’ shape and uniform nuclei with high viability. The labeled cells integrated into an RPE monolayer without compromising the formation of functional tight junctions, as evidenced by the high TER.
The label could be detected in continuously propagating cell cultures for at least 4 weeks. By contrast, the fluorescent signal remained strong for over 5 weeks in quiescent cultures, even when the RPE was co-cultured with unlabeled cells. In co-culture experiments, labeled and unlabeled cell suspensions were mixed 50:50 and plated at high density. After 4 weeks, 30% of the cells were labeled, and there was a 1 week lag (relative to control cultures) in the establishment of a TER. These results suggest the labeling protocol or the label itself might reduce plating efficiency or lengthen the cell cycle. It appeared that once the cultures became confluent and the cells stopped proliferating, the proportion of labeled cells did not change for the remainder of the experiment. When transplanted into the rabbit subretinal space, the labeled cells could be followed for several months, consistent with previous observations that the retinal environment normally limits RPE proliferation.27, 54 Similarly, Hasbold et al.,32 found that labeled lymphocytes retained the label for at least 6 months.
In most of the cultured cells, the label appeared to be confined to the nucleus, consistent with reports in other cell types that the label covalently binds stable structures in the nucleus.28 In some cells, a bright punctate signal was found in the cytoplasm. The latter pattern of localization appeared to predominate in the transplanted RPE. The CFSE may have bound structures in the cytoplasm of RPE that don't turnover or turnover slowly, such as melanin granules or phagocytic bodies. Notably, the retina overlying the transplanted RPE displayed a normal morphology in histopathology, fundus exam and angiography. There was no evidence of atrophy of the outer nuclear layer or accumulation of debris in the subretinal space. These observations suggest that the transplanted RPE was functional and capable of phagocytizing shed outer segments.
We developed a novel transplantation method to overcome several disadvantages of earlier techniques. The external (posterior trans-scleral) approach requires the disinsertion of a rectus muscle and rotation of the globe, which makes it impossible to directly monitor the injection of the transplanted cells. Further, the choroid, the Bruch's membrane and the RPE are damaged.7, 58, 59 Previous internal (anterior transvitreal) approaches damage the sclera and the vitreous and require two or three ports, which increases the risk of injury.59, 60 The open sky approach causes trauma to the retina, vitreous, and anterior chamber.4 It would be difficult to translate these techniques to human.
The advantage of our technique is that it required only a single 30 gauge needle be introduced into the vitreous cavity. There was no need for a conjunctival flap and scleroectomy. Instead of a contact lens, we preferred using a 90D preset lens with the surgical microscope to make the visual field wider. By avoiding vitrectomy, the vitreous was not removed and only one port was required for the needle instead of three to accommodate a light source and irrigation. Further, the retinal entrance was sealed by a sterile air bubble, which prevented reflux of the transplanted cells into the vitreous. A similar technique can readily be used in humans. The injection volume of only 25μl was too small to significantly increase the intraocular pressure, and the scar at the injection site was small. The relative ease of the procedure led to a higher rate of success with fewer complications.
This model provides a means to test the efficacy of transplantation strategies. The strong, non-quenchable CFSE signal from the transplanted RPE was readily observed by cSLO. When combined with angiography, OCT and fundus photography, the fate of the grafts could be followed in the living animal for months. By using immunohistochemistry to reveal the expression and distribution of key proteins, it will be possible to determine whether functional interactions were re-established between the grafted cells and the choroid and neural retina. An important aspect is the relationship between the characteristics of the cultured cells and the efficacy of their transplantation. The efficacy of freshly isolated newborn rabbit RPE would be a benchmark for evaluating secondary cultures, RPE derived from adults, different methods of culturing RPE and RPE transplanted in sheets versus in cell suspension. These experiments might determine the extent to which dedifferentiation can occur in culture and still be reversed when the RPE is reintroduced to its normal environment. The analysis of redifferentiation would have to include the major functions of the RPE: phagocytosis, retinoid metabolism and the establishment of a blood-retinal barrier. The rabbit model promises to be a bridge between laboratory investigation of these issues and clinical therapy. One goal of such an approach would be to identify the markers that are important for evaluating the quality of RPE cultures prior to transplantation.
Acknowledgements
This work was supported by National Natural Science foundation of China NO: 30271395 and NO: 30772381(SP) and the Heilongjiang Natural Science Funds NO:D0241 (SP) and NIH EY EY008694 (LJR). The authors thank Dr. Ron Adelman (Yale University ) for helpful discussions.
References
- 1.West SK. Looking forward to 20/20: a focus on the epidemiology of eye diseases. Epidemiol Rev. 2000;22:64–70. doi: 10.1093/oxfordjournals.epirev.a018025. [DOI] [PubMed] [Google Scholar]
- 2.Aramant RB, Seiler MJ, Ball SL. Successful cotransplantation of intact sheets of fetal retina with retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:1557–64. [PubMed] [Google Scholar]
- 3.Crafoord S, Algvere PV, Kopp ED, Seregard S. Cyclosporine treatment of RPE allografts in the rabbit subretinal space. Acta Ophthalmol Scand. 2000;78:122–9. doi: 10.1034/j.1600-0420.2000.078002122.x. [DOI] [PubMed] [Google Scholar]
- 4.Gouras P, Flood MT, Kjedbye H, Bilek MK, Eggers H. Transplantation of cultured human retinal epithelium to Bruch's membrane of the owl monkey's eye. Curr Eye Res. 1985;4:253–65. doi: 10.3109/02713688509000857. [DOI] [PubMed] [Google Scholar]
- 5.Lane C, Boulton M, Marshall J. Transplantation of retinal pigment epithelium using a pars plana approach. Eye. 1989;3(Pt 1):27–32. doi: 10.1038/eye.1989.4. [DOI] [PubMed] [Google Scholar]
- 6.Li L, Turner JE. Optimal conditions for long-term photoreceptor cell rescue in RCS rats: the necessity for healthy RPE transplants. Exp Eye Res. 1991;52:669–79. doi: 10.1016/0014-4835(91)90019-b. [DOI] [PubMed] [Google Scholar]
- 7.Li LX, Turner JE. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988;47:911–7. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- 8.Little CW, Castillo B, DiLoreto DA, Cox C, Wyatt J, del Cerro C, et al. Transplantation of human fetal retinal pigment epithelium rescues photoreceptor cells from degeneration in the Royal College of Surgeons rat retina. Invest Ophthalmol Vis Sci. 1996;37:204–11. [PubMed] [Google Scholar]
- 9.Little CW, Cox C, Wyatt J, del Cerro C, del Cerro M. Correlates of photoreceptor rescue by transplantation of human fetal RPE in the RCS rat. Exp Neurol. 1998;149:151–60. doi: 10.1006/exnr.1997.6642. [DOI] [PubMed] [Google Scholar]
- 10.Lopez R, Gouras P, Kjeldbye H, Sullivan B, Reppucci V, Brittis M, et al. Transplanted retinal pigment epithelium modifies the retinal degeneration in the RCS rat. Invest Ophthalmol Vis Sci. 1989;30:586–8. [PubMed] [Google Scholar]
- 11.Seaton AD, Sheedlo HJ, Turner JE. A primary role for RPE transplants in the inhibition and regression of neovascularization in the RCS rat. Invest Ophthalmol Vis Sci. 1994;35:162–9. [PubMed] [Google Scholar]
- 12.Algvere PV, Berglin L, Gouras P, Sheng Y, Kopp ED. Transplantation of RPE in age-related macular degeneration: observations in disciform lesions and dry RPE atrophy. Graefes Arch Clin Exp Ophthalmol. 1997;235:149–58. doi: 10.1007/BF00941722. [DOI] [PubMed] [Google Scholar]
- 13.Binder S, Stanzel BV, Krebs I, Glittenberg C. Transplantation of the RPE in AMD. Prog Retin Eye Res. 2007;26:516–54. doi: 10.1016/j.preteyeres.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 14.da Cruz L, Chen FK, Ahmado A, Greenwood J, Coffey P. RPE transplantation and its role in retinal disease. Prog Retin Eye Res. 2007;26:598–635. doi: 10.1016/j.preteyeres.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Braun M, Kage A, Heimann K, Schraermeyer U. Retinal pigment epithelial cells from Royal College of Surgeons dystrophic rats can take up melanin granules. Graefes Arch Clin Exp Ophthalmol. 1999;237:67–71. doi: 10.1007/s004170050196. [DOI] [PubMed] [Google Scholar]
- 16.Crafoord S, Algvere PV, Seregard S, Kopp ED. Long-term outcome of RPE allografts to the subretinal space of rabbits. Acta Ophthalmol Scand. 1999;77:247–54. doi: 10.1034/j.1600-0420.1999.770301.x. [DOI] [PubMed] [Google Scholar]
- 17.Ye J, Wang HM, Ogden TE, Ryan SJ. Allotransplantation of rabbit retinal pigment epithelial cells double-labelled with 5-bromodeoxyuridine (BrdU) and natural pigment. Curr Eye Res. 1993;12:629–39. doi: 10.3109/02713689309001842. [DOI] [PubMed] [Google Scholar]
- 18.Lund RD, Kwan AS, Keegan DJ, Sauve Y, Coffey PJ, Lawrence JM. Cell transplantation as a treatment for retinal disease. Prog Retin Eye Res. 2001;20:415–49. doi: 10.1016/s1350-9462(01)00003-9. [DOI] [PubMed] [Google Scholar]
- 19.Shiragami C, Matsuo T, Shiraga F, Matsuo N. Transplanted and repopulated retinal pigment epithelial cells on damaged Bruch's membrane in rabbits. Br J Ophthalmol. 1998;82:1056–62. doi: 10.1136/bjo.82.9.1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dunn KC, Aotaki-Keen AE, Putkey FR, Hjelmeland LM. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–69. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- 21.Gabrielian K, Oganesian A, Patel SC, Verp MS, Ernest JT. Cellular response in rabbit eyes after human fetal RPE cell transplantation. Graefes Arch Clin Exp Ophthalmol. 1999;237:326–35. doi: 10.1007/s004170050240. [DOI] [PubMed] [Google Scholar]
- 22.Gouras P, Algvere P. Retinal cell transplantation in the macula: new techniques. Vision Res. 1996;36:4121–5. doi: 10.1016/s0042-6989(96)00180-0. [DOI] [PubMed] [Google Scholar]
- 23.Jiang LQ, Jorquera M, Streilein JW, Ishioka M. Unconventional rejection of neural retinal allografts implanted into the immunologically privileged site of the eye. Transplantation. 1995;59:1201–7. [PubMed] [Google Scholar]
- 24.Zhang X, Bok D. Transplantation of retinal pigment epithelial cells and immune response in the subretinal space. Invest Ophthalmol Vis Sci. 1998;39:1021–7. [PubMed] [Google Scholar]
- 25.Gouras P, Lopez R, Brittis M, Kjeldbye H. The ultrastructure of transplanted rabbit retinal epithelium. Graefes Arch Clin Exp Ophthalmol. 1992;230:468–75. doi: 10.1007/BF00175936. [DOI] [PubMed] [Google Scholar]
- 26.Lai CC, Gouras P, Doi K, Lu F, Kjeldbye H, Goff SP, et al. Tracking RPE transplants labeled by retroviral gene transfer with green fluorescent protein. Invest Ophthalmol Vis Sci. 1999;40:2141–6. [PubMed] [Google Scholar]
- 27.Morstyn G, Pyke K, Gardner J, Ashcroft R, de Fazio A, Bhathal P. Immunohistochemical identification of proliferating cells in organ culture using bromodeoxyuridine and a monoclonal antibody. J Histochem Cytochem. 1986;34:697–701. doi: 10.1177/34.6.3517148. [DOI] [PubMed] [Google Scholar]
- 28.Dumitriu IE, Mohr W, Kolowos W, Kern P, Kalden JR, Herrmann M. 5,6-carboxyfluorescein diacetate succinimidyl ester-labeled apoptotic and necrotic as well as detergent-treated cells can be traced in composite cell samples. Anal Biochem. 2001;299:247–52. doi: 10.1006/abio.2001.5415. [DOI] [PubMed] [Google Scholar]
- 29.Paramore CG, Turner DA, Madison RD. Fluorescent labeling of dissociated fetal cells for tissue culture. J Neurosci Methods. 1992;44:7–17. doi: 10.1016/0165-0270(92)90108-p. [DOI] [PubMed] [Google Scholar]
- 30.Weston SA, Parish CR. New fluorescent dyes for lymphocyte migration studies. Analysis by flow cytometry and fluorescence microscopy. J Immunol Methods. 1990;133:87–97. doi: 10.1016/0022-1759(90)90322-m. [DOI] [PubMed] [Google Scholar]
- 31.Fujioka H, Hunt PJ, Rozga J, Wu GD, Cramer DV, Demetriou AA, et al. Carboxyfluorescein (CFSE) labelling of hepatocytes for short-term localization following intraportal transplantation. Cell Transplant. 1994;3:397–408. doi: 10.1177/096368979400300506. [DOI] [PubMed] [Google Scholar]
- 32.Hasbold J, Gett AV, Rush JS, Deenick E, Avery D, Jun J, et al. Quantitative analysis of lymphocyte differentiation and proliferation in vitro using carboxyfluorescein diacetate succinimidyl ester. Immunol Cell Biol. 1999;77:516–22. doi: 10.1046/j.1440-1711.1999.00874.x. [DOI] [PubMed] [Google Scholar]
- 33.Groszer M, Erickson R, Scripture-Adams DD, Lesche R, Trumpp A, Zack JA, et al. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 2001;294:2186–9. doi: 10.1126/science.1065518. [DOI] [PubMed] [Google Scholar]
- 34.Gruber HE, Leslie KP, Ingram JA, Hanley EN., Jr Optimization of 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester for labeling human intervertebral disc cells in vitro. Biotech Histochem. 2000;75:118–23. doi: 10.3109/10520290009066489. [DOI] [PubMed] [Google Scholar]
- 35.Li X, Dancausse H, Grijalva I, Oliveira M, Levi AD. Labeling Schwann cells with CFSE-an in vitro and in vivo study. J Neurosci Methods. 2003;125:83–91. doi: 10.1016/s0165-0270(03)00044-x. [DOI] [PubMed] [Google Scholar]
- 36.Del Priore LV, Tezel TH, Kaplan HJ. Survival of allogeneic porcine retinal pigment epithelial sheets after subretinal transplantation. Invest Ophthalmol Vis Sci. 2004;45:985–92. doi: 10.1167/iovs.03-0662. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Zhuo Y, Fukuhara M, Rizzolo LJ. Effects of culture conditions on heterogeneity and the apical junctional complex of the ARPE-19 cell line. Invest Ophthalmol Vis Sci. 2006;47:3644–55. doi: 10.1167/iovs.06-0166. [DOI] [PubMed] [Google Scholar]
- 38.Peng S, Rahner C, Rizzolo LJ. Apical and basal regulation of the permeability of the retinal pigment epithelium. Invest Ophthalmol Vis Sci. 2003;44:808–17. doi: 10.1167/iovs.02-0473. [DOI] [PubMed] [Google Scholar]
- 39.Gonzalez-Mariscal L, Chavez de Ramirez B, Cereijido M. Effect of temperature on the occluding junctions of monolayers of epithelioid cells (MDCK) J Membr Biol. 1984;79:175–84. doi: 10.1007/BF01872121. [DOI] [PubMed] [Google Scholar]
- 40.Hunt RC, Dewey A, Davis AA. Transferrin receptors on the surfaces of retinal pigment epithelial cells are associated with the cytoskeleton. J Cell Sci. 1989;92(Pt 4):655–66. doi: 10.1242/jcs.92.4.655. [DOI] [PubMed] [Google Scholar]
- 41.Hu DN, Ritch R, McCormick SA, Pelton-Henrion K. Isolation and cultivation of human iris pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:2443–53. [PubMed] [Google Scholar]
- 42.Hollborn M, Kohen L, Wiedemann P, Enzmann V. The influence of pro-inflammatory cytokines on human retinal pigment epithelium cell receptors. Graefes Arch Clin Exp Ophthalmol. 2001;239:294–301. doi: 10.1007/s004170100263. [DOI] [PubMed] [Google Scholar]
- 43.McKechnie NM, Boulton M, Robey HL, Savage FJ, Grierson I. The cytoskeletal elements of human retinal pigment epithelium: in vitro and in vivo. J Cell Sci. 1988;91(Pt 2):303–12. doi: 10.1242/jcs.91.2.303. [DOI] [PubMed] [Google Scholar]
- 44.Thumann G, Bartz-Schmidt KU, El Bakri H, Schraermeyer U, Spee C, Cui JZ, et al. Transplantation of autologous iris pigment epithelium to the subretinal space in rabbits. Transplantation. 1999;68:195–201. doi: 10.1097/00007890-199907270-00006. [DOI] [PubMed] [Google Scholar]
- 45.Rizzolo LJ. Development and role of tight junctions in the retinal pigment epithelium. Int Rev Cytol. 2007;258:195–234. doi: 10.1016/S0074-7696(07)58004-6. [DOI] [PubMed] [Google Scholar]
- 46.Ban Y, Rizzolo LJ. A culture model of development reveals multiple properties of RPE tight junctions. Mol Vis. 1997;3:18. < http://www.molvis.org/molvis/v3/ban>. [PubMed] [Google Scholar]
- 47.Rahner C, Fukuhara M, Peng S, Kojima S, Rizzolo LJ. The apical and basal environments of the retinal pigment epithelium regulate the maturation of tight junctions during development. J Cell Sci. 2004;117:3307–18. doi: 10.1242/jcs.01181. [DOI] [PubMed] [Google Scholar]
- 48.Chang CW, Roque RS, Defoe DM, Caldwell RB. An improved method for isolation and culture of pigment epithelial cells from rat retina. Curr Eye Res. 1991;10:1081–6. doi: 10.3109/02713689109020348. [DOI] [PubMed] [Google Scholar]
- 49.Gamm DM, Melvan JN, Shearer RL, Pinilla I, Sabat G, Svendsen CN, et al. A novel serum-free method for culturing human prenatal retinal pigment epithelial cells. Invest Ophthalmol Vis Sci. 2008;49:788–99. doi: 10.1167/iovs.07-0777. [DOI] [PubMed] [Google Scholar]
- 50.Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Molecular Vision. 2001;7:14–9. < http://www.molvis.org/molvis/v7/a3/>. [PubMed] [Google Scholar]
- 51.Maminishkis A, Chen S, Jalickee S, Banzon T, Shi G, Wang FE, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–24. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu JG, Gallemore RP, Bok D, Lee AY, Frambach DA. Localization of NaK ATPase on cultured human retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1994;35:3582–8. [PubMed] [Google Scholar]
- 53.Gallemore RP, Hughes BA, Miller SS. Retinal pigment epithelial transport mechanisms and their contributions to the electroretinogram. Prog Ret Eye Res. 1997;16:509–66. [Google Scholar]
- 54.Wang H, Leonard DS, Castellarin AA, Tsukahara I, Ninomiya Y, Yagi F, et al. Short-term study of allogeneic retinal pigment epithelium transplants onto debrided Bruch's membrane. Invest Ophthalmol Vis Sci. 2001;42:2990–9. [PubMed] [Google Scholar]
- 55.De Clerck LS, Bridts CH, Mertens AM, Moens MM, Stevens WJ. Use of fluorescent dyes in the determination of adherence of human leucocytes to endothelial cells and the effect of fluorochromes on cellular function. J Immunol Methods. 1994;172:115–24. doi: 10.1016/0022-1759(94)90384-0. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka K, Koga Y, Taniguchi K, Kamikaseda K, Nomoto K. T cell recruitment from the thymus to the spleen in tumor-bearing mice. I. Analysis of recruited cells by surface markers. Cancer Immunol Immunother. 1986;22:37–42. doi: 10.1007/BF00205714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wongpichedchai S, Weiter JJ, Weber P, Dorey CK. Comparison of external and internal approaches for transplantation of autologous retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1992;33:3341–52. [PubMed] [Google Scholar]
- 58.Sheedlo HJ, Li LX, Turner JE. Functional and structural characteristics of photoreceptor cells rescued in RPE-cell grafted retinas of RCS dystrophic rats. Exp Eye Res. 1989;48:841–54. doi: 10.1016/0014-4835(89)90067-5. [DOI] [PubMed] [Google Scholar]
- 59.Thumann G, Aisenbrey S, Schraermeyer U, Lafaut B, Esser P, Walter P, et al. Transplantation of autologous iris pigment epithelium after removal of choroidal neovascular membranes. Arch Ophthalmol. 2000;118:1350–5. doi: 10.1001/archopht.118.10.1350. [DOI] [PubMed] [Google Scholar]
- 60.Wen J, McKenna KC, Barron BC, Langston HP, Kapp JA. Use of superparamagnetic microbeads in tracking subretinal injections. Mol Vis. 2005;11:256–62. [PubMed] [Google Scholar]