Abstract
Background. Icodextrin is a glucose polymer derived by hydrolysis of cornstarch. The different biocompatibility profile of icodextrin-containing peritoneal dialysis (PD) solutions may have a positive influence on peritoneal host defence. Furthermore, cases of sterile peritonitis potentially associated with icodextrin have been reported.
Methods. The primary objective of this multicentre, longitudinal, observational, non-interventional, prospective cohort study, which included 722 PD patients, was to evaluate the incidence of overall peritonitis in patients treated with icodextrin-containing PD solutions (Extraneal™) used during one long-dwell exchange/day compared with those treated with non-icodextrin-containing PD solutions. The secondary objective was to determine if culture-negative peritonitis rates differed between patients treated with icodextrin from two independent manufacturers. All peritonitis episodes were assessed by a Steering Committee in a blind manner.
Results. There was no significant difference between icodextrin-treated and control patients in the adjusted overall, culture-positive or culture-negative peritonitis rates. When stratified by the icodextrin supplier, there was no significant difference in the adjusted rate of culture-negative peritonitis episodes between groups.
Conclusion. Subjects receiving icodextrin as part of their PD regimen experienced neither a higher rate of culture-negative peritonitis nor a lower rate of infectious peritonitis compared with non-icodextrin users. There was no significant influence of the icodextrin raw material supplier on peritonitis rates.
Keywords: biocompatibility, glucose degradation products, glucose polymer, peptidoglycan, sterile peritonitis
Introduction
Icodextrin, a glucose polymer derived by hydrolysis of cornstarch, is used as an alternative osmotic agent in peritoneal dialysis (PD) solutions. Due to its high molecular weight and slow absorption, icodextrin provides sustained peritoneal ultrafiltration for up to 16 h by the mechanism of colloidal osmosis. It is, therefore, especially suitable for the nighttime dwell in patients on continuous ambulatory peritoneal dialysis (CAPD) and for the daytime dwell in patients on automated peritoneal dialysis (APD). Several studies report a significantly improved peritoneal fluid removal, increased clearances of small solutes and middle molecules, and a significant decrease of total body water when icodextrin is prescribed for the long dwell instead of conventional glucose solutions [1–4]. Accordingly, icodextrin has been used as a salvage therapy in PD patients with refractory volume overload who were otherwise about to be transferred to haemodialysis [5,6]. Furthermore, some, but not all, studies suggested a positive influence of icodextrin on hyperlipidaemia, on metabolic control in diabetic PD patients and on the course of residual renal function [3,6–11]. Because of these potential advantages, icodextrin is a dialysis solution widely prescribed to PD patients.
Among the most important side effects of icodextrin, skin rash [12,13] and sterile peritonitis [14] have to be considered. On the other hand, it has been questioned if, due to the lower content of glucose degradation products and the iso-osmolality with plasma, icodextrin may have a positive influence on peritoneal host defence [15–17]. Infectious peritonitis still constitutes one of the most important causes of morbidity, technique failure and mortality in PD patients [18–20]. The primary objective of this study was to investigate possible differences in peritonitis rates (both infectious and sterile) between PD patients treated with icodextrin and patients treated exclusively with non-icodextrin solutions. The secondary objective was to determine if there was a relationship between the incidence of culture-negative peritonitis and the icodextrin raw material supplier.
Subjects and methods
This multicentre, longitudinal, observational, non-inter- ventional prospective cohort study was conducted at 27 centres in seven European countries (Austria, Belgium, France, Germany, Spain, Switzerland and UK). The maximum observation period was 2 years (inclusion of patients lasted from 25 March 2003 to 22 December 2005). Both incident and prevalent PD patients (eligible age ≥18 and ≤ 75 years) treated either with CAPD or APD (with or without icodextrin) were included. Patients who had a past episode of cloudy effluent possibly associated with icodextrin or were only treated by one single dwell of icodextrin (which was not part of an APD or CAPD regimen) were excluded from this study. Patients currently on icodextrin after re-challenge were eligible. Prevalent patients had been on PD treatment for at least 3 months prior to study inclusion. Incident patients were defined as those who were on PD for <3 months at the time of inclusion. All patients provided written informed consent before inclusion. The study was conducted in accordance with the general ethical principles as expressed in the Declaration of Helsinki and in conformance with the International Conference on Harmonisation Guidelines for Good Clinical Practice. Furthermore, written approval of the Local Research Ethics Committee was obtained by local investigators when required.
Study design
Since this was an observational, non-interventional study, dialysis modality and solutions were chosen by the investigator according to the individual patient's needs. Based on their prescription at enrolment, patients were categorized as belonging to the icodextrin group or the control group. The icodextrin group consisted of patients who were prescribed icodextrin (Extraneal, Baxter Healthcare) once a day for the single long dwell (nighttime dwell in CAPD patients, daytime dwell in APD patients), with any other solutions prescribed for the remaining exchanges. Patients prescribed CAPD or APD without icodextrin constituted to the control group. All PD solutions used for treatment during the trial were obtained from routine commercial sources. Icodextrin raw material was provided by two suppliers (supplier A in Spain and Switzerland and supplier B in Austria, Belgium, France, Germany and UK). Prescription of concomitant medications was left to the physicians involved in patient care and was based on clinical requirements.
Study objectives
The primary objective of this study was to evaluate the incidence of overall peritonitis (infectious and sterile) in PD patients treated with icodextrin for one long dwell exchange per day as compared with patients treated exclusively with non-icodextrin solutions. The secondary objective was to evaluate if the incidence of culture-negative peritonitis between the icodextrin group and the control group differed, based on the two independent icodextrin suppliers.
Definition of peritonitis and peritonitis resolution
For the blinded data review by the study Steering Committee (see below), peritonitis was defined as a cloudy effluent with >100 leukocytes/mm3. Each peritonitis episode was further classified, depending on dialysate culture (culture positive or culture negative).
Resolution of peritonitis was defined as the date of the last dose of antibiotics (in cases where antibiotics were used), or the date the symptoms disappeared (in cases where no antibiotics were used), and/or the day the cell count was <100/mm3 (if available). Relapsing peritonitis was defined as an episode with the same organism or a sterile episode that occurred within 4 weeks of completion of antibiotic therapy of a prior peritonitis episode [21].
Since this was an observational study, diagnostic measures (including culture technique), choice of antibiotic therapy and duration of treatment of peritonitis were left to each investigator, dependent on each centre's standard practice.
Collection and analysis of data
In the case of peritonitis, the following data were collected for each patient: start and end dates of peritonitis episodes, clinical symptoms (e.g. fever, abdominal pain, nausea, diarrhoea), dialysate cell count, culture results, need for and duration of hospitalization, need for and type of antibiotic therapy, duration of therapy, time until peritonitis resolved, outcome of peritonitis and need for switch to haemodialysis. Besides peritonitis, no other adverse events had to be reported by investigators in the case report forms, but the investigators were asked to report serious adverse events per standard pharmacovigilance routine.
All data recorded on the case report forms were double entered into an electronic database using a clinical data management system. Computerized data-cleaning checks were used, together with manual reviews, to check for discrepancies and to ensure the consistency of the data. An electronic audit trail was used to track all data changes in the database.
A Steering Committee, comprising four independent investigators from four different countries participating in this study and three nephrologists of Baxter, reviewed all collected data and performed individual causality assessments for all peritonitis episodes. These members reviewed each peritonitis episode while blinded to both the interpretation of the other committee members and the solution used by the patient. The presence of Baxter nephrologists in the Steering Committee was considered to combine a local and specific (independent nephrologists) with a general and broad (Baxter nephrologists) perspective and overview of the peritonitis cases. Baxter nephrologists certainly had the most extensive experience with the icodextrin-associated sterile peritonitis as they have thoroughly reviewed all cases reported through the pharmacovigilance system to Baxter during these years as well as discussed a great deal of these cases personally with the involved nephrologists throughout Europe. Each of the independent nephrologists had both a long-term clinical experience with PD and their own clinical experience with icodextrin-associated sterile peritonitis episodes in their dialysis units. This combination of Baxter and independent nephrologists enabled us to bring depth and soundness to the review process. However, to be not decisive when the independent nephrologists agreed on their vote the three Baxter nephrologists had the right to only one combined vote. In contrast to this ‘agreed upon’ one vote by Baxter nephrologists, each independent nephrologist provided his/her own vote, resulting in a total of five votes per peritonitis episode.
During the two study Steering Committee meetings, a causal assessment was assigned to each peritonitis episode (for association with dialysis solution) based on the WHO causality definitions (the following definitions were used for classification: certain, probably/likely, possible, unlikely, conditional, not related, not assessable). If the culture of dialysate was positive the diagnosis was of an infectious peritonitis. Infectious peritonitis episodes were classified as not related to icodextrin. If the culture was negative but the patient presented with a clinical picture compatible with infectious peritonitis (fever, severe abdominal pain) and responded to antibiotic therapy, the episode was classified as culture-negative peritonitis not related to icodextrin. In the case of disagreement among members of the Steering Committee, the treatment blind was broken and the clinical assessment was discussed until a final decision was reached, based on majority vote (with Baxter nephrologists’ vote being counted as one vote only), and documented. The independent Steering Committee nephrologists were not paid for their committee work, but were only reimbursed for their travel expenses related to attending the two meetings.
Sample size calculation
Based on clinical reports and centre standards the average pre-study peritonitis rate among non-icodextrin-treated PD patients was estimated to be one episode every 28 patient months. Assuming an annual dropout rate of 20% (40% in 2 years), a power of 80% and a type I error rate of 0.025 (which was chosen to allow for sub-group analyses), it was estimated that a sample of 250 patients in the non-icodextrin arm and 500 patients in the icodextrin arm would be needed to detect an overall peritonitis rate increase of just over 50% in the icodextrin arm. To achieve these sample sizes, a 2:1 sampling ratio of icodextrin to non-icodextrin users had to be selected. To minimize any bias during inclusion of prevalent patients, investigators were asked to select patients with the shortest interval between the start of PD and study enrolment (minimum 3 months) first, followed by patients with a longer PD history. When assuming that icodextrin-treated patients were distributed equally between both icodextrin suppliers, a sample size of 500 patients in the icodextrin arm (250 from each manufacturer) was also determined as necessary to detect, with an estimated power of 70%, a 56% rate increase in peritonitis rate. Therefore, a total of 750 patients were estimated as an adequate sample size with a target goal of accruing ∼600 prevalent patients and ∼150 incident patients. No sample size calculation was performed for analyses of the rate of culture-negative peritonitis episodes, as this was a secondary endpoint.
Statistics
For the primary objective (overall peritonitis rate) an intent-to-treat (ITT) analysis was performed comparing peritonitis rates between treatment groups (icodextrin and control) using Poisson regression techniques (gamma-Poisson regression model or standard Poisson model with over dispersion) according to the solutions used for treatment at the time of enrolment, regardless of any subsequent changes that may have occurred in treatment solution utilization [22,23]. Peritonitis rates are expressed as the number of episodes per patient year and results are summarized in terms of both unadjusted (crude) and adjusted peritonitis rates along with adjusted rate ratios (ARR) and corresponding 95% confidence intervals. All comparisons between the icodextrin and control groups were adjusted for the baseline characteristics of age, gender, treatment modality (CAPD or APD) and diabetic status. The gamma-Poisson model (also referred to as the negative binomial model) also adjusts or accounts for subject-to-subject variability in individual peritonitis rates. Additionally, an as-treated analysis was performed in which episodes of peritonitis and time at risk were computed according to the actual treatment solution and treatment modality a subject was receiving, with treatment solution and treatment modality serving as time-dependent covariates. Patients who dropped out due to transfer to haemodialysis, death or kidney transplantation were censored at the time of dropout. Secondary ITT and as-treated analyses were also performed, in which the comparisons of culture-negative peritonitis stratified by the manufacturer were adjusted for multiplicity using Bonferroni corrected confidence intervals and P-values. All analyses utilized robust standard errors to accommodate possible intra-subject correlation in cases requiring the use of time-dependent covariates.
For discrete variables, the Pearson chi-square and Fisher's exact test were used to compare subjects’ baseline characteristics (e.g. gender, age groupings, diabetic status) stratified by their baseline solution (icodextrin versus non-icodextrin). Student t-tests and Wilcoxon rank sum tests were used to compare baseline differences between icodextrin and non-icodextrin treatment groups for continuous measurements (e.g. baseline age). Lifetable techniques were used to compute and compare median resolution times associated with the duration of peritonitis episodes. To accommodate those subjects with more than one episode of peritonitis, a Cox proportional hazards model with robust standard errors was also used to analyse and compare the distribution of resolution times for peritonitis. All analyses were done using the SAS statistical software package (SAS Inc., Cary, NC, USA, version 9.1); in particular, the GENMOD procedure was used for the gamma-Poisson regression analysis of peritonitis rates.
Results
Patient flow
A total of 722 patients (586 prevalent and 136 incident patients) were enrolled in this study, corresponding to a total of 10 860 patient-months. Numbers of APD and CAPD patients were almost equal (369 and 353, respectively). According to the solutions used for treatment at the time of enrolment, there were 456 patients in the icodextrin group and 266 patients in the control group. Of the 456 patients treated with icodextrin, 241 patients used icodextrin provided by supplier A, whereas 215 used icodextrin provided by supplier B. During the observation period, some patients changed from icodextrin to non-icodextrin solutions or vice versa. As a result, in the as-treated analysis these patients were counted in both the icodextrin and control groups. This is reflected in the number of patients reported for the as-treated analysis which is higher than that reported for the ITT analysis (icodextrin group, n = 543; control group, n = 307).
Baseline characteristics (Table 1)
Table 1.
Baseline characteristics of included patients
| Icodextrin group | Control group | P-value | |
|---|---|---|---|
| (n = 456) | (n = 266) | ||
| Age (years) | 54.4 ± 15.1 | 53.7 ± 14.3 | 0.397 |
| Female/male (%) | 34.6/65.4 | 48.9/51.1 | <0.001 |
| Time on peritoneal dialysis (months) | 23.0 ± 22.8 | 18.4 ± 22.4 | <0.001 |
| CAPD/APD (%) | 39.5/60.5 | 65.0/35.0 | <0.001 |
| Diabetes (%) | 30.5 | 18.4 | <0.001 |
| History of peritonitis (%) | 34.6 | 24.1 | 0.003 |
| History of exit site infection (%) | 27.0 | 20.7 | 0.058 |
| History of tunnel infection (%) | 5.3 | 3.8 | 0.358 |
There was no significant difference in the mean age or age distribution (data not shown) between treatment groups. Compared with the control group, patients in the icodextrin group had a significantly longer time on PD, and were more likely to be male, diabetic or on APD. There were, however, no significant differences in the type of diabetes or the mean time from diagnosis of diabetes to enrolment in the study (data not shown). Significantly more patients in the icodextrin group had a history of peritonitis as compared with control subjects, whereas there was no difference in the history of exit site and tunnel infections (Table 1). The time since the last episode of peritonitis, exit site infection or tunnel infection to the enrolment date was similar between the groups (data not shown).
Comparison of overall peritonitis rates between the icodextrin and the control group
In total, 365 peritonitis episodes were reported. Neither in the ITT analysis nor in the as-treated analysis was there a significant difference in the overall peritonitis rate between patients in the icodextrin group and control patients when adjusted for subject-to-subject variability, baseline characteristics and treatment modality (Table 2). There were only 21 relapses, which were included in the analysis.
Table 2.
Overall peritonitis rates (episodes/year; 95% CI) and adjusted rate ratio (95% CI) of the icodextrin and the control group
| Icodextrin group | Control group | Adjusted rate ratio | P-value | |
|---|---|---|---|---|
| Intent-to-treat (ITT) analysis | ||||
| Patient number (n) | 456 | 266 | ||
| Overall peritonitis episodes (n) | 229 | 136 | ||
| Unadjusted rate (episodes/year) | 0.409 | 0.393 | ||
| Adjusteda rate (episodes/year) | 0.407 (0.351; 0.472) | 0.406 (0.324; 0.508) | 1.003 (0.762; 1.321) | 0.981 |
| As-treated analysis | ||||
| Patient number (n) | 543 | 307 | ||
| Overall peritonitis episodes (n) | 257 | 108 | ||
| Unadjusted rate (episodes/year) | 0.415 | 0.379 | ||
| Adjusteda rate (episodes/year) | 0.417 (0.363; 0.480) | 0.386 (0.303; 0.492) | 1.080 (0.819; 1.424) | 0.595 |
aAdjusted for subject-to-subject variability, baseline characteristics (gender, age and diabetic status) and either baseline treatment modality and baseline solution (ITT analysis) or time-dependent treatment modality and time-dependent solution (as-treated analysis).
According to the protocol the eligible age was ≤75 years. However, 19 patients older than 75 were included in the study (received sponsor approval). Exclusion of these patients from the ITT or the as-treated analysis of adjusted overall peritonitis rates had no influence on study results (data not shown). Furthermore, inclusion or exclusion of six patients with prior peritonitis episodes that were possibly related to icodextrin yielded comparable results (data not shown).
The median time to resolution of the peritonitis episode was 14 days in both treatment groups. No statistically significant differences in the duration of the peritonitis episode were observed between icodextrin-treated and control patients or between CAPD patients and APD patients (data not shown).
Comparison of peritonitis rates by culture results
Only as-treated analyses were performed for these calculations. Within the observation period, 303 of the 365 peritonitis episodes were culture-positive (211 episodes in 543 patients in the icodextrin group and 92 episodes in 307 patients in the control group). Dialysate cultures were negative in 62 of the total of 365 episodes of peritonitis (17%). The rate of culture-negative peritonitis was 18% in the icodextrin group (46 of 257 episodes) and 15% in the control group (16 of 108 episodes). Forty (7.4%) of the 543 patients of the icodextrin group and 16 (5.2%) of the 307 control patients had at least one episode of culture-negative peritonitis (P = 0.226).
No significant differences between treatment groups were observed in the adjusted rates of culture-positive or culture-negative peritonitis (Table 3).
Table 3.
Rates of culture-positive and culture-negative peritonitis (episodes/year; 95% CI) in PD patients of the icodextrin and the control group (as-treated analysis)
| Icodextrin group | Control group | Adjusted rate ratio | P-value | |
|---|---|---|---|---|
| (Patients: n = 543) | (Patients: n = 307) | |||
| Culture-positive peritonitis | ||||
| Peritonitis episodes (n) | 211 | 92 | ||
| Unadjusted rate (episodes/year) | 0.340 | 0.323 | ||
| Adjusteda rate (episodes/year) | 0.331 (0.284; 0.386) | 0.329 (0.254; 0.427) | 1.006 (0.750; 1.349) | 0.969 |
| Culture-negative peritonitis | ||||
| Peritonitis episodes (n) | 46 | 16 | ||
| Unadjusted rate (episodes/year) | 0.074 | 0.056 | ||
| Adjusteda rate (episodes/year) | 0.078 (0.057; 0.107) | 0.052 (0.030; 0.091) | 1.498 (0.779; 2.880) | 0.233 |
aAdjusted for subject-to-subject variability, baseline characteristics (gender, age and diabetic status), time-dependent treatment modality and time-dependent solution.
Comparison of peritonitis rates by icodextrin supplier
When the adjusted overall peritonitis rates between patients in the icodextrin group and those in the control group were analysed per icodextrin raw material supplier, no statistically significant differences were observed in either the ITT or the as-treated analysis (Figure 1).
Fig. 1.
Analysis of overall peritonitis rates in episodes (ep)/year: icodextrin versus control group, by supplier
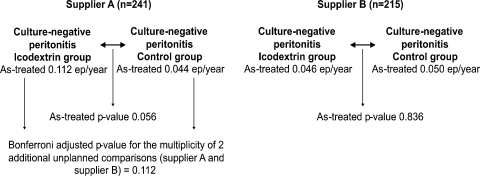
When stratified by the supplier, the adjusted rates of culture-negative peritonitis episodes tended to be higher in the icodextrin group compared with the control group for supplier A (not significant), whereas there was no difference between treatment groups for supplier B (Figure 2). It is interesting to note that five of the culture-negative peritonitis episodes in the icodextrin group occurred in only two patients of one investigational centre that was supplied by provider A. Considering that this study is strictly a prospective observational and unblinded study not powered to analyse the incidence of culture-negative peritonitis episodes, it is possible that any number of unmeasured, centre-specific factors (e.g. related to laboratory techniques or unmeasured patient characteristics) may account for some or all of these differences.
Fig. 2.
Analysis of culture-negative peritonitis rates in episodes (ep)/year: icodextrin versus control group, by supplier
Association of peritonitis episodes with icodextrin
After a blind review by each individual member of the Steering Committee there was unanimous concordance between independent nephrologists and Baxter nephrologists in 293 of the 365 cases of peritonitis (80.3%) reported and reviewed in this study. In 47 of the remaining 72 cases the independent nephrologists either had together a unanimous vote (n = 28) or the majority of the independent nephrologists had a vote which prevailed against the isolated Baxter nephrologists’ vote and one of the independent nephrologists’ different vote (n = 19). There were six cases with a tie and 19 cases where all the blinded reviews led to different interpretations among all voters. In these 25 cases (6.8%), the final decision on the causal assessment was taken after discussion among all members in a meeting, where the Baxter vote was still considered as one only, while each independent nephrologist again had his/her own vote. All the information of the Steering Committee meetings and votes has been documented.
Of the 365 peritonitis episodes reported, 364 episodes were classified by the Steering Committee. Of these 364 episodes, 355 were classified as not related to the solution prescribed (icodextrin or non-icodextrin). Three culture-negative episodes were classified as unlikely related to icodextrin (hospitalization was required in one of these three cases). Six episodes (all culture negative), which occurred in four patients, were classified as possibly related to icodextrin treatment. None of these episodes required hospitalization. Five of the six possibly related peritonitis episodes and two of the three unlikely related sterile peritonitis episodes occurred at one single investigational site, with one patient experiencing three of these six peritonitis episodes (data not shown). One of the 365 peritonitis episodes was not classified by the Steering Committee because of a lack of sufficient available information.
Withdrawals and deaths
Excluding patients who were withdrawn when the study ended (December 2005), a total of 421 subjects (282 in the icodextrin group, 139 in the control group) withdrew early from the study. This dropout rate of 49.5 per 100 patient-years in the icodextrin group and 39.4 withdrawals per 100 patient-years in the control group was higher than the 20% annual withdrawal rate expected in the sample size calculation. When considering the actual peritonitis rate and total number of treatment months reported in the present trial, this higher dropout rate had little impact on the overall power of the study to detect a difference in peritonitis rates of just over 50% between groups (observed power after considering the higher dropout rate: 76% versus target power when assuming a 20% annual dropout rate: 80%).
Peritonitis was the reason for study discharge in 37/456 patients in the icodextrin group (8.1%) and in 17/266 patients in the control group (6.4%). A total of 97 of the 722 enrolled subjects died during the observation period: 67/456 patients (14.7%) in the icodextrin group and 30/266 patients (11.3%) in the control group. None of these deaths were considered to be related to the use of the prescribed dialysis solutions. Since this was an observational study, no further data on reason of deaths were requested.
There was no significant difference between the icodextrin group and the control group in the percent of patients withdrawn from the study because of peritonitis (P = 0.396), death (P = 0.194) or any other reason, including kidney transplantation (23.5% versus 23.7%, P = 0.947) and transfer to haemodialysis (10.3% versus 6.8%, P = 0.109).
Discussion
The use of icodextrin-containing dialysis solutions optimizes peritoneal ultrafiltration, allows better fluid control as compared with standard solutions [3], improves quality of life [24] and prolongs technique survival of PD patients [5,6]. An icodextrin-containing dialysis solution has an acidic pH. However, in contrast to glucose-based standard solutions both its iso-osmolality to serum and the lower content of glucose degradation products may improve the biocompatibility profile of icodextrin. Accordingly, a subanalysis of data from the European APD Outcome Study showed that peritoneal function was better preserved in anuric APD patients using icodextrin during the daytime dwell as compared with those treated with a glucose solution [25]. Furthermore, it could be hypothesized that the greater proliferation of mesothelial cells [26] and improvement of phagocytic function [15–17] reported by some authors after exposure to icodextrin as compared with standard solutions may have a positive impact on decreasing infectious peritonitis rates. However, a previous randomized study conducted in 1995 did not demonstrate a statistically significant difference in the risk of peritonitis between CAPD patients treated with icodextrin and those using glucose-based standard solutions [27]. These data cannot be transferred to the present PD treatment regimens because the PD technique has improved and the number of APD patients has increased since that time. These factors may account for the fact that the peritonitis rate of both icodextrin- and non-icodextrin-treated patients in this observational trial was markedly lower than that in the randomized study published by Gokal et al. more than 10 years ago [27]. More importantly, the peritonitis rates found in the present study in both treatment groups were lower than the maximum acceptable target of 1 episode/18 treatment months and 1 episode/24 treatment months recommended by recent guidelines [21,28]. We found no difference in overall or culture-positive peritonitis rates between patients treated with icodextrin and those using exclusively non-icodextrin solutions, which is in agreement with the above-mentioned earlier results, as well as some more recent studies [2,17]. Additionally, in the present trial, the duration of peritonitis was similar in icodextrin-treated and control patients. However, because of differences in local treatment practices and the variability in definition of peritonitis resolution used by different investigators, these latter results should be interpreted with caution.
Besides infectious peritonitis, the question if icodextrin influences the incidence of culture-negative peritonitis remains. A higher incidence of culture-negative peritonitis may reflect inappropriate culture techniques used for microbiological analysis of dialysate samples [21]. Episodes of sterile peritonitis have also been reported that were caused by endotoxin contamination of the dialysis solution, intraperitoneal chemical agents (including glucose degradation products and several drugs), allergic mechanisms (including eosinophilic peritonitis) or, rarely, by peritoneal metastases, pancreatitis and abdominal lymphomas [29–34]. Between November 2001 and July 2002, a markedly increased incidence of sterile peritonitis associated with icodextrin treatment was reported [35–41]. These cases were caused by a peptidoglycan (released from Alicyclobacillus acidocaldarius, a thermophilic acidophilic Gram-positive organism contaminating the cornstarch used for icodextrin production) [41]. Peptidoglycans induce pro-inflammatory cytokine production of mononuclear cells, though to a much lower extent than endotoxins [42]. After implementation of routine serial monitoring of icodextrin solutions for peptidoglycan during the manufacturing process, the frequency of icodextrin-associated peritonitis decreased dramatically within a few months [41]. Nevertheless, a few recent cases have been reported [43]. Besides common causes of culture-negative peritonitis (e.g. peritonitis with false-negative culture or atypical infection) several other reasons for these newer cases have been considered, including sensitization of patients to peptidoglycan or icodextrin, or individual reaction of some patients to a level of peptidoglycan below the detectable threshold [44,45]. However, it must be taken into account that sterile peritonitis has always existed with glucose-based PD solutions, even long before the introduction of icodextrin [46,47]. An increased incidence of culture-negative peritonitis may result in unnecessary use of antibiotics or even catheter removal. According to international guidelines, the rate of culture-negative peritonitis should be <20% [21]. The incidence of 17% for culture-negative peritonitis found in the present trial meets this recommendation. Furthermore, in this large population of PD patients from seven European countries, the rate of culture-negative peritonitis did not differ between icodextrin-treated and control patients, who were mainly prescribed glucose-based PD solutions.
The incidence rate of peritonitis in icodextrin-treated patients between the two suppliers was not analysed because each icodextrin supplier provided an entire country, making it impossible to provide a rationale to stratify by country. Therefore, a difference between the two providers may primarily reflect country-specific differences in peritonitis rate, diagnosis and treatment of peritonitis, patient selection and/or laboratory techniques. However, when stratified by the icodextrin supplier, no statistically significant differences were observed in adjusted overall and culture-negative peritonitis rates between patients in the icodextrin group compared with patients in the control group. Based on these results it appears that a significant influence of the supplier on peritonitis rates in icodextrin-treated patients is unlikely.
This study has certain limitations such as a non-randomized study design and a heterogeneous patient population. However, randomization of patients into a control group without icodextrin was not acceptable to several centres because of the fear of overhydration, especially in patients with high peritoneal transport rates. Therefore, a randomized design would have significantly reduced the number of patients available for enrolment. Furthermore, a randomized controlled trial is inappropriate for accurate measurement of the frequency of rare events [48].
There were more diabetic patients and patients with longer duration of dialysis before enrolment in the icodextrin group, and other than diabetic status, no data on co-morbidity were requested in this observational study. Therefore, despite adjustment for age, gender, PD modality and diabetic status a selection bias cannot be excluded. For example, patients with higher co-morbidity, including those with diabetes, have faster peritoneal solute transport rates [49] and are therefore more likely to receive icodextrin. Regardless of dialysis prescription, these patients may also have a higher risk of infectious peritonitis [50,51]. Consequently, a higher peritonitis rate (if anything) could be expected in icodextrin-treated patients compared with control patients, which, however, was not the case in this study.
As mentioned above sterile peritonitis may be of different aetiologies, but a significant part of these episodes are infectious cases that remain culture-negative. If a patient with culture-negative peritonitis responds to antibiotic therapy, it is highly likely that this is an infectious episode. However, even in this case an association with icodextrin (or any other dialysis solution) cannot be completely ruled out. A spectrum of diagnostic measures would have to be available to evaluate the pathogenesis of culture-negative peritonitis and its possible association with icodextrin. Performing all these tests in each episode of sterile peritonitis, however, goes beyond the scope of an observational trial including almost 30 centres. Instead of that, the Steering Committee, after a blinded review, classified each episode of peritonitis (based on clinical data and routine laboratory results provided by each investigator). Based on these votes, an association with icodextrin could not be completely excluded in <10 out of 365 peritonitis episodes. It has to be considered that sterile peritonitis usually is a sporadic event. Therefore, the fact that we did not find a significant difference in peritonitis rates between icodextrin and non-icodextrin patients or between the two providers does not preclude that in future other cases or even clusters of sterile peritonitis (associated with icodextrin or any other dialysis solution) could reappear.
In summary, this study further supports that icodextrin provided by both raw material suppliers is safe to use as a single daily exchange that is part of a PD regimen, as being done daily by >10 000 patients in Europe today. There was neither a higher rate of culture-negative peritonitis nor a lower rate of infectious peritonitis experienced in subjects receiving icodextrin as part of CAPD or APD regimens compared with non-icodextrin users.
Acknowledgments
The study was sponsored by Baxter Healthcare. Monique van Bree and Gerda Sabbe are acknowledged for their operational support in the conduct of the study and in organization of the study Steering Committee meetings.
Conflict of interest statement. A.V., C.R., C.M. and P.W. are members of the study Steering Committee. The independent Steering Committee nephrologists were not paid any honorarium, but only the travel expenses to attend the two meetings. A.V. has received consulting and lecture fees and travel funding from Baxter, Gambro and Fresenius (manufacturers of dialysis solutions) unrelated to this trial. He has also been a principal investigator in clinical trials funded by Baxter and Fresenius. C.R. did not receive consulting fees, lecture fees or travel grants from manufacturers of dialysis solutions. C.M. has only received consulting fees from Gambro. P.W. has received consulting fees from Baxter unrelated to this trial. A.R.C. has received travel funding from Baxter and Fresenius and research funding from Baxter unrelated to this trial. B.M., S.v.d.H. and J.C.D.F. are employed by Baxter Healthcare. E.V. was employed by Baxter Healthcare at the time of this analysis. The results presented in this paper have not been published previously in whole or part, except in abstract form. The paper is not under consideration by any other journal.
Appendix. Sites and members of the Extraneal Peritonitis Study Group
Dr Ana Rodríguez Carmona, Dr Miguel Pérez Fontán, Hospital Juan Canalejo, La Coruña, Spain
Dr M. Auxiliadora Bajo Rubio, Dr Gloria del Peso Gilsanz, Hospital La Paz, Madrid, Spain
Prof. Dr Andreas Vychytil, Dr Elisabeth Dittrich, Dr Tatjana Lilaj, Medical University of Vienna, Austria
Dr Nicanor Vega Diaz, Hospital Dr Negrín, Las Palmas de Gran Canaria, Spain
Prof. Jürg Steiger, Dr Michael Mayr, University Hospital Basel, Basel, Switzerland
Prof. Eric Goffin, UCL St-Luc, Brussels, Belgium
Dr Catherine Michel, Dr Latifa Azeroual, Dr Gilles Hufnagel, CHRU Bichat, Paris, France
Dr Dominique Pagniez, Dr Eric Boulanger, Dr Claude Moranne, CHRU de Lille, Hôpital Calmette, Lille, France
Dr Patricia de Sequera, Dr Jesús Benito, Hospital Universitario Principe de Asturias, Madrid, Spain
Dr Christian Verger, CH Pontoise, Pontoise, France
Dr Cesar Remón Rodríguez, Dr Pedro Quirós Ganga, Mrs Mercedes Tejuca Marenco, Hospital Puerto Real, Cadiz, Spain
Prof. Dr Johannes Roob, Dr Günther Enzinger, University Hospital Graz, Graz, Austria
Dr Françoise Heibel, Dr Larbi Bencheikh, CHRU de Strasbourg Hôpital Civil, Strasbourg, France
Dr José Antonio Sánchez Tomero, Dr Antonio Cirugeda, Hospital La Princesa, Madrid, Spain
Dr Gorka Garcia Erauzkin, Hospital de Cruces, Vizcaya, Spain
Dr María Teresa Rivera, Dr Teresa Tenorio Cañamas, Hospital Ramón y Cajal, Madrid, Spain
Dr Paul Ford Williams, Ipswich Hospital, Ipswich, United Kingdom
Dr Sandrine Genestier, CH Colmar, Colmar, France
Dr Agnès Caillette-Beaudoin, Dr Cora Denicola, CH Lucien Hussel, Vienne, France
Priv.-Doz. Dr Andreas Fußhoeller, Dr Firuseh Farokhzad, University of Düsseldorf, Düsseldorf, Germany
Dr Paloma Gallar, Dr Olímpia Ortega Marcos, Dr Isabel Rodriguez Villarreal, Hospital Severo Ochoa, Leganes, Spain
Dr Odile Rivault, L’Anider, Le Petit Quevilly, France
Dr Juan Manuel Lopez Gomez, Hospital General Universitario Gregorio Marañon, Madrid, Spain
Dr Yves Durand, CHRU de Nancy, Hôpitaux de Brabois, Nancy, France
Dr Michel Burnier, Dr Georges Halabi, Dr P. Meier, Dr G. Nseir, Dr Phan, Centre Hospitalier Universitaire, Lausanne, Switzerland
Dr Michael Zellweger, Dr Nicola Marangon, Dr Pierre-Yves Martin, Dr Pierre Alain Triverio, Hôpital Universitaire de Genève, Geneva, Switzerland
Dr Belkacem Issad, CHRU La Pitié Salpétrière, Paris, France.
References
- 1.Plum J, Gentile S, Verger C, et al. Efficacy and safety of a 7.5% icodextrin peritoneal dialysis solution in patients treated with automated peritoneal dialysis. Am J Kidney Dis. 2002;39:862–871. doi: 10.1053/ajkd.2002.32009. [DOI] [PubMed] [Google Scholar]
- 2.Wolfson M, Piraino B, Hamburger RJ, et al. A randomized controlled trial to evaluate the efficacy and safety of icodextrin in peritoneal dialysis. Am J Kidney Dis. 2002;40:1055–1065. doi: 10.1053/ajkd.2002.36344. [DOI] [PubMed] [Google Scholar]
- 3.Davies SJ, Woodrow G, Donovan K, et al. Icodextrin improves the fluid status of peritoneal dialysis patients: results of a double-blind randomized controlled trial. J Am Soc Nephrol. 2003;14:2338–2344. doi: 10.1097/01.asn.0000083904.12234.27. [DOI] [PubMed] [Google Scholar]
- 4.Finkelstein F, Healy H, Abu-Alfa A, et al. Superiority of icodextrin compared with 4.25% dextrose for peritoneal ultrafiltration. J Am Soc Nephrol. 2005;16:546–554. doi: 10.1681/ASN.2004090793. [DOI] [PubMed] [Google Scholar]
- 5.Wilkie ME, Plant MJ, Edwards L, et al. Icodextrin 7.5% dialysate solution (glucose polymer) in patients with ultrafiltration failure: extension of CAPD technique survival. Perit Dial Int. 1997;17:84–87. [PubMed] [Google Scholar]
- 6.Johnson DW, Arndt M, O'Shea A, et al. Icodextrin as salvage therapy in peritoneal dialysis patients with refractory fluid overload. BMC Nephrol. 2001;2:2. doi: 10.1186/1471-2369-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bredie SJ, Bosch FH, Demacker PN, et al. Effects of peritoneal dialysis with an overnight icodextrin dwell on parameters of glucose and lipid metabolism. Perit Dial Int. 2001;21:275–281. [PubMed] [Google Scholar]
- 8.Gradden CW, Ahmad R, Bell GM. Peritoneal dialysis: new developments and new problems. Diabet Med. 2001;18:360–363. doi: 10.1046/j.1464-5491.2001.00455.x. [DOI] [PubMed] [Google Scholar]
- 9.Sisca S, Maggiore U. Beneficial effect of icodextrin on the hypertriglyceridemia of CAPD patients. Perit Dial Int. 2002;22:727–729. [PubMed] [Google Scholar]
- 10.Martikainen T, Teppo AM, Gronhagen-Riska C, et al. Benefit of glucose-free dialysis solutions on glucose and lipid metabolism in peritoneal dialysis patients. Blood Purif. 2005;23:303–310. doi: 10.1159/000086553. [DOI] [PubMed] [Google Scholar]
- 11.Adachi Y, Nakagawa Y, Nishio A. Icodextrin preserves residual renal function in patients treated with automated peritoneal dialysis. Perit Dial Int. 2006;26:405–407. [PubMed] [Google Scholar]
- 12.Goldsmith D, Jayawardene S, Sabharwal N, et al. Allergic reactions to the polymeric glucose-based peritoneal dialysis fluid icodextrin in patients with renal failure. Lancet. 2000;355:897. doi: 10.1016/S0140-6736(99)05327-1. [DOI] [PubMed] [Google Scholar]
- 13.Divino Filho JC. Allergic reactions to icodextrin in patients with renal failure. Lancet. 2000;355:1364–1365. [PubMed] [Google Scholar]
- 14.Gokal R. Icodextrin-associated sterile peritonitis. Perit Dial Int. 2002;22:445–448. [PubMed] [Google Scholar]
- 15.de Fijter CW, Verbrugh HA, Oe LP, et al. Biocompatibility of a glucose-polymer-containing peritoneal dialysis fluid. Am J Kidney Dis. 1993;21:411–418. doi: 10.1016/s0272-6386(12)80270-8. [DOI] [PubMed] [Google Scholar]
- 16.Thomas S, Schenk U, Fischer FP, et al. In vitro effects of glucose polymer-containing peritoneal dialysis fluids on phagocytic activity. Am J Kidney Dis. 1997;29:246–253. doi: 10.1016/s0272-6386(97)90037-8. [DOI] [PubMed] [Google Scholar]
- 17.Posthuma N, ter Wee P, Donker AJ, et al. Peritoneal defense using icodextrin or glucose for daytime dwell in CCPD patients. Perit Dial Int. 1999;19:334–342. [PubMed] [Google Scholar]
- 18.Pérez Fontán M, Rodríguez-Carmona A, Garcia-Naveiro R, et al. Peritonitis-related mortality in patients undergoing chronic peritoneal dialysis. Perit Dial Int. 2005;25:274–284. [PubMed] [Google Scholar]
- 19.Krishnan M, Thodis E, Ikonomopoulos D, et al. Predictors of outcome following bacterial peritonitis in peritoneal dialysis. Perit Dial Int. 2002;22:573–581. [PubMed] [Google Scholar]
- 20.Fourtounas C, Savidaki E, Dousdabanis P, et al. Peritonitis during the first year after commencement of peritoneal dialysis has an impact on technique survival and patient morbidity. Adv Perit Dial. 2006;22:50–54. [PubMed] [Google Scholar]
- 21.Piraino B, Bailie GR, Bernardini J, et al. Peritoneal dialysis-related infections recommendations: 2005 update. Perit Dial Int. 2005;25:107–131. [PubMed] [Google Scholar]
- 22.Vonesh EF. Estimating rates of recurrent peritonitis for patients on CAPD. Perit Dial Bull. 1985;5:59–65. [Google Scholar]
- 23.Vonesh EF. Modelling peritonitis rates and associated risk factors for individuals on continuous ambulatory peritoneal dialysis. Stat Med. 1990;9:263–271. doi: 10.1002/sim.4780090309. [DOI] [PubMed] [Google Scholar]
- 24.Guo A, Wolfson M, Holt R. Early quality of life benefits of icodextrin in peritoneal dialysis. Kidney Int. 2002;62(Suppl 81):S72–S79. doi: 10.1046/j.1523-1755.62.s81.10.x. [DOI] [PubMed] [Google Scholar]
- 25.Davies SJ, Brown EA, Frandsen NE, et al. Longitudinal membrane function in functionally anuric patients treated with APD: data from EAPOS on the effects of glucose and icodextrin prescription. Kidney Int. 2005;67:1609–1615. doi: 10.1111/j.1523-1755.2005.00243.x. [DOI] [PubMed] [Google Scholar]
- 26.Bajo MA, Selgas R, Castro MA, et al. Icodextrin effluent leads to a greater proliferation than glucose effluent of human mesothelial cells studied ex vivo. Perit Dial Int. 2000;20:742–747. [PubMed] [Google Scholar]
- 27.Gokal R, Mistry CD, Peers EM. Peritonitis occurrence in a multicenter study of icodextrin and glucose in CAPD. MIDAS Study Group. Multicenter investigation of icodextrin in ambulatory dialysis. Perit Dial Int. 1995;15:226–230. [PubMed] [Google Scholar]
- 28.Dombros N, Dratwa M, Feriani M, et al. European best practice guidelines for peritoneal dialysis. 3 peritoneal access. Nephrol Dial Transplant. 2005;20(Suppl 9):ix8–ix12. doi: 10.1093/ndt/gfi1117. [DOI] [PubMed] [Google Scholar]
- 29.Freiman JP, Graham DJ, Reed TG, et al. Chemical peritonitis following the intraperitoneal administration of vancomycin. Perit Dial Int. 1992;12:57–60. [PubMed] [Google Scholar]
- 30.Coronel F, Martin-Rabadan P, Romero J. Chemical peritonitis after intraperitoneal administration of amphotericin B in a fungal infection of the catheter subcutaneous tunnel. Perit Dial Int. 1993;13:161–162. [PubMed] [Google Scholar]
- 31.Thakur SS, Unikowsky B, Prichard S. Eosinophilic peritonitis in CAPD: treatment with prednisone and diphenhydramine. Perit Dial Int. 1997;17:402–403. [PubMed] [Google Scholar]
- 32.Mangram AJ, Archibald LK, Hupert M, et al. Outbreak of sterile peritonitis among continuous cycling peritoneal dialysis patients. Kidney Int. 1998;54:1367–1371. doi: 10.1046/j.1523-1755.1998.00110.x. [DOI] [PubMed] [Google Scholar]
- 33.Tuncer M, Sarikaya M, Sezer T, et al. Chemical peritonitis associated with high dialysate acetaldehyde concentrations. Nephrol Dial Transplant. 2000;15:2037–2040. doi: 10.1093/ndt/15.12.2037. [DOI] [PubMed] [Google Scholar]
- 34.de Freitas DG, Gokal R. Sterile peritonitis in the peritoneal dialysis patient. Perit Dial Int. 2005;25:146–151. [PubMed] [Google Scholar]
- 35.Heering P, Brause M, Plum J, et al. Peritoneal reaction to icodextrin in a female patient on CAPD. Perit Dial Int. 2001;21:321–322. [PubMed] [Google Scholar]
- 36.Montagnac R, Slingeneyer A, Schillinger F. Aseptic peritonitis: role of icodextrin. Nephrol Dial Transplant. 2001;16:435–436. doi: 10.1093/ndt/16.2.435. [DOI] [PubMed] [Google Scholar]
- 37.Reichel W, Schulze B, Dietze J, et al. A case of sterile peritonitis associated with icodextrin solution. Perit Dial Int. 2001;21:414–415. [PubMed] [Google Scholar]
- 38.Williams PF, Foggensteiner L. Sterile/allergic peritonitis with icodextrin in CAPD patients. Perit Dial Int. 2002;22:89–90. [PubMed] [Google Scholar]
- 39.Goffin E, Scheiff JM. Transient sterile chemical peritonitis in a CAPD patient using icodextrin. Perit Dial Int. 2002;22:90–91. [PubMed] [Google Scholar]
- 40.Basile C, De Padova F, Montanaro A, et al. The impact of relapsing sterile icodextrin-associated peritonitis on peritoneal dialysis outcome. J Nephrol. 2003;16:384–386. [PubMed] [Google Scholar]
- 41.Martis L, Patel M, Giertych J, et al. Aseptic peritonitis due to peptidoglycan contamination of pharmacopoeia standard dialysis solution. Lancet. 2005;365:588–594. doi: 10.1016/S0140-6736(05)17908-2. [DOI] [PubMed] [Google Scholar]
- 42.Nakagawa Y, Maeda H, Murai T. Evaluation of the in vitro pyrogen test system based on proinflammatory cytokine release from human monocytes: comparison with a human whole blood culture test system and with the rabbit pyrogen test. Clin Diagn Lab Immunol. 2002;9:588–597. doi: 10.1128/CDLI.9.3.588-597.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rozenberg R, Magen E, Weissgarten J, et al. Icodextrin-induced sterile peritonitis: the Israeli experience. Perit Dial Int. 2006;26:402–405. [PubMed] [Google Scholar]
- 44.Enia G, Mandalari A, Ventura G, et al. Sterile icodextrin-associated peritonitis may induce hypersensitivity and recurrent peritonitis on re-challenge. Nephrol Dial Transplant. 2003;18:626. doi: 10.1093/ndt/18.3.626. [DOI] [PubMed] [Google Scholar]
- 45.Goffin E. Aseptic peritonitis and icodextrin. Perit Dial Int. 2006;26:314–316. [PubMed] [Google Scholar]
- 46.Lee S, Schoen I. Eosinophilia of peritoneal fluid and peripheral blood associated with chronic peritoneal dialysis. Am J Clin Pathol. 1967;47:638–640. doi: 10.1093/ajcp/47.5.638. [DOI] [PubMed] [Google Scholar]
- 47.Karanicolas S, Oreopoulos DG, Izatt S, et al. Epidemic of aseptic peritonitis caused by endotoxin during chronic peritoneal dialysis. N Engl J Med. 1977;296:1336–1337. doi: 10.1056/NEJM197706092962309. [DOI] [PubMed] [Google Scholar]
- 48.Jager KJ, Stel VS, Wanner C, et al. The valuable of observational studies to nephrology. Kidney Int. 2007;72:671–675. doi: 10.1038/sj.ki.5002397. [DOI] [PubMed] [Google Scholar]
- 49.Davies SJ, Phillips L, Naish PF, et al. Quantifying comorbidity in peritoneal dialysis patients and its relationship to other predictors of survival. Nephrol Dial Transplant. 2002;17:1085–1092. doi: 10.1093/ndt/17.6.1085. [DOI] [PubMed] [Google Scholar]
- 50.Vychytil A, Lorenz M, Schneider B, et al. New strategies to prevent staphylococcus aureus infections in peritoneal dialysis patients. J Am Soc Nephrol. 1998;9:669–676. doi: 10.1681/ASN.V94669. [DOI] [PubMed] [Google Scholar]
- 51.Chow KM, Szeto CC, Leung CB, et al. A risk analysis of continuous ambulatory peritoneal dialysis-related peritonitis. Perit Dial Int. 2005;25:374–379. [PubMed] [Google Scholar]