Abstract
Background. Extending the administration interval of erythropoiesis-stimulating agents (ESAs) represents an opportunity to improve the efficiency of anaemia management in patients with chronic kidney disease (CKD). However, effective haemoglobin (Hb) maintenance can be challenging with epoetin alfa and epoetin beta administered at extended intervals. C.E.R.A., a continuous erythropoietin receptor activator, has a unique pharmacologic profile and long half-life (∼130 h), allowing administration at extended intervals. Phase III results have demonstrated that C.E.R.A. administered once every 4 weeks effectively maintains stable Hb levels in patients with CKD on dialysis.
Methods. STRIATA (Stabilizing haemoglobin TaRgets in dialysis following IV C.E.R.A. Treatment for Anaemia) was a multicentre, open-label randomized phase III study to evaluate the efficacy and safety of intravenous C.E.R.A. administered once every 2 weeks (Q2W) for Hb maintenance following direct conversion from darbepoetin alfa (DA). Adult patients on dialysis receiving stable intravenous DA once weekly (QW) or Q2W were randomized (1:1) to continue their current DA regimen (n = 156) or receive intravenous C.E.R.A. Q2W (n = 157) for 52 weeks. Doses were adjusted to maintain Hb levels within ± 1.0 g/dl of baseline and between 10.0 and 13.5 g/dl. The primary endpoint was the mean Hb change between baseline and the evaluation period (weeks 29–36).
Results. Most patients (>80%) received DA QW before randomization. The mean (95% CI) difference between C.E.R.A. and DA in the primary endpoint was 0.18 g/dl (−0.05, 0.41), within a pre-defined non-inferiority limit. C.E.R.A. was clinically non-inferior to DA (P < 0.0001) in maintaining Hb levels. Both treatments were well tolerated.
Conclusions. Stable Hb levels were successfully maintained in patients on haemodialysis directly converted to Q2W intravenous C.E.R.A. from DA.
Keywords: anaemia, C.E.R.A., darbepoetin alfa, dialysis, haemoglobin
Introduction
Anaemia secondary to chronic kidney disease (CKD) is associated with increased hospitalization and decreased survival [1,2], increased burden of cardiovascular disease [3,4] and reduced quality of life [5,6]. Despite improvements, large observational studies such as the Dialysis Outcomes and Practice Patterns Study (DOPPS) indicate that anaemia remains prevalent in patients receiving dialysis; therefore, there is a need to increase the proportions of patients achieving guideline haemoglobin (Hb) targets [7]. With the growing prevalence of CKD [8,9], new approaches are required to improve the efficiency of anaemia management without increasing the workload of healthcare staff.
Extended administration intervals represent an opportunity to simplify treatment and improve the efficiency of anaemia management. For example, the preliminary results of a recent observational study indicated that extending the administration interval from three times weekly to once weekly (QW) was associated with substantial time savings [10]. It was estimated that 350 h of physician/nurse time per year could be saved in a centre with 50 dialysis patients. Extended administration intervals may also offer benefits for patients with CKD. If administration intervals could be successfully extended beyond QW for all patients, the resulting time savings could enable healthcare providers to spend more time focusing on other aspects of CKD management, including patient education, and to address other modifiable risk factors, such as hypertension and mineral balance.
C.E.R.A., a continuous erythropoietin receptor activator, is indicated in the European Union for once-monthly maintenance of Hb levels in patients with CKD. C.E.R.A. has different receptor binding characteristics to established erythropoiesis-stimulating agents (ESAs) [11,12] and a long half-life of ∼130 h following intravenous and subcutaneous administration [13]. In phase II and phase III studies in patients with CKD on dialysis, C.E.R.A. effectively maintained stable Hb levels when administered once every 4 weeks (Q4W) [14–16].
STRIATA (Stabilizing haemoglobin TaRgets in dialysis following IV C.E.R.A. Treatment for Anaemia) was a multicentre, open-label randomized phase III study designed to investigate the efficacy and safety of intravenous C.E.R.A. administered once every 2 weeks (Q2W) for Hb maintenance in patients on dialysis who converted directly from darbepoetin alfa (DA) QW or Q2W. We report the primary efficacy and safety results from STRIATA.
Patients and methods
Patients
Adult patients with chronic renal anaemia receiving adequate haemodialysis (Kt/V ≥1.2 or urea reduction ratio ≥ 65%) or peritoneal dialysis (weekly Kt/V ≥1.8) for ≥ 12 weeks and intravenous DA therapy at the same administration interval (either QW or Q2W) for ≥8 weeks were eligible for screening.
Patients had stable mean baseline Hb levels (difference between mean individual Hb values at the beginning and end of the run-in period ≤1 g/dl) of between 10.5 and 13.0 g/dl. The values for Hb entry criteria were within the ranges recommended by anaemia treatment guidelines at the time the study was designed [17,18]. Adequate iron status (serum ferritin ≥100 ng/ml or transferrin saturation ≥20% or hypochromic red cells <10%) was also required for study entry.
Patients were excluded if they had non-renal causes of anaemia (e.g. folic acid or vitamin B12 deficiency, haemolysis and haemoglobinopathies), C-reactive protein >30 mg/l or life expectancy <12 months.
The study was conducted in accordance with the Declaration of Helsinki, International Conference on Harmonisation Good Clinical Practice guidelines and was approved by local ethics committees. All study participants provided written informed consent.
Study drugs
C.E.R.A. (F. Hoffmann-La Roche Ltd, Basel, Switzerland) was provided as a sterile injectable solution in 2 ml single-use glass vials containing 1 ml solution. The solution was available in 50, 100, 200, 400 and 1000 μg/ml concentrations.
DA (Amgen Inc., Thousand Oaks, USA) was obtained commercially in pre-filled syringes.
Study design
STRIATA was a multicentre, parallel-group, open-label, randomized phase III study designed to investigate the efficacy and safety of intravenous C.E.R.A. Q2W for Hb maintenance in patients on dialysis who converted directly from intravenous DA QW or Q2W.
After a 4-week run-in period during which patients continued to receive DA, patients were randomized (1:1) to receive intravenous C.E.R.A. Q2W or to continue their intravenous DA regimen (Figure 1). Patients were randomly assigned to one of the two treatment groups by a central randomization centre. For each study centre, randomization numbers were allocated sequentially to each patient in the order in which they were enrolled.
Fig. 1.
Study design. DA, darbepoetin alfa; R, randomization; QW, once weekly; Q2W, once every 2 weeks.
The starting doses of C.E.R.A. were selected based on the results of phase II studies and calculated by patient according to their DA dose before randomization (Table 1). A period of 28 weeks after the first dose of study drug was used for dose titration. This was followed by an 8-week efficacy evaluation period (weeks 29–36), and a further 16-week safety observation period (weeks 37–52; Figure 1).
Table 1.
Starting doses of C.E.R.A.
| Previous DA dosage (μg/week) | C.E.R.A. starting dosage (μg/2 weeks) |
|---|---|
| <40 | 60 |
| 40–80 | 100 |
| >80 | 180 |
DA, darbepoetin alfa.
During the dose-titration and evaluation periods, doses of trial medication were adjusted according to Hb response. The aim was to maintain individual patients’ Hb within ±1.0 g/dl of their baseline level, and to maintain absolute Hb values for the overall study population between 10.0 and 13.5 g/dl. During the safety observation period, doses were adjusted to maintain the Hb levels within 11–13 g/dl. It should be noted that the target Hb ranges chosen for this study were agreed by consensus of the Steering Committee based on contemporary anaemia treatment guidelines; the study was completed before the publication of the CHOIR study which prompted safety concerns regarding the maintenance of Hb levels >13 g/dl [19].
Dose adjustments were permitted for safety at any point during the study. Unless safety concerns dictated otherwise, dose adjustments were performed at the next scheduled dosing day so that the administration interval remained unchanged throughout the study. In addition, dose adjustments for C.E.R.A. were not performed more frequently than once every 4 weeks. Dose adjustments for DA were performed according to its approved labelling (i.e. 25% dose increase or decrease based on the Hb value), but maintaining the target Hb and the interval between dose adjustments the same as for C.E.R.A. Patients received intravenous iron supplementation as required throughout the run-in and treatment periods.
Study parameters
The primary efficacy parameter was the change in mean Hb levels between baseline and the evaluation period. Secondary efficacy parameters were the proportion of patients maintaining Hb within ± 1 g/dl of baseline during the evaluation period and the incidence of red blood cell (RBC) transfusions during the dose-titration and evaluation periods. Additional efficacy parameters included mean Hb over time, mean Hb by study period, intrapatient Hb variability (mean within-patient standard deviations for Hb) during the dose titration, evaluation and safety observation periods and the number of patients requiring dose adjustments. Safety parameters included adverse event (AE) reporting, vital signs, laboratory haematology and blood chemistry (including iron), immunogenicity testing and electrocardiograms.
Assessments
Patients were assessed weekly during the screening/ baseline, dose-titration and evaluation periods, and every other week during the safety observation period. Hb levels, blood pressure, heart rate, iron status and other safety laboratory parameters were assessed throughout the study. Blood samples were collected for anti-erythropoietin antibody testing at weeks 1, 13, 29 and 41 and at the final visit.
AEs were recorded during the randomized treatment period and RBC transfusions and iron supplementation were recorded throughout the study.
Statistical analysis
A sample size of 264 patients (≥132 patients per treatment group) was required to test with 90% power the hypothesis that C.E.R.A. Q2W was non-inferior to DA (primary efficacy parameter). This assumes a true difference between the C.E.R.A. and DA groups not greater than 0.3 g/dl, a common standard deviation for both groups of 1.0 g/dl and a drop-out/major protocol violation rate of 20%.
Non-inferiority was tested by calculating the difference in the primary efficacy parameter (mean Hb change between baseline and the evaluation period) for patients randomized to treatment with C.E.R.A. and DA. Two-sided 95% confidence intervals (CIs) for this difference were calculated by analysis of covariance (ANCOVA), using treatment group as the independent variable and adjusting for covariates that might influence Hb response (baseline Hb and geographic region). Non-inferiority of C.E.R.A. versus DA could be concluded if the lower limit of the 95% CI was ≥−0.75 g/dl. In setting the −0.75 g/dl Hb threshold for the lower limit of the 95% CI (around the mean group difference), it was expected that the observed mean difference between the groups would be markedly <0.75 g/dl (according to the anticipated level of variability) and at a level not regarded as being clinically significant.
The primary efficacy analysis was conducted on the per-protocol (PP) population (patients without major protocol violations or withdrawal) and was confirmed on the intent-to-treat (ITT) population (all randomized patients).
Secondary and additional efficacy analyses were performed on the ITT population and summarized using descriptive methods. The last observation carried forward approach was used for missing data. In the case of RBC transfusions, the Hb value measured before the transfusion was used. Safety assessments, which included AE reporting, laboratory assessments of iron parameters and blood chemistry and monitoring of vital signs, were examined in the safety population (all patients who received at least one dose of study medication and had a safety follow-up assessment), and group summary statistics were calculated.
Results
Patients
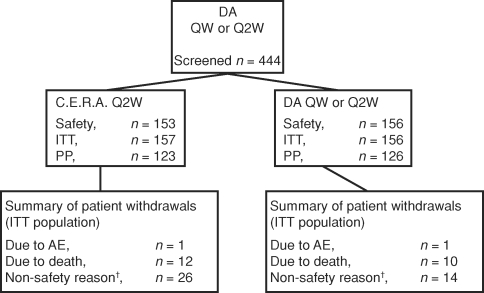
Patients were recruited from 48 centres in 12 countries in Europe, Australia and Canada. The disposition of enrolled patients is shown in Figure 2. The ITT population comprised 313 patients who were randomized to C.E.R.A. Q2W (n = 157) or DA at their continued weekly dose and interval (n = 156). The PP population comprised 249 patients. The main reasons for exclusion from the PP population were less than five Hb values measured during evaluation (n = 37), inadequate iron status at baseline and during evaluation or no valid iron assessments (n = 29) and RBC transfusions within weeks 20–32 (n = 17). In addition, four patients in the C.E.R.A. group withdrew before receiving any study medication [protocol violation (n = 1), refused treatment (n = 1), failed to return (n = 2)] and were not included in the safety population.
Fig. 2.
Patient populations and disposition. †Non-safety reasons were kidney transplantation (C.E.R.A., n = 14; DA, n = 6), refusal of treatment (C.E.R.A., n = 4; DA, n = 3) and failure to return (C.E.R.A., n = 2). The remaining 11 patients (C.E.R.A., n = 6; DA, n = 5) withdrew for reasons that included patient vacation, patient decision, patient instability (poor medical condition), protocol violation, discontinuation of dialysis, enrolled in nocturnal haemodialysis study and starting home dialysis. DA, darbepoetin alfa; QW, once weekly; Q2W, once every 2 weeks; ITT, intent-to-treat; PP, perprotocol; AE, adverse event.
Most patients (n = 249) completed the study. Reasons for premature withdrawal from the study included death (12 and 10 patients in the C.E.R.A. and DA groups, respectively), transplantation (14 and 6 patients treated with C.E.R.A. and DA, respectively), refusal of treatment (four patients in the C.E.R.A. group and three patients in the DA group) and AEs (one patient in each group).
Baseline characteristics were similar between treatment groups (Table 2) and were generally representative of the dialysis population. The primary causes of CKD in each treatment group were diabetes (24.0%), glomerulonephritis (23.0%) and hypertension/large vessel disease (21.4%) (Table 2). Before randomization, 82.2% of patients in the C.E.R.A. group and 84.0% DA group were receiving DA at weekly intervals (Table 2). There were slightly more male patients in the C.E.R.A. group (Table 2). Mean Hb levels at baseline were similar in patients randomized to C.E.R.A. (12.0 g/dl) and DA (11.9 g/dl). Most patients were undergoing haemodialysis with arteriovenous fistula for vascular access and had been receiving dialysis for ∼3.5 years (Table 2); one patient was receiving peritoneal dialysis (DA group). Arteriovenous grafts and catheters were slightly more common in the DA group (Table 2). Similar proportions of patients in each group received angiotensin-converting enzyme inhibitors and angiotensin-II receptor antagonists.
Table 2.
Baseline characteristics (intent-to-treat population)
| C.E.R.A. (n = 157) | DA (n = 156) | |
|---|---|---|
| Male, n (%) | 100 (64) | 81 (52) |
| Mean age, year (± SD) | 62.4 (16.17) | 61.8 (14.74) |
| Mean weight, kg (± SD) | 68.9 (16.69) | 70.32 (16.50) |
| Mean Hb, g/dl (± SD) | 12.0 (0.7) | 11.9 (0.7) |
| Median TSAT, % (IQR) | 28.4 (22.3–35.3) | 28.0 (21.6–33.5) |
| Median ferritin, μg/l (IQR) | 367.8 (216–547) | 382.3 (233–596) |
| Primary cause of CKD (incidence ≥5%) (%) | ||
| Diabetes | 25 | 22 |
| Glomerulonephritis | 25 | 21 |
| Hypertension/large vessel disease | 24 | 19 |
| Interstitial nephritis/pyelonephritis | 8 | 16 |
| Undefined aetiology | 6 | 9 |
| Secondary glomerulonephritis/vasculitis | 6 | 6 |
| Polycystic kidney disease | 4 | 5 |
| Other hereditary/congenital disease | 5 | <1 |
| Previous DA schedule, n (%) | ||
| QW | 129 (82.2) | 131 (84.0) |
| Q2W | 28 (17.8) | 25 (16.0) |
| Mean Kt/Va (± SD) | 1.57 (0.33) | 1.57 (0.34) |
| Vascular access type, n (%) | ||
| Arteriovenous fistula | 132 (84.1) | 117 (75.5) |
| Arteriovenous graft | 18 (11.5) | 26 (16.8) |
| Indwelling (tunnelled) catheter | 7 (4.5) | 12 (7.7) |
| Median time since first dialysis (days) | 1254 | 1319 |
aSafety population.
DA, darbepoetin alfa; SD, standard deviation; Hb, haemoglobin; TSAT, transferrin saturation; IQR, interquartile range; CKD, chronic kidney disease; QW, once weekly; Q2W, once every 2 weeks.
Efficacy evaluation
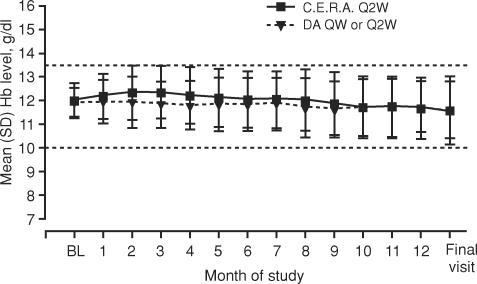
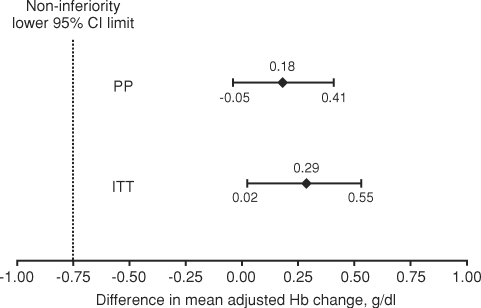
Mean Hb levels during the evaluation period were similar between treatment groups (Table 3; Figure 3): 12.1 g/dl for C.E.R.A. and 11.8 g/dl for DA (Table 3). Adjusted mean changes in Hb between baseline and the evaluation period (PP population) were 0.06 g/dl for C.E.R.A. and −0.12 g/dl for DA with the mean (95% CI) difference between groups being 0.18 g/dl (−0.05, 0.41) (Figure 4). The lower limit of the 95% CI was considerably greater than the pre-defined −0.75 g/dl non-inferiority threshold, demonstrating that C.E.R.A. was non-inferior to DA for the maintenance of Hb levels (P < 0.0001). Comparable results were obtained when the test was repeated in the ITT population (Figure 4).
Table 3.
Mean Hb by study period (per-protocol population)
| Period | n | Mean (SD) Hb level (g/dl) |
|---|---|---|
| Baseline (weeks −4 to 0) | ||
| C.E.R.A. | 123 | 12.0 (0.7) |
| DA | 126 | 11.9 (0.6) |
| Titration (weeks 0 to 28) | ||
| C.E.R.A. | 123 | 12.3 (0.7) |
| DA | 126 | 11.9 (0.7) |
| Evaluation (weeks 29 to 36) | ||
| C.E.R.A. | 123 | 12.1 (1.0) |
| DA | 126 | 11.8 (1.0) |
| Safety (weeks 37 to 52) | ||
| C.E.R.A. | 120 | 11.7 (1.1) |
| DA | 124 | 11.7 (1.0) |
DA, darbepoetin alfa; Hb, haemoglobin; SD, standard deviation.
Fig. 3.
Mean monthly Hb values (intent-to-treat population). Darbepoetin alfa, DA; QW, once weekly; Q2W, once every 2 weeks; Hb, haemoglobin; SD, standard deviation; BL, baseline.
Fig. 4.
Non-inferiority test for treatment differences. CI, confidence interval; ITT, intent-to-treat; PP, per-protocol; Hb, haemoglobin. P < 0.0001 for both comparisons.
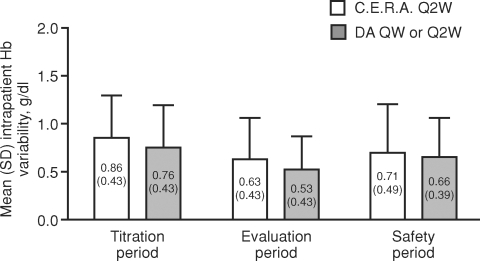
During the evaluation period, mean Hb values were maintained within +1 g/dl of baseline in a similar proportion of patients treated with C.E.R.A. and DA (65.5 and 71.8% of patients in the C.E.R.A. and DA groups, respectively, P = 0.2502, chi-square test). Assessment of the mean standard deviation of Hb revealed similar intrapatient Hb variability in the C.E.R.A. and DA groups during the titration (0.86 versus 0.76 g/dl), evaluation (0.63 versus 0.53 g/dl) and safety observation (0.71 versus 0.66 g/dl) periods (Figure 5). These findings are consistent with the primary efficacy assessment and support the view that Hb control was successfully maintained following conversion to C.E.R.A. in patients who were predominantly (>80%) receiving QW DA before randomization.
Fig. 5.
Individual stability of Hb mean levels (intent-to-treat population). No formal test was conducted to assess the statistical significance of between-group differences; however, the overlapping SD bars suggest any difference would not be significant. DA, darbepoetin alfa; QW, once weekly; Q2W, once every 2 weeks; Hb, haemoglobin; SD, standard deviation.
Iron parameters were well maintained in both treatment groups (Table 4). Concomitant iron supplementation was administered to 92% of patients treated with C.E.R.A. and 93% of those receiving DA. The most frequently used iron supplements were iron sucrose and ferrous gluconate.
Table 4.
Median ferritin and transferrin saturation by study period (safety population)
| Period | Median (IQR) ferritin (ng/ml) | Median (IQR) TSAT (%) |
|---|---|---|
| Baseline (weeks −4 to 0) | ||
| C.E.R.A. | 368 (216–543) | 28.2 (22.0–35.0) |
| DA | 382 (231–596) | 28.0 (21.9–33.5) |
| End of evaluation (week 36) | ||
| C.E.R.A. | 357 (223–569) | 29.0 (22.5–36.7) |
| DA | 402 (251–635) | 25.9 (21.0–32.0) |
| End of study (week 52) | ||
| C.E.R.A. | 405 (282–614) | 25.9 (21.0–32.0) |
| DA | 400 (241–577) | 25.8 (19.5–34.0) |
DA, darbepoetin alfa; IQR, interquartile range; TSAT, transferrin saturation.
Similar proportions of patients required at least one RBC transfusion during the dose-titration and evaluation periods: C.E.R.A. (12.4%) and DA (10.3%).
Assessment of doses of trial medication revealed that baseline median doses were 0.49 μg/kg/week [interquartile range (IQR): 0.43–0.73] for C.E.R.A. and 0.44 μg/kg/week (0.27–0.69) for DA. During the evaluation period, the median dose in the C.E.R.A. group was 0.35 μg/kg/week (0.19–0.54): this remained stable at the end of the study [0.35 μg/kg/week (0.16–0.58)]. The median dose of DA was 0.40 μg/kg/week (0.25–0.74) during the evaluation period and 0.44 μg/kg/week (0.28–0.81) at the end of the study. Similar proportions of patients (90.2% C.E.R.A. and 89.7% DA) required adjustments in the dose of study medication. The median (IQR) number of dose adjustments per patient throughout the entire study period was 6 (3–9) in the C.E.R.A. group and 5 (3–9) in the DA group.
Safety and tolerability
The overall incidences of AEs, serious AEs and AEs leading to withdrawal and deaths were similar between the two treatment groups and typical of the patient population (Table 5). At least one AE was experienced by 88% and 92% of patients in the C.E.R.A. and DA groups, respectively, although most events were mild or moderate in intensity. The most commonly reported AEs were diarrhoea, nasopharyngitis and influenza (Table 5). AEs leading to withdrawal occurred in one patient in each treatment group (hypertensive encephalopathy in the C.E.R.A. group and peripheral ischaemia in the DA group).
Table 5.
Overall and most frequent adverse events (≥8% of patients) (safety population)
| Number (%) | ||
|---|---|---|
| C.E.R.A. | DA | |
| (n = 153) | (n = 156) | |
| Diarrhoea | 24 (16) | 16 (10) |
| Nasopharyngitis | 19 (12) | 16 (10) |
| Influenza | 19 (12) | 12 (8) |
| Fluid overload | 14 (9) | 13 (8) |
| Cough | 14 (9) | 12 (8) |
| Hypertension | 13 (8) | 12 (8) |
| Back pain | 11 (7) | 13 (8) |
| Vomiting | 10 (7) | 13 (8) |
| Upper respiratory tract infection | 9 (6) | 12 (8) |
| Headache | 8 (5) | 12 (8) |
| Angina pectoris | 12 (8) | 7 (4) |
| Pyrexia | 12 (8) | 6 (4) |
| Any adverse event | 135 (88.2) | 143 (91.7) |
| Serious adverse events | 71 (46.4) | 75 (48.1) |
| Adverse events leading to withdrawal | 1 (0.7) | 1 (0.6) |
| Deathsa | 13 (8.5) | 12 (7.7) |
aIncludes three deaths of patients who had been withdrawn for other reasons.
DA, darbepoetin alfa.
Serious AEs were experienced by 46% and 48% of patients in the C.E.R.A. and DA groups, respectively. Serious AEs were considered to be related to study treatment in one patient in the C.E.R.A. group (arteriovenous graft thrombosis) and three patients in the DA group (arteriovenous graft thrombosis, arteriovenous fistula site haemorrhage and cerebral infarction).
Twenty-five deaths occurred in the study (13 C.E.R.A. patients and 12 DA patients; Tables 5 and 6), including three deaths of patients who had been withdrawn for other reasons (one patient in the C.E.R.A. group and two patients in the DA group). None of these deaths were considered to be related to study treatment. Table 6 shows all causes of death during the study. It should be emphasized that the majority of deaths in patients treated with both C.E.R.A. and DA were associated with maximum Hb levels <11 g/dl within the 2 weeks before death.
Table 6.
Listing of all deaths during the study ranked by maximum Hb level within the 2 weeks before death (safety population)
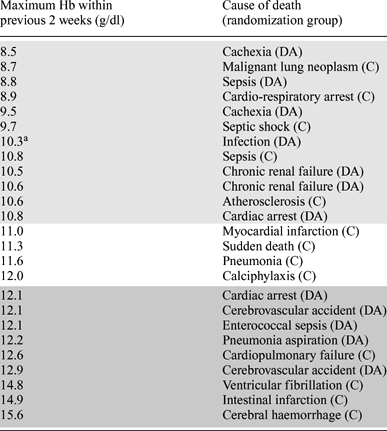 |
aMaximum Hb level within the 4 weeks before death. Light shading denotes maximum Hb levels <11 g/dl within the 2 weeks before death; no shading denotes maximum Hb levels between 11 and 12 g/dl; dark shading maximum Hb levels >12 g/dl.
C, C.E.R.A.; DA, darbepoetin alfa.
There were no clinically relevant changes in vital signs or laboratory parameters during the study period. Study drug-related antibodies were not detected in any patient.
Discussion
These findings from STRIATA demonstrate that patients receiving haemodialysis can be successfully converted to Q2W intravenous C.E.R.A. from intravenous DA maintenance therapy. In addition, the results confirm that the simple dosing conversion scheme based on previous DA dose was effective in providing stable Hb maintenance for patients converting from DA to Q2W C.E.R.A. Patients in STRIATA successfully maintained mean Hb levels within the 10.0–13.5 g/dl target range, with the primary efficacy analysis demonstrating non-inferiority of C.E.R.A. relative to DA (P < 0.0001) for Hb maintenance in patients receiving dialysis.
Important features of this study are the randomized, parallel-group design and the inclusion of patients with considerable co-morbidities, which can be considered representative of the general dialysis population. The study design was conceived and developed with the intention of testing the hypothesis that intravenous C.E.R.A. is non-inferior to intravenous DA for Hb maintenance in patients on dialysis. The study recruited patients who had undergone previous anaemia treatment to Hb levels within the target ranges recommended by contemporary guidelines [17,18], and the aim was to maintain Hb levels within similar target ranges following randomization.
Since the completion of STRIATA, new evidence has become available which has prompted safety concerns over the targeting of Hb levels >13 g/dl [19,20]. While no correlations between Hb concentrations and safety outcomes were observed in STRIATA, the study was not designed to address such questions. The results from STRIATA suggest that Q2W intravenous C.E.R.A. can maintain Hb levels within a specified target range as effectively as intravenous DA.
During the evaluation period, Hb was maintained within ±1 g/dl of baseline in approximately two-thirds of patients, and intrapatient Hb variability, a measure of individual stability of Hb levels, was comparable between treatment groups during the titration, evaluation and safety observation periods. It is noteworthy that while patients in the reference group, who had previously been maintained on DA, continued to be successfully maintained, those patients randomized to C.E.R.A. also maintained stable Hb levels despite the change in therapy and, in most patients, treatment interval.
RBC transfusions, which were assessed as a secondary efficacy parameter, were low and similar across treatment groups supporting the non-inferiority result obtained in the primary efficacy analysis. The observed transfusion rates of 10–12% are consistent with those reported by other investigators in a similar patient population [21]. C.E.R.A. was generally well tolerated, with a similar safety profile to that of DA. Most AEs were considered to be unrelated to study medication and were consistent with common AEs associated with this patient population. Considered together, these findings indicate that Q2W intravenous C.E.R.A. offers comparable Hb control with similar safety and tolerability to the comparator DA.
Anaemia is a common complication of CKD, and despite advances in care since the introduction of the first ESAs, further improvements in the proportions of patients achieving and maintaining guideline targets for Hb concentration are still possible [7,22]. Moreover, within the context of clinical practice, there is a need to optimize the efficiency of anaemia management to ensure that nephrology clinics can continue to provide effective care for a growing patient population. One approach to realizing such efficiency is to reduce the administration frequency of ESAs. Preclinical and pharmacokinetic studies indicate that the pharmacokinetic and pharmacodynamic profile of C.E.R.A. may enable administration at extended intervals [12,13]. This hypothesis is supported by the results from STRIATA, as well as other phase III [15,16] and phase II [14] clinical studies, in which intravenous and subcutaneous C.E.R.A. effectively maintained Hb levels in patients with CKD on dialysis when administered Q4W.
In summary, results from STRIATA confirm that stable Hb levels can be maintained with comparable variability in patients with CKD on haemodialysis who convert to intravenous C.E.R.A. Q2W from intravenous DA QW or Q2W. The time savings estimated to arise from reductions in administration frequency [10] could represent an important benefit in clinical practice, facilitating greater efficiency of anaemia management and enabling healthcare providers to devote more time to other aspects of patient care. Although the debate surrounding optimal Hb targets is ongoing and further evolution of anaemia treatment guidelines is anticipated, the findings from STRIATA should offer confidence that intravenous C.E.R.A. can maintain stable Hb levels within a pre-specified target range.
Acknowledgments
STRIATA was funded by F. Hoffmann-La Roche Ltd, Basel, Switzerland. Data analyses were conducted by the sponsor. Responsibility for opinions, conclusions and interpretation of data lies with the authors. Medical writing support during the preparation of this paper was provided by Emma Marshman, Mark Waterlow and Annete Njue-Doswell at Prime Medica Ltd, supported by F. Hoffmann-La Roche Ltd.
Conflict of interest statement. Data from this study have been presented previously in the following published abstract: Canaud B, Braun J, Locatelli F et al; on behalf of the STRIATA study investigators: Intravenous (IV) C.E.R.A. (continuous erythropoietin receptor activator) administered once every 2 week maintains stable haemoglobin (Hb) levels in patients with chronic kidney disease (CKD) on dialysis. Nephrol Dial Transplant 2006; 21: iv157 (abstract SP425).
Professor Canaud has received consultancy fees from Amgen, Baxter, F. Hoffmann-La Roche Ltd, Fresenius Medical Care, Janssen-Cilag, Medcomp and Shire; he does not hold stock in any company with interests in nephrology. The Institut de Recherche et de Formation en Dialyse affiliated to the Service de Néphrologie has received research grants from several companies including F. Hoffmann-La Roche Ltd.
Dr Mingardi has received honoraria and consultancy fees from F. Hoffmann-La Roche Ltd; he does not hold stock in any company with interests in nephrology. The Unita’ Operativa di Nefrologia e Dialisi, Ospedali Riuniti di Bergamo has received research grants from F. Hoffmann-La Roche Ltd.
Professor Braun has received honoraria and research grants from Amgen, F. Hoffmann-La Roche Ltd and Genzyme; he does not hold stock in any company with interests in nephrology. KFH-Dialysezentrums, Nuernberg, has received research grants from F. Hoffmann-La Roche Ltd.
Professor Aljama has received consultancy fees from Amgen, F. Hoffmann-La Roche Ltd, Janssen-Cilag and Shire; he does not hold stock in any company with interests in nephrology. The Hospital Reina Sofia, Servicio de Nefrologia, has received research grants from Amgen, F. Hoffmann-La Roche Ltd, Janssen-Cilag and Shire.
Professor Kerr has received honoraria and consultancy fees from Amgen Australia, Fresenius Medical Care and F. Hoffmann-La Roche Ltd; he does not hold stock in any company with interests in nephrology. The Department of Nephrology, Monash Medical Centre has received research grants from Amgen Australia and F. Hoffmann-La Roche Ltd.
Professor Locatelli has received honoraria and consultancy fees from Amgen, Astellas, F. Hoffmann-La Roche Ltd and Shire Pharmaceuticals; he does not hold stock in any company with interests in nephrology. The Divisione di Nefrologia e Dialisi, Azienda Ospedale di Lecco has received research grants from Amgen, F. Hoffmann-La Roche Ltd and Shire Pharmaceuticals.
Professor Villa has no financial relationships with any pharmaceutical company and does not hold stock in any company with interests in nephrology.
Dr Van Vlem has received honoraria and consultancy fees from Amgen, F. Hoffmann-La Roche Ltd, Janssen-Cilag and Novartis; he does not hold stock in any company with interests in nephrology. The Department of Nephrology, Dialysis and Hypertension, O.L. Vrouw Ziekenhuis has received research grants from Amgen, F. Hoffmann-La Roche Ltd, Janssen-Cilag and Novartis.
Dr McMahon has received honoraria and consultancy fees from Amgen, AstraZeneca, Bristol-Myers-Squibb, F. Hoffmann-La Roche Ltd, Ortho Biotech, Novartis and Sanofi-Aventis; he does not hold stock in any company with interests in nephrology. The University of Alberta Hospital has received research grants from Amgen, AstraZeneca, F. Hoffmann-La Roche Ltd and Ortho Biotech.
Ms Kerloëguen and Dr Beyer are employees of F. Hoffmann-La Roche Ltd; they do not hold stock in any company with interests in nephrology.
Appendix
STRIATA study investigators: P. Aljama, R. Bigazzi, A. H. Bock, J. Bonal Bastons, C. Bovy, J. Braun, B. Canaud, A. Cases, J. H. Christensen, J.-M. Cisterne, A. L. M. de Francisco, H. Dieperink, E. Gago, F. Giacardy, E. Goffin, T. Hannedouche, H. Holzer, P. Jaeger, J. Jastrzebski, D. Johnson, S. Jolly, L. Kairaitis, P. G. Kerr, V. Kliem, P. Koskinen, F. Locatelli, A. W. McMahon, G. Mingardi, G. Mortis, O. V. Oestergaard, G. Remuzzi, S. Roger, D. Sanz Guajardo, V. Savica, S. D. Soroka, M. Suranyi, J. M. Tabernero, C. Tielemans, P. Urena Torres, B. Van Vlem, G. Villa, B. von Albertini, R. Walker, C. Warholm, T. Weinreich, L. G. Weiss, C. Wijeyesinghe, G. K. T. Wong, J. M. Zacharias.
References
- 1.Li S, Foley RN, Collins AJ. Anemia, hospitalization, and mortality in patients receiving peritoneal dialysis in the United States. Kidney Int. 2004;65:1864–1869. doi: 10.1111/j.1523-1755.2004.00584.x. [DOI] [PubMed] [Google Scholar]
- 2.Regidor DL, Kopple JD, Kovesdy CP, et al. Associations between changes in hemoglobin and administered erythropoiesis-stimulating agent and survival in hemodialysis patients. J Am Soc Nephrol. 2006;17:1181–1191. doi: 10.1681/ASN.2005090997. [DOI] [PubMed] [Google Scholar]
- 3.Silverberg D. Outcomes of anaemia management in renal insufficiency and cardiac disease. Nephrol Dial Transplant. 2003;18(Suppl 2):ii7–ii12. [PubMed] [Google Scholar]
- 4.Muntner P, He J, Astor BC, et al. Traditional and nontraditional risk factors predict coronary heart disease in chronic kidney disease: results from the atherosclerosis risk in communities study. J Am Soc Nephrol. 2005;16:529–538. doi: 10.1681/ASN.2004080656. [DOI] [PubMed] [Google Scholar]
- 5.Gerson A, Hwang W, Fiorenza J, et al. Anemia and health-related quality of life in adolescents with chronic kidney disease. Am J Kidney Dis. 2004;44:1017–1023. doi: 10.1053/j.ajkd.2004.08.024. [DOI] [PubMed] [Google Scholar]
- 6.Perlman RL, Finkelstein FO, Liu L, et al. Quality of life in chronic kidney disease (CKD): a cross-sectional analysis in the Renal Research Institute-CKD study. Am J Kidney Dis. 2005;45:658–666. doi: 10.1053/j.ajkd.2004.12.021. [DOI] [PubMed] [Google Scholar]
- 7.Locatelli F, Pisoni RL, Akizawa T, et al. Anemia management for hemodialysis patients: Kidney Disease Outcomes Quality Initiative (K/DOQI) guidelines and Dialysis Outcomes and Practice Patterns study (DOPPS) findings. Am J Kidney Dis. 2004;44(Suppl 2):27–33. doi: 10.1053/j.ajkd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 8.Xue JL, Ma JZ, Louis TA, et al. Forecast of the number of patients with end-stage renal disease in the United States to the year 2010. J Am Soc Nephrol. 2001;12:2753–2758. doi: 10.1681/ASN.V12122753. [DOI] [PubMed] [Google Scholar]
- 9.Roderick P, Davies R, Jones C, et al. Simulation model of renal replacement therapy: predicting future demand in England. Nephrol Dial Transplant. 2004;19:692–701. doi: 10.1093/ndt/gfg591. [DOI] [PubMed] [Google Scholar]
- 10.De Cock E, Van Bellingham L, Standaert B. Assessing provider time for anaemia management of dialysis patients using time & motion methods: a multi-centre observational study in Europe. Value Health. 2002;5:581. [Google Scholar]
- 11.Macdougall IC. CERA (continuous erythropoietin receptor activator): a new erythropoiesis-stimulating agent for the treatment of anemia. Curr Hematol Rep. 2005;4:436–440. [PubMed] [Google Scholar]
- 12.Jarsch M, Brandt M, Lanzendörfer M, et al. Comparative erythropoietin receptor binding kinetics of C.E.R.A. and epoetin-beta determined by surface plasmon resonance and competition binding assay. Pharmacology. 2008;81:63–69. doi: 10.1159/000109166. [DOI] [PubMed] [Google Scholar]
- 13.Macdougall IC, Robson R, Opatrna S, et al. Pharmacokinetics and pharmacodynamics of intravenous and subcutaneous continuous erythropoietin receptor activator (C.E.R.A.) in patients with chronic kidney disease. Clin J Am Soc Nephrol. 2006;1:1211–1215. doi: 10.2215/CJN.00730306. [DOI] [PubMed] [Google Scholar]
- 14.Locatelli F, Villa G, de Francisco ALM, et al. on behalf of the BA16286 Study Investigators Effect of a continuous erythropoietin receptor activator (C.E.R.A.) on stable haemoglobin in patients with CKD on dialysis: once monthly administration. Curr Med Res Opin. 2007;23:969–979. doi: 10.1185/030079907x182103. [DOI] [PubMed] [Google Scholar]
- 15.Levin NW, Fishbane S, Valdés Cañedo F, et al. Intravenous methoxy polyethylene glycol-epoetin beta for haemoglobin control in patients with chronic kidney disease who are on dialysis: a randomised non-inferiority trial (MAXIMA) Lancet. 2007;370:1415–1421. doi: 10.1016/S0140-6736(07)61599-2. [DOI] [PubMed] [Google Scholar]
- 16.Sulowicz W, Locatelli F, Ryckelynck J-P, et al. on behalf of the PROTOS study investigators Once-monthly subcutaneous C.E.R.A. maintains stable hemoglobin control in patients with chronic kidney disease on dialysis and converted directly from epoetin one to three times weekly. Clin J Am Soc Nephrol. 2007;2:637–646. doi: 10.2215/CJN.03631006. [DOI] [PubMed] [Google Scholar]
- 17.Locatelli F, Aljama P, Bárány P, et al. European Best Practice Guidelines Working Group Revised European best practice guidelines for the management of anaemia in patients with chronic renal failure. Nephrol Dial Transplant. 2004;19(Suppl 2):ii1–ii47. doi: 10.1093/ndt/gfh1032. [DOI] [PubMed] [Google Scholar]
- 18.National Kidney Foundation K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease. 2000. Am J Kidney Dis. 2001;37(Suppl 1):S182–S238. doi: 10.1016/s0272-6386(01)70008-x. [DOI] [PubMed] [Google Scholar]
- 19.Singh AK, Szczech L, Tang KL, et al. CHOIR Investigators Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 20.Drueke TB, Locatelli F, Clyne N, et al. CREATE Investigators Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355:2071–2084. doi: 10.1056/NEJMoa062276. [DOI] [PubMed] [Google Scholar]
- 21.Tolman C, Richardson D, Bartlett C, et al. Structured conversion from thrice weekly to weekly erythropoietic regimens using a computerized decision-support system: a randomized clinical study. J Am Soc Nephrol. 2005;16:1463–1470. doi: 10.1681/ASN.2004080688. [DOI] [PubMed] [Google Scholar]
- 22.Port FK, Pisoni RL, Bragg-Gresham JL, et al. DOPPS estimates of patient life years attributable to modifiable hemodialysis practices in the United States. Blood Purif. 2004;22:175–180. doi: 10.1159/000074938. [DOI] [PubMed] [Google Scholar]