Abstract
Background. Despite recognized risks associated with hyperphosphataemia in patients with chronic kidney disease (CKD) Stage 5 on dialysis, the achievement of target levels of serum phosphate is poor. It is likely that this is partly due to poor adherence by patients to their phosphate-binder treatment regimens, which often comprise large daily tablet burdens.
Methods. In this multicentre, open-label trial, patients on a stable dialysis regimen were screened while receiving phosphate-binder therapy, then entered into a washout phase. Patients with serum phosphate > 1.78 mmol/L after washout entered into the main 12-week treatment phase (N = 367), during which they were treated to target [Kidney Disease Outcomes Quality Initiative (K/DOQI)]: 1.13–1.78 mmol/L; 3.5–5.5 mg/dL) with lanthanum carbonate monotherapy. Efficacy variables included serum phosphate levels and the percentage of patients with serum phosphate control. Safety and tolerability assessments were also conducted.
Results. Mean serum phosphate levels were significantly reduced following 12 weeks of lanthanum carbonate monotherapy versus previous phosphate-binder therapy. The mean number of phosphate-binder tablets being taken per day at screening was 7.6, but during treatment with lanthanum carbonate, most patients were taking doses of up to 3000 mg/day, achievable with 3 × 1000 mg tablets per day (maximum of 6).
Conclusion. These findings suggest that lanthanum carbonate monotherapy offers effective control of serum phosphate and, due to a low tablet burden, may help to simplify the management of hyperphosphataemia in patients with CKD Stage 5.
Keywords: dialysis, hyperphosphataemia, lanthanum carbonate, phosphate binder, tablet burden
Introduction
Hyperphosphataemia is an established risk factor for cardiovascular mortality, renal osteodystrophy and secondary hyperparathyroidism in patients with chronic kidney disease (CKD) Stage 5 on dialysis, and the need for adequate control of serum phosphate is well recognized [1–3]. Despite this, the evidence suggests that target levels of serum phosphate are not consistently being met. Indeed, the Dialysis Outcomes and Practice Patterns Study (DOPPS) revealed that less than half of the patients (40.8%) achieved the Kidney Disease Outcomes Quality Initiative (K/DOQI) target range for serum phosphate (1.13–1.78 mmol/L; 3.5–5.5 mg/dL) [4]. Given the demonstrated efficacy of phosphate binders in clinical trials [5–9], it is unlikely that the failure to achieve targets is entirely due to a lack of availability of effective treatments. A number of other factors may contribute to the poor achievement of serum phosphate targets, including lack of patient understanding of the consequences of hyperphosphataemia, variable dietary management and poor adherence to phosphate-binder treatment regimens.
Poor adherence to medication is an increasingly recognized problem among patients with CKD Stage 5 [10]. These patients are subject to large daily tablet burdens and may be prescribed >10 different medications [11]. Typically, the tablet burden can be expected to be two- to threefold higher than the total number of prescribed medications per day [12]. Without doubt, the cumulative effect of multiple dosing regimens can impose a confusing and possibly overwhelming burden on a patient. In addition, patients with CKD Stage 5 are often elderly and the condition has been associated with an increased rate of cognitive decline compared with individuals with preserved renal function [13]
Phosphate-binder therapy contributes a considerable proportion of the daily tablet burden for patients with CKD Stage 5, and for some binders, nine or more tablets are required per day to treat elevated serum phosphate levels in certain patients [14,15]. Furthermore, combination phosphate-binder therapy is commonly adopted in an attempt to minimize costs, avoid excessive elevation of serum calcium levels and achieve control of serum phosphate to target levels [1]. However, this practice may be counterproductive if it reduces the adherence of patients to their phosphate-binder regimens as a result of increased tablet burden or complexity of the treatment regimen.
Lanthanum carbonate (FOSRENOL®, Shire Pharmaceuticals, Basingstoke, UK) is an effective non-calcium-based phosphate binder [16,17], for which efficacy, safety and tolerability profiles have been demonstrated in both short- and long-term clinical studies [18–22]. Importantly, it has proven to be effective with a relatively low daily tablet burden, with the majority of patients requiring a single tablet taken during each meal [20].
In this prospective, multicentre, open-label study, we switched patients from their previous phosphate-binder therapy (which frequently consisted of more than one agent) to lanthanum carbonate monotherapy, in order to investigate the effect on serum phosphate control and dose requirements. Although this study was designed to assess phosphate control using higher doses of lanthanum carbonate in a clinical practice setting, other data were captured, including the tablet burden attributable to phosphate binders.
Subjects and methods
Inclusion/exclusion criteria
The study included male and female CKD Stage 5 patients aged at least 18 years, who had received dialysis for two consecutive months prior to the study and had a current requirement for treatment of hyperphosphataemia. Females of childbearing potential were required to have a negative serum pregnancy test prior to entering the study.
Patients were excluded from the study if they required continued treatment with aluminium-, calcium- or magnesium-containing compounds into the study treatment period. Exclusion criteria also included: significantly abnormal laboratory values, serum transaminases [alanine aminotransferase (ALT) and aspartate aminotransferase (AST)] elevated to more than three times the upper limit of normal, intact parathyroid hormone (PTH) >85 pmol/L (800 pg/mL) or clinically significant, uncontrolled concurrent illness.
Study design
This was a multicentre, open-label trial carried out in 49 centres across Europe, Israel and Canada, designed to assess whether daily doses of lanthanum carbonate > 3000 mg offer improved rates of serum phosphate control. Patient eligibility was assessed during a screening period of up to 1 week. A complete medical history was taken and demographic information recorded at this point, along with vital signs, pre-dialysis blood profile and concomitant medications.
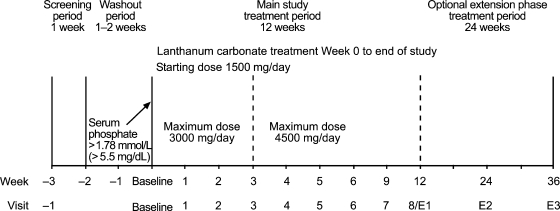
The study design is summarized in Figure 1. Patients receiving prior phosphate binder(s) discontinued their treatment and entered a 1- to 2-week washout period. Serum phosphate levels were reviewed after each week. Patients whose serum phosphate levels rose above 1.78 mmol/L were eligible to begin lanthanum carbonate treatment and considered to be at baseline at this point. Those who did not demonstrate this level of serum phosphate after 2 weeks were excluded from the study. Patients who were not receiving any phosphate-binder treatment and had serum levels above 1.78 mmol/L entered the treatment period immediately following the screening period.
Fig. 1.
Study design. Note: Week 12 was the end of study visit (visit 8) for patients who did not continue into the extension phase and was the first visit of the extension phase (visit E1) for patients who chose to continue into the extension phase. Week E3 was the end of study visit for patients who continued into the extension phase.
Patients began lanthanum carbonate treatment at a total daily recommended dose of 1500 mg/day (tablets containing 250, 500, 750 and 1000 mg elemental lanthanum were available). The daily dose was divided equally between meals, with tablets to be chewed and taken immediately after food, and could be increased by 750 mg/day each week to achieve optimal control of serum phosphate levels, to a maximum of 3000 mg/day by week 3 and 4500 mg/ day by week 5 (daily dose could also be decreased). The target serum phosphate range was 1.13–1.78 mmol/L (3.5–5.5 mg/dL), with investigators requested to aim for the middle of this range. Once target levels were reached, patients received stable dosage for the remainder of the treatment period.
The primary efficacy variable was the percentage of patients with controlled serum phosphate. The primary comparison was the control rate at week 3, when patients could have been titrated up to 3000 mg/day, compared with the rate at week 5, when patients could have been titrated up to 4500 mg/day.
Patients who completed the main 12-week study were eligible to continue into an optional 24-week extension phase. Patients entering the extension phase maintained their dose at week 12 levels but dosage could be adjusted at any time to achieve optimal control of serum phosphate levels.
Pre-dialysis vital signs, post-dialysis weight, concomitant medications and adverse events (AEs) were assessed at baseline, weekly for treatment weeks 1–6 and at weeks 9, 12 (end of main study), 24 and 36 (end of extension phase). For haemodialysis patients, blood samples were collected pre-dialysis at the first dialysis session of the week. For peritoneal dialysis patients, study visits were scheduled for a Monday or Tuesday. Full blood profiles were conducted and pre-dialysis plasma lanthanum levels assessed at baseline and weeks 3, 5, 12 and 36 (partial blood profiles were conducted at all other visits).
From the full blood profiles conducted at screening, baseline and at weeks 3, 5, 12 and 36, primary (serum phosphate) and secondary [calcium, intact PTH and calcium × phosphate product (Ca × P)] efficacy variables were assessed. For all patients, serum-intact PTH was measured using a DPC Immulite iPTH kit. Routine physical examinations and laboratory measurements were taken. Liver function tests were carried out, measuring ALT, AST, gamma glutamyl transferase (GGT), alkaline phosphatase and bilirubin levels.
Adverse events were recorded from the time that informed consent was signed until the end of treatment exposure and for the 30 days following the last exposure to the study medication. Treatment-emergent adverse events (TEAEs) were defined as those that occurred during this period and were not present prior to the first dose of study medication, or were present prior to the first dose of study medication but the severity increased during treatment. A serious adverse event (SAE) was defined as any untoward medical occurrence that resulted in death, was life-threatening, required hospitalization, resulted in persistent or significant disability/incapacity or was a congenital abnormality/birth defect. Investigators recorded whether they considered AEs/SAEs to be unrelated, possibly related or probably related to the study medication.
Patient populations
Safety population
Safety population included all patients who received at least one dose of study medication. Screening and baseline characteristics were reported for this population, as well as assessments of tolerability and safety.
Intention-to-treat (ITT) population
The primary efficacy population included all patients who received at least one dose of lanthanum carbonate and had at least one post-baseline phosphate measurement. Primary and secondary efficacy data were reported for this population.
Statistics
It was anticipated that at week 3 of lanthanum carbonate treatment (maximum dose 3000 mg/day), ∼50% of patients would be controlled, and that at higher doses (maximum dose 4500 mg, week 5), a clinically important effect would result in 55–60% of patients being controlled. It was estimated that 388 patients would be needed at week 3 and week 5 to provide 90% power to detect a minimum clinically important difference of 5% in control rates between doses, with 5% statistical significance determined using a two-sided test. Continuous variables were summarized using the number of observations, number of missing observations, mean, standard deviation (SD), 95% confidence interval (95% CI), median, minimum and maximum. The standard error (SE) was presented where appropriate. Categorical data were summarized using the number of observations, number of missing observations and percentages. Changes from screening were analysed using the one-sample t-test. McNemar's test of paired proportions was used to determine differences in control rates between weeks 3 and 5. All statistical testing was two sided and at the 5% level of significance. All estimates of treatment effect were presented with two-sided 95% CIs. The last observation carried forward (LOCF) analysis was applied for the main study (to week 12) and for patients who entered the optional extension phase (to week 36).
Results
Patient disposition
In total, 477 patients were screened, 367 of whom entered the main study. Of these, 366 received treatment, 274 completed the 12-week period, while 93 withdrew. Reasons for discontinuation in the main study are presented in Table 1.
Table 1.
Primary reasons for discontinuation from the main study
| Primary reason | Number of patients |
|---|---|
| Adverse events/serious adverse events | 37 |
| Patient request | 22 |
| Kidney transplant | 7 |
| Death | 4 |
| Calcium–phosphate product levels violated | 3 |
| Calcium levels violated | 2 |
| Non-compliance | 2 |
| Serum phosphate levels violated | 2 |
| Lost to follow-up | 1 |
| Other | 13 |
Two hundred and eleven patients entered the 24-week extension phase. Of these, 210 received treatment, 159 completed the study, while 52 withdrew. The most common reasons for discontinuation were AEs/SAEs (17 patients), patient request (13 patients) and kidney transplant (9 patients). TEAEs resulting in withdrawal from the study were reported for 9.5% of patients during the extension phase.
Patient demographics and other baseline characteristics
Baseline characteristics are shown in Table 2. The majority of patients entering the main study were male, Caucasian and had been on dialysis for 2 years or more [median (range) time since starting dialysis, 2.42 (0.2–33.3) years]. Approximately one-sixth had received renal transplants (16.1%) and the most frequent known causes underlying CKD Stage 5 were diabetes (23.2%) and glomerulonephritis (21.6%).
Table 2.
Baseline characteristics for all patients entering the main study and extension phase
| Characteristic | Main study n = 366 | Extension phase n = 211 |
|---|---|---|
| Sex, n (%) | ||
| Female | 115 (31.4) | 57 (27.0) |
| Male | 251 (68.6) | 154 (73.0) |
| Ethnic origin, n (%) | ||
| Caucasian | 307 (83.9) | 183 (86.7) |
| Black | 4 (1.1) | 4 (1.9) |
| Hispanic | 5 (1.4) | 4 (1.9) |
| Asian | 12 (3.3) | 4 (1.9) |
| Other | 2 (0.5) | 1 (0.5) |
| Missinga | 36 (9.8) | 15 (7.1) |
| Age and height, mean (SD) [range] | ||
| Age (years) | 59.5 (13.81) [24–85] | 57.9 (14.53) [27–85] |
| Weight (kg) | 73.1 (15.47) [33–128] | 74.1 (11.53) [33–128] |
aLocal legislation prevented study centres in France from recording ethnic or racial information.
Medication use at screening
The number of medications that patients were receiving at the screening visit ranged from 2 to 27 per patient (mean, 10.2).
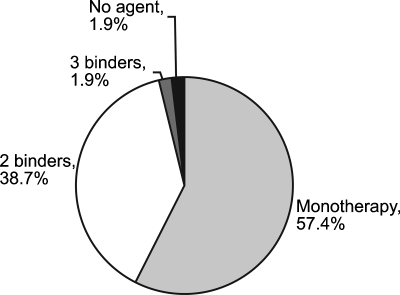
At screening, a large proportion of the ITT population (40.7%) were receiving more than one phosphate-binding agent. Another 57.4% were receiving monotherapy, and 1.9% were not receiving any phosphate binder (Figure 2). Calcium carbonate was the most commonly used binder (60.2%), followed by sevelamer hydrochloride (55.7%), calcium acetate (11.4%) and aluminium-based binders (10.9%). These figures demonstrate that combination phosphate-binder use was prevalent in this patient population. The mean total daily phosphate-binder tablet count for the ITT population in the main study, whether on monotherapy or combination therapy, was 7.6 tablets (range, 1–28). For patients who required one, two or three agents the mean (range) daily tablet count was 5.3 (1–15), 10.5 (2–28) and 13.1 (9–21) tablets, respectively.
Fig. 2.
Categorization of previous phosphate-binder therapy for the main study population (ITT population).
Phosphate levels and control rates
At screening, 34.9% of patients met the K/DOQI serum phosphate target range, falling to 7.0% after washout. After 3 weeks of lanthanum carbonate treatment, 39.1% of patients met the K/DOQI targets, with the control rate 43.6% at week 5. The percentage of patients with controlled serum phosphate at week 5 was observed to be higher than that at week 3, but it was not statistically significant and was less than the predefined level of clinical importance. Forty-eight percent of patients were controlled to K/DOQI by week 12, and this was statistically significant (P = 0.026) when compared with screening levels. Of those patients whose serum phosphate was not controlled on previous phosphate binder(s), 26.4% achieved control on lanthanum carbonate.
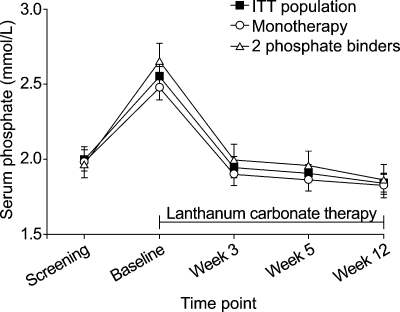
At screening, when patients were still using their previous phosphate binder, the mean (95% CI) serum phosphate level was 1.99 (1.92, 2.06) mmol/L. During the 1- to 2-week washout period, serum phosphate levels increased as expected, and then decreased with lanthanum carbonate treatment. At week 12, the mean (95% CI) serum phosphate level was 1.84 (1.78, 1.90) mmol/L. The mean reduction from screening in serum phosphate levels after 12 weeks (for patients with both screening and week 12 measurements) on lanthanum carbonate treatment was significant [mean (SE) change from screening, −0.13 (0.05) mmol/L; P = 0.007 using the one-sample t-test based on the 98.1% patients receiving phosphate binders at screening]. Furthermore, mean serum phosphate levels improved with 12 weeks of lanthanum carbonate monotherapy regardless of whether patients were previously receiving monotherapy (1.83 versus 1.98 mmol/L) or combination binder therapy (1.87 versus 1.97 mmol/L) (Figure 3).
Fig. 3.
Mean (95% CI) serum phosphate levels for the ITT population (n = 359) and patients previously treated with one (n = 204) or two (n = 137) phosphate binders.
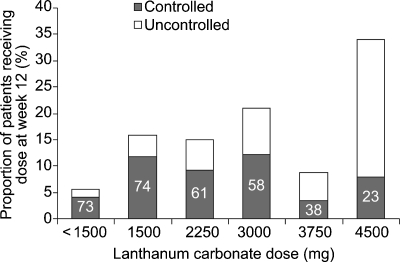
The distribution of dose levels at week 12 and percentages of patients controlled to K/DOQI targets at each dose are shown in Figure 4. Although the tablet burden was not recorded in this study, most patients (57.1%) were receiving doses that could be achievable with 3 tablets per day (≤ 3000 mg/day), with the remainder on doses achievable with 6 tablets per day (median dose, 3 tablets per day).
Fig. 4.
Distribution of lanthanum carbonate dose levels at Week 12 (ITT population). For each dose, the proportion of patients with serum phosphate levels controlled to K/DOQI targets is shown by the split bar; the number inside the ‘controlled’ bar demonstrates the percentage of patients controlled at that particular dose.
Of the 48% of patients whose serum phosphate levels fell within the target range by week 12, more than three quarters (77.1%) were taking daily lanthanum carbonate doses of ≤ 3000 mg.
Other mineral metabolism parameters
At screening, the mean (95% CI) serum total calcium level in the main study group was 2.35 (2.33, 2.37) mmol/L. Levels of calcium fell following washout to 2.27 (2.25, 2.29) mmol/L at baseline, but mean serum calcium rose back to a level similar to those observed at screening with lanthanum carbonate treatment [2.34 (2.32, 2.37) mmol/L at week 12]. Similar observations were made in patients taking part in the extension phase. Calcium levels corrected for albumin were almost identical to total calcium levels.
The mean (95% CI) level of Ca × P at screening was 4.65 (4.51, 4.80) mmol2/L2. Levels increased after washout to 5.77 (5.62, 5.93) mmol2/L2 at baseline, but then fell following commencement of lanthanum carbonate therapy to below those observed at screening [4.31 (4.16, 4.47) mmol2/L2 at week 12]. A similar change was seen in patients taking part in the extension phase.
At screening, the mean (95% CI) intact PTH level was 27.76 (25.29, 30.23) pmol/L but increased following the washout period to 38.63 (35.67, 41.59) pmol/L at baseline. Once lanthanum carbonate treatment began, mean PTH levels decreased [33.92 (31.14, 36.70) pmol/L at week 3] and remained relatively stable throughout treatment.
Compliance
The tablet intake was estimated using data detailing the amount of lanthanum carbonate dispensed, used and returned by each patient. The median compliance was 96.5%, suggesting that most patients adhered to their dosing regimens.
Concomitant use of calcium, cinacalcet and vitamin D supplementation
At baseline, the mean (SD) dialysate calcium concentration was 1.46 (0.40) mmol/L. Few patients required adjustments to dialysate calcium concentration (6.6%) or calcium supplementation (7.0%; single nighttime dose). Just over half (52.4%) of patients used a vitamin D preparation during the main study (45.7% of whom were treated with an active vitamin D derivative). The vitamin D schedules used were those of the routine clinical practice at each site and varied from occasional oral doses to intravenous administration at each dialysis session. No patient in the main study and one patient in the extension phase received concomitant cinacalcet treatment.
Safety and tolerability
Lanthanum carbonate treatment was generally well tolerated and caused no unexpected safety concerns. The TEAE profile was generally typical of a population with CKD Stage 5 receiving haemodialysis (Table 3). Although increasing the dose from 3000 to 4500 mg/day did not result in a statistically significant increase in the percentage of patients controlled, it had no effect on the number of TEAEs that occurred. Treatment emergent adverse events were reported for 145 (72.5%) and 114 (68.7%) patients receiving doses up to 3000 mg/day and >3000 mg/day, respectively.
Table 3.
Treatment-emergent adverse events (TEAEs) with an incidence of ≥5% in the main study (0–12 weeks) and extension phase (12–36 weeks), according to MedDRA preferred term
| Number (%) of patients | ||||
|---|---|---|---|---|
| MedDRA preferred term | Main study, N = 366 | Extension phase, N = 211 | ||
| Vomiting | 48 | (13.1) | 15 | (7.1) |
| Nausea | 45 | (12.3) | 9 | (4.3)a |
| Diarrhoea | 35 | (9.6) | 17 | (8.1) |
| Abdominal pain upper | 19 | (5.2) | 2 | (0.9)a |
| Nasopharyngitis | 24 | (6.6) | 23 | (10.9) |
| Total patients with ≥1 TEAE | 259 | (70.8) | 152 | (72.0) |
aValue included for completeness due to ≥5% incidence in other section of the study.
In the main study and extension phase, 14.2% and 7.6% of patients, respectively, reported SAEs. These were primarily infections, cardiac disorders and gastrointestinal disorders. Eight deaths occurred during the study, seven of which were considered to be unrelated to treatment. One was documented as possibly related to treatment, the most likely cause being reported as gastrointestinal bleeding caused by non-infectious enteritis, complicated by excessive anti-coagulation and possible myocardial infarction.
Treatment with lanthanum carbonate caused no clinically noteworthy important changes (clinical importance was determined by site investigators) in vital signs, haematological parameters or markers of liver function during the main study. Background plasma lanthanum levels were detectable at baseline and increased slightly following lanthanum carbonate treatment to 0.3–0.7 ng/mL during the main study and to 0.5–0.8 ng/mL during the extension phase. There was no evidence of dose dependence in these increases, and the levels are typical of those observed in other studies of lanthanum carbonate treatment [20,22].
Discussion
The data presented here demonstrate that patients can be treated with lanthanum carbonate monotherapy following either combination or monotherapy with other phosphate binders, and levels of serum phosphate are generally maintained or improved. There was no significant difference between the percentage of patients who achieved serum phosphate control at week 3 compared with week 5, when the maximum possible doses of lanthanum carbonate were 3000 mg/day and 4500 mg/day, respectively. The additional percentage of patients controlled on doses of 3750 and 4500 mg compared with 3000 mg was smaller than the pre-determined threshold for clinical importance. Hence, only a small proportion of patients benefited from increasing lanthanum carbonate doses above 3000 mg, supporting the current clinical practice of mainly utilizing doses in the region of 2250–3000 mg/day.
According to the protocol, titration of the dose of lanthanum carbonate up to 4500 mg/day was permitted in order to achieve control of serum phosphate to K/DOQI targets. Surprisingly, some patients did not achieve the K/DOQI target range for serum phosphate and yet were not titrated up to the maximum dose of 4500 mg/day, as permitted by and expected according to the protocol. With regard to dose titration, we consider that the behaviour of the investigators is likely to be related to their expectations for each particular patient. If one patient has achieved serum phosphate levels that the investigator considers to be a significant improvement, despite not achieving the specified target range, they may elect not to titrate further. It should also be considered that binding dietary phosphate alone cannot eradicate hyperphosphataemia. Phosphate may be released from the bone under the influence of PTH [24], and the patient must adhere to their dietary restrictions and dialysis regimen if phosphate control is to be optimal.
The medical history and disease characteristics of the patients enrolled in this study were typical of a dialysis population, but the data relating to concomitant medications highlight the considerable tablet burden that this disease generates. Patients were taking up to 27 different medications on entry to the study, many of which require administration more than once a day. Indeed, the tablet burden associated with phosphate-binder therapy alone was shown to be extensive, with between 1 and 28 tablets per day recorded at screening in this study.
Despite demonstrated use of phosphate binders in this population at screening, with a large proportion of patients receiving combination therapy, approximately two-thirds of patients (65.1%) had serum phosphate levels that were not controlled to K/DOQI targets. Given the efficacy that has been demonstrated in clinical trials [5,9], it can be speculated that this poor level of target achievement in clinical practice may be at least partly due to suboptimal levels of patient adherence to their phosphate-binder therapy. Failure of patients to adhere to their prescribed treatment regimens is believed to be related to both perceptual and practical barriers [23]. A poor understanding of what hyperphosphataemia is, and of the potential dangers of failing to control it adequately, coupled with a high daily tablet burden, would be expected to impact on the extent to which patients take their phosphate binders as prescribed. Minimizing the tablet burden associated with phosphate-binder therapy may help to improve the level of patient adherence to that treatment, as well as perhaps to other medications, by reducing the overall complexity of the daily dosing regimen.
The majority (77.1%) of patients who achieved serum phosphate control on lanthanum carbonate treatment did so on a dose of 3000 mg/day or less, which is equivalent to one tablet taken during each meal (3 per day). Given that the mean daily tablet burden attributable to phosphate-binder therapy prior to switching to lanthanum carbonate was 7.6 tablets, switching to lanthanum carbonate monotherapy clearly offered a reduction in average daily tablet burden in this study.
There is a tendency in practice to combine phosphate binders in an attempt to improve efficacy or minimize side effects, but this may be counterproductive, as combination phosphate-binder use can add to treatment complexity, which may in turn make it more difficult for patients to adhere. The reduction in tablet burden that lanthanum carbonate offers may therefore help to improve patient adherence, although a study of this design does not permit us to investigate this. Nevertheless, the data from this study support the practice of stopping other phosphate-binder therapy altogether and switching to lanthanum carbonate monotherapy, as tablet burden is reduced with no loss of efficacy or undesirable changes in serum calcium levels. Mean intact PTH levels were generally slightly above the upper limit of the K/DOQI-recommended range (16.5–33 pmol/L; 150–300 pg/mL) during treatment with lanthanum carbonate.
Treatment with lanthanum carbonate was generally well tolerated and did not generate any unexpected safety concerns compared with the screening population. The high affinity of lanthanum for phosphate ions and the low level of absorption from the gastrointestinal tract contribute to a minimal drug–drug interaction profile [17,25,26]
A limitation of this study was the lack of control group. However, a previous 6-month, randomized trial, comparing the efficacy of lanthanum carbonate with calcium carbonate, has demonstrated that lanthanum carbonate is an effective phosphate binder compared with standard therapy [19].
In conclusion, lanthanum carbonate as a monotherapy generated a statistically significant reduction in serum phosphate levels compared with previous phosphate-binder mono- or combination therapy in this study. In more than three quarters of patients who achieved serum phosphate control to K/DOQI targets during the main study, the dose at week 12 was not >3000 mg/day, which equates to just one tablet taken during each meal. These data demonstrate that switching to lanthanum carbonate monotherapy offers maintained or improved control of serum phosphorus with a reduced tablet burden. This may have positive implications for the adherence of patients with CKD Stage 5 to their phosphate-binder therapy.
Acknowledgments
We thank the following investigators for their contributions to the SPD405-313 clinical study. Oxford PharmaGenesis™ provided editorial support to the authors. The study was funded by Shire Pharmaceuticals.
Belgium: Dr J. C. Stolear, Réseau Hospitalier de Médicine Sociale, Tournai; Dr P. Arnouts, A. Z. Sint Jozef, Tournhout.
Canada: Dr R. Morton, Kingston General Hospital, Kingston; Dr M. Leblanc, Hôpital Maisonneuve-Rosemont, Montreal; Dr C. Lok, Toronto General Research Institute, Toronto; Dr P. Barre, Royal Victoria Hospital, Montreal; Dr A. B. Hodsman, St Joseph's Health Care Center, London; Dr G. Hercz, Humber River Regional Hospital, Toronto.
France: Professor M. Laville, Hôpital Edouard Herriot, Lyon; Professor B. Canaud, CHU – Hôpital Lapeyronie, Montpellier; Professor F. Berthoux, CHU St Etienne – Hôpital Nord, Saint-Etienne; Professor F. Mignon, Hôpital Bichat Claude Bernard, Paris; Dr V. de Precigout, Hôpital Pellegrin, Bordeaux; Dr. U. Torres, Clinique de l’Orangerie.
Germany: Professor H.-H. Neumayer, KfH Nierenzentrum, Berlin; Professor Dr. R. Brunkhorst, Klinikum Hannover, Hannover; Professor G. A. Müller, Georg-August-Universitat, Gottingen; Professor U. Kunzendorf, Universitätsklinikum Schleswig Holstein, Kiel; Professor Thaïs, UK Eppendorf, Hamburg; Professor H. Sperschneider, KfH Nierenzentrum, Jena; Professor R. Schmieder, KfH-Nierenzentrum, Nürnberg; Professor W. Dschietzig, Privates Dialysezentrum, Cottbus.
Greece: Dr C. Siamopoulos, University of Ioannina, Ioannina; Dr J. Papadakis, Hippokratio Hospital, Athens; Dr V. Vargemezis, General University Hospital of Alexandroupolis, Alexandroupolis.
Israel: Professor J. Silver, Hadassah Hospital, Jerusalem.
Italy: Professor F. Locatelli, Ospedale ‘A. Manzoni’, Lecco; Professor D. Brancaccio, Azienda Ospedaliera San Paolo, Milan; Professor F. P. Schena, Universitá degli Studi di Bari, Bari; Professor S. Mazzaferro, Divisione di Nefrologia Dipartimento Scienze Cliniche, Rome; Professor A. Albertazzi, Policlinico di Modena, Modena; Professor F. Malberti, Azienda Ospedaliera, Istituti Ospedalieri di Cremona, Cremona; Professor P. Messa, U.O. di Nefrologia e Dialisi, Milan.
Sweden: Dr A. Christenson, Universitetssjukhuset MAS, Malmo; Dr A. Alverstrand, Universitetssjukhuset Huddinge, Stockholm; Dr S. Jacobson, Universitetssjukhuset Solna, Stockholm.
Spain: Dr J. Montenegro, Hospital de Galdakao, Vizcaya; Dr V. Lorenzo, Hospital Universitario de Canarias, Tenerife; Dr D. Sanz-Guajardo, Hospital Puerta de Hierro, Madrid; Dr A. Palma Álvarez, Hospital Virgen de Macarena, Sevilla; Dr J. V. Torregrosa, Hospital Clinic I Provincal de Barcelona, Barcelona; Dr M. T. González, Hospital Bellvitge, Barcelona; Dr E. Grús, Fundación Hospital Alcorcón, Madrid.
UK: Dr A. Hutchison, Manchester Royal Infirmary, Manchester; Dr E. McGregor, Western Infirmary, Glasgow; Dr J. Bradley, Addenbrooke's Hospital, Cambridge; Dr D. Goldsmith, Guys and St. Thomas’ Hospital, London; Dr Medcalf, Leicester General Hospital, Leicester; Dr M. Thomas, Birmingham Heartlands Hospital, Birmingham.
Conflict of interest statement. A.J.H. and M.L. have received research funding from Shire Pharmaceuticals and are consultants to the company. Shire Pharmaceuticals also provided assistance with analysis and interpretation of the data. The results presented in this paper have not been published previously in whole or part, except in abstract format.
References
- 1.Eknoyan G, Levin A, Levin NW. Bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42:1–201. [Google Scholar]
- 2.Slatopolsky E, Delmez JA. Pathogenesis of secondary hyperparathyroidism. Am J Kidney Dis. 1994;23:229–236. doi: 10.1016/s0272-6386(12)80977-2. [DOI] [PubMed] [Google Scholar]
- 3.Block GA, Klassen PS, Lazarus JM, et al. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15:2208–2218. doi: 10.1097/01.ASN.0000133041.27682.A2. [DOI] [PubMed] [Google Scholar]
- 4.Young EW, Albert JM, Satayathum S, et al. Predictors and consequences of altered mineral metabolism: the dialysis outcomes and practice patterns study. Kidney Int. 2005;67:1179–1187. doi: 10.1111/j.1523-1755.2005.00185.x. [DOI] [PubMed] [Google Scholar]
- 5.Malluche HH, Monier-Faugere MC. Hyperphosphatemia: pharmacologic intervention yesterday, today and tomorrow. Clin Nephrol. 2000;54:309–317. [PubMed] [Google Scholar]
- 6.Almirall J, Campistol JM, Torras A, et al. Calcium carbonate as phosphate binder in dialysis. Lancet. 1987;2:799–800. doi: 10.1016/s0140-6736(87)92531-1. [DOI] [PubMed] [Google Scholar]
- 7.Clarkson EM, Luck VA, Hynson WV, et al. The effect of aluminium hydroxide on calcium, phosphorus and aluminium balances, the serum parathyroid hormone concentration and the aluminium content of bone in patients with chronic renal failure. Clin Sci. 1972;43:519–531. doi: 10.1042/cs0430519. [DOI] [PubMed] [Google Scholar]
- 8.Emmett M, Sirmon MD, Kirkpatrick WG, et al. Calcium acetate control of serum phosphorus in hemodialysis patients. Am J Kidney Dis. 1991;17:544–550. doi: 10.1016/s0272-6386(12)80496-3. [DOI] [PubMed] [Google Scholar]
- 9.Chertow GM, Burke SK, Lazarus JM, et al. Poly[allylamine hydrochloride] (RenaGel): a noncalcemic phosphate binder for the treatment of hyperphosphatemia in chronic renal failure. Am J Kidney Dis. 1997;29:66–71. doi: 10.1016/s0272-6386(97)90009-3. [DOI] [PubMed] [Google Scholar]
- 10.Horne R, Sumner S, Jubraj B, et al. Haemodialysis patients’ beliefs about treatment: implications for adherence to medication and fluid-diet restrictions. Int J Pharm Pract. 2001;9:169–175. [Google Scholar]
- 11.United States Renal Data System Annual Data Report 1998. Available at http://www.usrds.org/adr_1998.htm. (July 2007, date last accessed)
- 12.Mehrotra R. Case: Pill Burden and Adherence in Patients with End-Stage Renal Disease and Hyperphosphatemia. Available at http://www.medsitecme.com/(vugtx2npifdy04a4imsaxb55)/PrintCase.aspx?casestudyid=77&sceneid=1955. (29 May 2007, date last accessed)
- 13.Kurella M, Chertow GM, Fried LF, et al. Chronic kidney disease and cognitive impairment in the elderly: the health, aging, and body composition study. J Am Soc Nephrol. 2005;16:2127–2133. doi: 10.1681/ASN.2005010005. [DOI] [PubMed] [Google Scholar]
- 14.Genzyme Corporation Renagel Tablets (Sevelamer Hydrochloride) 400 & 800 mg Tablets; Prescribing Information. Available at http://www.renagel.com/docs/renagel_pi.pdf. (March 2007, date last accessed)
- 15.Fresenius Medical Care PhosLo® Capsules (Calcium Acetate)— Package Insert. Available at http://www.fda.gov/Cder/foi/nda/2001/021160_Phoslo_prntlbl.pdf. (April 2007, date last accessed)
- 16.Albaaj F, Hutchison AJ. Lanthanum carbonate for the treatment of hyperphosphataemia in renal failure and dialysis patients. Expert Opin Pharmacother. 2005;6:319–328. doi: 10.1517/14656566.6.2.319. [DOI] [PubMed] [Google Scholar]
- 17.de Freitas D, Donne RL, Hutchison AJ. Lanthanum carbonate–a first line phosphate binder? Semin Dial. 2007;20((4)):325–8. doi: 10.1111/j.1525-139X.2007.00301.x. [DOI] [PubMed] [Google Scholar]
- 18.Finn WF, Joy MS, Hladik G. Efficacy and safety of lanthanum carbonate for reduction of serum phosphorus in patients with chronic renal failure receiving hemodialysis. Clin Nephrol. 2004;62:193–201. doi: 10.5414/cnp62193. [DOI] [PubMed] [Google Scholar]
- 19.Hutchison AJ, Maes B, Vanwalleghem J, et al. Efficacy, tolerability, and safety of lanthanum carbonate in hyperphosphatemia: a 6-month, randomized, comparative trial versus calcium carbonate. Nephron Clin Pract. 2005;100:c8–c19. doi: 10.1159/000084653. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison AJ, Maes B, Vanwalleghem J, et al. Long-term efficacy and tolerability of lanthanum carbonate: results from a 3-year study. Nephron Clin Pract. 2006;102:c61–c71. doi: 10.1159/000088932. [DOI] [PubMed] [Google Scholar]
- 21.Joy MS, Finn WF. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis. 2003;42:96–107. doi: 10.1016/s0272-6386(03)00554-7. [DOI] [PubMed] [Google Scholar]
- 22.Finn WF. Lanthanum carbonate versus standard therapy for the treatment of hyperphosphatemia: safety and efficacy in chronic maintenance hemodialysis patients. Clin Nephrol. 2006;65:191–202. doi: 10.5414/cnp65191. [DOI] [PubMed] [Google Scholar]
- 23.Horne R. Beliefs and adherence to treatment: the challenge for research and clinical practice. In: Halligan PW, Aylward M, editors. The Power of Belief: Psychosocial Influence on Illness, Disability and Medicine. Oxford, UK: Oxford University Press; 2006. pp. 115–136. [Google Scholar]
- 24.Brown AJ, Slatopolsky E. Drug insight: vitamin D analogs in the treatment of secondary hyperparathyroidism in patients with chronic kidney disease. Nat Clin Pract Endocrinol Metab. 2007;3:134–144. doi: 10.1038/ncpendmet0394. [DOI] [PubMed] [Google Scholar]
- 25.Evans CH. Biochemistry of the Lanthanides. New York: Plenum; 1990. p. 219. [Google Scholar]
- 26.Pennick M, Dennis K, Damment SJ. Absolute bioavailability and disposition of lanthanum in healthy human subjects administered lanthanum carbonate. J Clin Pharmacol. 2006;46:738–746. doi: 10.1177/0091270006289846. [DOI] [PubMed] [Google Scholar]