Abstract
Background. Both the phenotypic alterations of parathyroid (PT) cells, e.g. down-regulation of the calcium-sensing receptor, and the increase of the PT cell number in nodular hyperplasia are the main causes of refractory secondary hyperparathyroidism. It is of great importance to prevent PT growth in an early stage.
Methods. To examine a more effective method of calcitriol therapy for the prevention of PT hyperplasia, we randomized haemodialysis patients with mild hyperparathyroidism to receive either daily orally administered calcitriol (n = 33) or intravenous calcitriol (n = 27) over a 12-month study period. Calcitriol was modulated so as to keep the serum intact PTH level between 100 and 150 pg/ml.
Results. Both groups showed similar reductions of the serum PTH level and similar increases in serum calcium. In both groups, there were no significant changes in the serum phosphate level. Long-term daily oral calcitriol therapy failed to prevent the increase of both maximum PT volume and total volume, as assessed by ultrasonography; however, intravenous calcitriol therapy successfully suppressed this progression. In the daily, oral group, both the bone-specific alkaline phosphatase (BAP) and the N-telopeptide cross-linked of type I collagen (NTX) significantly decreased, which was probably due to the PTH suppression. However, these bone metabolism markers remained stable in the intravenous group. The total dosage of calcitriol during the study was comparable in both groups.
Conclusions. These data indicate that intravenous calcitriol therapy in an early stage of secondary hyperparathyroidism is necessary to prevent PT growth and to keep a good condition of bone metabolism.
Keywords: calcium, calcitriol, growth, parathyroid, phosphate
Introduction
Secondary hyperparathyroidism is a serious complication in patients with renal failure. It has been reported that its aetiological factors include active vitamin D (VD) deficiency, hypocalcaemia and an excessive level of phosphate (Pi). To treat this disorder, VD supplementation and the administration of a Pi binder have been performed. However, the correction of these serological abnormalities does not inhibit the excessive secretion of parathyroid hormone (PTH) in some patients. Concerning parathyroid (PT) hyperplasia in humans, diffuse hyperplasia may histologically deteriorate to nodular hyperplasia [1]. In the latter, quantitative (an increase in the cell count) and qualitative [a decrease in the expressions of vitamin D receptors (VDR) and calcium-sensing receptors (CaSR)] changes are marked, which may contribute to the medical therapy-refractory condition [2,3,4,5]. There is a secondary hyperparathyroidism-related increase in the PTH level when the value of creatinine clearance becomes 30 ml/min or less. Usually, the PTH level is controlled with oral VD preparations in the early stage. Even after dialysis is started, therapy with oral VD preparations is continued in many Japanese patients with an intact PTH level of ∼300 pg/ml or less, although there are slight differences among hospitals. When PTH control is difficult, this therapy is switched to intravenous (IV) VD therapy. However, the control of serum calcium (Ca) and Pi levels gradually becomes difficult, making the continuation of intravenous VD therapy difficult. On echography, PT enlargement suggests nodular hyperplasia, often requiring parathyroidectomy and percutaneous ethanol injection therapy. Briefly, it is important to inhibit deterioration to nodular hyperplasia for long-term control of secondary hyperparathyroidism.
With respect to VD administration methods, previous clinical studies compared oral-pulse/daily-oral treatment with IV treatment [6,7,8,9,10,11,12]. However, a consensus regarding VD-related changes in the PT volume has not been reached. Fukagawa et al. [13,14] reported a decrease in the PT volume on echography after VD pulse therapy for 12 weeks. Quarles et al. [6] indicated that there were no changes in the PT volume on echography nor magnetic resonance imaging (MRI) after intravenous VD therapy. In the former study, the PT volume was relatively small; therefore, intravenous VD therapy in the phase of diffuse hyperplasia, with a small PT volume, may inhibit PT enlargement. In an experiment using 5/6 nephrectomized rats, bolus VD therapy immediately after renal failure induction inhibited PT enlargement [15,16].
Considering that conventional daily oral (DO) VD therapy does not inhibit PT enlargement, we conducted a prospective randomized study to investigate the inhibitory effects of intravenous VD therapy in the initial phase of secondary hyperparathyroidism, in which PT enlargement begins, on PT enlargement.
Methods
Patient selection
A total of 77 patients were recruited into the study from 23 haemodialysis units from January 2004 to November 2005. Entry criteria included a serum intact PTH concentration between 100 pg/ml and 300 pg/ml, and three times weekly haemodialysis for >6 months. Patients were excluded for the following reasons: prior treatment with calcitriol oral pulse or IV calcitriol, corrected calcium exceeding 10.5 mg/dl, serum phosphorus >6.5 mg/dl or serum alkaline phosphatase <115 U/l.
Study protocol
The study protocol consisted of an initial 2-week control period and a 12-month treatment period. All patients signed informed consent forms prior to entering the study. During the control period, all vitamin D oral drugs and injections were discontinued for 2 weeks. A total of 77 patients were initially enrolled. Of these patients, five failed to complete the entry criteria. Seventy-two patients were randomized to receive one of the following two protocols: daily orally calcitriol (DO group) or IV calcitriol (IV group, Rocaltrol® Injection: KIRIN Brewery Ltd., Tokyo, Japan). IV calcitriol was administered three times weekly at the end of each dialysis treatment procedure. The planned duration of treatment was 12 months. A restricted randomization technique was employed to assign these study participants to the treatment groups. At registration, we employed the minimization method and assigned the subjects to two groups so that there were no differences in gender nor the presence or absence of previous VD therapy. Using this method, 72 patients were randomized to the DO (N = 35) and IV (N = 37) group.
Calcitriol was started at an initial dose of 0.25 μg in the DO group and 0.5 μg in the IV group, respectively. The dose of calcitriol was increased or decreased by 0.5 μg/week to maintain the serum PTH level between 100 pg/ml and 150 pg/ml. Upward adjustments of calcitriol doses were not performed if the serum calcium and phosphorus levels exceeded 10.5 mg/dl and 5.5mg/dl, respectively. Severe hypercalcaemia (serum calcium >11.5 mg/dl for 2 months), a low PTH level (intact PTH <100 pg/ml for 2 months) and low bone metabolism markers (when all bone metabolism markers were below the lower limits of the normal ranges for 2 months) were treated by discontinuing calcitriol. If the cessation of calcitriol was necessary, it was restarted with reference to the dose before discontinuation. As a rule, sevelamer hydrochloride was used as a phosphate-adsorbing agent to maintain serum phosphate levels ranging from 3.5 to 5.5 mg/dl. However, when control with sevelamer hydrochloride alone was difficult, the agent was combined with other phosphate-binding agents (calcium carbonate, etc.). As a rule, the dialysate Ca level was established as 3.0 mEq/l. Combination therapy with VD preparations other than the test agent, ipriflavone, bisphosphonate or aluminium preparations was contraindicated. Patients in whom a corrected Ca level of >10.5 mg/dl, a phosphate level of >6 mg/dl or a Ca/phosphate product of >65 mg2/dl2 had persisted for 4 weeks were excluded from this study.
Of the 72 patients who entered the treatment protocol, 60 completed the planned 12 months. Two subjects requested to end the involvement for unspecified personal reasons; one patient was removed from the study because of uncontrolled hypercalcaemia; three subjects were excluded due to the deterioration of other diseases. The remaining patients were excluded from this study due to a lack of data.
Biochemical parameters
During the study protocol, serum-corrected calcium and phosphorus were measured weekly. Serum intact PTH and alkaline phosphatase were determined monthly, and both bone-specific alkaline phosphatase (BAP) and N-telopeptide cross-linked of type 1 collagen (NTX) were measured at the start and in the 3rd, 6th and 12th month. Serum FGF23 levels were assessed at the outset of this study. Serum calcium and phosphorus were measured by each dialysis unit. Serum BAP was measured by Osteolinks BAP (Quidel Corpora Serum, San Diego, CA, USA). Intact PTH was measured by the Allegro Intact PTH kit; Nichols Institute Diagnostics, San Juan Capistrano, CA, USA. Serum osteocalcin (OC) was measured by Osteocalcin Test Kokusai-F (International Reagents Corporation, Tokyo, Japan). Concerning OC, the sale of a measuring kit was discontinued during the study period, and this parameter was excluded. Serum NTX was measured by OSTEOMARK® NTx serum (Inverness Medical Professional Diagnostics, Princeton, NJ, USA). Serum FGF23 was measured by the FGF-23 ELISA Kit (KAINOS LABORATORIES, INC., Tokyo, Japan).
Parathyroid gland imaging
At the start of the study protocol and after 12 months of therapy, patients in both treatment groups were scheduled for imaging studies to assess the PT gland size. Of the 60 patients, pre- and post-imaging studies were successfully performed in 58 patients. The PT gland size was assessed by ultrasonography before and after the treatment. Most of measurements of the PT gland size were made by the authors (M. Taniguchi and M. Tokumoto). Eleven of 58 patients were assessed by the medical laboratory technician in a distant institution. The measuring methods were standardized as follows. The visualized glands were measured in the anterio-posterior (a), transverse (b) and longitudinal (c) dimensions to calculate PT volumes. We measured the PT size using the following formula:
Estimated PT volume = π/6 × a × b × c.
Statistical analysis
Data are expressed as the mean ± SD. All statistical analyses were conducted using the DOCTOR SPSS II program (SPSS Japan Inc., Japan). We used the Mann–Whitney U-test for unpaired non-parametric data and the Wilcoxon signed-ranks test for continuous variables, as appropriate. Similar comparisons of PT gland volume before and after treatment values within groups were done using the Wilcoxon signed-ranks test. Logistic regression models were applied to identify the factors that significantly affect the prognosis of PT gland enlargement. A P-value <0.05 was considered significant.
Results
Clinical features
As shown in Table 1, both the DO group and the IV group were similar with regard to age, gender, primary disease, haemodialysis duration, history of vitamin D therapy and dialysate calcium concentration. Concerning haematological markers, we measured the levels of corrected calcium, phosphate, alkaline phosphatase (ALP), intact PTH, BAP, OC and N-telopeptide cross-linked of type I collagen (NTX). There were no significant differences between the two groups. As the sale of an OC-measuring kit was discontinued during the study period, OC was excluded from the subsequent survey.
Table 1.
Clinical features
| OD group | IV group | P-value | |
|---|---|---|---|
| N | 33 | 27 | – |
| Age (years) | 66 ± 10 | 59 ± 12 | 0.235 |
| Gender (male/female) | 19/14 | 17/10 | 0.438 |
| Primary disease (DM/non-DM) | 5/28 | 2/25 | 0.309 |
| Haemodialysis duration (years) | 7.5 ± 5.9 | 7.1 ± 6.7 | 0.867 |
| History of vitamin D therapy (yes/no) | 25/8 | 22/5 | 0.583 |
| Dialysate calcium concentration | 2/31 | 7/20 | 0.065 |
| (2.5/3.0 mEq/l) | |||
| Biochemical parameters at entry | |||
| Corrected calcium (mg/dl) | 9.2 ± 0.7 | 9.3 ± 0.7 | 0.915 |
| Phosphate (mg/dl) | 4.6 ± 1.0 | 5.0 ± 1.0 | 0.126 |
| Alkaline phosphatase (U/l) | 236 ± 94 | 227 ± 65 | 0.101 |
| Intact PTH (pg/ml) | 230 ± 100 | 282 ± 167 | 0.209 |
| BAP (U/l) | 30 ± 13 | 26 ± 13 | 0.154 |
| OC (ng/ml) | 23 ± 13 | 19 ± 12 | 0.265 |
| NTX (nmol/l) | 137 ± 87 | 146 ± 92 | 0.818 |
| Parathyroid gland volume at entry | |||
| Maximum gland (mm3) | 49 ± 73 | 96 ± 215 | 0.298 |
| Total glands (mm3) | 65 ± 108 | 150 ± 292 | 0.169 |
Data are expressed as mean ± SD.
n, number of specimens; DM, diabetes mellitus; PTH, parathyroid hormone; BAP, bone-specific alkaline phosphatase; OC, osteocalcin; NTX, N-telopeptide cross-linked of type 1 collagen.
Changes in biochemical parameters
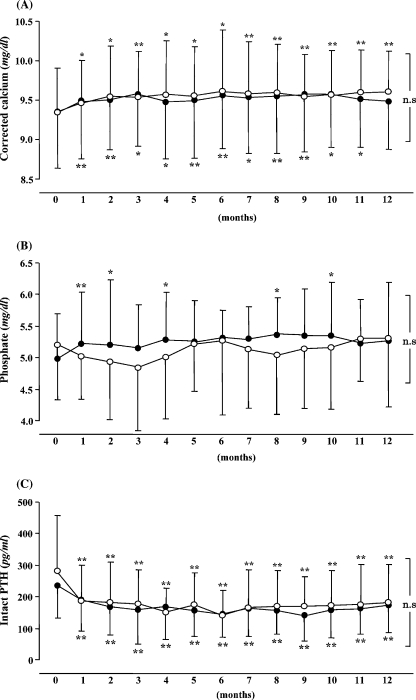
After administration, the serum-corrected Ca levels significantly increased in the two groups (Figure 1A). There were no changes in the serum Pi level in both groups (Figure 1B). In the two groups, the intact PTH levels significantly decreased after 1 month (Figure 1C). There were no significant differences in any parameters between the two groups. The total doses of VD during treatment were 91.5 ± 40.6 μg and 82.9 ± 31.2 μg in the DO and IV groups, respectively, with no significant difference. There were no differences in the doses of Ca carbonate and sevelamer administration between the two groups. No patient took calcimimetics.
Fig. 1.
Changes in serum biochemical parameters following treatment with daily oral and intravenous calcitriol for 12 months. (A) Effects of calcitriol therapy on serum-corrected calcium, (B) serum phosphate and (C) serum intact PTH concentrations. Calcitriol therapy was started at time zero. The daily oral (DO) group is represented by the closed circle ( ), whereas the intravenous (IV) group is represented by the open circle (◯). Data are expressed as mean ± SD. *P<0.05, **P<0.01 versus at time zero.
), whereas the intravenous (IV) group is represented by the open circle (◯). Data are expressed as mean ± SD. *P<0.05, **P<0.01 versus at time zero.
Parathyroid gland volume
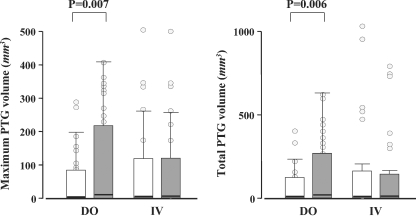
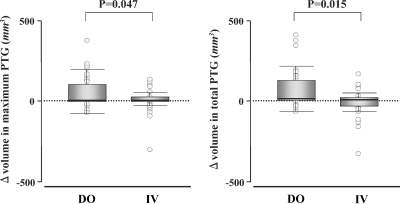
There were no significant differences in the maximum PT gland volume at the start of administration nor total volume between the two groups (Table 1). We additionally evaluated the PT volume 12 months after the start of administration (Figure 2). In the DO group, the maximum PT gland volume significantly increased (49 ± 73 mm3 → 101 ± 141 mm3, P = 0.007). In the IV group, there were no changes in the volume (96 ± 215 mm3 → 89 ± 170 mm3). In the DO group, the total volume also increased (65 ± 108 mm3 → 134 ± 196 mm3, P = 0.006). In the IV group, there was no significant increase (150 ± 292 mm3 → 135 ± 250 mm3). There was a significant difference in the change of the PT volume between the two groups (Figure 3). The changes of both maximum and total gland volume were significantly larger in the DO group than those in the IV group (P = 0.047 and 0.015, respectively).
Fig. 2.
Maximum and total parathyroid gland volume before (white bar) and after treatment (grey bar) with daily oral and intravenous calcitriol. DO, the daily oral group; IV, the intravenous group. Median values and interquartile ranges were given.
Fig. 3.
Changes of maximum and total parathyroid gland volume during the treatment with daily oral and intravenous calcitriol. DO, the daily oral group; IV, the intravenous group. Median values and interquartile ranges were given.
Examination of factors influencing parathyroid gland enlargement
In this study, we investigated factors influencing PT gland enlargement using a logistic regression model. Initially, in our univariate logistic regression analysis, the serum-corrected Ca and ALP levels were detected as factors influencing PT gland enlargement, as shown in Table 2 (P = 0.018 and 0.046, respectively). In particular, the odds ratio of corrected Ca was extremely high (3.028). Our multivariate analysis, in which the data were corrected with gender, primary disease, history of VD therapy and dialysate Ca concentrations, also showed that a higher serum-corrected Ca level at the start of administration promoted PT enlargement (Table 3). A study reported that FGF23 was a prognostic factor for refractory hyperparathyroidism [17]; therefore, we also reviewed this parameter. In our univariate analysis, the P-value of log FGF23 was 0.051, showing no significant difference. However, the number of patients was small, and we cannot rule out the possibility that FGF23 is a prognostic factor for PT enlargement.
Table 2.
Relative risk of parathyroid gland enlargement using univariate logistic regression models (n = 60)
| P-value | Odds ratio | 95% confidence interval | |
|---|---|---|---|
| Age (every 1 year) | 0.840 | ||
| Gender (male/female) | 0.266 | ||
| Primary disease (DM/non-DM) | 0.301 | ||
| Haemodialysis duration (every 1 year) | 0.224 | ||
| History of vitamin D therapy (yes/no) | 0.380 | ||
| Dialysate calcium (2.5/3.0 mEq/l) | 0.449 | ||
| Biochemical parameters at the start | |||
| Corrected calcium (every 1 mg/dl) | 0.018 | 3.028 | 1.214–7.552 |
| Phosphate (every 1 mg/dl) | 0.912 | ||
| Alkaline phosphatase (every 1 U/l) | 0.046 | 0.991 | 0.982–1.000 |
| Intact PTH (every 1 pg/ml) | 0.351 | ||
| BAP (every 1 U/l) | 0.185 | ||
| OC (every 1 ng/ml) | 0.650 | ||
| NTX (every 1 nmol/l) | 0.839 | ||
| FGF23 (every 1 ng/l) | 0.116 | ||
| Log FGF23 | 0.051 |
DM, diabetes mellitus; PTH, parathyroid hormone; BAP, bone-specific alkaline phosphatase; OC, osteocalcin; NTX, N-telopeptide cross-linked of type 1 collagen.
Table 3.
Relative risk of parathyroid gland enlargement using multivariate logistic regression models (n = 60)
| P–value | Odds ratio | 95% confidence interval | |
|---|---|---|---|
| Corrected calcium | 0.017 | 3.662 | 1.260–10.644 |
| (every 1 mg/dl) | |||
| Alkaline phosphatase | 0.043 | 0.987 | 0.975–1.000 |
| (every 1 U/l) |
Adjusted for gender, primary kidney disease, history of vitamin D therapy and dialysate calcium concentration.
Changes in bone metabolism markers
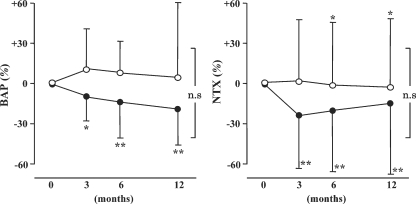
Markers of bone metabolism were expressed as the rates of change in BAP and NTX (Figure 4). In the DO group, the BAP level significantly decreased 6 months or more after the start of administration. However, there were no changes in the IV group. In the DO group, the NTX level significantly decreased 3 months or more after the start of administration (P <0.01). In the IV group, it significantly decreased after 12 months or more (P <0.05). However, the decrease was less marked than that in the DO group.
Fig. 4.
Changes in serum biochemical parameters following treatment with daily oral and intravenous calcitriol for 12 months. Effects of calcitriol therapy on serum bone-specific alkaline phosphatase (BAP) and N-telopeptide cross-linked of type 1 collagen (NTX). Calcitriol therapy was started at time zero. Data are expressed as mean ± SD. *P <0.05, **P <0.01 versus at time zero.
Discussion
In this study, intravenous VD therapy in the early stage inhibited the deterioration of PT hyperplasia. There was no significant difference in the total dose of calcitriol during the study period between the two groups; therefore, the difference in the administration method may have contributed to the inhibition of PT enlargement. These results suggest that intravenous VD therapy inhibits the deterioration from diffuse hyperplasia to nodular hyperplasia, a clinical problem. When the condition reaches nodular hyperplasia, its response to intravenous VD therapy is less marked due to quantitative (an increase in the cell count) and qualitative (decreases in the CaSR and VDR expressions) changes, requiring PT intervention in many cases. In our previous study regarding the PT gland after kidney transplantation, kidney transplantation did not cause quantitative nor qualitative changes in the nodular hyperplasia, but induced both quantitative (the enhancement of apoptosis) and qualitative (increases in the CaSR and VDR expressions) changes in the diffuse hyperplasia [18]. Briefly, these results suggest that intravenous VD therapy in the early stage maintains quality and quantity in diffuse hyperplasia, facilitating long-term conservative therapy. It may have any effect on the nodular hyperplasia qualitatively/quantitatively. An animal experiment showed that the bolus administration of VD in the early stage of renal failure inhibited PT hyperplasia, suppressing a decrease in VDR [15,19]. Furthermore, intravenous VD therapy may maintain the expression of CaSR, an aetiological factor for the medical treatment-refractory condition [20,21,22]. In another study that we performed, Ca/Pi control in patients with an intact PTH level of >300 pg/ml at the start of intravenous VD therapy was more difficult than in those with an intact PTH level of <300 pg/ml. It was also impossible to administer a sufficient dose of VD for a long period, requiring PT intervention in a high proportion of patients (data not shown). Poor Ca/Pi control may induce vascular calcification, leading to a poor prognosis. In contrast, the start of intravenous VD therapy in the phase of diffuse hyperplasia, in which the PTH level is low, may reduce the risk of PT intervention and achieve good Ca/Pi control, improving the prognosis.
In the present study, there were some important problems. Firstly, the target range of intact PTH level was comparably low. There was some risk of developing adynamic bone disease. In the K/DOQI guidelines [23], it is recommended that the intact PTH level in patients undergoing dialysis should be controlled to 150–300 pg/ml from the perspective of bone biopsy. When the intact PTH level exceeds 300 pg/ml, VD therapy, and if possible, intravenous VD therapy should be started. In Japan, the Japanese Society of Dialysis and Transplantation (JSDT) published guidelines on secondary hyperparathyroidism in 2006 [24]. It is recommended that the target intact PTH level should be established as 60–180 pg/ml with respect to the prognosis. In the adynamic bone disease, the reduced bone-buffer capacity would raise the serum levels of Ca and Pi, inducing vascular calcification. However, the present study revealed that both calcitriol therapies showed good Ca and Pi control (Ca × Pi product <55 mg2/dl2). This fact suggested that the target range of PTH level should be influenced by the prognosis. The second problem is the different effects to PT enlargement between daily, oral administration and IV administration. This finding was in agreement with previous studies [15], in which the prevention of PT enlargement in partial nephrecomized rat is more effectively suppressed when the same total dose of 1,25(OH)2D3 is given by intermittent bolus administration than by continuous infusion. The high peak serum levels of 1,25(OH)2D3 might be effective for preventing PT enlargement [25]; however, our study did not address this issue. The third problem is that the measurements of parathyroid gland size were made by some examiners in various centres. The accuracy of measurement might be unclear.
We investigated factors involved in PT enlargement using univariate and multivariate analyses. The results suggested the involvement of the serum Ca and ALP levels at registration. In particular, the former showed a high odds ratio. This may reflect a difference in the set point in the Ca–PTH relation curve. Briefly, the PTH level was high despite a high Ca level, suggesting that the condition had histologically reached nodular hyperplasia. Concerning the ALP level, the maintenance of the bone-buffer capacity may lead to a good Ca control. This may allow VD therapy at an increased dose, inhibiting PT enlargement. However, there was no such correlation with the BAP level; therefore, we cannot rule out the influence of liver dysfunction. Recently, Nakanishi et al. [17] reported that the blood FGF23 level was a prognostic factor for refractory hyperparathyroidism. However, in this study, there was no correlation between the blood FGF23 level and PT enlargement.
Previous studies have reviewed the actions of VD on bones. Many studies have reported that intravenous VD therapy relieved secondary hyperparathyroidism-related osteitis fibrosa [26,27,28]. However, it remains to be clarified which of the two factors, the direct actions of VD or a decrease in the PTH level, is involved in the action mechanism. In this study, in the DO group, bone absorption and formation significantly reduced. In the IV group, the bone formation marker was comparably constant, with no decrease, in spite of a decrease in the PTH level. Several in vitro studies indicated that VD enhanced ALP activity directly [29,30,31,32]. These facts suggest that the difference of VD treatment method may affect bone metabolism in vivo. However, we could not explain the discrepancy between the effects on bone.
This study showed that intravenous VD therapy in the early stage significantly inhibited the deterioration of PT hyperplasia. Intravenous VD therapy in the phase of diffuse hyperplasia may prevent the deterioration to nodular hyperplasia, avoiding parathyroidectomy. In addition, good control of the serum levels of PTH, Ca and Pi may inhibit vascular calcification, improving the prognosis. When echography shows PT enlargement in the presence of mild secondary hyperparathyroidism, intravenous VD therapy should be started in the early stage.
Acknowledgments
The authors wish to thank the investigators, staff and patients who participated in this study. The following investigators participated in this study: Ariyoshi T (Ariyoshi Clinic), Osato S (Osato Jin Clinic), Uchida M (Koyanagi Memorial Hospital), Kiyama S (Kiyama Naika), Kuma H (Kumajin Clinic), Nakamura S, Nakamura H (Kokura Daiichi Hospital), Tomiyoshi Y (Saga Prefectural Hospital Koseikan), Nakashima K (Sata Internal Circulatory Clinic), Shigematsu M, Koga Y (Shigematsu Clinic), Shimamatsu K (Shimamatsu Naika Iin), Shogakiuchi Y (Shin-ai Clinic), Hori K, Ogata C (Munakata Medical Association Hospital), Hattori F (Nagao Hospital), Katafuchi R (Gofukumachi Kidney Clinic, Harasanshin Hospital), Ohdera Y (Hirao Clinic), Fujimi S (Fukuoka Renal Clinic), Ikeda K, Kuroki Y (Fukuoka Red Cross Hospital), Hazuku A (Fujiyamato Spa Hospital), Maeda T (Maeda Hospital), Makino J (Makino Clinic), Miishima C (Miishima Clinic), Motomura K (Motomura Naika clinic) and Rikitake O (Rikitake Clinic).
Conflict of interest statement. None declared.
References
- 1.Tominaga Y, Takagi H. Molecular genetics of hyperparathyroid disease. Curr Opin Nephrol Hypertens. 1996;5:336–341. doi: 10.1097/00041552-199607000-00008. [DOI] [PubMed] [Google Scholar]
- 2.Kifor O, Moore FD, Jr, Wang P, et al. Reduced immunostaining for the extracellular Ca2+-sensing receptor in primary and uremic secondary hyperparathyroidism. J Clin Endocrinol Metab. 1996;81:1589–1606. doi: 10.1210/jcem.81.4.8636374. [DOI] [PubMed] [Google Scholar]
- 3.Gogusev J, Duchambon P, Hory B, et al. Depressed expression of calcium receptor in parathyroid gland tissue of patients with hyperparathyroidism. Kidney Int. 1997;51:328–336. doi: 10.1038/ki.1997.41. [DOI] [PubMed] [Google Scholar]
- 4.Fukuda N, Tanaka H, Tominaga Y, et al. Decreased 1,25-dihydro- xyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest. 1993;92:1436–1443. doi: 10.1172/JCI116720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rodriguez M, Caravaca F, Fernandez E, et al. Parathyroid function as a determinant of the response to calcitriol treatment in the hemodialysis patient. Kidney Int. 1999;56:306–317. doi: 10.1046/j.1523-1755.1999.00538.x. [DOI] [PubMed] [Google Scholar]
- 6.Quarles LD, Yohay DA, Carroll BA, et al. Prospective trial of pulse oral versus intravenous calcitriol treatment of hyperparathyroidism in ESRD. Kidney Int. 1994;45:1710–1721. doi: 10.1038/ki.1994.223. [DOI] [PubMed] [Google Scholar]
- 7.Bacchini G, Fabrizi F, Pontoriero G, et al. ‘Pulse oral’ versus intravenous calcitriol therapy in chronic hemodialysis patients. A prospective and randomized study. Nephron. 1997;77:267–272. doi: 10.1159/000190286. [DOI] [PubMed] [Google Scholar]
- 8.Peng SJ, Yang CS, Ferng SH, et al. A crossover comparison of intermittent oral and intravenous administration of calcitriol on the parathyroid hormone concentration in hemodialysis patients. Miner Electrolyte Metab. 1997;23:13–18. [PubMed] [Google Scholar]
- 9.Levine BS, Song M. Pharmacokinetics and efficacy of pulse oral versus intravenous calcitriol in hemodialysis patients. J Am Soc Nephrol. 1996;7:488–496. doi: 10.1681/ASN.V73488. [DOI] [PubMed] [Google Scholar]
- 10.Fischer ER, Harris DC. Comparison of intermittent oral and intravenous calcitriol in hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol. 1993;40:216–220. [PubMed] [Google Scholar]
- 11.Liou HH, Chiang SS, Huang TP, et al. Comparative effect of oral or intravenous calcitriol on secondary hyperparathyroidism in chronic hemodialysis patients. Miner Electrolyte Metab. 1994;20:97–102. [PubMed] [Google Scholar]
- 12.Indridason OS, Quarles LD. Comparison of treatments for mild secondary hyperparathyroidism in hemodialysis patients. Durham Renal Osteodystrophy Study Group. Kidney Int. 2000;57:282–292. doi: 10.1046/j.1523-1755.2000.00819.x. [DOI] [PubMed] [Google Scholar]
- 13.Fukagawa M, Okazaki R, Takano K, et al. Regression of parathyroid hyperplasia by calcitriol-pulse therapy in patients on long-term dialysis. N Engl J Med. 1990;323:421–422. doi: 10.1056/NEJM199008093230617. [DOI] [PubMed] [Google Scholar]
- 14.Fukagawa M, Kitaoka M, Yi H, et al. Serial evaluation of parathyroid size by ultrasonography is another useful marker for the long-term prognosis of calcitriol pulse therapy in chronic dialysis patients. Nephron. 1994;68:221–228. doi: 10.1159/000188261. [DOI] [PubMed] [Google Scholar]
- 15.Reichel H, Szabo A, Uhl J, et al. Intermittent versus continuous administration of 1,25-dihydroxyvitamin D3 in experimental renal hyperparathyroidism. Kidney Int. 1993;44:1259–1265. doi: 10.1038/ki.1993.377. [DOI] [PubMed] [Google Scholar]
- 16.Szabo A, Merke J, Beier E, et al. 1,25(OH)2vitamin D3 inhibits parathyroid cell proliferation in experimental uremia. Kindey Int. 1989;35:1049–1056. doi: 10.1038/ki.1989.89. [DOI] [PubMed] [Google Scholar]
- 17.Nakanishi S, Kazama JJ, Nii-Kono T, et al. Serum fibroblast growth factor-23 levels predict the future refractory hyperparathyroidism in dialysis patients. Kidney Int. 2005;67:1171–1178. doi: 10.1111/j.1523-1755.2005.00184.x. [DOI] [PubMed] [Google Scholar]
- 18.Taniguchi M, Tokumoto M, Matsuo D, et al. Persistent hyperparathyroidism in renal allograft recipients: vitamin D receptor, calcium-sensing receptor, and apoptosis. Kidney Int. 2006;70:363–370. doi: 10.1038/sj.ki.5001549. [DOI] [PubMed] [Google Scholar]
- 19.Denda M, Finch J, Brown AJ, et al. 1, 25-Dihydroxyvitamin D3 and 22-oxacalcitriol prevent the decrease in vitamin D receptor content in the parathyroid glands of uremic rats. Kidney Int. 1996;50:34–39. doi: 10.1038/ki.1996.283. [DOI] [PubMed] [Google Scholar]
- 20.Taniguchi M, Tokumoto M, Matsuo D, et al. Parathyroid growth and regression in experimental uremia. Kidney Int. 2006;69:464–470. doi: 10.1038/sj.ki.5000090. [DOI] [PubMed] [Google Scholar]
- 21.Brown AJ, Zhong M, Finch J, et al. Rat calcium-sensing receptor is regulated by vitamin D but not by calcium. Am J Physiol. 1996;270:F454–F460. doi: 10.1152/ajprenal.1996.270.3.F454. [DOI] [PubMed] [Google Scholar]
- 22.Canaff L, Hendy GN. Human calcium-sensing receptor gene. Vitamin D response elements in promoters P1 and P2 confer transcriptional responsiveness to 1,25-dihydroxyvitamin D. J Biol Chem. 2002;277:30337–30350. doi: 10.1074/jbc.M201804200. [DOI] [PubMed] [Google Scholar]
- 23.National Kidney Foundation K/DOQI Clinical Practice Guidelines. Am J Kidney Dis. 2003;42(Suppl 3):S1–S202. [PubMed] [Google Scholar]
- 24.Kazama JJ. Japanese society of dialysis therapy treatment guidelines for secondary hyperparathyroidism. Ther Apher Dial. 2007;11(suppl 1):S44–S47. doi: 10.1111/j.1744-9987.2007.00516.x. [DOI] [PubMed] [Google Scholar]
- 25.Salusky IB, Goodman WG, Horst R, et al. Pharmacokinetics of calcitriol in continuous ambulatory and cycling peritoneal dialysis patients. Am J Kidney Dis. 1990;XVI:126–132. doi: 10.1016/s0272-6386(12)80566-x. [DOI] [PubMed] [Google Scholar]
- 26.Andress DL, Norris KC, Coburn JW, et al. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989;321:274–279. doi: 10.1056/NEJM198908033210502. [DOI] [PubMed] [Google Scholar]
- 27.Cannella G, Bonucci E, Rolla D, et al. Evidence of healing of secondary hyperparathyroidism in chronically hemodialyzed uremic patients treated with long-term intravenous calcitriol. Kidney Int. 1994;46:1124–1132. doi: 10.1038/ki.1994.375. [DOI] [PubMed] [Google Scholar]
- 28.Akiba T, Kurihara S, Yamada T, et al. Intravenous calcitriol can increase bone mass in hemodialysis patients with osteitis fibrosa. Miner Electrolyte Metab. 1995;21:109–113. [PubMed] [Google Scholar]
- 29.Matsumoto T, Igarashi C, Takeuchi Y, et al. Stimulation by 1,25-dihydroxyvitamin D3 of in vitro mineralization induced by osteoblast-like MC3T3-E1 cells. Bone. 1991;12:27–32. doi: 10.1016/8756-3282(91)90051-j. [DOI] [PubMed] [Google Scholar]
- 30.Lomri A, Marie PJ, Tran PV, et al. Characterization of endosteal osteoblastic cells isolated from mouse caudal vertebrae. Bone. 1988;9:165–175. doi: 10.1016/8756-3282(88)90006-3. [DOI] [PubMed] [Google Scholar]
- 31.Manolagas SC, Burton DW, Deftos LJ, et al. 1,25-Dihydroxyvitamin D3 stimulates the alkaline phosphatase activity of osteoblast-like cells. J Biol Chem. 1981;256:7115–7117. [PubMed] [Google Scholar]
- 32.Halstead LR, Scott MJ, Rifas L, et al. Characterization of osteoblast-like cells from normal adult rat femoral trabecular bone. Calcif Tissue Int. 1992;50:93–95. doi: 10.1007/BF00297304. [DOI] [PubMed] [Google Scholar]