Abstract
Background. Japanese haemodialysis (HD) patients not only have a very low mortality and hospitalization risk but also low haemoglobin (Hb) levels. Internationally, anaemia is associated with mortality, hospitalization and health-related quality of life (QoL) measures of HD patients.
Methods. Longitudinal data collected from 1999 to 2006 from 60 to 64 representative Japanese dialysis units participating in the Dialysis Outcomes and Practice Patterns Study (DOPPS) were used to describe anaemia management practices and outcomes for Japanese HD patients.
Results. From 1999 to 2006, patient mean Hb increased from 9.7 g/dl to 10.4 g/dl, and the percentage of facilities with median Hb ≥10 g/dl increased from 27% to 75%. Hb was measured in the supine position for 90% of patients, resulting in substantially lower reported Hb values than those seen in other countries. As of 2006, erythropoietin (Epo) was prescribed to 83% of HD patients; mean Epo dose was 5231 units/week; intravenous (IV) iron use was 33% and median IV iron dose was 160 mg/month. Many patient- and facility-level factors were significantly related to higher Hb. A consistent overall pattern of lower mortality risk with higher baseline Hb levels was seen (RR = 0.89 per 1 g/dl higher Hb, P = 0.003). Facilities with median Hb ≥10.4 displayed a lower mortality risk (RR = 0.77, P = 0.03) versus facility median Hb <10.4 g/dl. Lower Hb levels were not significantly related to hospitalization risk, but were associated with lower QoL scores.
Conclusions. These results provide detailed information on anaemia management practices in Japan and the relationships of anaemia control with outcomes, with implications of anaemia management worldwide.
Keywords: anaemia, DOPPS, hospitalization, mortality, quality of life
Introduction
The number of dialysis patients in Japan has reached 250 000 [1], and Japan's prevalence of end-stage renal disease (ESRD) is one of the highest in the world. Many studies have shown that dialysis patients in Japan have a lower mortality and hospitalization risk than dialysis patients in other countries [2–4]. Several reasons for such a good prognosis among Japanese dialysis patients have been proposed, such as long treatment time for dialysis [5], greater use of arteriovenous fistulae for vascular access [6] and good nutritional status of patients [7].
Management of anaemia is an important factor associated with prognoses such as mortality, hospitalization and quality of life (QoL) in dialysis patients [8–10]. Japan's anaemia management stands in contrast to its low mortality rates: Japan has the lowest haemoglobin (Hb) level of all countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) [10], which may be attributable to the low dosages of erythropoietin (Epo) used in Japan and the influence of Japanese guidelines for treating renal anaemia [11]. To further improve the outcomes of Japanese dialysis patients, it is necessary to clarify the relationship between anaemia and prognosis in Japanese patients. In this paper, we examine DOPPS data on Japanese haemodialysis (HD) patients from three phases of the study (1999–2006). We describe many aspects of anaemia management practice in Japan and investigate whether baseline Hb levels are correlated with mortality, hospitalization and QoL of Japanese HD patients, as well as the relationship of pre-dialysis Epo treatment with the subsequent mortality risk.
Methods
Data sources
Assessment of anaemia management in Japan and related outcomes were based on data collected from randomly selected Japanese HD units participating in the DOPPS. These dialysis units were selected to be nationally representative of the various geographic regions in Japan, with 64 dialysis units participating in DOPPS I (1999–2001), 60 dialysis units in DOPPS II (2002–2004) and 61 facilities in DOPPS III (2006). In DOPPS I, 56% of facilities were private free-standing dialysis units, 42% were private hospital units and 2% were private general hospital units. The representation of dialysis units in DOPPS II was 43% from private units, 28% private hospitals, 3% private general hospitals, 3% private or national university hospitals and 22% official hospitals. The representation of dialysis units in DOPPS III was very similar to that seen in DOPPS II.
At each participating dialysis unit, baseline data on demographics, medical history, laboratory values, vascular access, anaemia measures, medications, vascular access and patient QoL were collected at the time of patient entry into the study. Thereafter, data on hospitalization, vascular access-related events, deaths and causes of death were collected throughout the study; data on longitudinal laboratory values and renal medication use were collected approximately every 4 months. Anaemia measures used for analysis included patient Hb concentrations, haematocrit, ferritin, transferrin saturation, iron, intravenous (IV) iron use, type and dose of Epo preparation and frequency of Epo administration. Nearly all DOPPS III data used for analyses were obtained before the 1 April 2006 change in the Japanese national policy for the reimbursement of Epo. Additional details of the DOPPS sampling plan and study methods have been previously described [12,13].
Statistical methods
Univariate analyses and t-tests were used to assess mean values and statistical significance concerning differences between groups. Logistic regression analysis was applied to investigate patient factors associated with the likelihood of having Hb >10.5 g/dl versus Hb ≤10.5 g/dl for patients with ESRD >180 days. Logistic models were adjusted for age, sex, body mass index (BMI), number of years with ESRD, country, study phase, serum levels of calcium, albumin, phosphorus and iron, and 13 summary comorbid conditions (coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, hypertension, diabetes, peripheral vascular disease, recurrent cellulitis/gangrene, lung disease, gastrointestinal bleeding within the previous 12 months, neurologic disease, psychiatric disorder and cancer). Vascular access type in use at study entry was found to be not significantly related to the outcomes and therefore was not included as a model covariate in the results shown. All logistic regression models used generalized estimating equations to account for clustering at the facility level, assuming a compound symmetry covariance structure. Mixed linear regression adjusted for patient characteristics and accounting for facility clustering effects was used to determine the relationship of Hb levels among patients with various patient factors and facility anaemia management practices.
Cox proportional hazards regression models were used to examine the relationship between mortality risk and a patient's baseline Hb concentration. Models were adjusted for age, sex, BMI, years with ESRD, single-pool Kt/V, phosphorus, calcium, albumin, the 13 summary comorbid conditions listed above and HIV/AIDS. Time at risk was defined as the time from patient entry into DOPPS until death, departure from the study or end of the study follow-up. The patient mortality risk was also evaluated with relationship to either the median Hb level of the patient's dialysis facility or whether the patient's dialysis facility had a median Hb level ≥10.4 g/dl (the midpoint of the DOPPS III Hb distribution in Japanese HD patients). Models using these predictors of facility-level practices were adjusted for the same factors as indicated above.
Cox models were also used to estimate the relationship between patient Hb concentrations and time to first hospitalization during the study period due to cardiovascular-related reasons, infection-related reasons or any cause. Cardiovascular-related hospitalization was defined as hospitalization due to or involving acute myocardial infarction, cardiac arrest, congestive heart failure, cardiomyopathy, valvular heart disease, atrial fibrillation, other arrhythmia, chest pain, hypertension, angina, cardiac catheterization, coronary angioplasty, coronary bypass graft, valve repair or replacement or cardioversion. Infection-related hospitalization was defined as hospitalization due to or involving septicaemia, pneumonia, endocarditis, meningitis, cellulitis/soft tissue infection and osteomyelitis. Hospitalization analyses were adjusted for the same factors used in mortality analyses. For the hospitalization analyses, time at risk was defined as the time from patient entry into DOPPS until first hospitalization event, departure from the study or end of the study follow-up. All Cox models used a robust estimator [14] to account for facility clustering.
Patient Hb concentrations were also related to patient mental and physical component summary scores for QoL (MCS, PCS), calculated from a standardized health-related SF-36 questionnaire that patients completed soon after study entry. Mixed linear regression was used to examine the associations between Hb concentrations and MCS and PCS scores. These models were adjusted for age, sex, years with ESRD, albumin and the 14 summary comorbidities noted above, and accounted for facility clustering effects.
All analyses were performed using SAS version 9.1 (SAS Institute, Cary, NC, USA) [15].
Results
The prevalence of selected patient comorbidities and other characteristics was determined at the start of each DOPPS phase covering the period from 1999 to 2006 (Table 1). The mean age of Japanese HD patients increased by 3.7 years over this period, from a mean of 58.7 years in 1999 to 62.4 years in 2006. Over the same period, the percentage of Japanese HD patients ≥75 years old rose from 11.1 to 17.8%, and the prevalence of diabetes mellitus and hypertension increased by 25–30%. The prevalence of cardiovascular disease also rose substantially: congestive heart failure rose fourfold, and coronary artery disease, peripheral arterial disease and other cardiovascular disease all rose by 40–50%. The prevalence of cancer and neurologic disease rose significantly too. Thus, the changes in anaemia control in Japan from 1999 to 2006 should be considered within the context of a patient population in which comorbidity burden and the fraction of elderly patients rose considerably over the same period.
Table 1.
Prevalence of selected patient characteristics for Japanese patients in the DOPPS, by study phase (1999–2006)
| DOPPS I (1999) | DOPPS II (2002) | DOPPS III (2006) | |
|---|---|---|---|
| n = 2157 | n = 1805 | n = 1826 | |
| Mean age (years) (±SD) | 58.7* (±12.4) | 61.1* (±13.0) | 62.4 (±12.5) |
| Male (%) | 61.9 | 59.9 | 60.1 |
| Mean years with end-stage renal disease | 7.45* | 7.06* | 8.02 |
| Mean body mass index (kg/m2) (±SD) | 20.4* (±2.86) | 20.6* (±3.03) | 21.0 (±3.21) |
| Diabetes as primary cause of ESRD (%) | 22.6* | 26.4* | 30.1 |
| Diabetes mellitus (any) (%) | 25.8* | 28.0* | 32.4 |
| Peripheral arterial disease (%) | 11.5* | 11.6* | 17.3 |
| Coronary artery disease (%) | 19.5* | 24.7* | 28.3 |
| Congestive heart failure (%) | 6.2* | 16.7* | 25.4 |
| Other cardiovascular disease (%) | 24.1* | 30.8 | 32.9 |
| Hypertension (%) | 56.4* | 66.3* | 72.9 |
| Cerebrovascular disease (%) | 12.6 | 14.4 | 13.4 |
| Lung disease (%) | 1.4* | 2.3 | 2.6 |
| Gastrointestinal bleeding in prior year (%) | 3.7 | 4.4 | 4.0 |
| Psychiatric disorder (%) | 2.6 | 3.4 | 3.6 |
| Neurologic disease (%) | 4.0* | 6.7* | 10.0 |
| Recurrent cellulitis/gangrene (%) | 2.1* | 3.4 | 4.0 |
| History of cancer (%) | 5.4* | 6.2* | 8.8 |
DOPPS, Dialysis Outcomes and Practice Patterns Study.
Results for each study phase based upon a prevalent cross-section of HD patients randomly selected for participation in DOPPS. Prevalence of comorbidities in a cross-section of patients with ESRD >180 days was very similar to that shown for all patients in the table.
*P < 0.05 for whether the DOPPS I or II value statistically differed from that of DOPPS III.
Hb levels in Japanese HD patients, 1999–2006
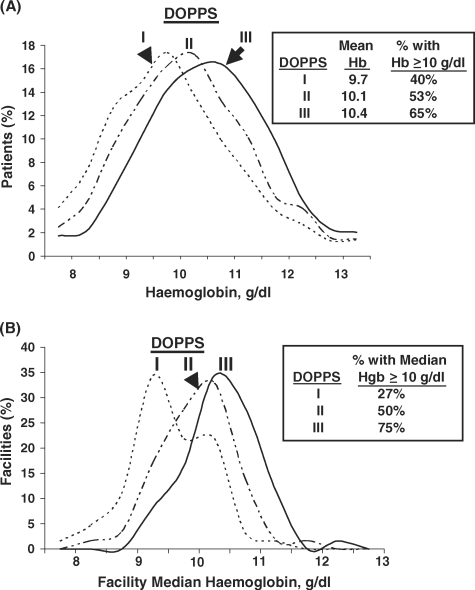
The distribution of Hb concentrations was determined for a prevalent cross-section of HD patients having chronic renal failure >180 days at the beginning of each DOPPS phase. As shown in Figure 1A, a substantial shift to higher Hb levels occurred over the period: The mean patient Hb concentration rose from 9.7 g/dl in 1999 to 10.4 g/dl in 2006 (P < 0.0001). The percentage of Japanese HD patients with Hb ≥10 g/dl increased from 40% in 1999 to 65% in 2006. Older HD patients were found to have considerably lower mean Hb concentrations in DOPPS III, with patients of age 18–44 having mean Hb of 10.73 g/dl compared with 10.58, 10.32 and 10.15 for patients aged 45–60, 61–75 and >75 years, respectively. Although significantly higher mean Hb levels were observed among younger Japanese HD patients, Epo use was significantly greater among older Japanese HD patients, ranging from 77 to 78% Epo use in patients 18– 60 years old versus 85% in 61–75 years old and 88% in patients >75 years old. No significant differences in weekly Epo dose or IV iron use were seen across these age groups.
Fig. 1.
(A) Distribution of haemoglobin concentrations among prevalent Japanese haemodialysis patients with ESRD >180 days in DOPPS I (1999), DOPPS II (2002) and DOPPS III (2006). The results for each study phase are based upon a point-prevalent cross-section of HD patients with ESRD >180 days in DOPPS I (n = 1996), DOPPS II (n = 1581) or DOPPS III (n = 1656). The standard deviation ranged from 1.35 in DOPPS I to 1.23–1.26 in DOPPS II and III. (B) Distribution of facility median haemoglobin concentrations among Japanese dialysis units in DOPPS I (1999), DOPPS II (2002), and DOPPS III (2006). Facility median haemoglobin concentrations were determined for a point-prevalent cross-section of HD patients having ESRD >180 days in each participating DOPPS dialysis unit at the start of each study phase; n = 64 facilities for DOPPS I, n = 60 for DOPPS II and n = 61 for DOPPS III.
In DOPPS III, information was also collected regarding the patient's position when blood samples were drawn for Hb measurement: 91% of patients were supine and 9% were seated. For 90% of prevalent patients in the DOPPS III cross-sectional sample, pre-dialysis session blood samples were taken on Monday or Tuesday, similar to that seen in DOPPS I and II (93%).
Anaemia control was also evaluated as a facility practice. Figure 1B shows the distribution of facility median Hb levels in participating facilities by study phase. In 1999, a bimodal distribution in the distribution of facility median Hb levels was evident, with only 27% of Japanese facilities having a median Hb concentration ≥10 g/dl. In contrast, by 2006, 75% of Japanese units had achieved a median Hb concentration ≥10 g/dl.
Multivariate logistic regression analyses (Table 2) were used to determine the likelihood of patients in prevalent cross-sections from each study phase to have Hb >10.5 g/ dl, the midpoint of the current recommended Hb target in Japan. Patients who were male or who had higher albumin or calcium levels were substantially more likely to have Hb >10.5 g/dl. In addition, patients with higher phosphorus or iron levels and patients with a higher BMI also displayed slightly higher odds of achieving Hb >10.5 g/dl, although these relationships were significant in only some of the study phases. In contrast to the above factors, patients who were older or had hypertension were significantly less likely to have Hb >10.5 g/dl. However, we found no significant differences in the likelihood of Hb >10.5 g/dl for patients 18–44 years old versus older age categories. Hypertensive patients were significantly less likely to achieve Hb >10.5, though they had a 1.3-fold higher adjusted odds of being given Epo (P = 0.03); they did not significantly differ from non-hypertensive patients in the weekly Epo dose level. When the adjusted logistic model was repeated using a cut point of 10.0 g/dl for patients in all DOPPS phases, the results obtained were very similar to those using a cut point of 10.5 g/dl.
Table 2.
Adjusted odds ratio (AOR) of having Hb >10.5 g/dl (versus ≤ 10.5) for Japanese HD patients with ESRD >180 days, by DOPPS study phase (1999–2006)
| AOR (Hb > 10.5 versus ≤ 10.5) | ||||
|---|---|---|---|---|
| Patient characteristic | DOPPS (all phases) | DOPPS I (1999) | DOPPS II (2002) | DOPPS III (2006) |
| (n = 4940) | (n = 1811) | (n = 1544) | (n = 1585) | |
| Male (versus female) | 1.41** | 1.55** | 1.35* | 1.35** |
| Serum albumin (per 0.5 g/dl higher) | 1.23** | 1.30* | 1.36** | 1.26** |
| Serum calcium (mg/dl) | 1.13** | 1.22** | 1.21** | 1.03 |
| Serum phosphorus (mg/dl) | 1.06** | 1.07 | 1.07 | 1.04 |
| Body mass index (per kg/m2) | 1.03* | 1.05* | 1.02 | 1.03 |
| Serum iron (per 5 μg/dl higher) | 1.02** | 1.00 | 1.05** | 1.02* |
| Age (per 10 years older) | 0.93* | 0.99 | 0.99 | 0.88** |
| Hypertension (yes versus no) | 0.78** | 0.67** | 0.82 | 0.83 |
DOPPS, Dialysis Outcomes and Practice Patterns Study.
Results for each study phase are based on a prevalent cross-section of HD patients having ESRD >180 days; AORs were estimated using logistic models that were adjusted for number of years with ESRD, coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, peripheral vascular disease, recurrent cellulitis/gangrene, diabetes, lung disease, GI bleed in prior year, neurologic disease, psychiatric disorder, cancer, and accounted for facility clustering effects; all-phase analysis was also adjusted for study phase. For patient factors included in the model but not shown in the table, no consistent significant relationship was seen with the odds of the patient having Hb >10.5 g/dl.
*P = 0.01–0.05, **P < 0.01.
Erythropoietin use
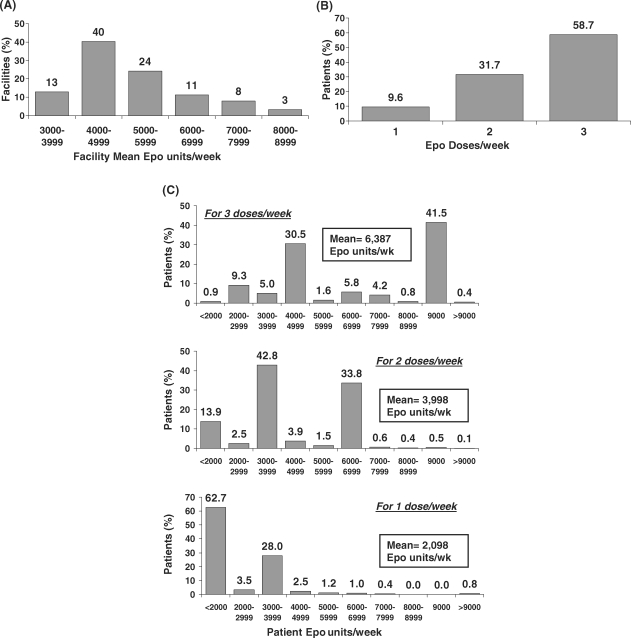
Table 3 shows results on Epo use, IV iron use and iron status. Epo was used by 83% of prevalent HD patients, with 99% of all such patients receiving it intravenously. The average Epo dose given to Japanese prevalent HD patients was 5176 units/week in DOPPS II and 5231 units/week in DOPPS III. However, the facility mean weekly Epo dose varied greatly across DOPPS III dialysis units (Figure 2A): 53% of facilities provided a mean Epo dose <5000 units/week, 24% a mean weekly dose of 5000–5999 units and 23% a mean weekly dose of 6000–9000 units.
Table 3.
Trends in anaemia and iron management therapies and measures for Japanese HD patients with ESRD >180 days, DOPPS II and III
| DOPPS II (2002) | DOPPS III (2006) | |
|---|---|---|
| n = 1634 | n = 1695 | |
| Epo use (%) | 83 | 83 |
| Mean Epoa (units/week) | 5176 | 5231 |
| Median Epoa (units/week) | 4500 | 4500 |
| IV iron use (%) | 32 | 33 |
| Mean total IV iron given per 4 months (mg) | b | 424 |
| Mean IV iron doses given per 4 months | 11.7 | 10.9 |
| % Patients for whom TSAT was reported | 41 | 45 |
| Mean (median) TSAT (%) (in facilities with ≥70% reporting of TSAT) | 27 (25) | 33 (26) |
| % Patients with TSAT<20% (in facilities with ≥70% reporting of TSAT) | 33 | 31 |
| % Patients for whom serum ferritin reported | 61 | 71 |
| Median serum ferritin (ng/ml) | 122 | 86 |
| % Patients with serum ferritin <50 ng/ml | 27 | 36 |
| % Patients with serum ferritin, 50–99 ng/ml | 17 | 18 |
| % Patients with serum ferritin, 100–499 ng/ml | 39 | 37 |
| % Patients with serum ferritin >499 ng/ml | 17 | 9 |
DOPPS, Dialysis Outcomes and Practice Patterns Study.
Data for each study phase based upon a prevalent cross-section of HD patients having ESRD>180 days.
aTo provide greater comparability between study phases, values >15 000 Epo units were excluded due to being far outside the typical distribution resulting in one value deleted in DOPPS III data and 23 from DOPPS II.
The DOPPS II mean of 5598 Epo units/week was obtained without excluding Epo values. Median Epo dose values were unaffected by this exclusion.
bDue to possible misinterpretation of study question, mean total IV iron data collected in DOPPS II were not used for analyses.
Fig. 2.
Epo dosing practices for Epo-treated HD patients in DOPPS III. (A) Distribution of facility mean Epo dose (Epo units/week) among DOPPS III dialysis units. Facility mean Epo dose (units/week) was calculated based on the number of Epo units/week given to Epo-treated patients from a point-prevalent cross-section of HD patients having ESRD >180 days in each participating DOPPS dialysis unit at the start of DOPPS III; n = 61 facilities. (B) Percentage of Epo-treated HD patients receiving one, two or three Epo doses per week in DOPPS III. Results are based upon a point-prevalent cross-section of Japanese HD patients having ESRD >180 days at the start of DOPPS III; n = 1412 patients. (C) Distribution of patient Epo doses (Epo units/week) in DOPPS III for patients receiving one, two or three Epo doses per week. Results are based on a point-prevalent cross-section of Japanese HD patients having ESRD >180 days at the start of DOPPS III; n = 1412 patients.
Substantial variability was also seen in the number of Epo doses given to patients per week (Figure 2B): 59% of patients received three doses, 32% received two doses and 10% received one dose. The Epo dosing patterns provided in Figure 2C display the most common weekly doses of Epo administered to Japanese HD patients and demonstrate the very large variation in Epo dosing strategies, from <2000 units/week to >9000 units/week.
Patient Hb concentrations were measured during the same month during which Epo use was reported, and indicated that, on average, patients with lower Hb levels were given a substantially greater average amount of Epo during the 4-week period (2193 more units of Epo given during 4 weeks for every 1 g/dl lower Hb, P < 0.0001; adjusted for demographics and 14 comorbidities, and restricted to patients with ESRD >180 days). The following month's Hb value was found to rise significantly in proportion to the amount of Epo given in the prior month (0.2 g/dl higher Hb rise per 2500 units/week more Epo given during the prior 4-week interval, P < 0.0001; adjusted for demographics, 14 comorbidities, IV iron given in the prior 4 months, and restricted to patients with ESRD >180 days). Over a 4-week period, most patients (∼90%) continued to receive the same number of Epo doses from week to week, except for nearly 15% of patients who were receiving one Epo dose per week in the first study week and had their prescriptions changed to two or three Epo doses per week by the fourth week of the study.
Patients receiving one Epo dose for each of the 4 weeks displayed an average decline of 0.33 g/dl in their mean Hb over a subsequent 3- to 5-week period (P < 0.0001). In contrast, patients receiving three Epo doses for each of 4 weeks displayed an average increase of 0.20 g/dl in their mean Hb (P < 0.0001). There was essentially no change in mean Hb over a 3- to 5-week period for patients receiving two Epo doses for each of the 4 weeks in the month prior to the second Hb measurement.
Intravenous iron use
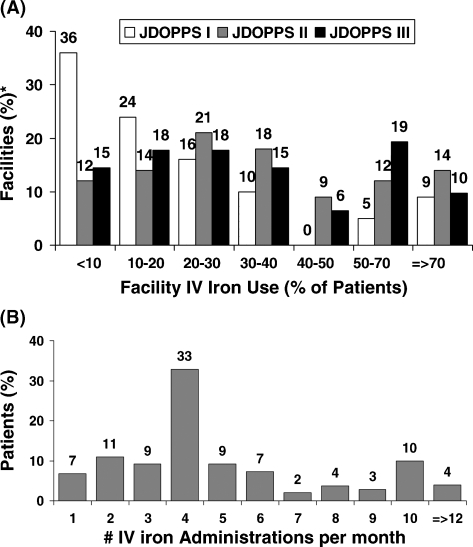
Intravenous (IV) iron use remained relatively constant in Japan, with 32% and 33% of prevalent HD patients receiving IV iron in 2002 and 2006, respectively (Table 3). However, as shown in Figure 3A, a wide variation is seen across Japanese dialysis units regarding the percentage of patients within a facility receiving IV iron. In 2006, 33% of Japanese dialysis facilities prescribed IV iron to <20% of facility patients during a 4-month period; 29% of facilities prescribed IV iron to ≥50% of patients during the same period.
Fig. 3.
(A) Distribution of facility IV iron use among Japanese dialysis units in DOPPS I (1999), DOPPS II (2002) and DOPPS III (2006). The percentage of patients within a facility using IV iron was determined for a point-prevalent cross-section of HD patients having ESRD >180 days in each participating DOPPS dialysis unit at the start of each study phase; n = 63 facilities for DOPPS I, n = 60 for DOPPS II and n = 61 for DOPPS III. (B) Distribution of the number of IV iron doses per month given to prevalent HD patients in DOPPS III. Results are based on a point-prevalent cross-section of Japanese HD patients having ESRD >180 days at the start of DOPPS III; analyses were restricted to patients receiving IV iron during the first month after study entry (n = 1336).
The number of IV iron doses administered monthly to patients varied greatly (Figure 3B). The median IV iron dose given to prevalent HD patients in Japan was 160 mg/month in DOPPS III, with ∼30% of iron recipients receiving this amount of IV iron. For the remaining patients receiving IV iron, 17% each were given 40–60 mg/month, 80– 150 mg/month, 180–280 mg/month or 320–520 mg/month.
Three major forms of IV iron were being given to HD patients in Japan in 2006: 46% of patients received saccharated ferric oxide (e.g. Fesin), 43% received a chondroitin sulfate/iron colloid mixture (e.g. Blutal) and 10% received cideferron (e.g. Ferricon). The most common IV iron dose amount was 40 mg/administration, given to ∼75% of Japanese HD patients receiving IV iron. Doses of 20 mg/administration were given to 6% of patients, and doses of 50 mg/administration were given to 10% of patients; this last group mostly received cideferron.
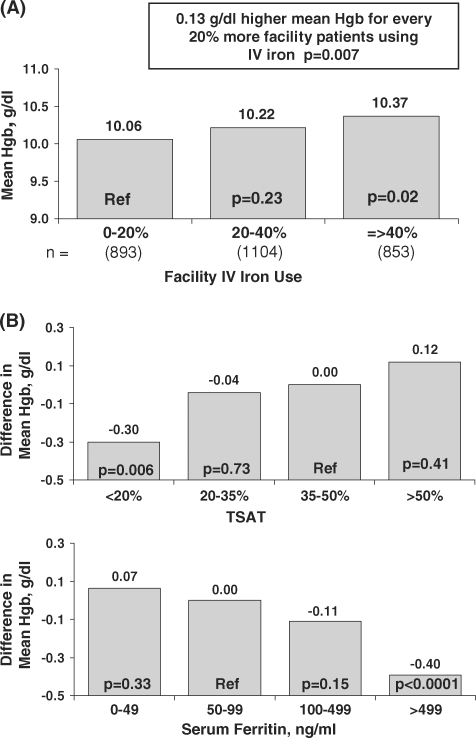
Mean Hb concentrations were found to be significantly higher in facilities administering IV iron to a larger fraction of patients (Figure 4A). Across all facilities, mean Hb concentrations were 0.067 g/dl higher for every 10% higher facility use of IV iron (P = 0.007).
Fig. 4.
Iron management and patient haemoglobin levels in DOPPS II and III. (A) Percentage of facility patients receiving IV iron and the mean haemoglobin concentration in Japanese HD patients having ESRD >180 days. The association between facility IV iron use and patient Hb levels was estimated using mixed linear regression models adjusted for age, sex, years with ESRD, coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, peripheral vascular disease, hypertension, recurrent cellulitis/gangrene, diabetes, lung disease, GI bleed in prior year, neurologic disease, psychiatric disorder, cancer, HIV and albumin. Facility IV iron use was calculated as the percentage of IV iron use in a cross-section of facility HD patients having ESRD >180 days in DOPPS II and III (n = 2850). (B) Difference in the mean haemoglobin concentration among patients by levels of ferritin (n = 3244) and percent transferrin saturation (TSAT) (n = 2850). The relationship was estimated using mixed linear regression models with the patient haemoglobin level as the outcome, adjusting for the same factors as in (A), as well as calcium, phosphorus and BMI. Results are based on a prevalent cross-section of DOPPS II and III HD patients having ESRD >180 days at the time of study initiation.
Iron management measures
Assessment of iron status was not routinely performed for all HD patients in Japan on a regular basis. In a point-prevalent cross-section of HD patients with ESRD >180 days, transferrin saturation (TSAT) was reported for only 41% and 45% of Japanese HD patients in DOPPS II and III, respectively; ferritin was reported for 61% and 71% of patients in DOPPS II and III, respectively (Table 3). However, Japanese facilities in DOPPS III differed greatly in whether TSAT or ferritin was measured. For example, both ferritin and TSAT were reported for ≥80% of patients at 39% of Japanese dialysis units. Ferritin but not TSAT was measured for ≥80% of patients at 21% of Japanese dialysis units. In contrast, at 16% of facilities, both TSAT and ferritin were measured for <20% of patients, with no TSAT, transferrin iron binding capacity or ferritin measurements reported for any patient in most of these facilities.
Multivariate regression analysis indicated that prevalent HD patients having TSAT <20% or with ferritin >499 ng/ml had significantly lower mean Hb concentrations (Figure 4B). As in previous international results from the DOPPS [10], higher ferritin concentrations were related to lower Hb concentrations across most of the ferritin concentration range.
Association of patient Hb levels with mortality risk, hospitalization risk and QoL
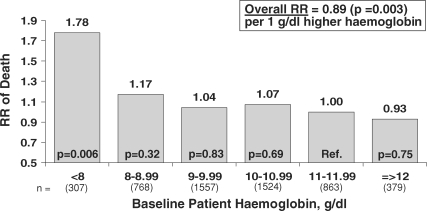
Crude mortality rates were observed to be higher in patients with lower baseline Hb levels ranging from 10.2% for patients with Hb <8 g/dl to 7.2% with Hb 8–9 g/dl and 5.0%–5.3% for Hb levels >9 g/dl. In multivariate analyses adjusted for numerous patient characteristics and laboratory values, higher baseline Hb levels were associated with a lower overall mortality risk when data from DOPPS I, II and III were combined: the relative risk (RR) of death was 11% lower for every 1 g/dl higher Hb concentration (P = 0.003) when all patient Hb values were considered (Figure 5). Although the mortality risk was consistently seen to be lower for patients with higher Hb levels, mortality risk was especially high for patients with Hb <8 g/dl (RR = 1.78, P = 0.006 versus Hb 11–12 g/dl). For patients with baseline Hb ≥8 g/dl, a 6% lower mortality risk was observed for every 1 g/dl higher Hb (P = 0.20). The mortality risk did not significantly differ for patients with Hb 8–11 g/dl or >12 g/dl compared to patients with Hb 11– 12 g/dl.
Fig. 5.
Baseline patient haemoglobin levels and subsequent mortality risk. Cox regression models were used to estimate the relationship between the mortality risk and levels of patient Hb either as a continuous variable (inset) or as categories of Hb. Results are based upon data combined from DOPPS phases I, II and III for patients having ESRD >180 days, and were adjusted for age, sex, BMI, years with ESRD, coronary artery disease, congestive heart failure, other cardiovascular disease, cerebrovascular disease, peripheral vascular disease, hypertension, recurrent cellulitis/gangrene, diabetes, lung disease, GI bleed in prior year, neurologic disease, psychiatric disorder, cancer, HIV, single-pool Kt/V, phosphorus, calcium, albumin, study phase and facility clustering effects (n = 5398). Mean study follow-up time was 1.53 years. When limiting the analysis to Hb ≥8 g/dl, the RR was smaller [RR = 0.94 per g/dl higher Hb, P = 0.20 (n = 5091)].
Hb control was also analysed as a facility practice. A trend towards the lower mortality risk was observed for patients treated in facilities having higher median Hb levels, although this overall relationship did not reach statistical significance in the fully adjusted Cox models (RR = 0.91 per 1 g/dl higher facility median Hb concentration, P = 0.24). However, patients treated in facilities with a median Hb concentration equal to or greater than the DOPPS III mean of 10.4 g/dl (n = 1381) displayed a 23% lower mortality risk (P = 0.03) than patients in facilities having a median Hb concentration <10.4 g/dl (n = 4017) in Cox models with full adjustment. In a separate analysis, no significant relationship was observed between the change in the country mean Hb level and country mortality rate in Japan from 1999 to 2006 (results not shown).
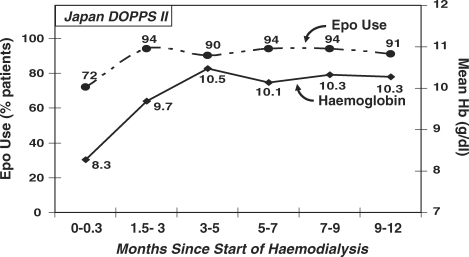
Data from a Japanese cohort of patients new to ESRD were analysed to determine the mean Hb level for patients when initiating HD, and how the level changed during the first year of HD. Patients initiated HD with a low mean Hb concentration of 8.3 g/dl, suggesting poor anaemia management before the onset of ESRD (Figure 6). Within 1.5–3 months of beginning HD, >90% of these incident HD patients were given Epo; the mean Hb level of this incident patient cohort rose from 8.3 g/dl to 10.5 g/dl within 3–5 months after beginning HD. Among Japanese patients entering DOPPS within 90 days of ESRD onset, the risk of mortality was significantly lower for patients who had received Epo before beginning HD (RR = 0.51, P = 0.04) than for those not receiving Epo, after adjusting for age, sex, race, time since ESRD onset, 14 comorbidities, facility clustering effects and whether the patient had been seen by a nephrologist >30 days before ESRD onset (N = 918). No significant association was observed between a patient's baseline Hb concentration and risk of all-cause hospitalization, cardiovascular-related hospitalization or infection-related hospitalization.
Fig. 6.
Time trend in Epo use and mean haemoglobin concentration for new ESRD patients after initiating haemodialysis. The percentage of patients receiving Epo and the mean haemoglobin concentration were determined at 6 points during the first year of haemodialysis for a cohort of new ESRD patients entering DOPPS II within 10 days of their first-ever dialysis treatment. The 6 time points for this analysis were 0–0.3 months, 1.5–3 months, 3.1–5 months, 5.1–7 months, 7.1–9 months and 9.1–12 months (n = 205). Hb (g/dl) = solid line, Epo use = dashed line.
An assessment was performed to determine the relationship of patient Hb levels with QoL, as measured by the PCS and MCS scores of the KDQOL SF-36 survey completed by patients at study entry. The PCS score was nearly 1.6 points lower for patients with Hb <8 g/dl than for patients with Hb 11–12 g/dl (P = 0.02). These results suggest that severe anaemia was associated with decreased physical functioning of Japanese HD patients. Similarly, the MCS score was 1.6 points lower for patients with Hb <8 g/dl versus Hb 11–12 g/dl, but this difference did not reach statistical significance (P = 0.07).
Discussion
Japan not only has the lowest HD patient mortality rate among the 12 countries participating in the DOPPS [2,3,16] but also displays the most conservative anaemia management program for HD patients among these 12 countries. Anaemia management practices observed in Japan should be considered in the context of Japanese anaemia management guidelines for HD patients. From 1990 through 2003, a haematocrit of 30% (Hb ∼10 g/dl) was generally recommended as a goal for HD patients treated with Epo. In 2004, the first anaemia management guidelines for Japanese HD patients were proposed by the Japanese Society for Dialysis Therapy (JSDT), which recommended a target Hb level of 10–11 g/dl for most chronic Japanese HD patients when measured at the first dialysis session of the week [11]. A higher Hb target of 11–12 g/dl was recommended for relatively young patients.
For ∼90% of HD patients in Japan, Hb measurements are taken while patients are in a supine position, and for ∼80% of patients, blood samples are taken during the first dialysis session of the week. In 2001, Inagaki et al. [17] reported haematocrit levels to be 7–8% higher (P < 0.01) for HD patients placed in a supine position for 5 versus 30 min. Gejyo et al. [11] showed that mean Hb levels were 10.7 g/dl when blood samples were taken in a seated position versus 10.1 g/dl when taken shortly thereafter in a supine position, among 99 Japanese HD patients. Similarly, Jacob et al. [18] studied 28 healthy subjects, observing that overall haematocrit was 11% higher in a standing versus supine position (37.7% versus 41.8%) and plasma volume was 17.9% lower in a standing versus supine position. These results are consistent with prior studies showing a 10–18% change in plasma volume in response to switching from a supine to upright posture [19–22], and with the underlying mechanism that standing up causes a net movement of fluid from intravascular to interstitial spaces, resulting in a decrease in plasma volume, and higher haematocrit percentages. Separately, Hb levels measured on the first dialysis session of the week have been seen to be slightly lower than Hb levels measured during mid-week dialysis sessions [11]. Taken together, these common practices of measuring Hb levels of Japanese HD patients result in lower reported Hb values than would be obtained in most other countries for the same level of anaemia control, since most other countries commonly measure Hb levels in a seated position during a mid-week dialysis session. An extrapolation from the JSDT anaemia guideline paper [11] suggests that a target of 10–11 g/dl in Japan may be equivalent to a target of ∼10.7–11.7 g/dl in another country measuring Hb levels in a seated position and during a mid-week session. Considering the substantial effect of blood sampling posture on observed Hb or haematocrit levels, an important consideration for future guideline recommendations would be to address this issue as part of anaemia management of patients receiving dialysis in supine versus upright positions.
Over the last 10 years, mean Hb levels have risen in many countries following the publication of new evidence-based guidelines for anaemia management that have recommended higher Hb levels for HD patients. This observation suggests that published guidelines and the guideline-related clinical discussions that typically follow guideline publication play substantial roles in affecting changes in practice and country mean Hb levels. The mean Hb levels observed in Japan from 1999 to 2006 align well with the recommended Hb targets at each time point, suggesting that these targets and possibly others may play an important role in stimulating the large improvement in anaemia control seen in Japan over this period. The mean Hb concentration among Japanese HD patients increased from 9.7 g/dl in 1999 to 10.4 g/dl in 2006. However, because of the blood sampling practices discussed above, the mean Hb concentration of 10.4 g/dl for Japanese patients may be equivalent to ∼11.1 g/dl for patients in a seated position during a mid-week dialysis session.
Even with a mean Hb concentration as high as 11.1 g/dl, the level of anaemia control is considerably lower in Japan than in most other DOPPS countries. Practice guidelines may contribute to the disparities between Hb attainment in Japan and other countries, although this is difficult to quantify. Nonetheless, there is considerable global debate regarding what is the best target Hb range for HD patients. In deciding upon the Hb target range for chronic HD patients in Japan, the JSDT [11] cited four main pieces of evidence: lower mean Hb levels in the general population in Japan compared with those in the US and Europe; results from a JSDT statistical survey and the work of Hirasawa et al. [23] showing a haematocrit level of 30–33% to be associated with the lowest mortality risk in Japanese HD patients; additional JSDT results showing the lowest mortality risk for patients aged 35–45 years to be observed at haematocrit 33–36%, and haematocrit 30.8–33.6% in Japan being nearly equivalent to 33–36% in Europe and the USA because of differences in method and timing of blood sample collection. In making its recommendations in 2004, the JSDT indicated the need to regularly review outcomes associated with different levels of anaemia control and the need for a large-scale prospective randomized clinical trial in the future to help further define the ideal Hb target range for chronic HD patients.
The rise in mean Hb in Japanese HD patients from 1999 to 2006 occurred broadly across Japan, with the percentage of facilities having median Hb ≥10 g/dl rising from 27% in 1999 to 75% in 2006. A relatively conservative anaemia management practice is seen for HD patients in Japan, employing an average weekly mean Epo dose of 5000–5500 units/week, with 32–33% of patients given IV iron during a 4-month period and receiving an average of ∼425 mg iron per 4-month period. However, as of early 2006, 35% of Japanese HD patients had Hb values below the recommended target minimum of 10 g/dl. We speculate that this finding may reflect non-adherence to the anaemia guideline, the Japanese policy restricting reimbursement to 9000 Epo units/week, and the packaging recommendation of 4500–6000 Epo units/week for maintenance doses.
Current anaemia management practices in Japan have resulted in substantially lower mean Hb levels for older HD patients. In contrast, very little difference in mean Hb concentrations is seen across age groups in other countries participating in DOPPS III (data not shown). The observed differences in anaemia control with older patients in Japan appear to be a result of anaemia management practices rather than age-related biological limitations.
Logistic regression analyses were used to examine the likelihood of Japanese HD patients achieving Hb of at least 10.5 g/dl, the midpoint of the current recommended target range. The results of these analyses suggest that two of the most important facility practices for achieving this Hb level are giving IV iron to a higher percentage of patients and providing a higher weekly dose of Epo. Indeed, facilities with greater IV iron use exhibited significantly higher mean Hb levels, and patients who were given three Epo doses per week displayed a significant and substantial rise in Hb levels in the subsequent month, compared with a decline or no change in mean Hb levels for patients receiving one or two Epo doses per week. Another important factor revealed by these analyses was that a higher level of anaemia control was consistently seen for Japanese patients having high albumin levels but not high ferritin levels. High albumin levels often serve as an indicator of good nutritional status in the absence of underlying inflammation. Other DOPPS analyses have shown that Japanese HD patients display the highest mean creatinine concentration and the lowest percentage of patients with a diagnosis of malnourishment among the 12 DOPPS countries [24], providing additional evidence of good nutritional care for HD patients in Japan. This level of nutritional care may be another reason for the ability of Japanese patients to attain higher Hb levels with relatively low doses of Epo.
In 2004, Pisoni et al. [10] described country variations in anaemia management indicators and practices across the 12 countries in the DOPPS. Those comparisons indicated that for nearly all measures, all other DOPPS countries achieved a higher level of anaemia control than Japan (ranging from 11.1 g/dl in France to 12.0 g/dl in Sweden). That study found IV iron use ranging from 53% in Italy to 89% in Belgium, Epo use ranging from 83% in France to 94% in the UK, Belgium and Sweden and mean weekly Epo doses ranging from 6846 in Germany to 17 360 in the USA. In contrasting the anaemia management practices in Japan with that of other countries, it is important to consider that Japan has the highest use of native arteriovenous fistulae (∼91%) and lowest use of central vein catheters (1%–2%) among all countries in the DOPPS. Since the risk of infection and inflammation is known to be strongly associated with catheter use [25] and catheter use is associated with lower Hb levels [10], this much lower use of catheters in Japan would be expected to decrease the exposure of Japanese patients to sources of inflammation, thereby supporting better anaemia control. As partial confirmation of this relationship, an evaluation of DOPPS II data revealed a much lower occurrence of septicaemia among HD patients in Japan versus those in any other DOPPS country (T Kawaguchi and RL Pisoni, work in progress).
Anaemia is a key factor associated with mortality of dialysis patients, and its importance has been pointed out in many studies [8–10,26,27]. Japan exhibits the lowest mean maintenance Hb level—but also the lowest mortality risk for HD patients—among the 12 DOPPS countries [2]. Even in Japan with its low mortality risk, anaemia is significantly associated with mortality, as shown by the overall lower mortality risk of RR = 0.89 (P = 0.003) for every 1 g/dl higher Hb level. Patients with baseline Hb <8 g/dl displayed a significantly higher mortality risk (RR = 1.78) than patients with Hb 11–12 g/dl. We found no significant difference in mortality risk for groups at different Hb levels. This result may be attributable to the very low mortality risk of Japanese dialysis patients and to the limited number of patients in each Hb group. However, the possibility of confounding by indication is inherent in patient-based analyses of observational data. This potential confounding makes it difficult to interpret how much of the observed mortality risk can be attributed to Hb levels per se and how much reflects the health status of sicker patients who have lower Hb levels arising from unmeasured comorbid conditions. In order to minimize the confounding by indication bias associated with patient-level analyses, the DOPPS was one of the first to publish a large, multi-centre facility-based finding indicating that patients displayed a significantly lower mortality risk if treated in facilities where a larger percentage of patients had Hb ≥11 g/dl [10]. This facility-based observation has been supported subsequently by Wolfe et al. [28], who showed significantly lower standardized mortality ratios in nearly 3000 US dialysis facilities having a higher percentage of patients with haematocrit ≥33%. These authors also showed that US facilities that had increased the fraction of patients with haematocrit ≥33% over a 4-year period displayed significant improvements in patient longevity over the same period. These results do not necessarily conflict with the findings from the CHOIR randomized trial [29], which showed a strong trend towards higher mortality risks for HD patients targeted to Hb 13.5 versus 11.3 g/dl, since the points of reference for the mortality comparisons differed considerably in the two studies.
Our observation of a 23% lower RR of death in facilities with mean Hb >10.4 g/dl (compared to facilities with mean Hb ≤10.4 g/dl) suggests a possible survival benefit for Japanese dialysis patients through the improvement of anaemia. A particular strength of this facility-based analysis is that it helps reduce the bias by indication that can exist in patient-based analyses. The facility-based result provides insight into how the mortality risk for patients at a facility is related to the facility's Hb management practices rather than being related to the Hb level of a particular patient.
We observed no significant relationship between the change in Japan's mean Hb level and mortality rate from 1999 to 2006. However, there has been a substantial increase in the mean patient age and in the comorbidity burden among Japanese HD patients over this time period (Table 1). Other practices may also have changed over this same period and exerted detrimental effects; the lack of correlation between the change in Hb control and country HD patient mortality rate does not exclude the possibility that improvements in anaemia control have had a beneficial effect.
Hospitalization risk was not markedly associated with Hb levels. Although the incidence of hospitalization of HD patients is lower in Japan than in any other DOPPS country, the percentage of patients hospitalized for non-medical reasons (such as social reasons) was the highest in Japan, which may modify the effect of Hb levels [2].
Anaemia is an important determinant factor for QoL, which is closely associated with mortality and hospitalization risk [30,31]. It has been reported that Japanese dialysis patients have high PCS scores compared with other DOPPS countries, but lower MCS scores than the USA [32]. Our study showed that patients with severe anaemia had significantly lower PCS scores, raising the question of whether poorer anaemia control may lead to lower PCS scores, or vice versa. The impact of anaemia control on PCS is particularly relevant considering the prior work showing PCS scores to be more strongly associated with HD patient mortality risk than a patient's albumin concentration [31].
Conclusion
This study provides a detailed description of anaemia management practices for HD patients in Japan, and relationships with outcomes. The anaemia management practices implemented in Japan provide an important perspective for assessing outcomes obtained through different anaemia management approaches worldwide. A large improvement in anaemia management has been seen in Japan from 1999 to 2006. However, on average, Hb levels in Japanese HD patients are low compared with those seen in other DOPPS regions.
Acknowledgments
We wish to acknowledge the great efforts and contributions of the study nurses, physicians, medical directors and patients from the Japanese dialysis units who have participated in DOPPS. We also gratefully acknowledge the expertise of Miles P. Finley for his editorial assistance.
Conflict of interest statement. The Japanese Dialysis Outcomes and Practice Patterns Study (Japan DOPPS) is supported by research grants from Kirin Pharma without restrictions on publications. Drs. Pisoni and Port are also investigators on the international DOPPS, which is supported by research grants from Amgen and Kirin Pharma without restrictions on publications.
References
- 1.Patient Registration Committee, Japanese Society for Dialysis Therapy An overview of regular dialysis treatment in Japan as of 31 December 2004. Ther Apher Dial. 2006;10:476–497. doi: 10.1111/j.1744-9987.2006.00440.x. [DOI] [PubMed] [Google Scholar]
- 2.Goodkin DA, Bragg-Gresham JL, Koenig KG, et al. Association of comorbid conditions and mortality in hemodialysis patients in Europe, Japan, and the United States: the Dialysis Outcomes and Practice Patterns Study (DOPPS) J Am Soc Nephrol. 2003;14:3270–3277. doi: 10.1097/01.asn.0000100127.54107.57. [DOI] [PubMed] [Google Scholar]
- 3.Held PJ, Brunner F, Odaka M, et al. Five-year survival for end-stage renal disease patients in the United States, Europe, and Japan, 1982–1987. Am J Kidney Dis. 1990;15:451–457. doi: 10.1016/s0272-6386(12)70363-3. [DOI] [PubMed] [Google Scholar]
- 4.Valderrabano F. Renal replacement therapy. What are the differences between Japan and Europe? Nephrol Dial Transplant. 1996;11:2151–2153. doi: 10.1093/oxfordjournals.ndt.a027129. [DOI] [PubMed] [Google Scholar]
- 5.Saran R, Bragg-Gresham J, Levin NW, et al. Longer treatment time and slower ultrafiltration in hemodialysis: associations with reduced mortality in the DOPPS. Kidney Int. 2006;69:1222–1228. doi: 10.1038/sj.ki.5000186. [DOI] [PubMed] [Google Scholar]
- 6.Saran R, Pisoni RL, Weitzel WF. Epidemiology of vascular access for hemodialysis and related practice patterns. Contrib Nephrol. 2004;142:14–28. doi: 10.1159/000074876. [DOI] [PubMed] [Google Scholar]
- 7.Pifer TB, McCullough KP, Port FK, et al. Mortality risk in hemodialysis patients and changes in nutritional indicators: DOPPS. Kidney Int. 2002;62:2238–2245. doi: 10.1046/j.1523-1755.2002.00658.x. [DOI] [PubMed] [Google Scholar]
- 8.Collins AJ, Li S, St Peter W, et al. Death, hospitalization, and economic associations among incident hemodialysis patients with hematocrit values of 36 to 39% J Am Soc Nephrol. 2001;12:2465–2473. doi: 10.1681/ASN.V12112465. [DOI] [PubMed] [Google Scholar]
- 9.Ofsthun N, Labrecque J, Lacson E, et al. The effects of higher hemoglobin levels on mortality and hospitalization in hemodialysis patients. Kidney Int. 2003;63:1908–1914. doi: 10.1046/j.1523-1755.2003.00937.x. [DOI] [PubMed] [Google Scholar]
- 10.Pisoni RL, Bragg-Gresham JL, Young EW, et al. Anemia management and outcomes from 12 countries in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44:94–111. doi: 10.1053/j.ajkd.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 11.Gejyo F, Saito A, Akizawa T, et al. 2004 Japanese society for dialysis therapy guidelines for renal anemia in chronic hemodialysis patients. Ther Apher Dial. 2004;8:443–459. doi: 10.1111/j.1774-9987.2004.00199.x. [DOI] [PubMed] [Google Scholar]
- 12.Pisoni RL, Gillespie BW, Dickinson DM, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): design, data elements, and methodology. Am J Kidney Dis. 2004;44(Suppl 2):S7–S15. doi: 10.1053/j.ajkd.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 13.Young EW, Goodkin DA, Mapes DL, et al. The Dialysis Outcomes and Practice Patterns Study (DOPPS): an international hemodialysis study. Kidney Int. 2000;57(Suppl 74):S74–S81. doi: 10.1046/j.1523-1755.2002.00387.x. [DOI] [PubMed] [Google Scholar]
- 14.Klein J, Moeschberger M. Survival Analysis Techniques for Censored and Truncated Data. New York: Springer; 1997. pp. 416–418. [Google Scholar]
- 15.SAS Institute . SAS/STAT User's Guide, Version 8. Vol. 2. Cary, NC: SAS Institute; 2000. [Google Scholar]
- 16.Nakai S, Niizato T, Shinoda T, et al. An overview of dialysis treatment in Japan as of Dec. 31, 2001. J Jpn Soc Dial Ther. 2003;36:1–31. [Google Scholar]
- 17.Inagaki H, Kuroda M, Watanabe S, et al. Changes in major blood components after adopting the supine position during haemodialysis. Nephrol Dial Transplant. 2001;16:798–802. doi: 10.1093/ndt/16.4.798. [DOI] [PubMed] [Google Scholar]
- 18.Jacob G, Raj S, Ketch T, et al. Postural pseudoanemia: posture-dependent change in hematocrit. Mayo Clin Proc. 2005;80:611–614. doi: 10.4065/80.5.611. [DOI] [PubMed] [Google Scholar]
- 19.Hinghofer-Szalkay H, Haas G, Oser H, et al. Monitoring fluid shifts in humans: application of a new method. Aviat Space Environ Med. 1989;60:23–28. [PubMed] [Google Scholar]
- 20.Fawcett JK, Wynn V. Effects of posture on plasma volume and some blood constituents. J Clin Pathol. 1960;13:304–310. doi: 10.1136/jcp.13.4.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bjerkhoel P, Lindgren P, Lundvall J. Protein loss and capillary protein permeability in dependent regions upon quiet standing. Acta Physiol Scand. 1995;154:311–320. doi: 10.1111/j.1748-1716.1995.tb09915.x. [DOI] [PubMed] [Google Scholar]
- 22.Lundvall J, Bjerkhoel P, Quittenbaum S, et al. Rapid plasma volume decline upon quiet standing reflects large filtration capacity in dependent limbs. Acta Physiol Scand. 1996;158:161–167. doi: 10.1046/j.1365-201X.1996.521294000.x. [DOI] [PubMed] [Google Scholar]
- 23.Hirasawa Y, Suzuki M, Itami N, et al. Maintenance hematocrit levels and mortality in hemodialysis patients with renal anemia receiving recombinant human erythropoietin (rHuEPO) treatment (rHuEPO survey) J Jpn Soc Dial Ther. 2003;36:1265–1272. [Google Scholar]
- 24.Akizawa T, Young EW, Maroni BJ, et al. Nutritional status across three continents: the dialysis outcomes and practice patterns study (Abstract) J Am Soc Nephrol. 2000;11:254A–255A. [Google Scholar]
- 25.Combe Ch, Pisoni RL, Port FK, et al. Dialysis outcomes and practice patterns study: données sur l’utilisation des cathéters veineux centraux en hémodialyse chronique. Nephrologie. 2001;22:379–384. [PubMed] [Google Scholar]
- 26.Robinson BM, Joffe MM, Berns JS, et al. Anemia and mortality in hemodialysis patients: accounting for morbidity and treatment variables updated over time. Kidney Int. 2005;68:2323–2330. doi: 10.1111/j.1523-1755.2005.00693.x. [DOI] [PubMed] [Google Scholar]
- 27.Locatelli F, Pisoni RL, Combe C, et al. Anaemia in haemodialysis patients of five European countries: association with morbidity and mortality in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2004;19:121–132. doi: 10.1093/ndt/gfg458. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe RA, Hulbert-Shearon TE, Ashby VB, et al. Improvements in dialysis patient mortality are associated with improvements in urea reduction ratio and hematocrit, 1999 to 2002. Am J Kidney Dis. 2005;45:127–135. doi: 10.1053/j.ajkd.2004.09.023. [DOI] [PubMed] [Google Scholar]
- 29.Singh AK, Szczech L, Tang KL, et al. Correction of anemia with Epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355:2085–2098. doi: 10.1056/NEJMoa065485. [DOI] [PubMed] [Google Scholar]
- 30.Mapes DL, Bragg-Gresham JL, Bommer J, et al. Health-related quality of life in the Dialysis Outcomes and Practice Patterns Study (DOPPS) Am J Kidney Dis. 2004;44(Suppl 2):54–60. doi: 10.1053/j.ajkd.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 31.Mapes DL, Lopes AA, Satayathum S, et al. Health-related quality of life as a predictor of mortality and hospitalization: the Dialysis Outcomes and Practice Patterns Study (DOPPS) Kidney Int. 2003;64:339–349. doi: 10.1046/j.1523-1755.2003.00072.x. [DOI] [PubMed] [Google Scholar]
- 32.Fukuhara S, Lopes AA, Bragg-Gresham JL, et al. Health-related quality of life among dialysis patients on three continents: the dialysis outcomes and practice patterns study. Kidney Int. 2003;64:1903–1910. doi: 10.1046/j.1523-1755.2003.00289.x. [DOI] [PubMed] [Google Scholar]