Summary
The aspartic protease cathepsin D (cath-D) is a key mediator of induced-apoptosis and its proteolytic activity has been generally involved in this event. During apoptosis, cath-D is translocated to the cytosol. Since cath-D is one of the lysosomal enzymes which requires a more acidic pH to be proteolytically-active relative to the cysteine lysosomal enzymes such as cath-B and -L, it is therefore open to question whether cytosolic cath-D might be able to cleave substrate(s) implicated in the apoptotic cascade. Here we have investigated the role of wild-type cath-D and its proteolytically-inactive counterpart over-expressed by 3Y1-Ad12 cancer cells during chemotherapeutic-induced cytotoxicity and apoptosis, as well as the relevance of cath-D catalytic function. We demonstrate that wild-type or mutated catalytically-inactive cath-D strongly enhances chemo-sensitivity and apoptotic response to etoposide. Both wild-type and mutated inactive cath-D are translocated to the cytosol, increasing the release of cytochrome c, the activation of caspases-9 and -3 and the induction of a caspase-dependent apoptosis. In addition, pre-treatment of cells with the aspartic protease inhibitor, pepstatin A, does not prevent apoptosis. Interestingly therefore, the stimulatory effect of cath-D on cell death is independent of its catalytic activity. Overall, our results imply that cytosolic cath-D stimulates apoptotic pathways by interacting with a member of the apoptotic machinery rather than by cleaving specific substrate(s).
Keywords: Antineoplastic Agents, Phytogenic; pharmacology; Apoptosis; Caspase 3; metabolism; Caspase 9; metabolism; Catalysis; Cathepsin D; metabolism; Cytochromes c; metabolism; Cytosol; metabolism; Drug Resistance, Neoplasm; Etoposide; pharmacology; Humans; Neoplasms; drug therapy; metabolism; pathology; Pepstatins; pharmacology; Protease Inhibitors; pharmacology; Tumor Cells, Cultured
Introduction, Results and Discussion
Cathepsin D (cath-D) is a ubiquitous lysosomal aspartic protease extensively reported as being an active player in breast cancer (1,2). More recently, cath-D has also been discovered as a key mediator of apoptosis induced by many apoptotic agents such as IFN-γ, Fas/APO, TNF-α (3), oxidative stress (4–8), adriamycin and etoposide (9, 10), as well as staurosporine (11). The role of cath-D in apoptosis has been linked to the lysosomal release of mature 34 kDa cath-D into the cytosol leading in turn to the mitochondrial release of cytochrome c (cyt c) into the cytosol (4–7, 11, 12), activation of pro-caspases (5, 11–13), in vitro cleavage of Bid at pH 6.2 (13), or Bax activation independently of Bid cleavage (14). Numerous studies have shown that pepstatin A, an aspartic protease inhibitor, could partially delay apoptosis induced by IFN-γ and Fas/APO (3), staurosporine (11, 14, 15), TNF-α (3, 13, 16), serum deprivation (17), oxidative stress (5–8) or even when cath-D was micro-injected (12). These authors have thus concluded that cath-D plays a key role in apoptosis mediated via its catalytic activity. However, cath-D is one of the lysosomal enzymes which requires a more acidic pH to be proteolytically active, relative to the cysteine lysosomal enzymes such as cath-B and -L. Acidification of the cytosol down to pH values of about 6.7–7 is a well-documented phenomenon in apoptosis (18). In vitro cath-D can cleave its substrates up to a pH of 5.8, but not above (19). Hence, it is predictable that the proteolytic activity of cytosolic cath-D would be drastically impaired under adverse pH conditions unfavourable for its catalytic function. It is therefore open to question whether cath-D might be able to cleave cytosolic substrate(s) implicated in the apoptotic cascade. In accordance with this proposal, it was recently described that pepstatin A did not prevent the death of cells treated by etoposide, doxorubicin, anti-CD95 or TNF-α (10, 16, 20). Furthermore, pepstatin A did not suppress Bid cleavage or pro-caspases-9 and -3 activation by photodynamic therapy in murine hepatoma cells (21). Finally, deficiency in cath-D activity did not alter cell death induction in CONCL fibroblasts (20).
In the present work, we investigated the role of wild-type cath-D and its proteolytically-inactive counterpart over-expressed by cancer cells in chemotherapeutic-induced cytotoxicity and apoptosis, and more precisely the relevance of cath-D catalytic function. We used the 3Y1-Ad12 cancer cell line - stably transfected with either wild-type cath-D or catalytically-inactive D231Ncath-D or an empty vector - as a tumor model of cath-D over-expression. Cath-D, D231Ncath-D and mock-transfected (control) 3Y1-Ad12 transfected cell lines were first treated with increasing concentrations of the topoisomerase II-inhibitor etoposide for two doubling time (e.g. 4 days) (Figure 1). Cell viability curves revealed that cath-D significantly enhanced (5-fold) the chemo-sensitivity of cancer cells exposed to etoposide concentrations ranging from 100 nM to 1 μM, compared to control cells. Interestingly, D231Ncath-D provided the same decrease in viability as cath-D (Figure 1). We therefore conclude that cath-D over-expressed by cancer cells increases their cytotoxic responses to chemotherapeutic agents independently of its catalytic activity. To dissect out the mechanism involved, we first determined whether chemo-sensitivity induced by over-expressed cath-D might be the consequence of an enhancement of apoptosis. Control, cath-D and D231Ncath-D cancer cells were first treated with 10 μM etoposide for 24 h and their nuclei were stained with DAPI (Figure 2A). Cancer cells over-expressing wild-type cath-D or D231Ncath-D exhibited characteristic apoptotic morphological features, such as cell shrinkage, chromatin condensation, and the formation of apoptotic bodies, whereas control cancer cells presented only few apoptotic nuclei (Figure 2A). Control, cath-D and D231Ncath-D cancer cells were then treated with increasing low (Figure 2B, left panel) or higher (Figure 2B, right panel) concentrations of etoposide for 24 h and apoptotic status was analysed by an ELISA that detects histones associated with fragmented DNA. Both wild-type cath-D and D231Ncath-D enhanced apoptosis in a dose-dependent manner (Figure 2B). A 3-fold increase in apoptosis was observed with 1 μM etoposide in cath-D and D231Ncath-D cells compared to control cells (Figure 2B, left panel). Similar results were obtained over a wide range of etoposide concentrations, indicating that cath-D increased apoptosis for a large scale of etoposide treatment, e.g. from 75 nM to 50 μM (Figure 2B ). We finally examined the DNA fragmentation by TUNEL staining in cell cultures treated with 10 μM etoposide for 24 h and found that the nuclei of the cath-D and D231Ncath-D transfected cells were positively stained by TUNEL, whereas almost no TUNEL-positive cells were seen in control cells (Figure 2C, top panel). Quantification of the TUNEL assay, performed with cells treated with either 1 or 10 μM etoposide, then revealed a significant (10–30 fold) increase of apoptosis in both cath-D and D231Ncath-D cells, compared to control cells (Figure 2C, bottom panel). Moreover, pre-treatment of wild-type cath-D cells with 100 μM pepstatin A for 16 h did not inhibit the increase of apoptosis induced by cath-D (Figure 2D, left panel). Furthermore, pepstatin A had no effect on apoptosis enhanced by catalytically-inactive D231Ncath-D (Figure 2D, left panel). Time-course experiments of cath-D cells pre-treated with pepstatin A confirmed its inefficiency to reverse cath-D-induced apoptosis (Figure 2D, right panel). We verified that cath-D catalytic activity was inhibited by 100 μM pepstatin A in cath-D cell extracts from untreated or etoposide-treated cells (Figure 2E). Overall, our results demonstrate that cath-D over-expressed by cancer cells enhances etoposide-induced apoptosis independently of its catalytic activity. Cath-D has been described as being readily translocated to the cytosol in response to various apoptotic stimuli (6, 7, 10, 11, 13, 14). We therefore investigated the subcellular distribution of wild-type and D231Ncath-D by immunostaining (Figure 3 A) and by cell fractionation (Figure 3B). In untreated cath-D or D231Ncath-D cells, immunostaining of cath-D displayed a punctuate distribution using immunofluorescence microscopy, consistent with a lysosomal location (Figure 3A). By contrast, in etoposide-treated cath-D or D231Ncath-D cells, cath-D displayed a mixed punctuate/diffuse distribution, suggesting that a portion of it was released from lysosomes into the cytosol (Figure 3A). Western blot analysis confirmed that the mature (34 kDa) form of cath-D was indeed present in the cytosolic fractions obtained from wild-type cath-D or D231Ncath-D cells treated for 1 h with etoposide, following permeabilization of the plasma membranes with digitonin (Figure 3B). Cyt c has been shown to be released from mitochondria to the cytosol during etoposide-induced apoptosis (22). A typical mitochondrial pattern was observed by immunofluorescence analysis of cyt c in untreated control, cath-D or D231Ncath-D cells, as well as in control cells treated with 10 μM etoposide (Figure 4A). By contrast, in cath-D or D231Ncath-D cells exposed to 10 μM etoposide, translocation of cyt c from the mitochondria into the cytosol was observed (Figure 4A). These results indicate that cath-D over-expressed by cancer cells contributes to mitochondrial cyt c release in etoposide-treated cells independently of its catalytic function. We then investigated whether cath-D plays a role in the activation of caspases-9 and -3 since these caspases have been shown to play a pivotal role in etoposide-induced apoptosis (22). As illustrated in Figure 4B, caspases-9 and -3 were both activated in cath-D and D231Ncath-D cells treated with 10 μM etoposide whereas almost no signal could be detected in control cells in similar conditions. To confirm that cath-D participates in the activation of caspases during etoposide-induced apoptosis, cath-D or D231Ncath-D were either left untreated or pre-treated first with the general caspase inhibitor, Z-VAD-FMK, and then with 10 μM etoposide (Figure 5A). TUNEL assays revealed that wild-type or D231Ncath-D enhanced a caspase-dependent apoptosis since etoposide-induced apoptosis was significantly inhibited by Z-VAD-FMK treatment (Figure 5A). In parallel experiments, we verified that Z-VAD-FMK efficiently inhibited caspase-3 activation in our cells (Figure 5B). Overall, our study has demonstrated that the over-expression of both wild-type and D231cath-D by cancer cells can enhance the cytotoxicity of chemotherapeutic agents and their apoptotic response to etoposide treatment. Both active and inactive cath-D are rapidly released into the cytosol after etoposide treatment and this phenomenon is closely associated with cyt c release and caspase-dependent apoptotic events. In conclusion, our work (23) implicates a role for cath-D independent of its catalytic function both in chemotherapeutic-induced cytotoxicity and in etoposide-induced apoptosis, and we suggest that an adequate strategy to identify cytosolic cath-D interacting protein(s) might be the two-yeast hybrid approach.
Figure 1. The over-expression of both catalytically-active and -inactive cath-D by 3Y1-Adl2 cancer cells enhances etoposide-induced cytotoxicity.
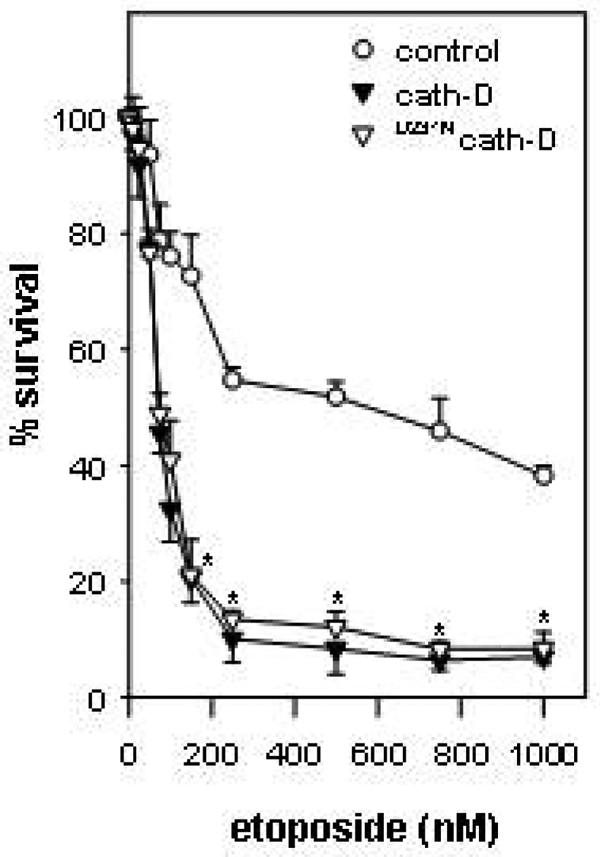
Cells were treated or not with increasing concentrations of etoposide and survival was evaluated by MTT. *, p<0.0005 versus control cells (Student’s t-test).
Figure 2. Catalytically-active and -inactive cath-D amplify etoposide-induced apoptosis.
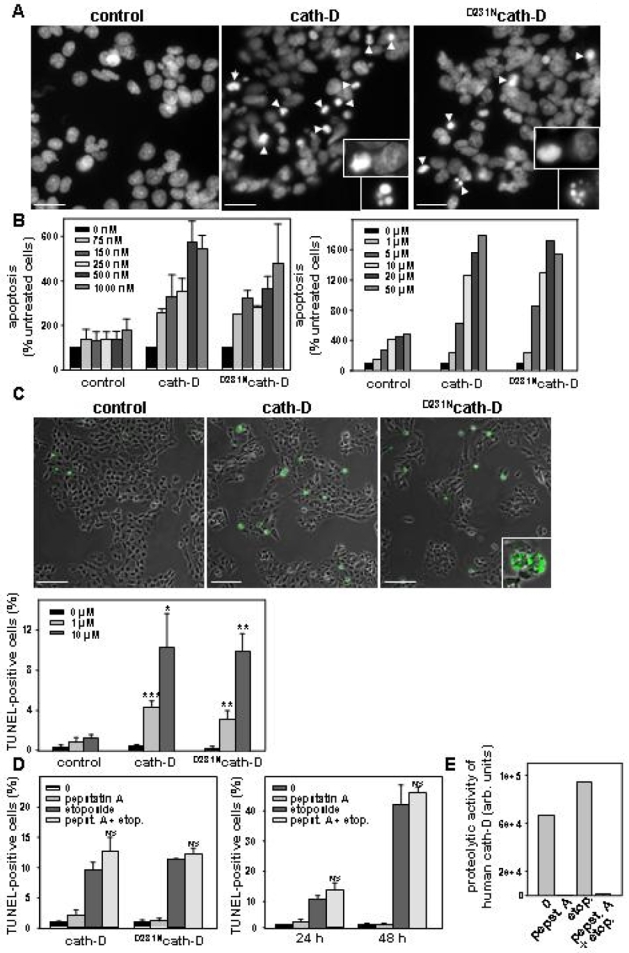
(A) Staining of nuclei with DAPI. Arrows indicate condensed chromatin and apoptotic bodies. Insets show DNA condensation and apoptotic bodies at a higher magnification. Bars, 23 μm. (B) ELISA apoptosis. Apoptosis induction was quantified on pooled floating and adherent cells using a cell death ELISA. (C) TUNEL assay. Representative immunocytochemistry of apoptotic cells (top panel). Apoptosis was analysed on adherent cells and floating cells cytospun on glass slides using the TUNEL method. Inset shows apoptotic bodies. For quantification of apoptotic cells (bottom panel), three fields for each experimental condition were chosen by random sampling and the total number of cells and the number of TUNEL positive nuclei were counted from adherent and cytospun floating cells. The results are expressed as a % of the estimated total number of cells present in the field. *, p<0.01, **, p<0.025 and ***, p<0.0025 versus control cells (t-test). Bars, 69 μm. (D) Effect of pepstatin A on cath-D-induced apoptosis. Cells were pre-treated or not with pepstatin A and then were treated or not with 10 μM etoposide for 24 h (left panel). NS (non significant), p<0.1 and 0.15 versus etoposide for cath-D and D231Ncath-D cells (t-test). Time-course analysis of apoptosis in the presence or absence of 100 μM pepstatin A was performed with cath-D cells (right panel). p<0.25 versus etoposide (t-test). (E) Effect of pepstatin A on cath-D catalytic activity analysed using a fluorogenic substrate.
Figure 3. Catalytically-active and -inactive cath-D are released early in etoposide-induced apoptosis.
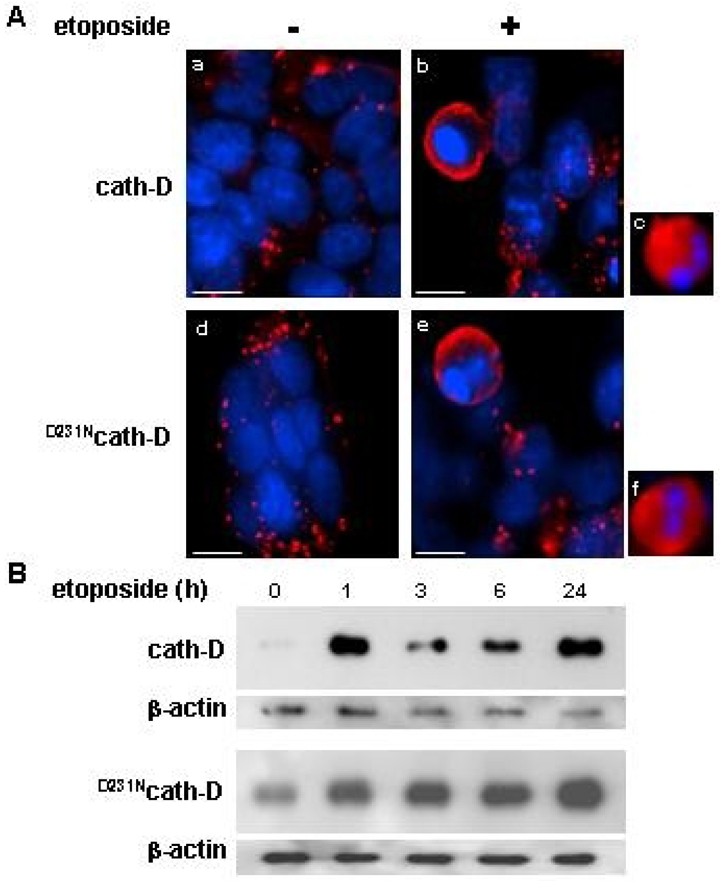
(A) Immunofluorescence of cath-D. Cells were either exposed to 10 μM etoposide for 24 h (b, c, e, f) or left untreated (a, d). Cells were incubated first with anti-human cath-D M1G8 mouse monoclonal antibody followed by a RITC-conjugated goat anti-mouse antibody. Bars, 7.4 μM. (B) Release of cytosolic cath-D. Western blotting of wild-type cath-D (top panel) and D231Ncath-D (bottom panel) in cytosols extracted from cells either exposed to 10 μM etoposide. Cytosol was extracted using 20 μg/ml and 45 μg/ml digitonin for cath-D and D231Ncath-D cells, respectively, for 10 min as previously described (11). Cath-D immunoblotting was performed using an anti-human cath-D monoclonal mouse antibody followed by an anti-mouse peroxidase-conjugated antibody.
Figure 4. Catalytically-active and -inactive cath-D induce mitochondrial release of cyt c and activation of caspases-9 and -3.
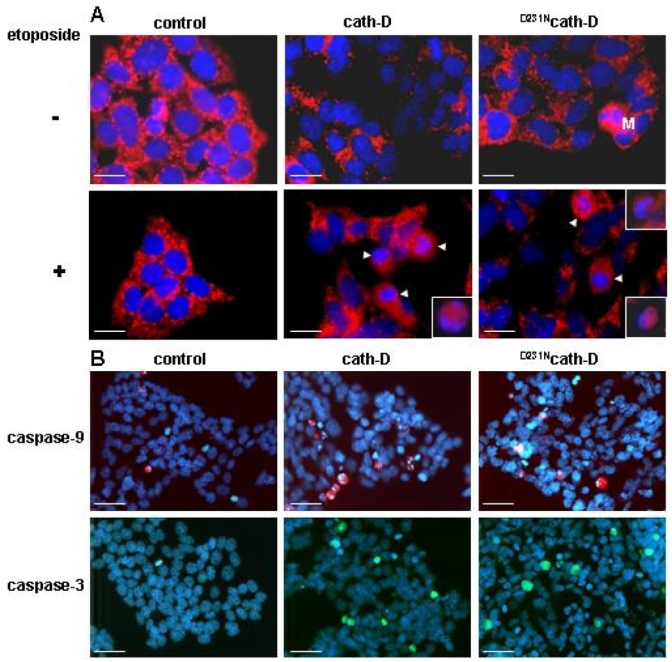
(A) Cyt c was analyzed by immunofluorescence using an anti-rat cyt c mouse monoclonal antibody followed by a RITC-conjugated goat anti-mouse antibody. Bars, 16 μm. (B) Activation of caspase-9 and -3 was analyzed by immunofluorescence using an anti-rat cleaved caspase-9 rabbit polyclonal antibody or an anti-rat cleaved caspase-3 rabbit polyclonal antibody followed by a TexasRed-conjugated or FITC-conjugated goat anti-rabbit antibody. Bars, 43 μm.
Figure 5. Catalytically-active and -inactive cath-D increase caspase-dependent apoptosis.
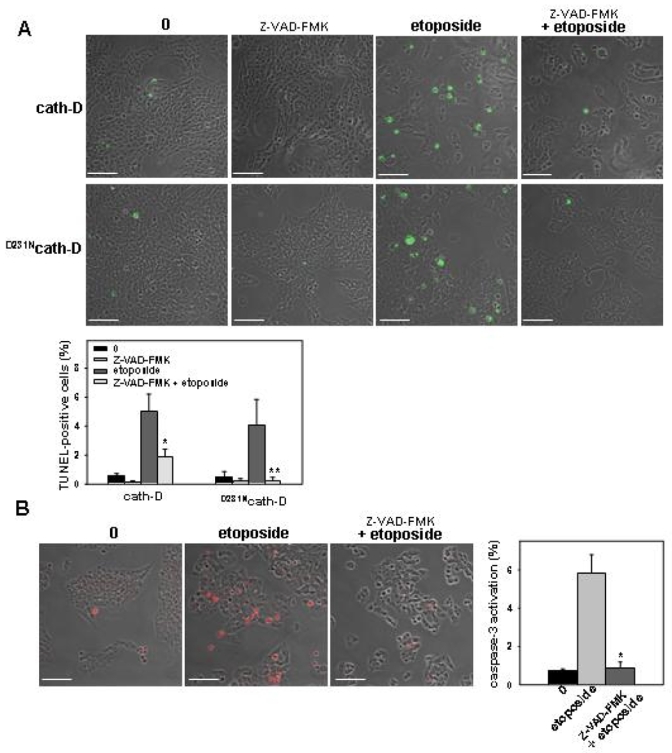
(A) Effect of Z-VAD-FMK on apoptosis. Cath-D (top panel) and D231Ncath-D (bottom panel) cells were first either pre-treated for 1 h with 100 μM Z-VAD-FMK or left untreated and were then either left untreated again or were treated with 10 μM etoposide for 24 h. Bars, 69 μm. *, p<0.05 and **, p<0.025 versus etoposide for cath-D and D231Ncath-D cells, respectively (t-test). (B) Effect of Z-VAD-FMK on caspase-3 activation Cells were pre-treated or not for 1 h with 100 μM Z-VAD-FMK and were then treated or not with 10 μM etoposide for 24 h. Bars, 69 μm. *, p<0.0025 versus etoposide treatment (t-test).
Acknowledgments
We thank Dr Karin Öllinger (Linkoping University, Sweden) for her kind help and valuable discussions concerning the digitonin method, Jean-Yves Cance for the photographs, Nicole Lautredou-Audouy (Centre de Ressources en Imagerie Cellulaire) for confocal microscopy and Nadia Kerdjadj for her secretarial assistance. This work was supported by Institut National de la Santé et de la Recherche Medicale, University of Montpellier I, Association pour la Recherche sur le Cancer (grant 3344, E Liaudet-Coopman) and Ligue Nationale contre le Cancer who provided a fellowship for Melanie Beaujouin.
References
- 1.Rochefort H. The prognostic value of cathepsin D in breast cancer. A long road to the clinic. Eur J Cancer. 1996;32A:7–8. doi: 10.1016/0959-8049(95)00637-0. [DOI] [PubMed] [Google Scholar]
- 2.Liaudet-Coopman E, Beaujouin M, Derocq D, et al. Cathepsin D: newly discovered functions of a long-standing aspartic protease in cancer and apoptosis. Cancer Letters. 2006;237:167–179. doi: 10.1016/j.canlet.2005.06.007. [DOI] [PubMed] [Google Scholar]
- 3.Deiss LP, Galinka H, Berissi H, et al. Cathepsin D protease mediates programmed cell death induced by interferon gamma, Fas/APO-1 and TNF alpha. EMBO J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 4.Roberg K, Ollinger K. Oxidative stress causes relocation of the lysosomal enzyme cathepsin D with ensuing apoptosis in neonatal rat cardiomyocytes. Am J Pathol. 1998;152:1151–1156. [PMC free article] [PubMed] [Google Scholar]
- 5.Ollinger K. Inhibition of cathepsin D prevents free-radical-induced apoptosis in rat cardiomyocytes. Arch Biochem Biophys. 2000;373:346–351. doi: 10.1006/abbi.1999.1567. [DOI] [PubMed] [Google Scholar]
- 6.Roberg K. Relocalization of cathepsin D and cytochrome c early in apoptosis revealed by immunoelectron microscopy. Lab Invest. 2001;81:149–158. doi: 10.1038/labinvest.3780222. [DOI] [PubMed] [Google Scholar]
- 7.Kagedal K, Johansson U, Öllinger K. The lysosomal protease cathepsin D mediates apoptosis induced by oxidative stress. FASEB J. 2001;15:1592–1594. doi: 10.1096/fj.00-0708fje. [DOI] [PubMed] [Google Scholar]
- 8.Takuma K, Kiriu M, Mori K, et al. Roles of cathepsins in reperfusion-induced apoptosis in cultured astrocytes. Neurochem Int. 2003;42:153–159. doi: 10.1016/s0197-0186(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 9.Wu GS, Saftig P, Peters C, et al. Potential role of cathepsin D in p53-dependent tumor suppression and chemosensitivity. Oncogene. 1998;16:2177–2183. doi: 10.1038/sj.onc.1201755. [DOI] [PubMed] [Google Scholar]
- 10.Emert-Sedlak L, Shangary S, Rabinovitz A, et al. Mol Cancer Ther. 2005;4:733–742. doi: 10.1158/1535-7163.MCT-04-0301. [DOI] [PubMed] [Google Scholar]
- 11.Johansson AC, Steen H, Öllinger K, et al. Cathepsin D mediates cytochrome c release and caspase activation in human fibroblast apoptosis induced by staurosporine. Cell Death Differ. 2003;10:1253–1259. doi: 10.1038/sj.cdd.4401290. [DOI] [PubMed] [Google Scholar]
- 12.Roberg K, Kagedal K, Ollinger K. Microinjection of cathepsin d induces caspase-dependent apoptosis in fibroblasts. Am J Pathol. 2002;161:89–96. doi: 10.1016/S0002-9440(10)64160-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinrich M, Neumeyer J, Jakob M, et al. Cathepsin D links TNF-induced acid sphingomyelinase to Bid-mediated caspase-9 and -3 activation. Cell Death Differ. 2004;11:550–563. doi: 10.1038/sj.cdd.4401382. [DOI] [PubMed] [Google Scholar]
- 14.Bidère N, Lorenzo HK, Carmona S, et al. Cathepsin D triggers Bax activation, resulting in selective apoptosis-inducing factor (AIF) relocation in T lymphocytes entering the early commitment phase to apoptosis. J Biol Chem. 2003;278:31401–31411. doi: 10.1074/jbc.M301911200. [DOI] [PubMed] [Google Scholar]
- 15.Terman A, Neuzil J, Kagedal K, et al. Decreased apoptotic response of inclusion-cell disease fibroblasts: a consequence of lysosomal enzyme missorting? Exp Cell Res. 2002;274:9–15. doi: 10.1006/excr.2001.5441. [DOI] [PubMed] [Google Scholar]
- 16.Demoz M, Castino R, Cesaro P, et al. Endosomal-lysosomal proteolysis mediates death signalling by TNFalpha, not by etoposide, in L929 fibrosarcoma cells: evidence for an active role of cathepsin D. Biol Chem. 2002;383:1237–1248. doi: 10.1515/BC.2002.137. [DOI] [PubMed] [Google Scholar]
- 17.Shibata M, Kanamori S, Isahara K, et al. Participation of cathepsins B and D in apoptosis of PC 12 cells following serum deprivation. Biochem Biophys Res Commun. 1998;251:199–203. doi: 10.1006/bbrc.1998.9422. [DOI] [PubMed] [Google Scholar]
- 18.Matsuyama S, Llopis J, Deveraux QL, et al. Changes in intramitochondrial and cytosolic pH: early events that modulate caspase activation during apoptosis. Nat Cell Biol. 2000;2:318–325. doi: 10.1038/35014006. [DOI] [PubMed] [Google Scholar]
- 19.Capony F, Morisset M, Barrett AJ, et al. Phosphorylation, glycosylation, and proteolytic activity of the 52-kD estrogen-induced protein secreted by MCF7 cells. J Cell Biol. 1987;104:253–262. doi: 10.1083/jcb.104.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tardy C, Tyynela J, Hasilik A, et al. Stress-induced apoptosis is impaired in cells with a lysosomal targeting defect but is not affected in cells synthesizing a catalytically inactive cathepsin D. Cell Death Differ. 2003;10:1090–1100. doi: 10.1038/sj.cdd.4401272. [DOI] [PubMed] [Google Scholar]
- 21.Reiners JJ, Jr, Caruso JA, Mathieu P, et al. Release of cytochrome c and activation of pro-caspase-9 following lysosomal photodamage involves Bid cleavage. Cell Death Differ. 2002;9:934–944. doi: 10.1038/sj.cdd.4401048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sawada M, Nakashima S, Banno Y, et al. Ordering of ceramide formation, caspase activation, and Bax/Bcl-2 expression during etoposide-induced apoptosis in C6 glioma cells. Cell Death Differ. 2000;7:761–772. doi: 10.1038/sj.cdd.4400711. [DOI] [PubMed] [Google Scholar]
- 23.Beaujouin M, Baghdiguian S, Glondu-Lassis M, et al. Overexpression of both catalytically active and -inactive cathepsin D by cancer cells enhances apoptosis-dependent chemo-sensitivity. Oncogene. 2006;25:1967–73. doi: 10.1038/sj.onc.1209221. [DOI] [PMC free article] [PubMed] [Google Scholar]


