Abstract
Airborne factors in a conventionally managed racing stable and markers of pulmonary inflammation in the stabled horses were investigated on 3 occasions at 6-month intervals, including 2 winter periods and the intervening summer period. The stable measurements included inside and outside ambient temperature and relative humidity, levels of total and respirable dust, endotoxin, and 1,3-β-glucan. Horses (n = 12) were examined in detail clinically as well as by endoscopy, bronchoalveolar lavage (BAL) cytology, and real-time polymerase chain reaction (RT-PCR) analysis of BAL-cells for IL-6 and IL-10 mRNA.
Indoor measurements showed low dust levels irrespective of season. Inhalable dust, as well as 1,3-β-glucan, were higher during the winter stabling period, whereas endotoxin levels were higher during summer. Complete data from all sampling occasions to be used for further evaluation was obtained for only 8 of the horses. There was a trend for elevation of BAL neutrophils in the horses during winter stabling that coincided with a 3.7-fold increased expression of IL-6 mRNA in BAL cells (P = 0.014). Compared to summer sampling, IL-10 mRNA expression was significantly upregulated in only 1 of the winter sampling occasions, implicating influence on immune regulation by factor/s apart from seasonal differences. Our findings suggest up-regulation of innate immunity in the airways of stabled horses; in particular involving IL-6 in association with mild elevations in respirable dust, 1,3-β-glucan, and/or cold ambient air. However, given that this study was observational, other unmeasured environmental factors associated with winter stabling need to be considered.
Résumé
Les facteurs d’origine aérienne présents dans une écurie pour chevaux de course gérée de manière conventionnelle et les marqueurs d’inflammation chez les chevaux hébergés ont été étudiés à trois occasions à six mois d’intervalle (deux périodes d’hiver ainsi que la période d’été les séparant). Les données prises à l’écurie incluaient les températures ambiantes intérieure et extérieure et l’humidité relative, les niveaux de poussière totale et respirable, d’endotoxine et de 1,3-β-glucan. Les chevaux (n = 12) ont été soumis à un examen clinique détaillé ainsi que par endoscopie, une cytologie d’un lavage broncho-alvéolaire (BAL) et une analyse par réaction d’amplification en chaîne par la polymérase en temps réel des cellules du BAL pour la détection d’ARNm d’IL-6 et d’IL-10.
Les mesures prises à l’intérieur montraient de faibles niveaux de poussière indépendamment de la saison. Les quantités de poussière respirable ainsi que le 1,3-β-glucan étaient plus élevées durant la période d’hiver, alors que les niveaux d’endotoxine étaient plus élevés durant l’été. Les informations complètes pour tous les échantillonnages n’ont été obtenues que pour 8 chevaux. Lors de l’hébergement des chevaux durant la période d’hiver, on notait une tendance à la hausse des neutrophiles du BAL qui coïncidait avec une augmentation de 3,7 fois l’expression d’ARNm d’IL-6 dans les cellules du BAL (P = 0,014). Comparativement au résultat obtenu lors de l’échantillonnage d’été, l’expression d’ARNm pour IL-10 était significativement augmentée pour seulement un des échantillonnages d’hiver, indiquant ainsi l’influence sur la régulation immunitaire de facteur(s) autre(s) que des différences saisonnières. Nos résultats suggèrent une régulation à la hausse de l’immunité innée dans les voies respiratoires des chevaux mis à l’écurie; en particulier l’association impliquant IL-6 et les légères augmentations de poussière respirable, de 1,3-β-glucan, et/ou l’air froid ambiant. Toutefois, compte tenu qu’il s’agissait d’une étude observationnelle, d’autres facteurs environnementaux non-mesurés associés avec la mise à l’écurie en hiver doivent être considérés.
(Traduit par Docteur Serge Messier)
Introduction
Recurrent airway obstruction (RAO, heaves, animal model of asthma) and inflammatory airway disease (IAD) in horses are major reasons for reduced performance and persistent cough in stabled horses (1–3). Recurrent airway obstruction is characterized by neutrophilic airway inflammation, reversible airway obstruction, and bronchial hyperresponsiveness (4). Studies indicate that the pathogenesis of RAO is multifactorial, with a genetic predisposition (5–7) in combination with exposure to specific airborne allergens or pro-inflammatory agents. The syndrome of IAD is associated with a milder grade of respiratory tract inflammation in which non-infectious agents from the stable environment may be key factors contributing to the inflammation (3,5,8).
Early studies of RAO with inhalation challenge implicated molds as causal agents (9,10). However, natural challenge with poor quality hay and straw was a more potent inducer of inflammation (9), suggesting additional factors from the stable environment that can contribute to airway inflammation. Pirie et al (11–13) used nebulized whole and fractionated hay dust suspensions to evaluate the inhaled dust components initiating pulmonary neutrophil recruitment in equine species. In more recent studies, they have shown that inhaled endotoxin together with organic dust particles induces dose dependent neutrophil accumulation in equine lung (14–16). While there is a clear connection between exposure to highly dusty stable environments and increased airway inflammation in horses, studies on the influence of air quality that can be expected in a working stable environment on markers of respiratory tract inflammation in horses are lacking.
The objectives of this study were to examine qualitative differences in indoor stable air during winter versus late summer conditions in a typical racing stable, and assess whether or not air quality or season was associated with clinically detectable respiratory signs or alterations to selected biomarkers of inflammation in horses.
Materials and methods
Study design
Twelve standardbred trotters in a conventional racing stable were investigated during 2 consecutive winter stabling periods (February 2004, March 2005) and once following a prolonged period at pasture in the intervening summer (September 2004). The study was approved by the Ethical Committee for Animal Experiments, Uppsala, Sweden (diary number C 235/3).
Stable environment and stable management
The stable measured 12 × 30 m, housed a total of 18 horses, and was without either supplemental heating or mechanical ventilation. It had a wooden-frame construction, concrete floor, insulated metal roof, and wooden outer walls with an inside lining of Plyfa (16 mm) board up to a height of 2 m. The stalls were divided by wood plank walls with upper steel bars, and with sliding doors of the same construction. There were 2 entrances to the stable, one at each end, with doors that were generally kept open during cleaning and training sessions, but otherwise closed during winter. The horses were bedded on straw, fed 3 times a day with haylage and pelleted fodder and had their stalls cleaned daily each morning, when most of the horses were outdoors. During winter, all horses were outside in paddocks for 4–7 h depending on training and weather conditions, and during summer, they were at pasture for up to 12 each day, but stabled at night.
Sampling for air quality was performed on 3 occasions at approximately 6-month intervals (February 2004, September 2004, March 2005), during the same week that the horses were evaluated. Stable air measurements were collected over 4–7 h, beginning at 07:00, while normal activities took place in the stable (cleaning stalls, feeding, cleaning and training horses). Horses were always present in the stable during the sampling period, at which time the number of stabled horses varied depending on daily activities, such as feeding and training. Samples were taken both in the stalls during cleaning and in the corridor. The indoor stable environment was assessed for total and respirable dust, endotoxin and 1,3-β-glucan, temperature, and relative humidity.
Air sampling
All air sampling was performed with pumps (SKC, Eighty Four, Pennslyvania, USA) placed in the breathing zone at approximately 1–1.5 m above ground level. Air sampling results were obtained from 3 points in the stable corridor; one at each exit and one in the middle. Pumps were placed just outside boxes with the filter unit attached to the steel bars. Samples of total and respirable dust-in-air were collected in separate cassettes using a 25-mm (pore size 0.8 μm) membrane filter. Total-dust samplers have open-faced cassettes with no size selection. For respirable dust samples, a metal cyclone separator (SKC) was used before the filter cassette, to remove particles > 3.5 μm in size. The equipment was attached to the clothing of personnel in the breathing zone while they worked in the stable, and was placed in a stationary position while they worked elsewhere. Samples were taken at a flow rate of 2 L/min for 4–7 h. Airborne endotoxin and 1,3-β-glucan were sampled in separate cassette-holders with 25 mm nucleopore filters (pore size 0.4 μm, at 2.0 L/min for 4 h).
Sample preparation and analysis
All dust samples were analyzed by a gravimetric method, and the organic proportion calculated after combustion of the filter and weighing of the remaining inorganic material (Occupational and Environmental Medicine Laboratory, Orebro University Hospital, Sweden). The detection limit was 0.1 mg/sample and results are expressed as mg/m3.
For analysis of endotoxin and 1,3-β-glucan the filters were extracted with pyrogen-free water. Endotoxin was determined by the Department of Environmental Medicine, University of Gothenburg (February 2004) and the Department of Infection Control, Uppsala University Hospital (September 2004) using the kinetic turbidi-metric method with the Limulus test (Associates of Cape Cod, East Falmouth, Massachusetts, USA) and Endosafe (Charles River Endosafe, Charleston, South Carolina, USA), respectively. The results are expressed as ng/m3 with a detection limit of 0.147 ng/m3.
The amount of 1,3-β-glucan was determined by the department of Environmental Medicine using the Limulus test with glucan specific lysate (Associates of Cape Cod) in the chromogenic, kinetic version. The results are expressed as ng/m3 with a detection limit of 0.1 ng/m3.
Horses — Clinical examination and sampling techniques
Twelve standarbred trotters (8 mares, 3 geldings, 1 stallion) aged 4.25 ± 1.4 y [mean ± (standard deviation) s, range: 3–7 y] from the group of 18 were included in the study, with selection based on the probability that they would remain in the same stable over the 1-year study period. All investigations on horses were performed at the nearby veterinary teaching hospital. All horses received detailed clinical and respiratory examinations according to previously published methods (17), including chest auscultation following rebreathing, routine blood sample analysis for white and red blood cell parameters (Cell-Dyn 3500; Abbott Laboratories, North Chicago, Illinois, USA), plasma fibrinogen (Konelab 30, Thermo Fisher Scientific, Waltham, Massachusetts, USA), arterial blood gas partial pressures (PaO2 and PaCO2) and pH (i-STAT; Abbott Laboratories, North Chigaco, Illinois, USA). Tracheal mucus or nasal discharge, when present, was scored as grades 1 to 3, and the color was observed. The amount of mucus was graded according following scale: grade 1 = single mucus clumps in ventral trachea, grade 2 = thin stream of mucus in ventral trachea, and grade 3 = larger stream of mucus in trachea, even mucus accumulation in lateral and ventral walls. Additionally, all horses had an upper and lower airway endoscopy (VES vet vision, XiON matrix FX 100, endoscope PV-G28; XiON Medical, Berlin, Germany), and bronchoscopically guided bronchial biopsy and BAL [3 × 100 mL sterile isotonic saline solution (at 37°C)]. The recovered BAL fluid was placed on ice, 30 mL was removed for immediate cytological examination and the remainder prepared and stored at −80°C for real-time polymerase chain reaction (RT–PCR) analysis.
BAL sample preparation and cytological examination
For cytological evaluation of BAL samples, cytospin preparations were prepared using 2 different concentrations of BAL-fluid. The 1st was prepared by centrifuging a 10-mL aliquot at 400 × g for 5 min and re-suspending the resulting cell pellet with 50 μL albumin solution (1 g bovine serum albumin and 0.002 g NaN3 dissolved in 10 mL of 0.9% NaCl and stored at 4°C). The noncentrifuged preparation was made by adding 50 μL of the albumin solution to a 250-μL aliquot of the BAL-fluid. Cytospin preparations with 100 μL aliquots were prepared in the cytocentrifuge cassettes. After the smears had dried, they were stained with modified Wright stain in an automatic stainer (Hematec; Bayer Diagnostic Division, Tarrytown, New York, USA). All smear evaluations were performed by one cytologist, who was blinded to the clinical aspects of this study. When possible, 400 cells were counted (200 cells on each slide) and the average number was used. Epithelial cells, which often appear in aggregates, and non-intact cells were excluded from the differential count. Total leukocyte counts were determined using Cell-Dyn 3500 (Abbott Laboratories). If the optical and impedance leukocyte counts of the Cell-Dyn disagreed, manual counts were performed using a counting chamber.
RNA extraction and one step real-time PCR
The BAL samples were centrifuged at 500 × g for 5 min; the resulting cell pellets were resuspended in 1 mL STE buffer (0.1 M NaCl, 1 mM EDTA, 10 mM Tris; pH 8.0) and re-centrifuged (500 × g, for 5 min). The pellets were lysed in Trizol (Sigma-Aldrich AB, Stockholm, Sweden) and stored at −80°C. The total RNA was isolated according to the manufacturer’s instructions shortly before performing the RT-PCR and then stored at −80°C for further use.
Quantification of equine IL-6 and IL-10 mRNA expression was performed by one step quantitative RT-PCR using a RotorGene3000 system (Corbett Research, Sydney, Australia). Primer and probe sequences used were designed from nucleotide sequence for this study. The following nucleotide sequences were used for IL-10: forward primer 5′ to 3′: TTC AGC AGG GTG AAG ACT TTC, reverse primer 5′ to 3′, CCT GGC AAC CCA GGT AAC CCT TA, and probe 5′ to 3′ TGT TGA ACG GGT CCC TGC TGG AGG ACT. Primers used for IL-6: forward primer 5′ to 3′: AGC AAG TGT GAA AAC AGC AAG, reverse primer 5′ to 3′, CAT CAG GCA GGT CTC CTG AT, and probe CTG GCA GAA AAC AAC CTG AAT CTT CCA. The RT-PCR reactions (25 μL final volume) contained 0.5 mM of each dNTP, 2.5 mM MnOAc2, 2.5 units of rTth polymerase (Applied Biosystems, Foster City, California, USA), 40 ng of total RNA and the primer/probe sets of interest. The single step reaction was run at 42°C for 5 min and 60°C for 20 min followed by 45 cycles at 95°C for 5 s and 60°C for 30 s.
All samples were run in triplicate and nontemplate controls were included in each run. The RNA levels of the target genes were normalized against GAPDH RNA levels (forward primer 5′ to 3′: AAG TGG ATA TTG TCG CCA TCA AT, reverse primer 5′ to 3′ AAC TTG CCA TGG GTG GAA TC, and probe 5′ to 3′ TGA CCT CAA CTA CAT GGT CTA CAT GTT TCA) (18) and the comparative CT (2−ΔΔCT) method was used for calculating relative cytokine mRNA expression (19). The ΔCT value is the difference between the CT value for the target gene and housekeeping gene, and the ΔΔCT value is the difference between ΔCT value for sampling B and the ΔCT value for sampling it is compared against. The PCR efficiencies, determined by assaying serial dilutions of RNA, were approximately equal for the target genes and the housekeeping genes.
Statistics
Concentrations of environmental parameters were generally presented as median values and, where appropriate, as mean ± standard deviation (s). Descriptive statistics from each sampling period were calculated and the horse data were treated as being nonparametric when examining for differences between sampling periods. Three horses had clinically significant elevations of BAL eosinophils (11%, 33%, 37%) during the summer sampling and were therefore excluded from all further analysis (17). Two horses were not available for sampling on all occasions; one was missing for the 2nd winter period (March) and the other for the summer sampling period (September). The horse missing for the summer sampling period was therefore also excluded from the cytokine mRNA analyses.
The Wilcoxon signed-rank test was used to assess statistical differences among individual sampling occasions, and to compare differences between seasons after averaging the results for the 2 winter samplings. The Wilcoxon signed-rank test was used also for comparison of relative cytokine expression where the theoretic median value 1 was used (theoretically no difference between provocation and remission or ΔΔCT = 0, 2−ΔΔCT = 20 = 1). A 2-tailed test was used with P < 0.05 set as level of significance. Statistical analyses were performed using a commercially available statistical software program (MINITAB; Minitab, State College, Pennsylvania, USA).
Results
Hygienic measurements in the stable environment
During winter sampling, the outdoor morning temperature was approximately −5°C, and the indoor stable temperature was only slightly higher (3 to 5°C). When sampling in late summer, the outdoor and indoor temperatures were the same (15.0°C). Outdoor and indoor relative humidities were similar (42.5% to 71%) with the highest level being in winter.
Results from indoor measurements are shown in Table I. Total and respirable dust measurements in the stable air were generally low and consistently below the upper limits recommended for humans (20) (Table I); measurements were only slightly higher during winter sampling. The organic dust level approximated 70% (range: 0.4–0.8 mg/m3) of the total dust level (data not shown), which is well under the upper acceptable limit for humans (5 mg/m3) (20) and for horses (10 mg/m3) (21). In the first winter sampling, levels of endotoxin and 1,3-β-glucan were 5 ng/m3 and 85 ng/m3, respectively, whereas during summer sampling, endotoxin was higher at 14 ng/m3 but 1,3-β-glucan lower at 21 ng/m3 (median values). Endotoxin and 1,3-β-glucan from the second winter sampling (March 2005) were unavailable because of problems at the laboratory.
Table I.
Stable air measurements
| Winter 2004 | Summer 2004 | Winter 2005 | |
|---|---|---|---|
| Total dust (mean; mg/m3) | 1.07 | 0.88 (range: 0.67–1.27) | 0.82 (range: 0.68–0.96) |
| Respirable dust (mean; mg/m3) | 1.21 (range: 0.8–1.6) | 0.27 (range: 0.24–0.31) | 0.48 (range: 0.45–0.51) |
| Endotoxin (median; ng/m3) | 4.6 (range: 1.9–6.9) | 15 (range: 9–16) | ND |
| 1,3-β-Glucan (median; ng/m3) | 85 (range: 24–121) | 21 (range: 19–27) | ND |
| Temperature | |||
| In stable (mean; °C) | 3 | 15 | 6 |
| Outdoors (mean; °C) | −5 | 15 | −5 |
| Relative humidity | |||
| In stable (%) | 67 | 55 | 43 |
| Outdoors (%) | 69 | nd | 43 |
ND — no data
Horses — Clinical examination and hematology
While the data from the 3 horses that had elevated eosinophils in BAL in September did not alter the clinical or cytokine mRNA findings, they were deemed to have subclinical respiratory disease and thus excluded from all analyses and interpretation of results reported hereafter. Of the clinical parameters measured, only the respiratory rate differed over the seasons, being lower at September measurement compared with the winter mean (P = 0.021). There was no significant difference over the seasons in all other clinical parameters, including other vital signs, blood parameters, PaO2 and subjectively graded appearance of tracheal mucus. Hematology revealed only minor variations that were of no clinical relevance.
Bronchoalveolar lavage cytology
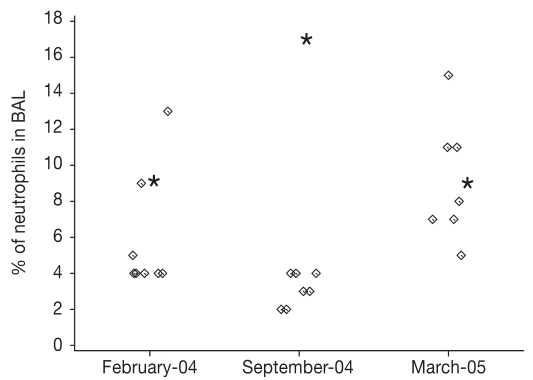
The median for neutrophil percentage in BAL during winter (median February 2004, 4.0% and March 2005, 8.5%) was more than double the level obtained in September (7.5% versus 3.5%). The difference did not reach significance (winter mean/September, P = 0.141) due to 1 outlier (Figure 1), but by removing it from the analysis the seasonal difference was clearly significant (P = 0.022). Neutrophil percentage during the separate winter months did not differ P = 0.151. Even after exclusion of data from the 3 horses with pronounced pulmonary eosinophilia [reported elsewhere, (17)], the percentage of eosinophils in BAL fluid was significantly higher in the summer (summer mean, 2.25% ± 2.05 s, median 1.0%), versus combined winter samples (mean, of 0.13% ± 0.35 s, median 0.0%, P = 0.022), which was presumably related to seasonal management factors such as increased parasite burden rather than stable air particle differences.
Figure 1.
Neutrophil percentage in BAL at three sampling occasions. The outlier with IAD is marked with an asterisk (*).
Both percentage and absolute cell numbers from the remaining cell types in BAL were statistically similar between winter mean and summer sampling. However, when winter samplings were compared separately against September samplings, March 2005 the (winter) sampling had significantly lower mast cells and eosinophils (P = 0.022 for both). The February 2004 sampling, however, had a strong trend for higher absolute number of cells in BAL, but this failed to reach significance (P = 0.05).
One step real-time PCR
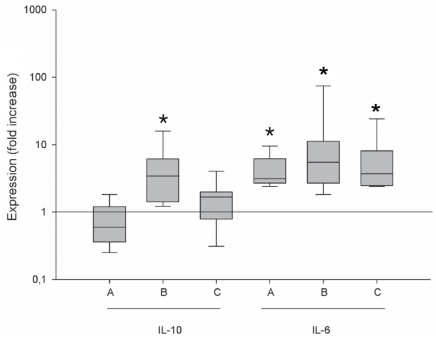
Gene expression of IL-6 mRNA in BAL cells was significantly enhanced during winter periods compared with summer sampling, with median winter values being 3.1-fold (P = 0.014) increased in February 2004 over September 2004, and median values being 5.5-fold greater in March 2005 than September 2004 (P = 0.022). When winter results were combined there was a 3.7-fold increase over September (P = 0.014) (Figure 2). In contrast, the median IL-10 mRNA expression was 3.4-fold higher during March sampling compared with September (P = 0.036) but there was no significant difference between September and February (Figure 2). There was no correlation of IL-6 with IL-10 mRNA cytokine expression, either when mean values or individual values were compared. Furthermore, there was no correlation between absolute number and percentage of neutrophils, eosinophils, or mast cells in BAL with expression of either IL-6 or IL-10 mRNA.
Figure 2.
The relative (fold) increase of cytokine mRNA expression of IL-6 and IL-10 in horse BAL cells is shown with interquartile range at different sampling occasions compared to September sampling. The horizontal line represents the median value (no change). A — February versus September, B — March versus September, C — winter mean versus September. * Significant difference in median value from 1 (significant up/down regulation).
Discussion
The most prominent difference between winter and summer in BAL samples was the increased expression of IL-6 mRNA during both winter periods which coincided with a trend for neutrophilia in BAL. A pluripotent cytokine with both pro-inflammatory and anti-inflammatory properties (22,23) the production of IL-6 is induced by a variety of different substances. For example, endotoxins induce IL-6 production both in vitro and in vivo (24,25). Interestingly, airborne endotoxin levels in this study were low compared with earlier studies in a low dust stable environment (26), and the winter values in this study were lower than our summer values. The horses herein were examined in the late stabling period (late winter) when they had been exposed to endotoxin levels in the stable environment over a prolonged continuous period, rather than the brief exposure as that of experimental inhalation studies (15). Thus, the cumulative effect of prolonged continuous exposure to low endotoxin levels cannot be excluded as causal for the increased IL-6mRNA expression in BAL in the late stabling period.
In an earlier study, IL-6 bioactivity was not detected in BAL fluid from horses with heaves during severe exacerbation after exposure to moldy hay (24). However, Laan et al (27) showed that inhalation provocation of RAO-susceptible horses and control horses with both hay dust suspension and with Aspergillus fumigatus antigen increased IL-6 expression in alveolar macrophages. In our study, total IL-6 mRNA produced by all BAL cell types and from racing horses without history of underlying inflammatory disease was measured. Thus, the results herein with increased IL-6 production could reflect differences in cumulative exposure to inhaled endotoxins during stabling period compared to after pasture, since summer conditions allow for far greater time spent outdoors. On the other hand, increased IL-6 mRNA has also been shown in BAL cells after cold weather exercise (28,29), which may partly explain increased expression in our study during the winter months.
Human tracheobronchial epithelial cell production of IL-6 has also been shown to affect mucus production in airways by stimulating mucin genes MUC5B and MUC5AC (30). Indeed, equine MUC5AC is expressed in horse airways and may play an important part in mucus hyper-secretion observed in RAO horses (5). However, our horses did not show increased amount of mucus in trachea during winter periods despite elevated expression of IL-6. The biological importance of the small but significant elevation in BAL eosinophils in September remains uncertain as the contribution of eosinophils to the entire BAL cell population recovered was minimal, and the increase did not appear to affect IL-6 mRNA expression in BAL cells.
There are contradictory results in human studies regarding IL-6 association with eosinophilic inflammation (31–34), probably partly related to differences in patient populations under study. Thus it is unclear whether the increased IL-6 mRNA in BAL during winter reflected stable environment or cold weather exercise.
The altered expression of IL-10 in total BAL cell mRNA in this study was less consistent than IL-6, with only March 2005 being significantly different by 3.8-fold over September 2004. A pleiotropic cytokine, IL-10 is secreted by activated CD4+ and CD8+ subsets of lymphocytes with immunoregulatory properties in humans with both TH1 and TH2 cell induced inflammatory processes and with production of pro-inflammatory cytokines in macrophages (22,35). The functional role of IL-10 in human asthma and airway responsiveness and equine RAO is still unclear. Laan et al (27) showed a slight increase in IL-10 mRNA expression from isolated alveolar macrophages in BAL 6 h after lipopolysaccharide (LPS) nebulization challenge in RAO-susceptible horses, but not in controls. This increase could not be measured after inhalation challenge with hay dust suspension (27) or after provocation with Aspergillus fumigatus antigen (36). Another study (37) showed that inhalation with LPS led to elevated IL-10 mRNA expression after 6 h in peripheral white blood cells in horses. However, factors other than inhaled agents from indoor stable environment, which can induce an inflammatory response, may affect cytokine mRNA expression. Davis et al (28) showed that TH2 cytokines IL-4, IL-5 and immunomodulatory IL-10 mRNA expression in BAL cells were up-regulated after cold weather exercise (−5°C), suggesting mast cell activation in combination with release of neurokinins as a source of these cytokines. In the same study, the BAL cytology was unaffected 5 h post-exercise, but IL-10 mRNA in BAL cells was up-regulated 10-fold suggesting local suppression of cell-mediated immunity in equine lung post cold-air exercise (28). In a later study (29) they showed that increased IL-10 expression was still present 48 h after exercise while breathing cold air. At the same time they also found a modest increase of neutrophils in BAL 24 h post exercise (29).
In this study, we used standardbred racehorses in training and racing at outdoor temperatures; they were sampled twice during the winter period and once after pasture. Up-regulation of IL-10 in winter due to regular exercise at cold weather and long-term exposure to stable environment may be expected, especially when IL-6 expression indicated increased inflammatory response during the winter period. On the other hand, from our summer measurements even after exclusion of the horses with marked pulmonary eosinophilia, a trend for increased eosinophil percentage in BAL persisted. Since IL-10 is also produced also by eosinophils, IL-10 mRNA expression might have been up-regulated due to parasite-induced (38) or idiopathic eosinophilia during summer measurements, which may have interfered with our overall comparison of IL-10 mRNA expression between summer and winter months. Kinetic studies of IL-10 in BAL cells (39) from mild asthmatics showed a significant correlation between eosinophilic concentrations in BAL fluid and IL-10 expression in BAL cells. Thus, there could be several explanations for the increased IL-10 expression during March 2005 but not February 2004 measurements and further studies are needed to better define the immunomodulatory properties of IL-10 within equine airways.
The tendency for elevated resting respiratory rate during winter sampling was likely related to examination of the horses in a higher ambient temperature at our clinic in contrast with the colder environment at the home stable, to which they were accustomed. While not reaching statistical significance, there was a consistent trend for increased percentage of neutrophils in BAL during winter sampling, compatible with a cytologic diagnosis of IAD.
In examining the stable environment measurements in more detail, all parameters were found to be within the published acceptable levels. Several studies have shown that type and hygienic quality of feed and bedding material have a great influence on dust concentration in stable air and are also correlated with clinical signs, pulmonary function, and neutrophil influx in RAO-susceptible horses (8,40–46). Given that the stable we examined fed haylage and had straw for bedding, the environment can be considered moderately dusty (40,42,43). When results from different stable environment studies are compared other factors need to be considered, such as the location of the sampling equipment (respiratory zone of the horses or in the stable corridor), type of ventilation, air humidity, stabling routines and time of day for sample collection as well as type of sampling devices and methods of analysis. The values of total and respirable dust obtained in this study, while consistently below upper limits for humans (20), were relatively high compared with earlier studies (26,40,43,47,48). Earlier studies have shown higher particle load during cleaning of boxes and daytime activity in the stable, compared with the time of day when the stable is free from activity (43,48,49). In our study, the samples were collected during normal daily stable routines, when feeding and stable cleaning can partly explain relatively high particle load compared with recent studies with stables having the same type of feeding and bedding material (40,43,46,48,50,51). Since most of the horses in our study were in paddocks during these activities, horses in general were probably exposed to lower dust levels during afternoon/night time stabling. Not surprisingly, the air particle load was higher during the winter stabling period than in the summer since horses spend more time in the stable during winter and natural ventilation is likely less effective during that season (47). In this pilot study, the respirable dust collected in the breathing zone of the personnel combined with stationary measurements was below the upper limits recommended for humans (20). Collection of both the total and respirable dust in the background and from breathing zones of horses would have been desirable as higher values are expected in the breathing zone compared with those of stationary measurements.
Components of the inhaled dust in stables that can have a substantial influence on pulmonary inflammation include endotoxin and 1,3-β-glucan. The role of endotoxin in equine RAO has mainly been studied by Pirie et al (14–16), who concluded that endotoxin present in the stable environment is a likely contributing factor to neutrophilic airway inflammation in horses. However, at present, there are no official guidelines regarding threshold values for either endotoxin or 1,3-β-glucan in horse stables. McGorum et al (26) showed that concentrations in horse stables varied greatly, and may be related to stable routines and hygiene as well as sampling and analysis methods used (52). In this study airborne endotoxin levels were lower than those reported by McGorum (26), and indeed lower in the one winter sampling when compared to summer. Due to loss of one of the winter seasons’ 1,3-β-glucan analyses, interpretation of the role of this airborne particle regarding season or airway inflammation remains unclear.
In conclusion, it appears that during winter stabling conditions horses have up-regulation of IL-6 mRNA that coincides with a trend for increased neutrophils in the BAL. This up-regulation was also associated with increased respirable dust and 1,3-β-glucan but curiously, lower airborne endotoxin, in the stable air. The next step will be to define whether there are specific air quality parameters in horse stables during winter that can be individually or collectively responsible for this up-regulation.
Acknowledgments
We thank C-H Bergström and his personnel at Lunda Gård, Uppsala, Sweden, for making this study possible and Mikael Berg, Department of Molecular Biosciences, Swedish University of Agricultural Sciences for providing technical resources. The Växjö Society of Animal Rights, the Swedish Veterinary Society, and the Swedish Research Council for Environment, Agricultural Sciences and Spatial Planning (FORMAS) provided funding for this study.
References
- 1.Robinson NE. International Workshop on Equine Chronic Airway Disease. Michigan State University 16–18 June 2000. Equine Vet J. 2001;33:5–19. doi: 10.2746/042516401776767412. [DOI] [PubMed] [Google Scholar]
- 2.Robinson NE. Inflammatory airway disease: Defining the syndrome. Conclusions of the Havemeyer Workshop. Equine vet Educ. 2003;15:61–63. [Google Scholar]
- 3.Couetil LL, Hoffman AM, Hodgson J, et al. Inflammatory airway disease in horses, ACVIM consensus statement. J Vet Intern Med. 2007;21:356–361. doi: 10.1892/0891-6640(2007)21[356:iadoh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 4.Robinson NE, Derksen FJ, Olszewski MA, Buechner-Maxwell VA. The pathogenesis of chronic obstructive pulmonary disease of horses. Br Vet J. 1996;152:283–306. doi: 10.1016/s0007-1935(96)80101-1. [DOI] [PubMed] [Google Scholar]
- 5.Gerber V, Robinson NE, Luethi S, Marti E, Wampfler B, Straub R. Airway inflammation and mucus in two age groups of asymptomatic well-performing sport horses. Equine Vet J. 2003;35:491–495. doi: 10.2746/042516403775600424. [DOI] [PubMed] [Google Scholar]
- 6.Jost U, Klukowska-Rotzler J, Dolf G, et al. A region on equine chromosome 13 is linked to recurrent airway obstruction in horses. Equine Vet J. 2007;39:236–241. doi: 10.2746/042516407x171110. [DOI] [PubMed] [Google Scholar]
- 7.Ramseyer A, Gaillard C, Burger D, et al. Effects of genetic and environmental factors on chronic lower airway disease in horses. J Vet Intern Med. 2007;21:149–156. doi: 10.1892/0891-6640(2007)21[149:eogaef]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 8.Holcombe SJ, Jackson C, Gerber V, et al. Stabling is associated with airway inflammation in young Arabian horses. Equine Vet J. 2001;33:244–249. doi: 10.2746/042516401776249606. [DOI] [PubMed] [Google Scholar]
- 9.McGorum BC, Dixon PM, Halliwell RE. Responses of horses affected with chronic obstructive pulmonary disease to inhalation challenges with mould antigens. Equine Vet J. 1993;25:261–267. doi: 10.1111/j.2042-3306.1993.tb02960.x. [DOI] [PubMed] [Google Scholar]
- 10.McPherson EA, Lawson GH, Murphy JR, Nicholson JM, Breeze RG, Pirie HM. Chronic obstructive pulmonary disease (COPD) in horses: Aetiological studies: responses to intradermal and inhalation antigenic challenge. Equine Vet J. 1979;11:159–166. doi: 10.1111/j.2042-3306.1979.tb01330.x. [DOI] [PubMed] [Google Scholar]
- 11.Pirie RS, Collie DD, Dixon PM, McGorum BC. Evaluation of nebulised hay dust suspensions (HDS) for the diagnosis and investigation of heaves. 2: Effects of inhaled HDS on control and heaves horses. Equine Vet J. 2002;34:337–342. doi: 10.2746/042516402776249074. [DOI] [PubMed] [Google Scholar]
- 12.Pirie RS, Dixon PM, McGorum BC. Evaluation of nebulised hay dust suspensions (HDS) for the diagnosis and investigation of heaves. 3: Effect of fractionation of HDS. Equine Vet J. 2002;34:343–347. doi: 10.2746/042516402776249236. [DOI] [PubMed] [Google Scholar]
- 13.Pirie RS, McLachlan G, McGorum BC. Evaluation of nebulised hay dust suspensions (HDS) for the diagnosis and investigation of heaves. 1: Preparation and composition of HDS. Equine Vet J. 2002;34:332–336. doi: 10.2746/042516402776249092. [DOI] [PubMed] [Google Scholar]
- 14.Pirie RS, Collie DD, Dixon PM, McGorum BC. Inhaled endotoxin and organic dust particulates have synergistic proinflammatory effects in equine heaves (organic dust-induced asthma) Clin Exp Allergy. 2003;33:676–683. doi: 10.1046/j.1365-2222.2003.01640.x. [DOI] [PubMed] [Google Scholar]
- 15.Pirie RS, Dixon PM, Collie DD, McGorum BC. Pulmonary and systemic effects of inhaled endotoxin in control and heaves horses. Equine Vet J. 2001;33:311–318. doi: 10.2746/042516401776249732. [DOI] [PubMed] [Google Scholar]
- 16.Pirie RS, Dixon PM, McGorum BC. Endotoxin contamination contributes to the pulmonary inflammatory and functional response to Aspergillus fumigatus extract inhalation in heaves horses. Clin Exp Allergy. 2003;33:1289–1296. doi: 10.1046/j.1365-2745.2003.01651.x. [DOI] [PubMed] [Google Scholar]
- 17.Riihimaki M, Lilliehook I, Raine A, Berg M, Pringle J. Clinical alterations and mRNA levels of IL-4 and IL-5 in bronchoalveolar cells of horses with transient pulmonary eosinophilia. Res Vet Sci. 2008;85:52–55. doi: 10.1016/j.rvsc.2007.09.015. [DOI] [PubMed] [Google Scholar]
- 18.Ainsworth DM, Grunig G, Matychak MB, et al. Recurrent airway obstruction (RAO) in horses is characterized by IFN-gamma and IL-8 production in bronchoalveolar lavage cells. Vet Immunol Immunopathol. 2003;96:83–91. doi: 10.1016/s0165-2427(03)00142-9. [DOI] [PubMed] [Google Scholar]
- 19.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 20.The Swedish Work Environment Authority, AFS 2005:17: Swedish Board of Agriculture, 2003:12.
- 21.Regulations on farm animal housing DFS 2004:17: Swedish Animal Welfare Agency 2004:1.
- 22.Opal SM, DePalo VA. Anti-inflammatory cytokines. Chest. 2000;117:1162–1172. doi: 10.1378/chest.117.4.1162. [DOI] [PubMed] [Google Scholar]
- 23.Xing Z, Gauldie J, Cox G, et al. IL-6 is an antiinflammatory cytokine required for controlling local or systemic acute inflammatory responses. J Clin Invest. 1998;101:311–320. doi: 10.1172/JCI1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Giguere S, Viel L, Lee E, MacKay RJ, Hernandez J, Franchini M. Cytokine induction in pulmonary airways of horses with heaves and effect of therapy with inhaled fluticasone propionate. Vet Immunol Immunopathol. 2002;85:147–158. doi: 10.1016/s0165-2427(01)00420-2. [DOI] [PubMed] [Google Scholar]
- 25.Morris DD, Crowe N, Moore JN, Moldawer LL. Endotoxin-induced production of interleukin 6 by equine peritoneal macrophages in vitro. Am J Vet Res. 1992;53:298–1301. [PubMed] [Google Scholar]
- 26.McGorum BC, Ellison J, Cullen RT. Total and respirable airborne dust endotoxin concentrations in three equine management systems. Equine Vet J. 1998;30:430–434. doi: 10.1111/j.2042-3306.1998.tb04514.x. [DOI] [PubMed] [Google Scholar]
- 27.Laan TT, Bull S, Pirie R, Fink-Gremmels J. The role of alveolar macrophages in the pathogenesis of recurrent airway obstruction in horses. J Vet Intern Med. 2006;20:167–174. doi: 10.1892/0891-6640(2006)20[167:troami]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 28.Davis MS, Malayer JR, Vandeventer L, Royer CM, McKenzie EC, Williamson KK. Cold weather exercise and airway cytokine expression. J Appl Physiol. 2005;98:2132–2136. doi: 10.1152/japplphysiol.01218.2004. [DOI] [PubMed] [Google Scholar]
- 29.Davis MS, Williams CC, Meinkoth JH, et al. Influx of neutrophils and persistence of cytokine expression in airways of horses after performing exercise while breathing cold air. Am J Vet Res. 2007;68:185–189. doi: 10.2460/ajvr.68.2.185. [DOI] [PubMed] [Google Scholar]
- 30.Chen Y, Thai P, Zhao YH, Ho YS, DeSouza MM, Wu R. Stimulation of airway mucin gene expression by interleukin (IL)-17 through IL-6 paracrine/autocrine loop. J Bio Chem. 2003;278:17036–17023. doi: 10.1074/jbc.M210429200. [DOI] [PubMed] [Google Scholar]
- 31.Gerber V, Robinson NE, Venta RJ, Rawson J, Jefcoat AM, Hotchkiss JA. Mucin genes in horse airways: MUC5AC, but not MUC2, may play a role in recurrent airway obstruction. Equine Vet J. 2003;35:252–257. doi: 10.2746/042516403776148291. [DOI] [PubMed] [Google Scholar]
- 32.Virchow JC, Jr, Kroegel C, Walker C, Matthys H. Inflammatory determinants of asthma severity: Mediator and cellular changes in bronchoalveolar lavage fluid of patients with severe asthma. J Allergy Clin Immunol. 1996;98:S27–33. discussion S33–40. [PubMed] [Google Scholar]
- 33.Broide DH, Lotz M, Cuomo AJ, Coburn DA, Federman EC, Wasserman SI. Cytokines in symptomatic asthma airways. J Allergy Clin Immunol. 1992;89:958–967. doi: 10.1016/0091-6749(92)90218-q. [DOI] [PubMed] [Google Scholar]
- 34.Tutluoglu B, Atis S, Anakkaya AN, Altug E, Tosun GA, Yaman M. Sensitization to horse hair, symptoms and lung function in grooms. Clin Exp Allergy. 2002;32:1170–1173. doi: 10.1046/j.1365-2745.2002.01439.x. [DOI] [PubMed] [Google Scholar]
- 35.Quaedvlieg V, Henket M, Sele J, Louis R. Cytokine production from sputum cells in eosinophilic versus non-eosinophilic asthmatics. Clin Exp Immunol. 2006;143:161–166. doi: 10.1111/j.1365-2249.2005.02968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koulis A, Robinson DS. The anti-inflammatory effects of inter-leukin-10 in allergic disease. Clin Exp Allergy. 2000;30:747–750. doi: 10.1046/j.1365-2222.2000.00839.x. [DOI] [PubMed] [Google Scholar]
- 37.Laan TT, Bull S, Pirie RS, Fink-Gremmels J. Evaluation of cytokine production by equine alveolar macrophages exposed to lipopolysaccharide, Aspergillus fumigatus, and a suspension of hay dust. Am J Vet Res. 2005;66:1584–1589. doi: 10.2460/ajvr.2005.66.1584. [DOI] [PubMed] [Google Scholar]
- 38.van den Hoven R, Duvigneau JC, Hartl RT, Gemeiner M. Clenbuterol affects the expression of messenger RNA for inter-leukin 10 in peripheral leukocytes from horses challenged intrabronchially with lipopolysaccharides. Vet Res Commun. 2006;30:921–928. doi: 10.1007/s11259-006-3383-4. [DOI] [PubMed] [Google Scholar]
- 39.Davidson AJ, Hodgkinson JE, Proudman CJ, Matthews JB. Cytokine responses to Cyathostominae larvae in the equine large intestinal wall. Res Vet Sci. 2005;78:169–176. doi: 10.1016/j.rvsc.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Colavita AM, Hastie AT, Musani AI, et al. Kinetics of IL-10 production after segmental antigen challenge of atopic asthmatic subjects. J Allergy Clin Immunol. 2000;106:880–886. doi: 10.1067/mai.2000.110475. [DOI] [PubMed] [Google Scholar]
- 41.Clements JM, Pirie RS. Respirable dust concentrations in equine stables. Part 1: Validation of equipment and effect of various management systems. Res Vet Sci. 2007;83:256–262. doi: 10.1016/j.rvsc.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Vandenput S, Duvivier DH, Votion D, Art T, Lekeux P. Environmental control to maintain stabled COPD horses in clinical remission: effects on pulmonary function. Equine Vet J. 1998;30:93–96. doi: 10.1111/j.2042-3306.1998.tb04466.x. [DOI] [PubMed] [Google Scholar]
- 43.Vandenput S, Istasse L, Nicks B, Lekeux P. Airborne dust and aeroallergen concentrations in different sources of feed and bedding for horses. Vet Q. 1997;19:54–158. doi: 10.1080/01652176.1997.9694762. [DOI] [PubMed] [Google Scholar]
- 43.Woods PS, Robinson NE, Swanson MC, Reed CE, Broadstone RV, Derksen FJ. Airborne dust and aeroallergen concentration in a horse stable under two different management systems. Equine Vet J. 1993;25:208–213. doi: 10.1111/j.2042-3306.1993.tb02945.x. [DOI] [PubMed] [Google Scholar]
- 45.Vandenput S, Votion D, Duvivier DH, et al. Effect of a set stabled environmental control on pulmonary function and airway reactivity of COPD affected horses. Vet J. 1998;155:189–195. doi: 10.1016/s1090-0233(98)80018-x. [DOI] [PubMed] [Google Scholar]
- 46.Robinson NE, Karmaus W, Holcombe SJ, Carr EA, Derksen FJ. Airway inflammation in Michigan pleasure horses: Prevalence and risk factors. Equine Vet J. 2006;38:293–299. doi: 10.2746/042516406777749281. [DOI] [PubMed] [Google Scholar]
- 47.Clements JM, Pirie RS. Respirable dust concentrations in equine stables. Part 2: The benefits of soaking hay and optimising the environment in a neighbouring stable. Res Vet Sci. 2007;83:263–268. doi: 10.1016/j.rvsc.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 48.Rosenthal FS, Gruntman A, Couetil LL. A comparison of total, respirable, and real-time airborne particulate sampling in horse barns. J Occup Environ Hyg. 2006;3:599–605. doi: 10.1080/15459620600948557. [DOI] [PubMed] [Google Scholar]
- 49.Crichlow EC, Yoshida K, Wallace K. Dust levels in a riding stable. Equine Vet J. 1980;12:185–188. doi: 10.1111/j.2042-3306.1980.tb03422.x. [DOI] [PubMed] [Google Scholar]
- 50.Webster AJ, Clarke AF, Madelin TM, Wathes CM. Air hygiene in stables. 1: Effects of stable design, ventilation and management on the concentration of respirable dust. Equine Vet J. 1987;19:448–453. doi: 10.1111/j.2042-3306.1987.tb02641.x. [DOI] [PubMed] [Google Scholar]
- 51.Zeitler MH. Concentration and size distribution of air-borne dust particles in horse stables. Berl Munch Tierarztl Wochenschr. 1985;98:241–246. [PubMed] [Google Scholar]
- 52.Clarke AF. A review of environmental and host factors in relation to equine respiratory disease. Equine Vet J. 1987;19:435–441. doi: 10.1111/j.2042-3306.1987.tb02638.x. [DOI] [PubMed] [Google Scholar]
- 53.Jacobs RR. Analyses of endotoxins. International Journal Occupational Enviromental Health. 1997;3:42–48. [Google Scholar]