Abstract
Paracoccidioides brasiliensis is a dimorphic fungus that causes paracoccidioidomycosis, the most prevalent human deep mycosis in Latin America. The dimorphic transition from mycelium to yeast (M-Y) is triggered by a temperature shift from 25°C to 37°C and is critical for pathogenicity. Intracellular Ca2+ levels increased in hyphae immediately after temperature-induced dimorphism. The chelation of Ca2+ with extracellular (EGTA) or intracellular (BAPTA) calcium chelators inhibited temperature-induced dimorphism, whereas the addition of extracellular Ca2+ accelerated dimorphism. The calcineurin inhibitor cyclosporine A (CsA), but not tacrolimus (FK506), effectively decreased cell growth, halted the M-Y transition that is associated with virulence, and caused aberrant growth morphologies for all forms of P. brasiliensis. The difference between CsA and FK506 was ascribed by the higher levels of cyclophilins contrasted to FKBPs, the intracellular drug targets required for calcineurin suppression. Chronic exposure to CsA abolished intracellular Ca2+ homeostasis and decreased mRNA transcription of the CCH1 gene for the plasma membrane Ca2+ channel in yeast-form cells. CsA had no detectable effect on multidrug resistance efflux pumps, while the effect of FK506 on rhodamine excretion was not correlated with the transition to yeast form. In this study, we present evidence that Ca2+/calmodulin-dependent phosphatase calcineurin controls hyphal and yeast morphology, M-Y dimorphism, growth, and Ca2+ homeostasis in P. brasiliensis and that CsA is an effective chemical block for thermodimorphism in this organism. The effects of calcineurin inhibitors on P. brasiliensis reinforce the therapeutic potential of these drugs in a combinatory approach with antifungal drugs to treat endemic paracoccidioidomycosis.
The dimorphic pathogenic fungus Paracoccidioides brasiliensis is the etiological agent of paracoccidioidomycosis, an endemic disease in Latin America and the most prevalent human deep systemic mycosis found in Brazil, Colombia, and Venezuela (29). It is believed that infection by P. brasiliensis initiates when an individual inhales infectious microconidia from the saprophytic mycelial form present in the environment (29). Once in the lungs, the fungus initiates a process of morphological transition into the pathogenic yeast form (29). As with other pathogenic dimorphic fungi, the mycelium-to-yeast (M-Y) transition is essential for P. brasiliensis to establish the disease, given that P. brasiliensis lineages unable to differentiate into yeast cells are avirulent (9). The tight connection between morphological transition and virulence was recently supported by the work of Nemecek et al. (20), who provided strong evidence that there is a global control for the acquisition of both morphogenesis and pathogenicity traits common among dimorphic pathogenic fungi. The ability to grow at 37°C is considered an important virulence factor for all pathogenic fungi, and in P. brasiliensis, the transfer of a mycelial culture from 25°C to 37°C is sufficient to elicit the dimorphic transition (9).
About 10 million people are infected with P. brasiliensis, although only about 2% of those infected actually develop paracoccidioidomycosis (17). There are effective antifungal drugs against paracoccidioidomycosis, with different protocols for treatment depending on the form and severity of the disease and the age of the patient, but all have side effects when taken singularly and may have adverse interactions when taken with other drugs (38); additionally, the time of treatment is long and many patients quit before they are cured. Thus, there is a search for new medications or a combination of medications that could shorten the treatment time. The calcineurin pathway is a promising target for intervention with a combinatory therapy approach to treat invasive fungal infections (33).
Calcineurin is a Ca2+/calmodulin-dependent, serine/threonine-specific phosphatase essential for adaptation to environmental stresses, growth, morphogenesis, and pathogenesis in many fungal species (34). The enzyme consists of two subunits, the catalytic calcineurin A (Cna1p) and the Ca2+-binding regulatory subunit calcineurin B (Cnb1p) (30). Upon the increase of cytoplasmic free Ca2+, Ca2+/camodulin and Ca2+/Cnb1p bind to Cna1p, activating calcineurin by inducing a conformational change displacing an autoinhibitory domain from the active site (37). In Saccharomyces cerevisiae, calcineurin activation leads to the dephosphorylation and activation of the transcription factor Crz1p/Tcn1p (homolog of the mammalian nuclear factor NFATp), which translocates into the nucleus and induces the transcription of genes involved in cell survival and calcium homeostasis (5, 26, 39). In Neurospora crassa, the impairment of calcineurin function either chemically (with CsA or FK506) or using antisense RNA ceased the typical apical Ca2+ gradient of growing hyphae and arrested growth (27). The expression and activity of the Ca2+-permeable Cch1/Mid1 channel also is regulated by Ca2+ and calcineurin (3, 39). Cch1p/Mid1p mutants display a loss of viability and growth defects upon some stress stimuli (11, 25).
The rise of intracellular Ca2+ levels and the activation of calcineurin follow many different types of environmental changes and stresses in fungi, including temperature (23, 26), pH (1), ions (10, 15), antifungal drugs (24), and morphogenesis-inducing signaling (30). The activation of the Ca2+/calmodulin/calcineurin pathway induces fungi to perform the appropriate biochemical, epigenetic, and morphological changes required for their adaptation to stressful conditions. Depending on the species of the fungus, calcineurin performs a plethora of functions, including growth, germination, hyphal extension, development, and survival, under both normal and stressful conditions (34). The growth of Candida albicans in the presence of serum is dependent on calcineurin, which protects the fungus against high calcium stress (1, 2). In P. brasiliensis, Ca2+/calmodulin was shown to be essential for M-Y dimorphism using chemical inhibitors (7). Microarray analysis during the M-Y dimorphic transition of P. brasiliensis showed that the calcineurin B subunit was highly upregulated (22). The disruption of the CNA1 gene disabled the ability of Cryptococcus neoformans to grow at 37°C but not at 24°C (23). Even in the nonpathogenic yeast Saccharomyces cerevisiae, calcineurin is activated by environmental stresses and induces the transcription of genes related to cell survival in a Crz1p-dependent manner (5). Mutants lacking either the Cna1p or Cnb1p subunits of calcineurin were avirulent in animal models of cryptococcosis (23) and aspergillosis (6). The hyphal growth of Aspergillus fumigatus (6) and C. neoformans (4) mutants lacking functional CNA1 or CNB1 was severely affected at high temperatures.
Two known immunosupressor drugs, cyclosporine A (CsA) and tacrolimus (FK506), inhibit calcineurin activity. CsA and FK506 bind to the immunophilins cyclophilin A and FK506-binding protein (FKBP), respectively, and the drug-protein complex binds to the catalytic subunit of calcineurin, Cna1p, inhibiting calcineurin activity (8). The inhibition of calcineurin by CsA or FKBP depends on the relative concentrations of cyclophilin A and FKBP (36). As with CNA1 mutants, the calcineurin inhibitors CsA and FK506 also halted Cryptococcus neoformans growth at 37°C (23). Increased levels of calcineurin in Candida species increased resistance against azole antifungal drugs, whereas the impairment of calcineurin function produced synergistic fungicidal action with the azoles in both Candida (24, 38) and A. fumigatus (32). Therefore, efforts have been made in order to define a calcineurin target to be pharmacologically shut down in a combinatory approach with antifungal drugs to treat invasive fungal infections (33).
In this work, we provide evidence that (i) calcineurin is required early in the process of the temperature-induced dimorphism from M-Y and for the growth of P. brasiliensis, (ii) the transfer of mycelial cultures from 25°C to 37°C led to an increase in intracellular Ca2+, (iii) the chronic inhibition of calcineurin perturbed intracellular Ca2+ homeostasis during the M-Y transition and in yeast cells, and (iv) the chronic inhibition of calcineurin decreased the expression of the CCH1 calcium channel gene.
MATERIALS AND METHODS
Reagents.
Yeast extract, Bacto peptone, and dextrose for the YPD medium were purchased from Difco-BD. CsA, chymotrypsin, N-succinyl-alanyl-alanyl-prolyl-phenylalanyl 4-nitroanilide, pluronic acid, and rhodamine 123 were purchased from Sigma. Tacrolimus (FK506) was purchased from Janssen Labs. Fluo-3-AM (Fluo-3), 5-(and 6-)carboxy-2′,7′-dichlorofluorescein diacetate (DCFDA), and 1,2-bis(o-aminophenoxy) ethane-N,N,N′,N′-tetraacetic acid tetra (acetoxymethyl) ester (BAPTA) were purchased from Invitrogen-Molecular Probes. Zymolyase was purchased from Seikagaku Brewery (Japan).
Paracoccidioides brasiliensis isolate and culture.
Paracoccidioides brasiliensis isolate 18, which is the prototype of a highly virulent isolate (13), was provided by Z. P. Camargo, UNIFESP, Sao Paulo, Brazil. The fungus was grown at either 25°C or 37°C under rotation at 120 rpm in liquid YPD medium (1% yeast extract, 2% peptone, 2% dextrose) for 5 to 6 days until near the end of the exponential phase. The fungi were then inoculated in fresh medium at a 1:50 dilution (optical density at 600 nm, ca. 0.7) and grown for two more days, and then drugs were added at time zero (T0). For the transition experiments, mycelial cultures were transferred from 25°C to 37°C at T0 just after the addition of the drugs. Microscopy analysis of the morphological transition and both proliferation and viability assays were performed during the following 8 days of culture or longer.
Proliferation assay.
Proliferation was estimated by the dry weight of the fungus at different time points during the growth curve. For this, 1 ml of the culture was taken every day from T0 until the end of the experiment. The fungi were killed with sodium hypochlorite, centrifuged to drain off the liquid, and dried at 50°C. The dry pellet was weighted in microcentrifuge vials and plotted, discounting the weight of the empty vial.
Quantification of hyphal tip morphologies at different stages of the M-Y transition.
The hyphal tips of mycelia undergoing the transition to yeast cells were examined in a DMLB 100S Leica microscope under a bright field. A total of 200 hyphal tips were counted at ×1,000 magnification, under oil immersion, in random fields. Every tip in each field was counted and classified as having no hyphal tip enlargement, having a hyphal tip enlargement, or having a hyphal tip enlargement plus one or more buds or yeast cells.
Cell viability assay.
P. brasiliensis cells were washed in a sodium citrate buffer (50 mM, pH 5.0) plus 2% glucose, incubated at room temperature in 10 μM DCFDA for 20 min, and immediately analyzed under fluorescence microscopy.
Cytoplasmic calcium labeling.
P. brasiliensis cells were washed twice in Krebs-Ringer phosphate glucose and incubated for 20 min with or without 2 μg/ml CsA, 2 μg/ml FK506, 1 mM EGTA, or 5 μM BAPTA, followed by a 15-min incubation with 3 μM Fluo-3 (dissolved in dimethyl sulfoxide/2 μM pluronic acid; Invitrogen). The fungi were next washed in the same buffer and immediately analyzed under fluorescence microscopy.
Rhodamine 123 labeling.
P. brasiliensis cells were washed twice in Krebs-Ringer phosphate glucose and incubated for 20 min with or without 2 μg/ml CsA or 2 μg/ml FK506, followed by a 20-min incubation with 15 μM rhodamine 123. The fungi were next washed in the same buffer and immediately analyzed under fluorescence microscopy.
Real-time RT-qPCR analysis.
Real-time reverse transcriptase quantitative PCR (RT-qPCR) analysis for the CCH1 gene transcript was performed using 5′-TTGCGGAGAGGGTTGGAATGAGAT as the forward primer and 5′-ACATGCTGACGATGTTCCAGGAGA as the reverse primer. The primer sequences were based on the clone Pb50003-024G05 from the P. brasiliensis random sequence tag genomic library, which presented approximately 80% identity to Aspergillus fumigatus Af293 calcium channel subunit Cch1 (XM_747383) by BLAST analysis. The P. brasiliensis elongation initiation factor 3 gene was used as an endogenous control (22). Total RNA was extracted using Trizol reagent (Invitrogen) from mycelia, yeast, or fungus at the M-Y transition cultured for 48 h in the absence or presence of 2 μg/ml CsA. cDNAs were synthesized by reverse transcription using the Improm-II reverse transcriptase (Promega) as described by the manufacturer. Prior to conversion to cDNA, mRNA preparations were tested for DNA contamination by PCR using primers for an intronic region of the GP43 gene (19). Real-time RT-qPCRs were performed using Platinum Sybr green qPCR SuperMix-UDG (Invitrogen) with the standard cycling program for ABI instruments (50°C for 2 min; 95°C for 2 min; 40 cycles at 95°C for 15 s and 60°C for 30 s). The comparative 2−ΔΔCT method was used for relative quantification (16). Briefly, this method first normalizes the PCR signal (absolute quantification) of a target transcript in a treatment group to the PCR signal of an endogenous control in the same treatment group. The value obtained is then compared to that of an untreated control (which is always 1). mRNAs used for real-time RT-qPCRs were obtained from three independent experiments.
Immunophilin activity.
Peptidyl-prolyl cis-trans isomerase (PPI) activity was measured as previously described (14) with modifications. Approximately 100 ml of culture was harvested by centrifugation, and the cells were washed twice with cold water and once with buffer 1 (1 M mannitol, 10 mM K2HPO4 [pH 7.2], 10 mM EDTA, 0.1% sodium azide). The cells were resuspended in buffer 1 containing 50 mM β-mercaptoethanol. After a 15-min incubation at room temperature with shaking, the cells were harvested by centrifugation, resuspended in buffer 1 containing 5 mM β-mercaptoethanol and 5 μg/ml Zymolyase 100 T (or approximately 1,000 units/g cells), and incubated at 37°C until about 90% of the cells converted to spheroplasts (60 to 80 min). The digestion was stopped by the addition of an equal volume of ice-cold buffer 1, and spheroplasts were washed with ice-cold buffer 2 (0.6 M mannitol, 1 mM EDTA, 10 mM Tris-HCl [pH 7.4]). The pellet was resuspended in buffer 2 containing 1 mM phenylmethylsulfonyl fluoride, 2 μg/ml leupeptin, 2 μg/ml aprotinin, and 2 μg/ml pepstatin. The spheroplasts were mechanically broken using a Dounce homogenizer for up to 25 up-and-down strokes. Cell debris was pelleted by centrifugation for 10 min, and the total protein concentration in the supernatant was determined by the Lowry method. To test for PPI activity in the assay, 100 μg of total protein was added to a reaction mixture containing 100 mM Tris-HCl (pH 8.0) and 60 μM chymotrypsin in the absence (total PPI activity) or presence of 2 μg/ml CsA (to inhibit cyclophilin-dependent PPI activity) or 2 μg/ml FK506 (to inhibit FKBP-dependent PPI activity). The reaction was initiated after the addition of 20 μg/ml of peptide substrate N-succinyl-alanyl-alanyl-prolyl-phenylalanyl 4-nitroanilide. The reaction was monitored kinetically every 22.5 s over 12.4 min at room temperature in a Bio-Tek Synergy HT plate reader at 390 nm. The values from experimental samples were subtracted from those of background samples containing all regents except fungal protein to discount the spontaneous cleavage of the chymotrypsin substrate.
Statistical analysis.
Data are either the means or representative results of at least three similar repetitions, each performed in triplicate. Statistical analysis and comparisons were performed using paired Student's t tests.
RESULTS
Calcineurin inhibitor CsA impaired the growth of P. brasiliensis at 25°C and 37°C and affected the morphology of both yeasts and hyphae.
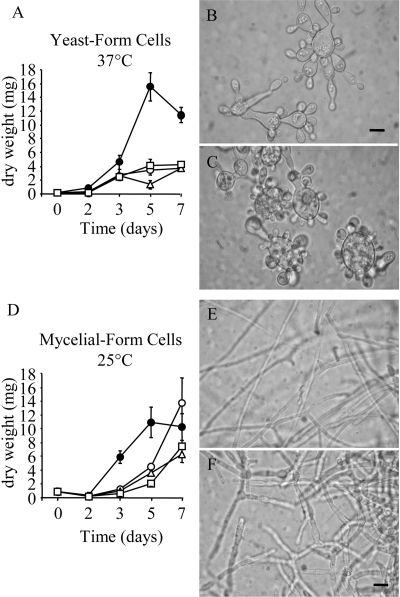
To test the effect of calcineurin inhibitors on fungal morphology and growth, different forms (mycelium at 25°C and yeast at 37°C) of P. brasiliensis were grown in the presence or absence of CsA. Yeast-form growth at 37°C was noticeably impaired at all CsA levels (0.5 μg/ml, 2 μg/ml, or 5 μg/ml; Fig. 1A). At 37°C, the control yeast cells (Fig. 1B) show multiple bud (primary blastoconidia) formation elongating away from the mother cell and forming pseudohyphae. Daughtering occurs at the apices of the mother cell, and daughter cells remain attached. Secondary blastoconidia form on apical buds opposite the original mother cell or on opposite sides of the apex of the first daughter cell.
FIG. 1.
Dose-dependent growth curves and representative photos of yeast and mycelial cells in the absence or presence of CsA. Growth rate of yeast cell culture at 37°C (A) and mycelial culture at 25°C (D) estimated by the dry weight of the fungus after culture for 7 days without (filled circles) or with 0.5 μg/ml (open circles), 2 μg/ml (open triangles), or 5 μg/ml (open squares) CsA. Bars indicate the means ± standard errors of the means of representative experiments (n = 3) made in triplicate. Yeast (B and C) or mycelial cells (E and F) after 5 days in culture without (B and E) or with 2 μg/ml CsA (C and F). Scale bar = 10 μm.
Cells treated with CsA (Fig. 1C) showed budding without neck elongation, and the number of daughter buds on a mother cell increased to dozens on a circumference. Emergent yeast buds showed a predominantly round shape with abundant intracellular granules. There was no secondary budding on the apices of the primary daughters and no pseudohyphal formations. The treatment of P. brasiliensis yeast cells with 0.5, 2, and 5 μg/ml CsA for 5 days produced less fungal dry weight.
At 25°C, CsA also altered mycelial growth. Though the mycelial form tolerated 0.5 μg/ml of CsA, higher concentrations of CsA caused an initial 50% reduction in cell growth (Fig. 1D). Control mycelial cells (Fig. 1E) are long and thin walled, with branching from the main stem occurring infrequently. At 2 μg/ml, CsA-treated mycelia (Fig. 1F) had shorter nodal lengths and an increased frequency of branching. Throughout the 5 days of culture, 2 μg/ml CsA caused an evident morphological alteration in hyphae, such as the enlargement of hyphal width, shortening of hyphal endings, roughening of the hyphal surface, and branching. The results imply that proper mycelial morphology also is governed by basal levels of activated calcineurin and that CsA also impacted hyphal morphology.
CsA and FK506 treatments induced a blockade of the M-Y transition, and though the calcineurin inhibitors did not cause cell death of P. brasiliensis, CsA suppressed the total dry weight of cells in culture.
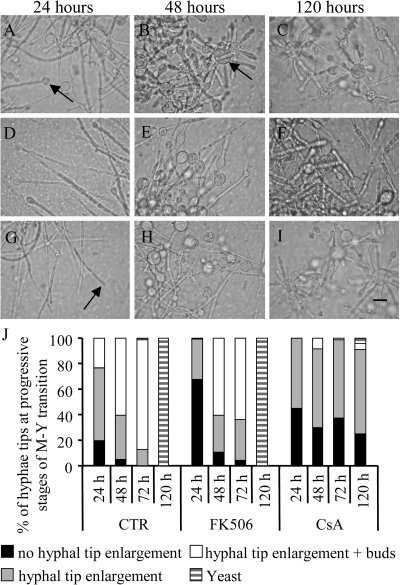
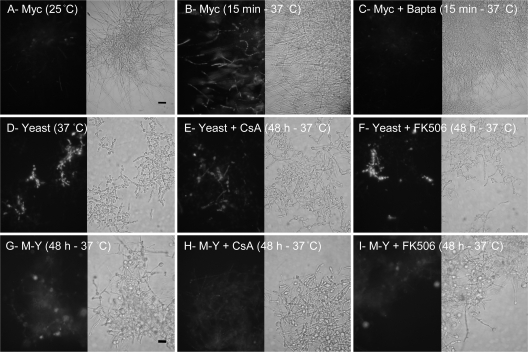
In order to show whether calcineurin is involved in the temperature-induced M-Y morphological transition of P. brasiliensis, we incubated mycelial forms with 2 μg/ml CsA or 2 μg/ml FK506 before shifting the temperature from 25°C to 37°C (Fig. 2). After 24 h, untreated hyphae shorten and thicken their cell walls, and approximately 50% of their hyphal tips become enlarged (Fig. 2A). By 48 h, the enlarged tips presented increasing numbers of secondary buds that elongate away from the initial enlarged formation (Fig. 2B). By 120 h, the transition was completed, and the characteristic multiple budding “steering wheel” yeast cells grow freely in the culture (Fig. 2C).
FIG. 2.
Effect of calcineurin inhibitors on morphology and rate of morphological hyphal tip progression through the M-Y transition stages during the M-Y transition of P. brasiliensis. (A to I) Morphology of the fungus during the M-Y transition at 24, 48, and 120 h at 37°C in the absence (Control; A to C) or presence of 2 μg/ml CsA (D to F) or FK506 (G to I). Arrows indicate hyphal tip enlargement (A), hyphal tip enlargement with buds or yeasts (B), or no hyphal tip enlargement (G). Scale bar = 10 μm. (J) Graphical summary of the rate of morphological changes during progression through the M-Y transition stages at 24, 48, 72, and 120 h at 37°C in the absence (CTR) or presence of calcineurin inhibitors (2 μg/ml CsA or FK506).
Under CsA treatment, mycelia underwent only the initial phenotypic changes typical of the M-Y transition, i.e., thickening of the hyphae, vacuolization of the cytoplasm, and enlargement of the hyphal tips (Fig. 2D and E). Though the cells were observed to day 15 (data not shown), the transition to the yeast form was blocked before the blastoconidial budding stage (Fig. 2F).
Although the other potent calcineurin inhibitor tacrolimus (FK506) showed no evident impairment of the growth or morphology of yeast or mycelia (not shown), FK506-treated hyphae presented a retardation of the temperature-induced M-Y transition rather than blockage (Fig. 2G to I and J). After 24 h, FK506-treated hyphae showed no hyphal tip enlargement (Fig. 2G). However, by 48 h, FK506-treated hyphae advanced in the M-Y transition process, and by 120 h, P. brasiliensis cells had undergone the complete M-Y transition and acquired a normal yeast-like morphology (Fig. 2I). Untreated and FK506-treated cells successfully transitioned to yeast-form cells by 120 h. However, after 120 h, CsA-treated cells were severely retarded: 70% entered the tip enlargement stage, 30% of P. brasiliensis cells remained at the mycelial stage, and no cells were observed in later stages of the yeast form. The results of Fig. 2 are numerically summarized as a census of hyphal tips and cell types (Fig. 2J).
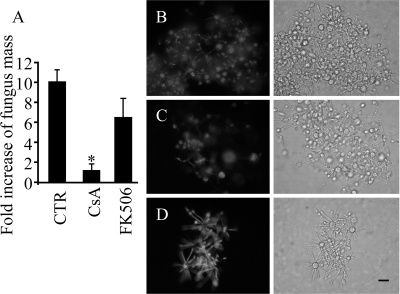
Despite severe loss in the biomass (Fig. 3A) and gross morphological aberration (Fig. 2), viability staining of P. brasiliensis during the M-Y transition indicated that cells treated with CsA were viable after 12 days (Fig. 3B and C) and, therefore, that CsA treatment was fungistatic for P. brasiliensis in culture. FK506 presented only a mild and transitory effect on both dimorphism and growth and did not affect viability (Fig. 3D).
FIG. 3.
Effect of calcineurin inhibitors on growth and viability during the M-Y transition of P. brasiliensis. (A) Fold increase of fungal mass (dry weight in mg) relative to the initial inoculum at 120 h of the M-Y transition at 37°C without or with 2 μg/ml CsA or FK506. Bars indicate the means ± standard errors of the means (n = 3). P was <0.01 for CsA versus the control. (B to D) Viability of fungus at 120 h of the M-Y transition at 37°C. Control (CTR; B) or with 2 μg/ml CsA (C) or FK506 (D). Cells were metabolically labeled with DCFDA (fluorescent). Exposure times were the same for all experimental groups (4 s). Scale bar = 20 μm.
Ca2+ chelators induced a transient blockade, whereas the addition of extracellular Ca2+ stimulated the M-Y transition of P. brasiliensis.
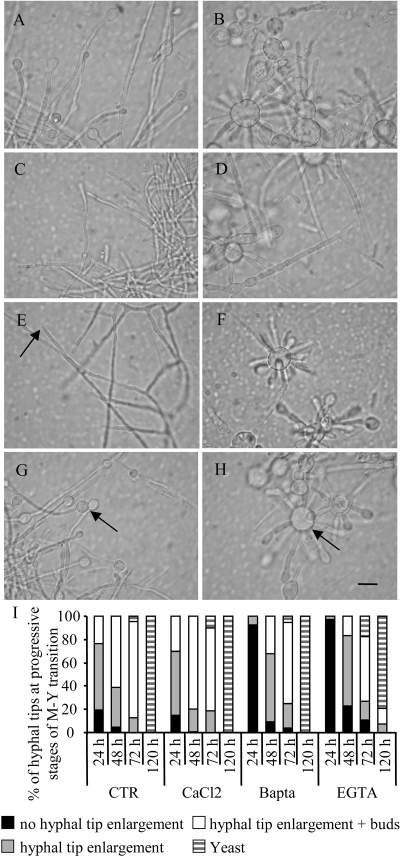
Because calcineurin activity is dependent on Ca2+, we investigated whether chelating this ion could mimic the effect of CsA on dimorphism (Fig. 4). Ca2+ chelators induced a transient blockade, whereas the addition of extracellular Ca2+ stimulated the M-Y transition of P. brasiliensis. Untreated hyphae showed tip swellings 24 h after commencement of the M-Y transition. Yeast-like hyphal enlarged tips plus multiple buds were seen at 72 h (Fig. 4A and B). An extracellular (EGTA) and a cell-permeable (BAPTA) Ca2+ chelator delayed the onset of the M-Y transition. After 24 h in the presence of 2 mM EGTA (Fig. 4C) or 5 μM BAPTA (Fig. 4E), P. brasiliensis hyphae retained mycelial-like morphology; however, EGTA- and BAPTA-treated mycelia eventually bypassed the blockade and underwent the transition to yeast cells (Fig. 4D and F, respectively). In contrast, when cultured in the presence of 5 mM CaCl2 (Fig. 4G and H), a physiological concentration in mammalian fluids, mycelia progressed slightly faster through the phases of the M-Y transition. By 48 h, the CaCl2-treated fungus showed 80% of hyphae at the blastoconidial budding stage (hyphal tip enlargement plus buds) against 60% in untreated fungus. After 48 h, no mycelial-like tip morphology was observed. Several micrographs were scanned, and the observations were summarized graphically in Fig. 4I.
FIG. 4.
Effect of Ca2+ and Ca2+ chelators on the M-Y transition of P. brasiliensis. Morphology of fungus during the M-Y transition at 37°C after 24 h (left panels) and 72 h (right panels) in the absence (A and B) or presence of 1 mM EGTA (C and D), 5 μM BAPTA (E and F), or 5 mM CaCl2 (G and H). Arrows indicate no hyphal tip enlargement (E), hyphal tip enlargement (G), or hyphal tip enlargement plus buds or yeasts (H). Scale bar = 10 μm. (I) Graphical summary of the rate of morphological changes during hyphal tip progression through the M-Y transition stages at 24, 48, 72, and 120 h in the absence (CTR) or presence of calcium chelators (1 mM EGTA or 5 μM BAPTA) or 5 mM CaCl2.
Raising the temperature from 25°C to 37°C is followed by an immediate increase in the cytoplasmic calcium levels in hyphae; the increase in Ca2+ levels was suppressed by CsA but not FK506.
Because the limiting step for calcineurin activation is the increase in cytoplasmic free Ca2+ (28) and because Ca2+ chelators transiently mimic the effect of CsA on dimorphism, we investigated whether intrahyphal Ca2+ levels increase in P. brasiliensis mycelia when the temperature is shifted to 37°C. Using the permeable Ca2+ fluorescent probe Fluo-3, we compared the fluorescence of a culture maintained at 25°C (Fig. 5A) with a culture shifted to 37°C (Fig. 5B). We observed a strong increase in cytoplasmic fluorescence 15 min after the temperature increase. Fluo-3 labeling was completely prevented by the preincubation of mycelia with 5 μM BAPTA (Fig. 5C), supporting the specific Fluo-3 labeling of the intracellular calcium. Fluo-3 labeling of mycelial cells treated with CsA or FK506 treatments was not depressed below the level of basal fluorescence (not shown).
FIG. 5.
Effect of temperature and calcineurin inhibitors on intrahyphal calcium levels. Fluorescence of mycelia after incubation with 3 μM Fluo-3 for 15 min at 25°C (A), for 15 min after the temperature shift to 37°C (B), or for 15 min after the temperature shift to 37°C preceded by incubation with 5 μM BAPTA for 20 min (C). Fluorescence of actively growing yeasts cultured at 37°C for 48 h and incubated for 15 min with 3 μM Fluo-3 in the absence (D) or presence of 2 μg/ml CsA (E) or 2 μg/ml FK506 (F). Fluorescence of cells at the M-Y transition cultured at 37°C for 48 h and incubated for 15 min with 3 μM Fluo-3 in the absence (G) or presence of 2 μg/ml CsA (H) or 2 μg/ml FK506 (I). Exposure times were the same for all experimental groups (4 s). Scale bar = 25 μm.
We found also that actively growing yeast have uniformly high intracellular Ca2+ levels (Fig. 5D), whereas fungus undergoing the M-Y transition had uneven distribution with a few structures showing high Ca2+ levels (Fig. 5G). Because there was no immediate change in intracellular calcium when CsA or FK506 was added to the mycelia at 25°C or 37°C (not shown), we measured the intracellular Ca2+ labeling 1 h, 6 h, 24 h, and 48 h after CsA treatment in both yeast cells and cells undergoing the M-Y transition. Until 24 h, there was no change in Fluo-3 labeling (not shown); however, after 48 h of chronic CsA treatment, Ca2+ levels in yeast cells had declined to levels slightly above those of mycelial cells (Fig. 5E). Chronic FK506 treatment did not cause a drop in intracellular Ca2+ levels in yeast cells (Fig. 5F).
Cells undergoing the M-Y transition show localized areas with high Ca2+ levels (Fig. 5G). These localized hotspots of high Ca2+ levels appear to be associated with enlarging hyphal tips. After 24 h, CsA-treated cells in the M-Y transition showed declines in Ca2+ levels, overall diminishment of fluorescence, and loss of Ca2+-localized zones (Fig. 5H). The overall fluorescence and distribution of calcium in FK506-treated cells in the M-Y transition appeared similar to that of the controls (Fig. 5I). This result was consistent with the low inhibitory effect of FK506 on cell morphology (Fig. 2). As the influx of calcium ions into the cytoplasm precedes calcineurin activation, and since FK506 was ineffective, the results imply that CsA is more effective for specifically silencing calcineurin signaling rather than blocking calcium influx. In sum, raising the temperature from 25°C to 37°C is followed by an immediate increase in cytoplasmic calcium levels in hyphae, and the chronic inhibition of calcineurin by CsA caused Ca2+ levels to peak at 24 h then drop to unstimulated levels by 48 h. We also concluded that neither CsA nor FK506 directly inhibits calcium uptake.
Chronic CsA treatment decreased gene expression of the plasma-membrane Ca2+ channel CCH1 mRNA in yeast cells.
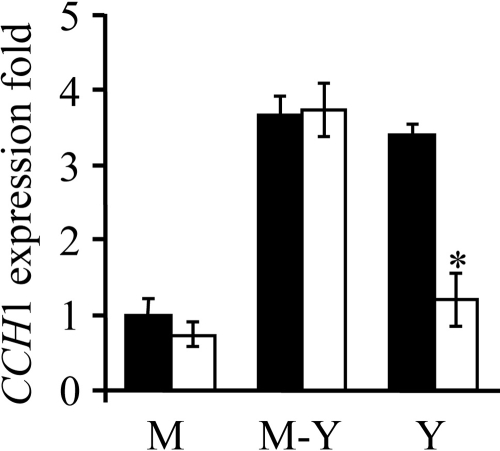
Among the various genes previously described to be regulated in a Ca2+- and/or calcineurin-dependent manner, the CCH1 gene coding for a plasma membrane Ca2+ channel had been identified in P. brasiliensis. We assayed for the expression of the plasma membrane Ca2+ channel CCH1 mRNA levels in mycelia, yeast, and cells undergoing the M-Y transition. Total mRNA was obtained from fungus either untreated or treated with 2 μg/ml CsA for 48 h and used for real-time PCR assays (Fig. 6). Untreated yeast cells or fungi at 48 h of the M-Y transition expressed about 3.5 times more CCH1 mRNA than mycelial cells. The chronic treatment of yeasts with CsA decreased by 50% the CCH1 mRNA levels of yeast cells after 48 h but did not change the expression pattern of the CCH1 gene in fungi at the M-Y transition or in mycelia. The decline in CCH1 mRNA levels correlates well with the decline of Fluo-3 fluorescence levels between 24 and 48 h after CsA treatment (Fig. 5).
FIG. 6.
Relative CCH1 gene expression of P. brasiliensis by real-time RT-PCR. Mycelia (M) at 25°C, yeast (Y) at 37°C, or fungus at the M-Y transition at 37°C was cultured for 48 h either in the absence (black bars) or presence (white bars) of 2 μg/ml CsA before mRNAs were extracted. Bars indicate the means ± standard errors of representative experiments made in triplicate. P was <0.01 for CsA versus the control untreated yeast.
Difference between cyclophilins and FKBP activity in mycelial forms of P. brasiliensis correlated with the distinct effectiveness of CsA and FK506 on M-Y dimorphism.
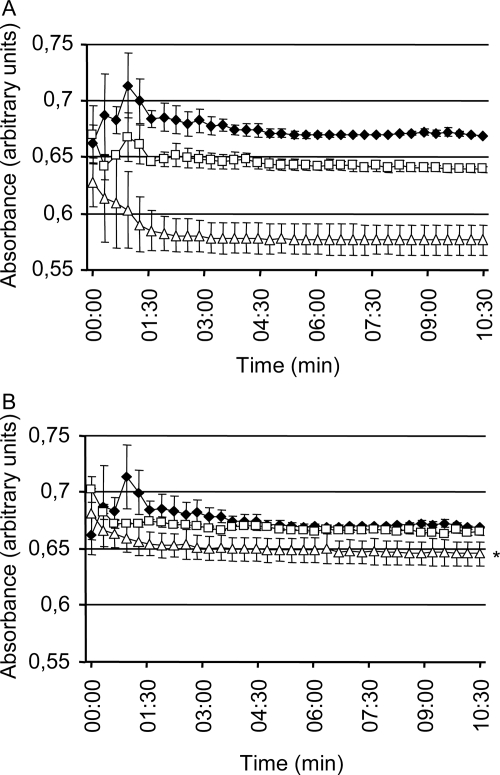
CsA was more effective than FK506 in inhibiting the P. brasiliensis M-Y transition. Since it is known that these drugs act on Cna1p indirectly by first forming a complex with an immunophilin, we assayed the cytoplasmic levels of FKBP and cyclophilin A to determine whether low inhibition of calcineurin activity by FK506 in P. brasiliensis was due to limited amounts of FKBP. As immunophilins are isomerases that racemize the cis-trans conformation of proline residues in peptides (12, 35, 36), we measured their activity by the release of a chromophore from a proline-containing peptide by chymotrypsin (Fig. 7). We prepared crude lysates from untreated or CsA- or FK506-treated mycelia and measured the cyclophilin isomerase activity in vitro. Total isomerase activity was reduced significantly by CsA but only slightly by FK506 pretreatment (Fig. 7A). The addition of CsA directly to crude extracts diminished in vitro proline isomerase activity slightly but not to the extent of pretreated cells; the addition of FK506 to crude extracts was ineffective at diminishing in vitro proline isomerase activity (Fig. 7B). When CsA or FK506 was added in vitro to crude extracts from either CsA- or FK506-treated mycelial cells, there was no further isomerase inhibition (not shown). The results imply strongly that FKBP levels are significantly lower than Cna1p or cyclophilin levels in live P. brasiliensis cells.
FIG. 7.
Effect of calcineurin inhibitors on PPI activity in protein extract of mycelial cells. (A) Proteins from the control mycelia (filled circles) or mycelia treated with 2 μg/ml CsA (open triangles) or with 2 μg/ml FK506 (open squares). (B) Protein extracts from the untreated control culture tested for PPI activity in the absence (filled circles) or presence of 2 μg/ml CsA (open triangles) or 2 μg/ml FK506 (open squares) added to the reaction mixture. Bars indicate the means ± standard errors of representative experiments made in triplicate. P was <0.05 for CsA versus control.
Mycelial MDR pumps were not sensitive to calcineurin inhibitors; yeast-expressed MDR pumps showed inhibition by FK506.
As it has been reported that CsA and FK506 target other cell proteins, it is possible that the effects we observed were not solely due to the inhibition of calcineurin by cyclophilins. Although used specifically as calcineurin inhibitors, both CsA and FK506 have an inhibitory effect over some members of the multidrug resistance (MDR) family of plasma membrane pumps (18). MDR proteins are transmembrane efflux transport proteins that pump structurally unrelated molecules out of cells (18). It has been reported that rhodamine 123 is transported by fungal MDR efflux transporters (31). Therefore, we investigated whether MDR efflux pumps were secondary targets of CsA or FK506.
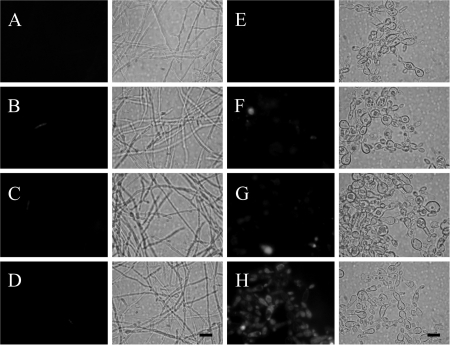
We incubated untreated and calcineurin-inhibited hyphal and yeast cells with rhodamine 123 (Fig. 8). We showed that unlabeled hyphae (Fig. 8A) and yeast cells (Fig. 8E) have low levels of natural rhodamine-like fluorescence. After staining with rhodamine 123, very few P. brasiliensis cells retained the stain in culture under untreated conditions (Fig. 8B and F), indicating MDR efflux pump activity. Rhodamine 123 efflux was not impaired by either CsA in either hyphal or yeast forms (Fig. 8C and G). FK506-treated mycelia were not stained, whereas FK506-treated yeast cells became fluorescent with rhodamine 123 (Fig. 8D and H). Hence, FK506 was a potent inhibitor of a yeast-form-specific MDR pump. The inhibition of one or more yeast-form-specific MDR pumps was not correlated with inhibition of the M-Y transition.
FIG. 8.
Effect of calcineurin inhibitors on MDR pumps. Mycelium (A to D) or yeast (E to H) cells were incubated for 20 min at 25°C or 37°C, respectively, in the absence or in the presence of 2 μg/ml CsA or 2 μg/ml FK506, followed by a 20-min incubation with 15 μM rhodamine 123. (A and E) Unlabeled fungi (fluorescence control); (B and F) with rhodamine 123 only; (C and G) with both CsA and rhodamine 123; (D and H) with both FK506 and rhodamine 123. Exposure times were the same for all experimental groups (4 s). Scale bar = 15 μm.
DISCUSSION
Calcineurin is implicated in the stress adaptation against environmental constraints and is critical for growth, morphogenesis, and pathogenesis in many fungal species (34). The broad calcineurin roles in the essential functions for the success of fungi in their environment make the calcineurin pathway a source of potential therapeutic targets to treat various mycoses. In this study, we provide evidence that calcineurin controls the morphology and growth of both noninfective mycelial forms and infective yeast cells of Paracoccidioides brasiliensis. Calcineurin regulates the morphological transition from M-Y triggered by a temperature shift to 37°C, a hallmark of pathogenicity.
Because the genetic manipulation of P. brasiliensis is still not a routine, we used CsA and tacrolimus (FK506) as pharmacological tools to study calcineurin function in this system. Interestingly, only CsA, not FK506, produced significant effects on growth and morphology in P. brasiliensis. Both CsA and FK506 require binding to specific immunophilins to suppress calcineurin activity. We hypothesize that the difference in performance between the two calcineurin inhibitors is due to inequalities in the relative levels of the immunophilins cyclophilin A and FKBP. Cyert demonstrated that the overexpression of cyclophilin A in S. cerevisiae increased its sensitivity to CsA, whereas decreased immunophilin expression abrogated sensitivity to either drug (5). Other studies concluded that levels of FKBP are at least 20 times lower than cyclophilin A in P. brasiliensis (21). In this study, we observed that approximately 16% of the in vivo cytoplasmic proline isomerase activity was inhibitable by CsA, while less than 4% of the activity was inhibited by FK506. Our results support our hypothesis that P. brasiliensis has higher levels of cyclophilin A relative to FKBP.
We present evidence that CsA suppresses the calcineurin signal transduction pathway. We also searched for additional CsA targets that could control morphogenesis and growth; we found that CsA did not act through a blockade of the MDR efflux pumps, and although CsA suppressed cyclophilin activity, the M-Y transition was preceded by and dependent on an intracellular rise in Ca2+ levels. Ca2+ chelators produced only a transient suppression of the M-Y dimorphism. We provide evidence that chronic CsA treatment lowered intracellular Ca2+ levels both in yeast cells and cells undergoing the M-Y transition.
In this study, we find that the plasma membrane Ca2+ channel Cch1p is a candidate for calcineurin regulation at the transcriptional level. The CCH1 mRNA transcript was lowered by chronic CsA in yeast cells of P. brasiliensis, but CsA did not prevent the temperature-induced spike in mRNA transcription at the onset of the M-Y transition. While not inhibited by CsA, Cch1p appears to have a half-life of about 48 h. In yeast cells, calcineurin activity maintains high Cch1p levels, which in turn maintained high levels of cytoplasmic calcium. When calcineurin induction is halted, calcium levels drop, as ambient Cch1p is destroyed. The results imply a dual control of Cch1p levels by a CsA-inhibitable factor (calcineurin) that maintains both the low basal levels in mycelia and high basal levels in yeast and a CsA-noninhibitable factor that controls the rapid increase in CCH1 mRNA levels after temperature induction. Aberrant yeast growth in CsA-containing media implies that there is a need for a basal-level expression of Cch1p by calcineurin. However, proper mycelial growth and the M-Y dimorphism depend on another CsA-inhibitable factor to control intracellular calcium levels.
Calcineurin has been previously implicated in stress-induced responses that lead to differentiation, proliferation at high temperatures, control of calcium homeostasis, and fitness to environmental changes in other fungi. Our results point to calcineurin control of thermodimorphism and growth of P. brasiliensis during the M-Y transition. Although the mechanisms behind calcineurin action in the various fungal species require more extensive investigation, this work supports findings for other fungal species of the importance of the calcineurin pathway in P. brasiliensis survival and virulence at 37°C.
We see a therapeutic potential of a combinatory approach using calcineurin pathway inhibitors together with antifungal drugs, which will lead to improved treatment regimens for chronic paracoccidioidomycoses. FK506 can be used to block drugs exported by the rhodamine-specific MDR efflux pump, and CsA can be used for the disruption of the transcription of multiple genes associated with yeast-form-specific growth and virulence.
Acknowledgments
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP).
We thank Francisco G. Nobrega for help with the laboratory facilities and suggestions. We also thank Priscila M. Correa, Maria C. Lopes, and Guilherme N. D. Souza for technical support.
Footnotes
Published ahead of print on 5 September 2008.
REFERENCES
- 1.Blankenship, J. R., F. L. Wormley, M. K. Boyce, W. A. Schell, S. G. Filler, J. R. Perfect, and J. Heitman. 2003. Calcineurin is essential for Candida albicans survival in serum and virulence. Eukaryot. Cell 2422-430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blankenship, J. R., and J. Heitman. 2005. Calcineurin is required for Candida albicans to survive calcium stress in serum. Infect. Immun. 735767-5774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bonilla, M., K. K. Nastase, and K. W. Cunningham. 2002. Essential role of calcineurin in response to endoplasmic reticulum stress. EMBO J. 212343-2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cruz, M. C., D. S. Fox, and J. Heitman. 2001. Calcineurin is required for hyphal elongation during mating and haploid fruiting in Cryptococcus neoformans. EMBO J. 201020-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cyert, M. S. 2003. Calcineurin signaling in Saccharomyces cerevisiae: how yeast go crazy in response to stress. Biochem. Biophys. Res. Commun. 3111143-1150. [DOI] [PubMed] [Google Scholar]
- 6.da Silva Ferreira, M. E., T. Heinekamp, A. Härtl, A. A. Brakhage, C. P. Semighini, S. D. Harris, M. Savoldi, P. F. de Gouvêa, M. H. de Souza Goldman, and G. H. Goldman. 2007. Functional characterization of the Aspergillus fumigatus calcineurin. Fungal Genet. Biol. 44219-230. [DOI] [PubMed] [Google Scholar]
- 7.de Carvalho, M. J. A., R. S. Amorim Jesuino, B. S. Daher, I. Silva-Pereira, S. M. de Freitas, C. M. A. Soares, and M. S. S. Felipe. 2003. Functional and genetic characterization of calmodulin from the dimorphic and pathogenic fungus Paracoccidioides brasiliensis. Fungal Genet. Biol. 39204-210. [DOI] [PubMed] [Google Scholar]
- 8.Derkx, P. M., and S. M. Madrid. 2001. The Aspergillus niger cypA gene encodes a cyclophilin that mediates sensitivity to the immunosuppressant cyclosporin A. Mol. Genet. Genomics 266527-536. [DOI] [PubMed] [Google Scholar]
- 9.Franco, M. 1987. Host-parasite relationships in paracoccidioidomycosis. J. Med. Vet. Mycol. 255-18. [DOI] [PubMed] [Google Scholar]
- 10.Garrett-Engele, P., B. Moilanen, and M. S. Cyert. 1995. Calcineurin, the Ca2þ/calmodulin-dependent protein phosphatase, is essential in yeast mutants with cell integrity defects and in mutants that lack a functional vacuolar Hþ-ATPase. Mol. Cell. Biol. 154103-4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hallen, H. E., and F. Trail. 2008. The L-type calcium ion channel Cch1 affects ascospore discharge and mycelial growth in the filamentous fungus Gibberella zeae (anamorph Fusarium graminearum). Eukaryot. Cell 7415-424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harding, M. W., A. Galat, D. E. Uehling, and S. L. Schreiber. 1989. A receptor for the immunosuppressant FK506 is a cis-trans peptidyl-prolyl isomerase. Nature 341758-760. [DOI] [PubMed] [Google Scholar]
- 13.Kashino, S. S., V. L. G. Calich, E. Burger, and L. M. Singer-Vermes. 1985. In vivo and in vitro characteristics of six Paracoccidioides brasiliensis strains. Mycopathologia 92173-178. [DOI] [PubMed] [Google Scholar]
- 14.Liu, J., and C. T. Walsh. 1990. Peptidyl-prolyl cis-trans-isomerase from Escherichia coli: a periplasmic homolog of cyclophilin that is not inhibited by cyclosporin A. Proc. Natl. Acad. Sci. USA 874028-4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu, M., P. Du, G. Heinrich, G. M. Cox, and A. Gelli. 2006. Cch1 mediates calcium entry in Cryptococcus neoformans and is essential in low-calcium environments. Eukaryot. Cell 51788-1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Livak, K. J., and T. D. Schmittgen. 2001. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 25402-408. [DOI] [PubMed] [Google Scholar]
- 17.McEwen, J. G., A. M. Garcia, B. L. Ortiz, S. Botero, and A. Restrepo. 1995. In search of the natural habitat of Paracoccidioides brasiliensis. Arch. Med. Res. 26305-306. [PubMed] [Google Scholar]
- 18.Mizuno, K., Y. Furuhashi, T. Misawa, M. Iwata, M. Kawai, F. Kikkawa, T. Kano, and Y. Tomoda. 1992. Modulation of multidrug resistance by immunosuppressive agents: cyclosporin analogues, FK506 and mizoribine. Anticancer Res. 1221-25. [PubMed] [Google Scholar]
- 19.Morais, F. V., T. F. Barros, M. K. Fukada, P. S. Cisalpino, and R. Puccia. 2000. Polymorphism in the gene coding for the immunodominant antigen gp43 from the pathogenic fungus Paracoccidioides brasiliensis. J. Clin. Microbiol. 383960-3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nemecek, J. C., M. Wüthrich, and B. S. Klein. 2006. Global control of dimorphism and virulence in fungi. Science 312583-588. [DOI] [PubMed] [Google Scholar]
- 21.Nicola, A. M., R. V. Andrade, and I. Silva-Pereira. 2005. Molecular chaperones in the Paracoccidioides brasiliensis transcriptome. Genet. Mol. Res. 4346-357. [PubMed] [Google Scholar]
- 22.Nunes, L. R., R. Costa de Oliveira, D. B. Leite, V. S. da Silva, E. dos Reis Marques, M. E. da Silva Ferreira, D. C. Ribeiro, L. A. de Souza Bernardes, M. H. Goldman, R. Puccia, L. R. Travassos, W. L. Batista, M. P. Nóbrega, F. G. Nobrega, D. Y. Yang, C. A. de Bragança Pereira, and G. H. Goldman. 2005. Transcriptome analysis of Paracoccidioides brasiliensis cells undergoing mycelium-to-yeast transition. Eukaryot. Cell 42115-2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Odom, A., S. Muir, E. Lim, D. L. Toffaletti, J. Perfect, and J. Heitman. 1997. Calcineurin is required for virulence of Cryptococcus neoformans. EMBO J. 162576-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Onyewu, C., J. R. Blankenship, M. Del Poeta, and J. Heitman. 2003. Ergosterol biosynthesis inhibitors become fungicidal when combined with calcineurin inhibitors against Candida albicans, Candida glabrata, and Candida krusei. Antimicrob. Agents Chemother. 47956-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paidhungat, M., and S. Garrett. 1997. A homolog of mammalian, voltage-gated calcium channels mediates yeast pheromone-stimulated Ca2+ uptake and exacerbates the cdc1(Ts) growth defect. Mol. Cell. Biol. 176339-6347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peiter, E., M. Fischer, K. Sidaway, S. K. Roberts, and D. Sanders. 2005. The Saccharomyces cerevisiae Ca2+ channel Cch1p/Mid1p is essential for tolerance to cold stress and iron toxicity. FEBS Lett. 5795697-5703. [DOI] [PubMed] [Google Scholar]
- 27.Prokisch, H., O. Yarden, M. Dieminger, M. Tropschug, and I. B. Barthelmess. 1997. Impairment of calcineurin function in Neurospora crassa reveals its essential role in hyphal growth, morphology and maintenance of the apical Ca2+ gradient. Mol. Gen. Genet. 256104-114. [DOI] [PubMed] [Google Scholar]
- 28.Rusnak, F., and P. Mertz. 2000. Calcineurin: form and function. Physiol. Rev. 801483-1521. [DOI] [PubMed] [Google Scholar]
- 29.San-Blas, G., G. Niño-Vega, and T. Iturriaga. 2002. Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med. Mycol. 40225-242. [DOI] [PubMed] [Google Scholar]
- 30.Sanglard, D., F. Ischer, O. Marchetti, J. Entenza, and J. Bille. 2003. Calcineurin A of Candida albicans: involvement in antifungal tolerance, cell morphogenesis and virulence. Mol. Microbiol. 48959-976. [DOI] [PubMed] [Google Scholar]
- 31.Sipos, G., and K. Kuchler. 2006. Fungal ATP-binding cassette (ABC) transporters in drug resistance & detoxification. Curr. Drug Targets 7471-481. [DOI] [PubMed] [Google Scholar]
- 32.Steinbach, W. J., R. A. Cramer, Jr., B. Z. Perfect, C. Henn, K. Nielsen, J. Heitman, and J. R. Perfect. 2007. Calcineurin inhibition or mutation enhances cell wall inhibitors against Aspergillus fumigatus. Antimicrob. Agents Chemother. 512979-2981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Steinbach, W. J., J. L. Reedy, R. A. Cramer, Jr., J. R. Perfect, and J. Heitman. 2007. Harnessing calcineurin as a novel anti-infective agent against invasive fungal infections. Nat. Rev. Microbiol. 5418-430. [DOI] [PubMed] [Google Scholar]
- 34.Stie, J., and D. Fox. 2008. Calcineurin regulation in fungi and beyond. Eukaryot. Cell 7177-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Takahashi, N., T. Hayano, and M. Suzuki. 1989. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature 337473-475. [DOI] [PubMed] [Google Scholar]
- 36.Wang, P., and J. Heitman. 2005. The cyclophilins. Genome Biol. 6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watanabe, Y., B. A. Perrino, and T. R. Soderling. 1996. Activation of calcineurin A subunit phosphatase activity by its calcium-binding B subunit. Biochemistry 35562-566. [DOI] [PubMed] [Google Scholar]
- 38.Yasuda, M. A. 2005. Pharmacological management of paracoccidioidomycosis. Expert Opin. Pharmacother. 6385-397. [DOI] [PubMed] [Google Scholar]
- 39.Yoshimoto, H., K. Saltsman, A. P. Gasch, H. X. Li, N. Ogawa, D. Botstein, P. O. Brown, and M. S. Cyert. 2002. Genome-wide analysis of gene expression regulated by the calcineurin/Crz1p signaling pathway in Saccharomyces cerevisiae. J. Biol. Chem. 27731079-31088. [DOI] [PubMed] [Google Scholar]