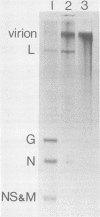
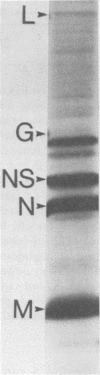
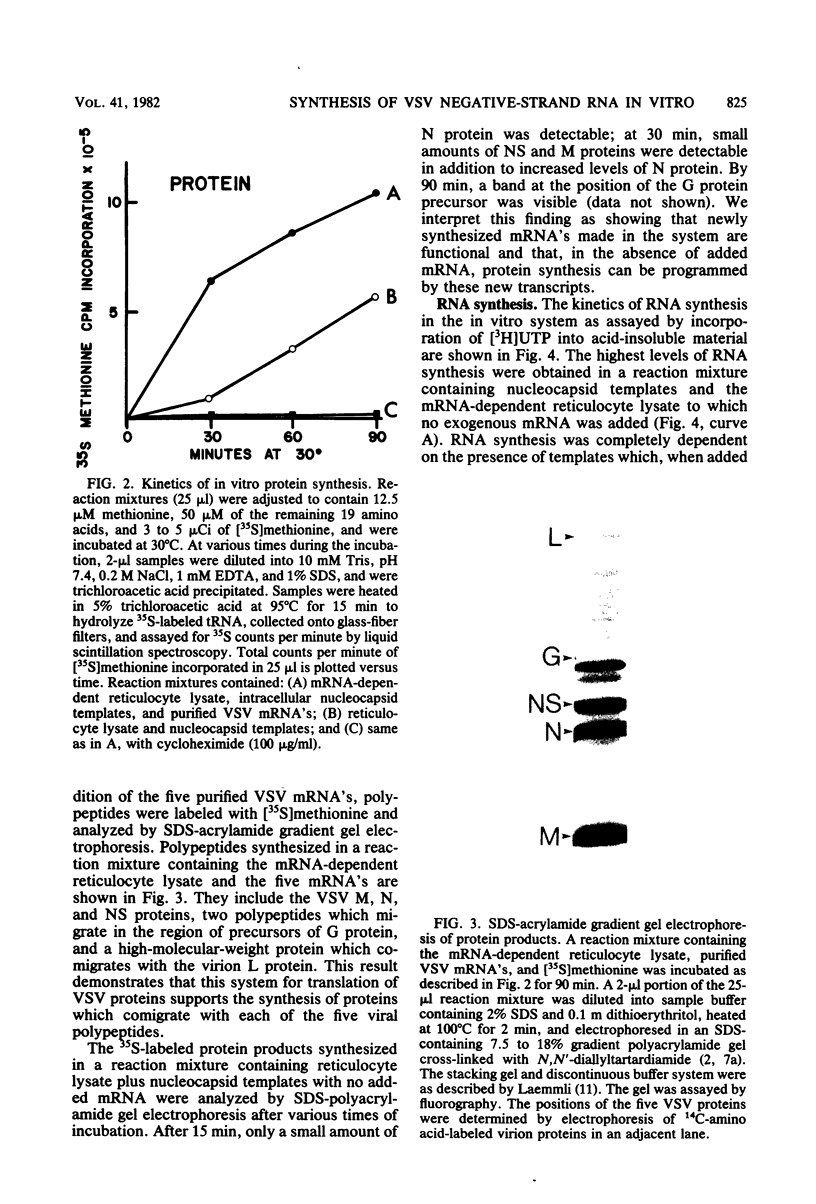
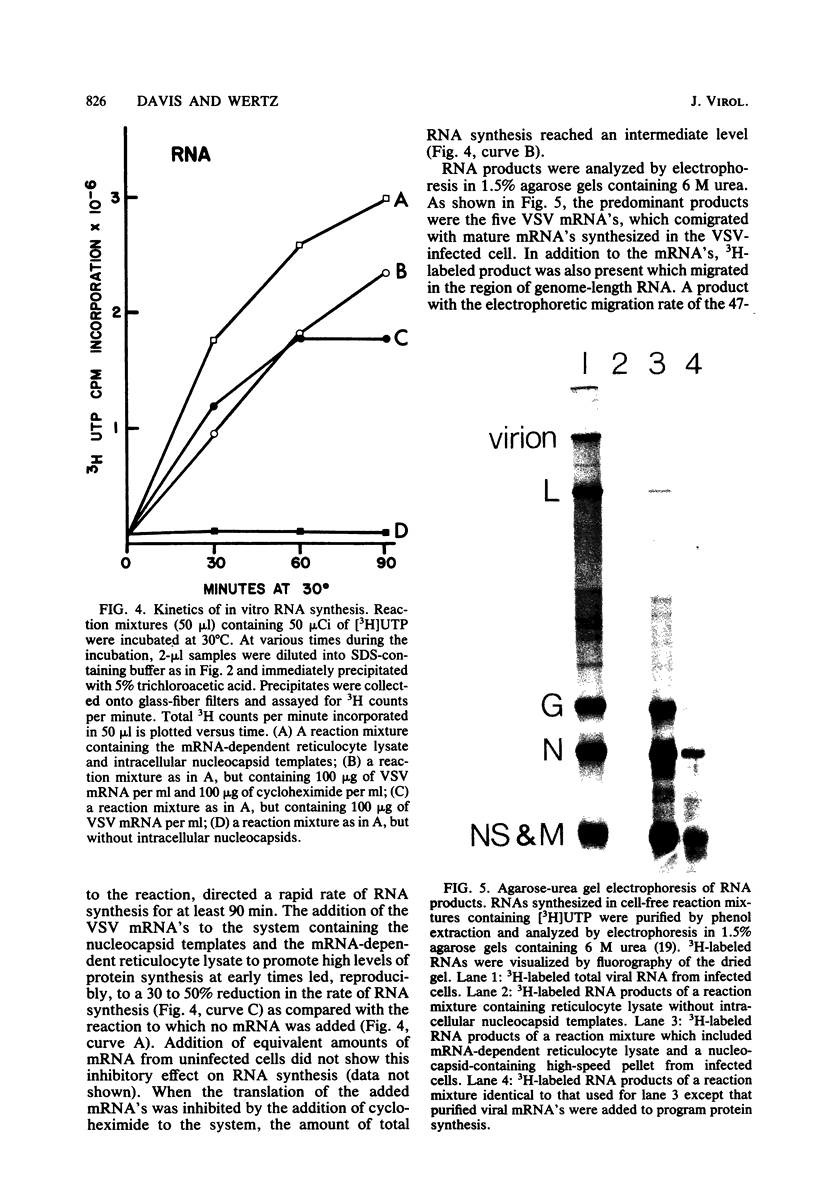
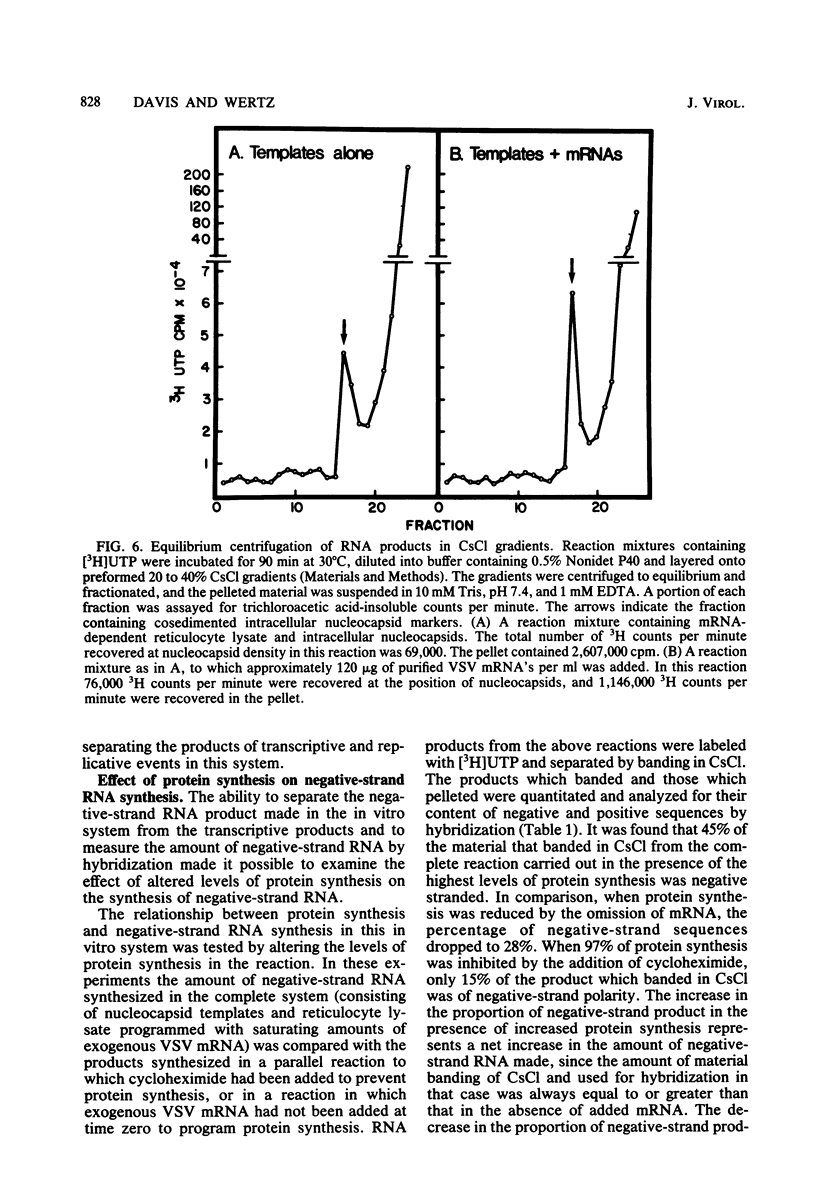
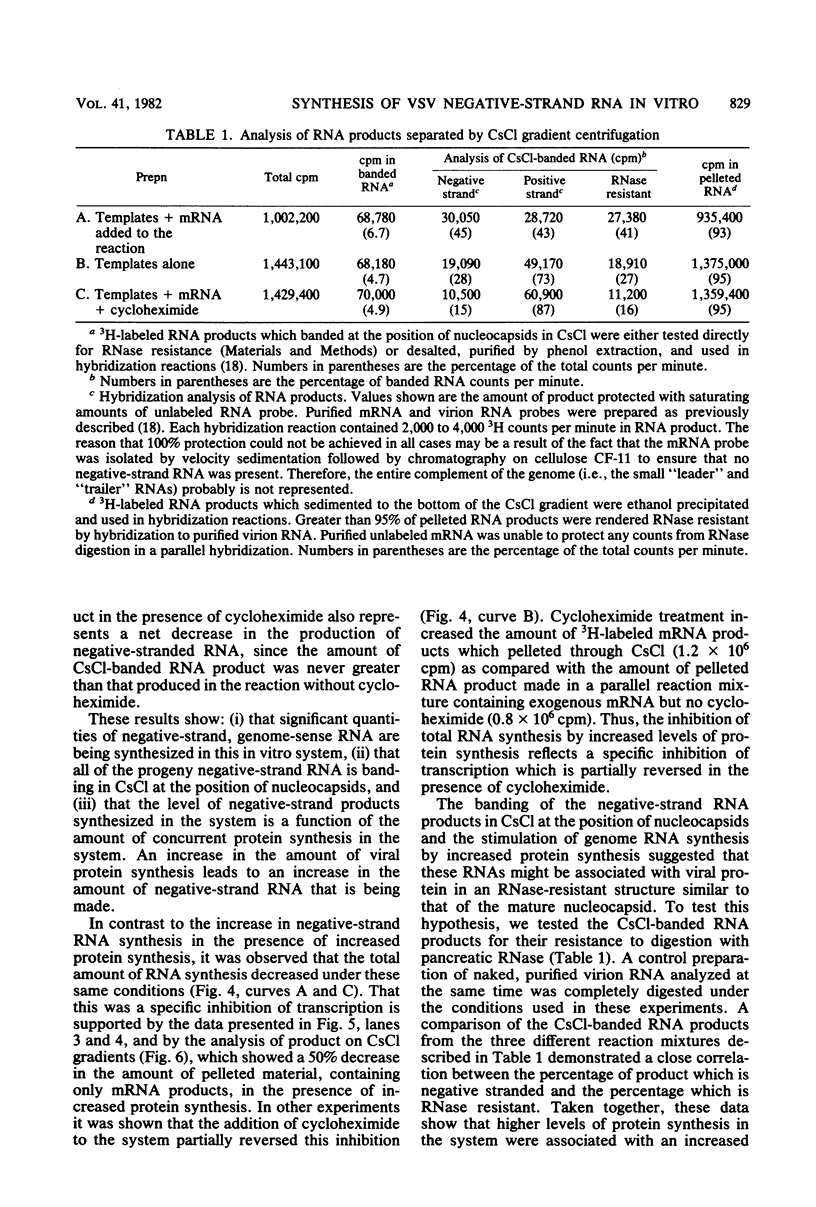
Abstract
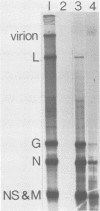
An in vitro system is described which supports the synthesis of vesicular stomatitis virus (VSV) negative-strand RNA. The major components of this system are (i) an mRNA-dependent rabbit reticulocyte lysate to carry out cell-free protein synthesis, (ii) the five VSV mRNAs to program VSV-specific protein synthesis, and (iii) nucleocapsids containing positive- and negative-strand genome-length RNA. The protein products synthesized in the system in response to addition of saturating amounts of the five VSV mRNA's included polypeptides which comigrated in acrylamide gels with the five VSV proteins. Approximately 200 pmol of protein per ml was synthesized during a 90-min reaction. The RNA products synthesized in the system included all five of the VSV mRNA's and, in addition, negative-strand, genome-sense RNA. All of the negative-strand RNA, which represented 2 to 5% of the total RNA product synthesized in vitro, banded in CsCl at the position of nucleocapsids. All of the mature mRNA's made in the system pelleted in CsCl. This technique allowed a clear separation of negative-strand product from the mRNA products and facilitated further analysis of the negative-strand product. The amount of negative-strand product produced in the system was shown to be a function of the amount of concurrent protein synthesis in the system. An increase in the level of protein synthesis led to an increase in the amount of negative-strand RNA synthesized, whereas inhibition of protein synthesis by cycloheximide resulted in a 70% inhibition of negative-strand synthesis. In contrast to the negative-strand RNA product, the amount of transcriptive product was decreased by 50% in the presence of maximum levels of viral protein synthesis. This inhibition was reversed by adding cycloheximide. Characterization of the negative-strand product by Northern blot analysis demonstrated that negative-strand product was being synthesized which hybridized to all five of the VSV mRNA's and, hence, that product representing all of the VSV cistrons was being made. This in vitro system offers an opportunity to study factors involved in the promotion of VSV genome replication as well as those responsible for the regulation of transcription.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alwine J. C., Kemp D. J., Stark G. R. Method for detection of specific RNAs in agarose gels by transfer to diazobenzyloxymethyl-paper and hybridization with DNA probes. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5350–5354. doi: 10.1073/pnas.74.12.5350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Ball L. A., White C. N. Coupled transcription and translation in mammalian and avian cell-free systems. Virology. 1978 Feb;84(2):479–495. doi: 10.1016/0042-6822(78)90264-7. [DOI] [PubMed] [Google Scholar]
- Batt-Humphries S., Simonsen C., Ehrenfeld E. Full-length viral RNA synthesized in vitro by vesicular stomatitis virus-infected HeLa cell extracts. Virology. 1979 Jul 15;96(1):88–99. doi: 10.1016/0042-6822(79)90175-2. [DOI] [PubMed] [Google Scholar]
- Both G. W., Moyer S. A., Banerjee A. K. Translation and identification of the viral mRNA species isolated from subcellular fractions of vesicular stomatitis virus-infected cells. J Virol. 1975 Apr;15(4):1012–1019. doi: 10.1128/jvi.15.4.1012-1019.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breindl M., Holland J. J. Studies on the in vitro transcription and translation of vesicular stomatitis virus mRNA. Virology. 1976 Aug;73(1):106–118. doi: 10.1016/0042-6822(76)90065-9. [DOI] [PubMed] [Google Scholar]
- Colonno R. J., Banerjee A. K. A unique RNA species involved in initiation of vesicular stomatitis virus RNA transcription in vitro. Cell. 1976 Jun;8(2):197–204. doi: 10.1016/0092-8674(76)90003-9. [DOI] [PubMed] [Google Scholar]
- Emerson S. U., Wagner R. R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J Virol. 1972 Aug;10(2):297–309. doi: 10.1128/jvi.10.2.297-309.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang A. S., Manders E. K. Ribonucleic acid synthesis of vesicular stomatitis virus. IV. Transcription by standard virus in the presence of defective interfering particles. J Virol. 1972 Jun;9(6):909–916. doi: 10.1128/jvi.9.6.909-916.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K., Favre M. Maturation of the head of bacteriophage T4. I. DNA packaging events. J Mol Biol. 1973 Nov 15;80(4):575–599. doi: 10.1016/0022-2836(73)90198-8. [DOI] [PubMed] [Google Scholar]
- Mellon M. G., Emerson S. U. Rebinding of transcriptase components (L and NS proteins) to the nucleocapsid template of vesicular stomatitis virus. J Virol. 1978 Sep;27(3):560–567. doi: 10.1128/jvi.27.3.560-567.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito S., Ishihama A. Function and structure of RNA polymerase from vesicular stomatitis virus. J Biol Chem. 1976 Jul 25;251(14):4307–4314. [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Rose J. K., Gallione C. J. Nucleotide sequences of the mRNA's encoding the vesicular stomatitis virus G and M proteins determined from cDNA clones containing the complete coding regions. J Virol. 1981 Aug;39(2):519–528. doi: 10.1128/jvi.39.2.519-528.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shih D. S., Shih C. T., Zimmern D., Rueckert R. R., Kaesberg P. Translation of encephalomyocarditis virus RNA in reticulocyte lysates: kinetic analysis of the formation of virion proteins and a protein required for processing. J Virol. 1979 May;30(2):472–480. doi: 10.1128/jvi.30.2.472-480.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soria M., Little S. P., Huang A. S. Characterization of vesicular stomatitis virus nucleocapsids. I. Complementary 40 S RNA molecules in nucleocapsids. Virology. 1974 Sep;61(1):270–280. doi: 10.1016/0042-6822(74)90261-x. [DOI] [PubMed] [Google Scholar]
- Stampfer M., Baltimore D., Huang A. S. Ribonucleic acid synthesis of vesicular stomatitis virus. I. Species of ribonucleic acid found in Chinese hamster ovary cells infected with plaque-forming and defective particles. J Virol. 1969 Aug;4(2):154–161. doi: 10.1128/jvi.4.2.154-161.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Davis N. L. RNase III cleaves vesicular stomatitis virus genome-length RNAs but fails to cleave viral mRNA's. J Virol. 1979 Apr;30(1):108–115. doi: 10.1128/jvi.30.1.108-115.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W., Davis N. Characterization and mapping of RNase III cleavage sites in VSV genome RNA. Nucleic Acids Res. 1981 Dec 11;9(23):6487–6503. doi: 10.1093/nar/9.23.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wertz G. W. Isolation of possible replicative intermediate structures from vesicular stomatitis virus-infected cells. Virology. 1978 Mar;85(1):271–285. doi: 10.1016/0042-6822(78)90431-2. [DOI] [PubMed] [Google Scholar]
- Wertz G. W., Levine M. RNA synthesis by vesicular stomatitis virus and a small plaque mutant: effects of cycloheximide. J Virol. 1973 Aug;12(2):253–264. doi: 10.1128/jvi.12.2.253-264.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]