Abstract
The Drosophila fusome is a germ cell-specific organelle assembled from membrane skeletal proteins and membranous vesicles. Mutational studies that have examined inactivating alleles of fusome proteins indicate that the organelle plays central roles in germ cell differentiation. Although mutations in genes encoding skeletal fusome components prevent proper cyst formation, mutations in the bag-of-marbles gene disrupt the assembly of membranous cisternae within the fusome and block cystoblast differentiation altogether. To understand the relationship between fusome cisternae and cystoblast differentiation, we have begun to identify other proteins in this network of fusome tubules. In this article we present evidence that the fly homologue of the transitional endoplasmic reticulum ATPase (TER94) is one such protein. The presence of TER94 suggests that the fusome cisternae grow by vesicle fusion and are a germ cell modification of endoplasmic reticulum. We also show that fusome association of TER94 is Bam-dependent, suggesting that cystoblast differentiation may be linked to fusome reticulum biogenesis.
INTRODUCTION
The Drosophila germ cell lineage has provided an experimentally manipulatable system for studying stem cell function and cell differentiation. Several key events during progression from stem cells to nurse cells and oocyte are dependent on the enigmatic fusome organelle found in germ cells. Thus defining how the structure and function of this large, complex organelle relate to its regulatory roles is an important goal for understanding germ cell differentiation (for review see de Cuevas et al., 1997).
Germ line stem cell division produces a stem cell and a cystoblast daughter (de Cuevas et al., 1997). The cystoblast will execute precisely four rounds of mitosis with incomplete cytokinesis to produce a 16-cell cyst of interconnected cells. One of these cells will become the oocyte and the other 15 will become nurse cells. During these divisions, the fusome changes from a sphere in stem cells and cystoblasts (Lin et al., 1994) into a highly branched and elongated organelle stretching through the entire syncytial cyst. Mutations in genes encoding fusome-associated proteins implicate the organelle in regulating cystoblast differentiation, cell cycle synchrony, spindle orientation, and oocyte determination (Yue et al., 1992; de Cuevas et al., 1996; McGrail and Hays, 1997; Ohlstein and McKearin, 1997).
Structurally, the fusome can be divided into two compartments: one is composed of membrane-associated skeletal proteins and one of membranous cisternae and small vesicles. To date, the membrane skeletal proteins α-Spectrin (α-Spc), β-Spectrin (β-Spc), Hu-li tao shao (Hts), and Ankyrin have been recognized as components of the organelle (Lin et al., 1994; de Cuevas et al., 1996). Inactivating mutations in β-Spc and Hts block fusome formation altogether but produce multicellular cysts that contain fewer than the normal number of nurse cells and often lack an oocyte (Yue et al., 1992; de Cuevas et al., 1996). Two additional proteins, Dynein and Cyclin A, have been identified as being fusome-associated. The product of the Dhc64C dynein gene is probably important for stable centrosome associations with the fusome because mutations in Dhc64C cause misoriented spindles and impaired cyst formation (McGrail and Hays, 1997). The fusome localization of Cyclin A is cell cycle dependent, but the significance of localization is not clear (de Cuevas et al., 1997).
Within the fusome, the central region is filled with membranous tubules and vesicles that resemble the cisternae of smooth and rough endoplasmic reticulum (McKearin, 1997). This network of fusome tubules represents the majority of cisternal structures in germ cells, and several authors (Büning, 1994; de Cuevas et al., 1997; McKearin, 1997) have speculated that fusome cisternae could be modified endoplasmic reticulum (ER) and, perhaps, Golgi apparatus. In stem cells, the cisternae occupy a modest region of the fusome core; however, as cystoblasts and cystocytes form and the fusome grows, the cisternae become very dense in the fusome and appear in the electron microscope as an extensive reticulum. Presumably expansion of the fusome reticulum reflects active vesicle transport to the fusome site and vesicle fusion to build the cisternae as occurs during the assembly and maintenance of ER and Golgi in animal cells (Warren and Wickner, 1996).
To date, only the Bam protein has been implicated in assembling the fusome reticulum. Bam is a novel protein of unknown function that is associated with the fusome throughout the organelle’s life (McKearin and Ohlstein, 1995). Inactivating mutations of the bag-of-marbles (bam) gene cause fusomes to develop with a very low density of cisternae, suggesting that the process of reticulum building is compromised (McKearin and Ohlstein, 1995); however, fusome skeletal proteins aggregate in bam mutant cells, just as they do in wild-type cells (McKearin and Ohlstein, 1995). Developmentally, loss of bam function blocks cystoblast differentiation and causes germ cells to proliferate in a stem cell-like state (McKearin and Spradling, 1990; McKearin and Ohlstein, 1995). Thus the consequences of disrupting fusome reticulum formation with bam mutations are more severe than the consequences of disrupting the fusome skeletal apparatus with mutations in genes such as hts or α-spectrin (Yue and Spradling, 1992; de Cuevas and Spradling, 1996). As a first step to discover fusome functions that might account for a role in cystoblast differentiation, we have begun to study the biochemical nature of the fusome reticulum by identifying protein components of the organelle. One such candidate is the recently identified Drosophila TER94 protein that is the probable orthologue of the Saccharomyces cerevisiae CDC48 and vertebrate TER proteins. This family of proteins is required for vesicle fusion for ER and Golgi biogenesis (Warren and Wickner 1996; Patel and Latterich, 1998). In this article we show that TER94 is a fusome constituent and that its association with the fusome depends on functional Bam protein.
MATERIALS AND METHODS
Cloning
The initial TER94 cDNA, which contained codons 549–801, was recovered from an ovarian library (Nusslein-Volhard, personal communication) that was screened by the yeast two-hybrid method (Brent and Finley, 1997) for Bam-interacting clones. Complete TER94 clones were obtained by screening a bacteriophage ovarian cDNA library (Stroumbakis et al., 1994) with the TER94 insert from the B42-TER94. Purified plaques were selected, and the inserts were recovered by PCR according to manufacturer’s instructions (Boehringer Mannheim, Indianapolis, IN) using primers from the 5′ and 3′ flanking regions of the bacteriophage lambda gt22 vector. Three clones had inserts of 3 kb. One was used for complete sequencing and subcloning into Bluescript(KS+). Sequence alignments were performed using BLAST search programs (Altschul et al., 1997) against the National Center for Biotechnical Information Databases. The sequence for TER94 appears as GenBank Accession no. AAC27447, which was deposited by Pinter et al. (1998); our sequence agreed with their archived sequence.
Antibody Production
TER94 fragments were subcloned into a GST fusion vector, and protein was expressed using the manufacturer’s standard protocol (Pharmacia, Piscataway, NJ). Bacterial extracts containing GST–TER94 were separated in 10% SDS-PAGE gels, and the bands of overexpressed recombinant proteins were recovered from gels stained with 3 M CuCl. CuCl was removed from gel slices by incubating with 0.25 M EDTA for several hours with multiple buffer exchanges. Antibodies against GST–TER94 were prepared by standard procedures at the Immunological Resources Center at University of Illinois-Urbana/Champaign. TER94 antibodies were also prepared by injecting peptide-KLH conjugates (NH2-CILRPGRLDQLIYIPLPDDKSREAILKANLKR-COOH) into mice.
Germ Line Clone Induction
P[FRT; w+]2R-G13 l(2)k15502 and P[FRT; w+]2R-G13 P[arm-LacZ] chromosomes were constructed by standard meiotic recombination methods. P[FRT; w+]2R-G13 P[ovoD1; w+] and P[hsFLP] chromosomes were obtained from L. Cooley (Yale University), and P[FRT; w+]42D P[arm-lacZ] was obtained from T. Xie and A. Spradling (Carnegie Institute). The ovoD1 transgene is a dominant female sterile mutation that causes egg chamber development to arrest near stage 4 (Oliver et al., 1990). Thus all nonrecombinant egg chambers will show the ovoD1 phenotype; recombinants will produce cells that eliminate the ovoD1 transgene and can therefore form egg chambers allowing the effects of other mutations on oogenesis to be evaluated. Three days after appropriate matings, recombination was induced by exposing embryos and larvae to a single 37°C heat shock for 2 h. Adult females were collected after eclosion and allowed to lay eggs for several days to score recombinant germ cells. Subsequently, ovaries were dissected and examined directly to score oogenic progress.
In Situ Hybridization and Chromosome Mapping
RNA in situ hybridization against whole ovaries was performed as described by Tautz and Pfeiffle (1989) and modified by Christerson and McKearin (1994). Chromosomal in situ hybridization was used for TER94 gene localization on salivary gland chromosome squashes (Atherton and Gall, 1972; Pardue, 1986). Digoxigenin-labeled probes were prepared from cDNA clones following manufacturer’s instructions (Boehringer Mannheim).
Immunoblots
Immunoblots were performed as described in Christerson and McKearin (1994); antibodies against TER94 peptide and fusion protein were used at a dilution of 1:2000. All sera tested produced the same banding pattern. Alkaline phosphatase-conjugated secondary antibodies and CSPD-Star chemiluminescent detection reagents were used according to manufacturer’s instructions (Tropix, Bedford, MA). Antisera were tested using protein samples from yeast cells expressing the B42-TER protein as well as from ovarian samples.
Immunohistochemistry
Protein localization studies for TER94 were performed as in Christerson and McKearin (1994) using the same dilution conditions described for immunoblots. In some cases, 0.2% Saponin was used instead of 0.2% Tween-20 in fixation and antibody incubation solutions with comparable results. Monoclonal anti–Hu-li tao shao (mAb1B1; Zaccai and Lipshitz, 1996) antiserum was used at 1:20; anti-Vasa antiserum (courtesy of P. Lasko, McGill University) was used at 1:500, and anti-Spectrin antiserum (courtesy of D. Branton, Harvard University) was used at 1:2500.
Sucrose Gradient Fractionation
Fifty pairs of ovaries were homogenized in 250 μl of sucrose dilution buffer (20 mM HEPES, pH 7.5, 1 mM DTE, 110 mM KOAc, 2 mM MgOAc, 1 μg/ml aprotinin, 1 μg/ml leupeptin, 1 μg/ml pepstatin, 0.5 mM PMSF). One milligram of total protein was layered on 12 ml of 5–30% linear sucrose gradient, cushioned on 1 ml of a 40% sucrose layer. The gradient was spun at 36,000 rpm for 16 h at 4°C in a Sorvall TH641 rotor. Fractions of 300–400 μl were collected and analyzed on Western blots.
RESULTS
Isolation of Drosophila TER94 cDNA Clones
Candidates for Bam-interacting partners were identified in a yeast protein–protein interaction screen (Brent and Finley, 1997). To prioritize efforts, the expression patterns of Bam-interacting clones were determined by ovarian in situ hybridization and used as an initial test of in vivo authenticity of the two-hybrid interaction. Because bam expression is limited to relatively few cells in the adult ovary (McKearin and Spradling, 1990), the test of coexpression provided a stringent assay for overlapping gene expression.
We used an incomplete TER94 cDNA clone obtained from a Drosophila ovarian library (see MATERIALS AND METHODS) to determine gene chromosomal position and to recover additional cDNAs with full-length ORFs (Brown and Kafatos, 1988; Stroumbakis et al., 1994). Chromosomal in situ hybridization placed TER94 at position 46D on the right arm of chromosome 2. We recovered a 3.0-kb TER94 cDNA clone that contained the entire protein encoding sequence, a complete 3′-UTR, and most or all of the 5′-UTR. While this work was in progress, Pinter et al. (1998) reported the cloning of TER94. Our molecular and immunological data agree with theirs. We will therefore limit description of sequence comparisons between TER94 and other proteins in the TER family to those points relevant for this work; additional data can be found in Pinter et al. (1998).
TER94 is related to Cdc48p from S. cerevisiae (Frohlich et al. 1991) and the TER/VCP/p97 proteins from vertebrates (Peters et al., 1990; Egerton et al., 1992; Zhang et al., 1994). Cdc48p and the vertebrate TER proteins have been identified as probable orthologues of one another based on sequence conservation, cellular localization, and biochemical activities (Zhang et al. 1994; Latterich et al. 1995). Alignment of the sequences shows that Drosophila TER94 is 83% identical to rat TERA and 67% identical to yeast Cdc48p (Pinter et al., 1998; our data). Genetic (Patel et al., 1998) and biochemical (Latterich et al., 1995) studies have linked Cdc48p/TER to cellular processes requiring organelle vesicle fusion such as endoplasmic reticulum biogenesis and nuclear membrane fusion.
TER94 Is an Essential Gene and Mutations Are Cell Lethal
Searching the Drosophila Genome Database with the TER94 sequence revealed that a P-element transposon had inserted into the 5′-UTR of the TER94 gene (e.g., l(2)03775). A second, lethal P-element insertion localized to 46D (l(2)k15502) and failed to complement l(2)03775. A chromosome that carries a deletion of the 46C-D region (Df(2R)X1) failed to complement both P-alleles. Excision of the P-element from l(2)k15502 fully reverted the lethal phenotype, indicating that the transposon is responsible for the mutant phenotype. In addition, the lethal P-alleles failed to complement five ethylmethane sulfonate-induced mutations that were mapped to the TER94 locus (E. Goldstein, personal communication).
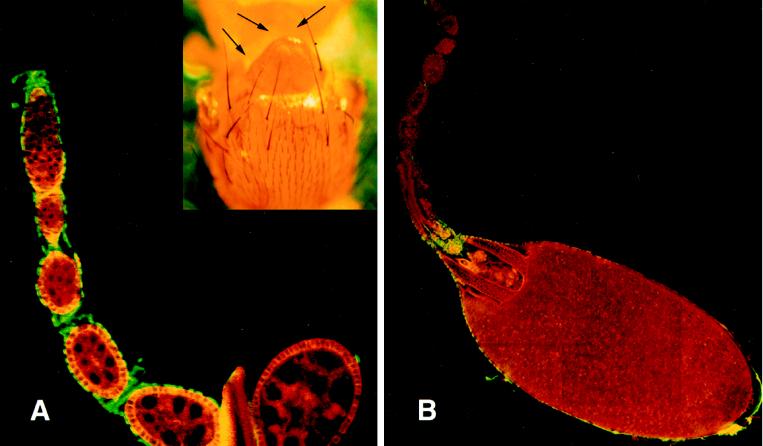
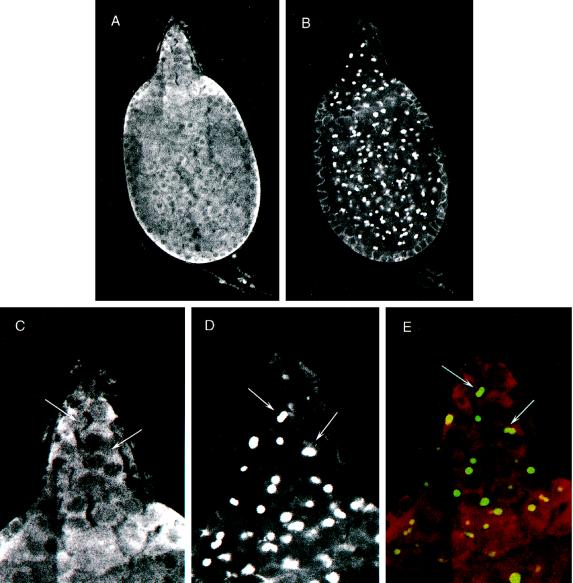
To evaluate the lethal phenotype at a cellular level, we constructed chromosomes that carried the appropriate markers and genetic elements for FLP/FRT-mediated recombination to induce mitotic clones (Xu and Rubin, 1993) in germ line cells. Initial experiments compared P[hsFLP]; P[FRT; w+]2R-G13/P[FRT; w+]2R-G13 P[ovoD1; w+] and P[hsFLP]; P[FRT; w+]2R-G13 l(2)k15502/P[FRT; w+]2R-G13 P[ovoD1; w+] animals (see MATERIALS AND METHODS). We found that, as expected, P[hsFLP]; P[FRT; w+]2R-G13/P[FRT; w+]2R-G13 P[ovoD1; w+] females laid fertile eggs and had a mixture of wild-type and ovoD1-like egg chambers in their ovaries. P[hsFLP]; P[FRT; w+]2R-G13 l(2)k15502/P[FRT; w+]2R-G13 P[ovoD1; w+] animals, however, did not lay eggs and had only ovoD1-like egg chambers. We considered two explanations for these results: (1) homozygous l(2)k15502 germ line stem cells produced egg chambers that were indistinguishable from ovoD1 chambers, or (2) homozygous l(2)k15502 germ line stem cells were inviable. To distinguish between these alternatives, we constructed P[hsFLP]; P[FRT; w+]2R-G13 P[arm-lacZ]/P[FRT; w+]2R-G13 l(2)k15502 flies and induced mitotic recombination. The P[arm-lacZ] transgene is a positive marker for nonrecombinant cells because it produces β-galactosidase ubiquitously (Lecuit and Cohen, 1997; Xie and Spradling, 1998); recombinant cells will be homozygous for l(2)k15502 and lacZ− negative. Ovaries from these heat-shocked females, however, contained only wild-type egg chambers that expressed β-galactosidase (Figure 1A). Ovaries from control animals (P[FRT; w+]2R-G13 P[arm-lacZ]/P[FRT; w+]2R-G13 P[ovoD1; w+]) that were treated in parallel contained mixtures of lacZ-positive ovoD1 egg chambers and wild type (Figure 1B).
Figure 1.
Mitotic clonal analysis of TER mutant allele. (A) Shown is an example of an ovariole dissected from a P[hsFLP]; P[FRT]2R-G13 l(2)k15502/P[FRT]2R-G13 [arm-LacZ]. Ovaries were reacted with anti-Spc antibodies (green) and LacZ antibodies (red) to detect recombination-induced clonal egg chambers that would appear LacZ negative. The inset shows the effects of mitotic recombination on somatic cells by showing the loss of scutellar bristles in a P[hsFLP]; P[FRT]2R-G13 l(2)k15502/P[FRT]2R-G13 [arm-LacZ]. (B) Shown is an ovariole from a P[hsFLP]; P[FRT]2R-G13 P[arm-lacZ]/P[FRT]2R-G13 P[ovoD1; w+]. Most egg chambers manifest the ovoD1 phenotype, but the stage 13 egg chamber shows that FRT-dependent recombination can produce wild-type follicles under the experimental conditions.
The failure to recover TER94 clones could mean that FLP-mediated recombination had failed or that cells homozygous for TER94 mutations were inviable and did not survive long enough to establish clones. As a positive assay for the occurrence of recombination, we examined bristles on the cuticles of P[hsFLP]; P[FRT]2R-G13 l(2)k15502/P[FRT]2R-G13 [arm-LacZ]. We reasoned that if the FLP-FRT recombination method was active and if TER94 mutations caused autonomous cell lethality, then these animals could produce small, survivable mutant clones, and some of these clones would eliminate a bristle. Bristle loss occurred infrequently in control animals that did not carry the FLP recombinase transgene (Table 1, rows B and D). Data in Table 1, row C demonstrated that recombination with chromosomes carrying mutations that were irrelevant for somatic cell development produced only background levels of bristle loss; however, we found that 86% of heat-shocked animals carrying the ter94 mutant chromosome were missing at least one major bristle on the head, scutellum, or sternopleurum. This frequency was dependent on FLP and the l(2)k15502 mutation because it was at least six times the occurrence of bristle loss in controls. The elevated frequency of bristle loss in nonheat-shocked animals suggests that the P[hsFLP] transgene is leaky. Only animals that were missing at least one bristle (Figure 1A, inset) were used for ovary examination by LacZ immunodetection.
Table 1.
Analysis of FLP/FRT-induced ter94 mutant clones in somatic tissue
| Genotype | HS | NHS | ||
|---|---|---|---|---|
| A | P[hsFLP] ; P[FRT] l(2)k15502 | 86% | 65% | |
| + P[FRT] P[arm-LacZ] | n = 179 | n = 156 | ||
| B | ±; P[FRT] l(2)k15502 | 11% | 14% | |
| Y P[FRT] P[arm-LacZ] | n = 106 | n = 154 | ||
| C | P[hsFLP] ; P[FRT] [ovoD1] | 13% | 4% | |
| + P[FRT] P[arm-LacZ] | n = 106 | n = 77 | ||
| D | ±; P[FRT] [ovoD1] | 2% | 8% | |
| Y P[FRT] P[arm-LacZ] | n = 53 | n = 50 | ||
Appropriate crosses were established to produce the progeny shown. Note that only females carry the transposon encoding the Flipase recombinase (e.g. [hsFLP]). The l(2)k15502 allele of the ter94 gene was used for production of mitotic clones. The percentage of animals of a given genotype that were missing at least one bristle was scored. P[FRT] is P[FRT; w+]2R-G13, l(2)k15502 is a lethal P-allele of ter94; [ovoD1] is P[ovoD1; w+]. P[hsFLP]; P[FRT; w+]2R-G13/P[FRT; w+]2R-G13 P[ovoD1; w+].
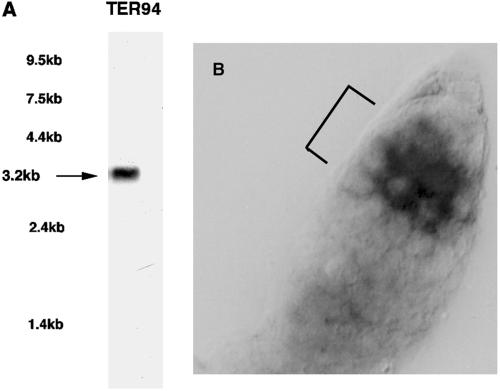
TER94 mRNA Is Up-regulated in Region 1
TER94 hybridized to a single 3.2-kb transcript that was abundant in ovaries on Northern blots (Figure 2A). RNA localization experiments showed that TER94 mRNA abundance increased dramatically in germarial Region 1 (for ovarian anatomy, see Spradling, 1993), beginning with probable cystoblasts (Figure 2B). Longer reaction development revealed that TER94 mRNA was present in all germ line and follicle cells. The RNA is present at baseline levels in germ line stem cells but begins to accumulate to higher levels in cystoblasts and the remaining Region 1 germ cells, which correspond to the dividing cystocytes. This pattern of accumulation closely parallels that of bam mRNA, which first becomes detectable in cystoblasts, remains in two-cell cysts, and then diminishes to undetectable levels by the time eight-cell cysts are formed (McKearin and Spradling, 1990). These findings raised the possibility that bam and TER94 expression might be coordinately regulated in Region 1 cystocytes.
Figure 2.
Molecular analysis of TER94 ovarian expression. (A) A radiolabeled probe derived from the TER94 cDNA clone was hybridized to Northern blots containing poly(A+) ovarian RNA. The positions of commercially available RNA markers are indicated at left. (B) A digoxigenin-labeled TER94 probe was hybridized against wild-type ovaries prepared for RNA in situ hybridization. The germaria are oriented such that the anterior end is upper right; the bracketed area in A shows germarial region 1.
TER94 Protein Accumulates in Fusomes
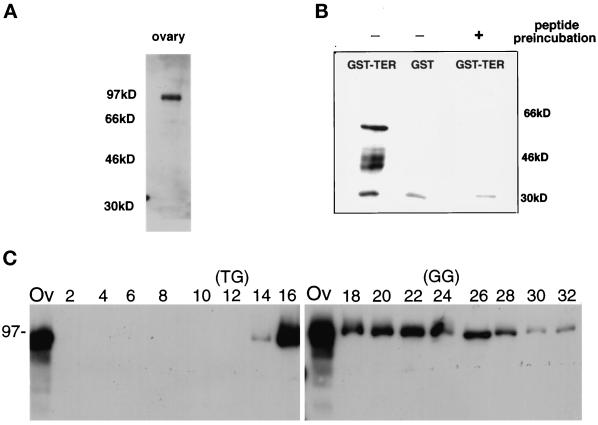
Antisera raised against a TER94 internal peptide (MATERIALS AND METHODS) reacted with bands of 94,000 Da in wild-type ovarian extracts (Figure 3A) and 57,000 Da in Escherichia coli cells expressing a fragment of TER94 as a GST-fusion protein (Figure 3B). As an additional test of antibody specificity, we determined that recognition of the immunoreactive GST–TER band could be blocked by preincubation with antigenic peptide (Figure 3B). TER94 polyclonal antisera raised against a recombinant protein reacted with the same bands as the anti-peptide antisera. The ovarian 94-kDa band is only slightly larger than the 89-kDa band predicted from conceptual translation of the TER94 cDNA and is similar to the apparent molecular masses of the vertebrate and Drosophila TER proteins (Patel and Latterich, 1998; Pinter et al. 1998). Both Cdc48p and vertebrate TERs oligomerize to form homohexameric complexes. When ovarian extracts were analyzed on native sucrose gradients, the peak of TER94 from flies sedimented at Mr ∼500,000, which is close to the expected size (Mr ∼530,000) for a homohexameric complex (Figure 3C).
Figure 3.
Immunological evaluation of TER94. (A) Western blot analysis of wild-type ovarian extract using antibodies raised against a TER94 peptide (MATERIALS AND METHODS) revealed a 94-kDa protein. The positions of molecular weight standards are shown on the left. (B) Immunoblot containing protein isolated from E. coli expressing either GST–TER94 ORF (lane 1; predicted molecular mass of 58 kDa) or GST alone (lane 2) was incubated with TER94 peptide antibodies (lanes 1 and 2). The multiple bands that appear near 46 kDa in lane 1 are probably breakdown products of the GST–TER94 recombinant protein. Lane 3 shows the results obtained when TER94 peptide antiserum was incubated with the antigenic peptide before incubating with a membrane carrying protein from E. coli expressing GST–TER94 ORF. The band at 30 kDa is a nonspecific reactivity because it is common in all samples and is not blocked by antigenic peptide incubation. (C) Protein was isolated from even-numbered fractions of a sucrose gradient and separated on two 10% SDS-PAGE gels. A sample of the input total ovarian protein (lane Ov) was included on each gel. The fraction in lane 2 was collected from the bottom, whereas lane 32 is from the top of the gradient. The corresponding immunoblot was incubated with TER94 antibodies to determine the fractions that contained TER94. A parallel sucrose gradient was run containing thyroglobulin (TG, Mr 670,000) and bovine gamma globulin (GG, Mr 158,000) to establish the position of known molecular weight standards.
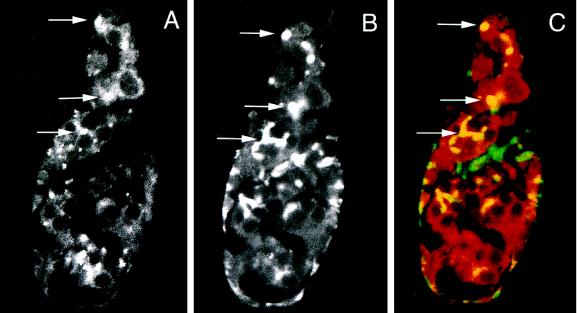
As expected from the patterns of RNA expression (Figure 2B), TER94 protein was present in both ovarian germ cells and somatic cells. Figure 4A is a confocal section of a wild-type germarium that demonstrates that TER94 was largely cytoplasmic in follicle and germ cells. Significantly, germ cells often contained one or several especially intense fluorescent signals (Figure 4A, arrows), suggesting that TER94 was distributed unevenly in the cytoplasm. In cystocytes in germarial Region 1, these were usually somewhat diffuse bright regions, whereas in more mature cystocytes the bright spots were more sharply defined.
Figure 4.
TER94 is present in the cytoplasm and in the fusome of early germ cells. (A–C) Wild-type ovaries were incubated with rat TER94 antibodies and monoclonal anti-Hts antibodies. Data were collected as single confocal sections and analyzed by standard confocal algorithms for signal overlap (Zeiss LSM-100 Operations Manual). The anterior end of the germarium is up in all panels. (A) The distribution of TER94 signal in a wild-type germarium is displayed. TER immunofluorescent is seen as both a faint, uniform signal and clusters of particularly bright signal at positions of fusomes (arrows). This optical section contains mostly germ cells and very few somatic cells. (B) Stem cell fusomes and fusomes were located by also incubating ovaries with Hts antibodies. The positions of the arrows correspond to those seen in A and allow a comparison of the regions of dense TER94 accumulation and stem cell fusome/fusome position. (C) Immunofluorescence for TER94 (red) and Hts (green) displayed simultaneously showed overlapping signals (yellow). Again, the position of the arrows corresponds to those in A and B. The arrowhead in C indicates an accumulation of Hts that does not colocalize with TER94. This material actually lies outside of any cells and may represent fusome or plasma membrane-associated Hts from cells disrupted during processing.
The number and positions of the TER-enriched regions suggested that they might correspond to fusomes. Figure 4B shows a confocal image of the fusome marker Hts (Zaccai and Lipshitz, 1996; Lin et al., 1994) in the same germarium shown in A. Note that stem cell fusomes in germ cells nearest the anterior tip appear as a single dot of intense staining (top arrow) (Lin et al., 1994; de Cuevas and Spradling, 1998), whereas those in a more posterior position (bottom arrow; i.e. more mature cysts) contain elongated, branched fusomes. Figure 4C shows the merged images of A and B. Significantly, stem cell fusomes and fusomes are yellow, indicating overlapping immunofluorescent signals and protein colocalization. Precise colocalization of TER94 and Hts was strongest in Region 1 germ cells and declined in regions containing mature cysts.
Because a fraction of TER is nuclear in yeast and mammals (Peters et al., 1990; Madeo et al., 1998), we looked carefully at Drosophila nuclei. Most germ cell nuclei were faintly TER94 positive (Figure 4A). We found many examples of strong nuclear and perinuclear staining in nonovarian somatic cells in larvae and adults (our unpublished results).
Distribution of TER94 Is Altered in bam Mutant Cells
Fusomes are the primary site of ER-like cisternae in young germ cells. If TER94 enrichment in fusomes represents accumulation at the fusome reticulum, TER94 distribution might be altered when the reticulum is not properly assembled. We had noted previously that Bam was a fusome-associated protein and that bam mutant fusomes were deficient in cisternae (McKearin and Ohlstein, 1995). We examined the distribution of TER94 protein in bam germ cells and found that it was distributed uniformly without signs of enrichment at the site of fusomes as was observed in wild-type germaria. Indeed, when the bam stem cell fusomes were visualized with Hts antibodies, it was clear that TER94 was no more abundant within or near stem cell fusomes than in any other cytoplasmic regions (Figure 5, compare A and B with C and D). Consistent with this conclusion, the merged images of TER94 and Hts distributions did not show immunofluorescent overlap (Figure 5E), indicating that bam fusomes did not accumulate detectable TER94.
Figure 5.
TER94 does not accumulate in stem cell fusomes and fusomes in bam mutant germ cells. Tumorous ovaries isolated from bam females were incubated with rat TER94 antibodies and monoclonal anti-Hts antibodies. (A) TER94 is uniformly distributed in the cytoplasm of bam mutant germ cells. Note that TER94 is not significantly enriched in any subcellular region. (B) This confocal section shows the positions of stem cell fusomes and fusomes in the same bam germarium. (C and D) A high-magnification view of the distribution of TER94 and Hts, respectively, in the anterior end of the germarium in A and B. The arrows indicate stem cell fusome positions; note that TER94 is not enriched at these positions. (E) This panel presents the merged projection of TER94 (red) and Hts (green) in the germarium’s anterior end. Stem cell fusomes do not show immunofluorescent overlap, indicating that TER94 does not become enriched.
TER94 was also enriched at a few sites that did not correspond to fusomes. We speculate that these may be sites of Golgi bodies or transport vesicles, although unambiguous identification requires additional reagents as markers. These extrafusome sites of TER94 enrichment were also abolished in bam mutant cells (Figure 5A).
The observation that TER94 fusome association is linked to Bam function can be explained by either a direct or indirect Bam dependent mechanism. Although loss of bam function might block fusome reticulum assembly before TER94 arrival, it is also possible that Bam recruits TER94 to the reticulum as part of the assembly process. This hypothesis has been difficult to test because Bam is a low-abundance protein in ovaries, and in vitro assays for Bam and TER94 interaction have produced inconsistent results. The interaction of Bam and TER94 as two-hybrid partners supports the hypothesis of in vivo interaction. Finding the Drosophila homologue of the S. cerevisiae protein Ufd3p as a second Bam interacting protein strengthened the significance of the Bam–TER94 interaction. Ghislain et al. (1996) have shown that Ufd3p and the yeast TER (i.e., Cdc48p) interact with one another directly and M. Latterich (personal communication) has determined that Ufd3p is required for efficient organelle vesicle fusion.
DISCUSSION
ER and Golgi Biogenesis Requires TER Proteins
TER94 is a recently identified Drosophila member of the AAA family of ATPases (Pinter et al., 1998). The fly protein has two conserved AAA domains that contain the canonical Walker motif ATP binding sites (Walker et al., 1982). Members of the AAA family have been implicated in diverse cellular processes, such as protein degradation, vesicle transport, and transcription (for review see Confaloneri and Duguet, 1995; Patel and Latterich, 1998). The extent of the sequence conservation and common oligomeric assembly between TER94 and vertebrate TERs and Cdc48p suggests that TER94 is the fly orthologue.
The ter94 gene is essential in flies for both organismal and cellular viability. This requirement means that we were unable to observe directly the consequences of loss of ter94 function. The high likelihood, however, that TER94 is the fly TER orthologue allows us to infer much about the function of the protein in flies by considering TER function in other organisms where the proteins have been extensively characterized. In S. cerevisiae, Latterich et al. (1995) showed that strong cdc48 mutations were lethal and that CDC48 protein was required for vesicle fusion associated with ER biogenesis. Vertebrate Cdc48p family members, the TER proteins, have been isolated from Xenopus (p97; Acharya et al., 1995; Rabouille et al., 1995), rat (TER; Zhang et al., 1994; Rabouille et al., 1995), and pig and rabbit liver cells (VCP; Frohlich et al., 1991; Acharya et al., 1995). In several cases (Zhang et al., 1994; Acharya et al., 1995; Latterich et al., 1995; Rabouille et al., 1995), TER proteins have been linked directly to organelle membrane fusion reactions. For example, in vertebrate cells, ER and Golgi organelles become fragmented into small vesicles during mitosis and are reassembled by fusion of homotypic vesicles after cytokinesis (Warren, 1993; Warren and Wickner, 1996). Biochemical reconstitution experiments showed that vesicle-enriched postmitotic cellular extracts recapitulated many stages of organelle biogenesis when purified TER was added (Acharya et al., 1995; Rabouille et al., 1995). The link between TER and cellular organelles in vertebrates was further strengthened by cellular fractionation experiments that showed VCP (porcine TER) associated with ER membranes (Frohlich et al., 1991). Additionally, electron microsocopic ultrastructural studies localized TER to the ER transitional zone in rat cells (Zhang et al., 1994).
The TER proteins collaborate with a many other polypeptides to catalyze organelle biogenesis (Patel and Latterich, 1998; Warren and Malhotra, 1998). One of these may be the PLAP family of proteins that associate with TERs (Ghislain et al., 1996) and are important for organelle vesicle fusion in at least one organism: yeast (M. Latterich, personal communication). Finding dPLAP as a Bam (and TER94; our unpublished observations) partner in two-hybrid tests suggests that these three proteins might form a complex in Drosophila germ cells.
Fusome Cisternae Might Be the Germ Cell’s ER
We have found that TER94 is abundantly expressed in the fusome and cytoplasm of germ line cells of the germarium. The fusome is the site of the majority of intracellular membrane tubules in the germ line stem cells, cystoblasts, and cystocytes, and the similarity of the fusome’s reticulum to ER cisternae has been noted previously (Storto and King, 1989; McKearin and Ohlstein, 1995). Some of the tubules appear studded with ribosomes like rough ER, whereas most resemble smooth ER. On the basis of these ultrastructural observations and the identification of TER94 as a component, we propose that the network of fusome tubules corresponds to a cell-specific modification of endoplasmic reticulum. Because ER functions are critical for cell viability, it is not surprising that TER94 inactivation was a cell lethal lesion.
Fusome Assembly as a Step in Differentiation
Mutations in fusome component genes have profound effects on the germ cell differentiation (Lin et al., 1994; de Cuevas et al., 1996; McGrail and Hays, 1997; McKearin and Ohlstein, 1997), suggesting that proper fusome biogenesis is a key step in germ line cyst differentiation. The phenotype is most dramatic in bam inactivating mutations that block fusome reticulum assembly and cystoblast differentiation. With this report, we have shown that one protein that is required for organelle vesicle fusion is enriched in the fusome. Furthermore, the accumulation of TER94 transcripts was accelerated in dividing cystocytes, suggesting that common signals might regulate fusome reticulum expansion and TER94 expression. Considering the central role that TER94 is likely to play in organelle vesicle fusion, the failure to accumulate TER94 in bam− fusomes could explain the failure to assemble tubules in the bam mutant organelles. The interaction of Bam and TER94 in two-hybrid assays supports the hypothesis that TER94 fusome enrichment depends on Bam but must be considered preliminary until we accumulate additional evidence of a biochemical association.
On the basis of data from our laboratory and others, we previously proposed that increased Bam expression is responsible for cystoblast differentiation in the stem cell mitotic daughter farthest from the terminal filament (McKearin and Ohlstein, 1995; Ohlstein and McKearin, 1997). Recent experiments from Xie and Spradling (1998) and Cox et al. (1998), suggested that signaling between ovarian somatic and germ line cells is essential for suppressing cystoblast differentiation in stem cells. The studies of Xie and Spradling (1998) implicated the Dpp-signaling pathway as an essential element of the suppressing mechanism and suggested that Bam expression might be one of the principal targets. In light of the data presented in this article, the effect of suppressing Bam production in the stem cell may be to delay bam-dependent fusome reticulum maturation until a proper cystoblast is born.
ACKNOWLEDGMENTS
We express special gratitude to Dr. D. Edwards for his assistance with the two-hybrid screen experiments and analysis. We thank E. Goldstein, M. Latterich, and D. Ruden for sharing information and ideas before publication. M. Kuhn provided valuable technical assistance for immunohistochemistry. B. Horazdovzky, M. Roth, L. Avery, H. Kramer, and two anonymous reviewers provided important critical comments on this manuscript. We thank present and past members of the McKearin and Wasserman labs, especially C. Lavoie, J. Maines, and B. Ohlstein, for their comments and insights during the development of this work. This work was supported by grant F31-GM17258 and National Institutes of Health grant GM-45820 to D.M.
REFERENCES
- Acharya U, Jacobs R, Peters JM, Watson N, Farquhar MG, Malhotra V. The formation of Golgi stacks from vesiculated Golgi membranes requires two distinct fusion events. Cell. 1995;83:895–904. doi: 10.1016/0092-8674(95)90269-4. [DOI] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atherton D, Gall J. Salivary gland squashes for in situ nucleic acid hybridization studies. Drosophila Inf Service. 1972;49:131–133. [Google Scholar]
- Brent R, Finley R. Understanding gene and allele function with two-hybrid methods. Annu Rev Genet. 1997;31:663–704. doi: 10.1146/annurev.genet.31.1.663. [DOI] [PubMed] [Google Scholar]
- Brown NH, Kafatos FC. Functional cDNA libraries from Drosophila embryos. J Mol Biol. 1988;203:425–437. doi: 10.1016/0022-2836(88)90010-1. [DOI] [PubMed] [Google Scholar]
- Büning J. The Insect Ovary: Ultrastructure, Previtellogenic Growth and Evolution. New York: Chapman & Hall; 1994. [Google Scholar]
- Christerson LB, McKearin DM. orb is required for anteroposterior and dorsoventral patterning during Drosophila oogenesis. Genes Dev. 1994;8:614–628. doi: 10.1101/gad.8.5.614. [DOI] [PubMed] [Google Scholar]
- Confaloneri F, Duguet M. A 200-amino acid ATPase module in search of a basic function. BioEssays. 1995;17:639–650. doi: 10.1002/bies.950170710. [DOI] [PubMed] [Google Scholar]
- Cox DN, Chao A, Baker J, Chang L, Qiao D, Lin H. A novel class of evolutionarily conserved genes defined by piwi are essential for stem cell self-renewal. Genes Dev. 1998;12:3715–3727. doi: 10.1101/gad.12.23.3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Cuevas M, Lee JK, Spradling AC. α-spectrin is required for germ line cell divisions and differentiation in the Drosophila ovary. Development. 1996;122:3959–3968. doi: 10.1242/dev.122.12.3959. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Lilly MA, Spradling AC. Germ line cyst formation in Drosophila. Annu Rev Genet. 1997;31:405–428. doi: 10.1146/annurev.genet.31.1.405. [DOI] [PubMed] [Google Scholar]
- de Cuevas M, Spradling AC. Morphogenesis of the Drosophila fusome and its implications for oocyte specification. Development. 1998;125:2781–2789. doi: 10.1242/dev.125.15.2781. [DOI] [PubMed] [Google Scholar]
- Egerton M, Ashe OR, Chen D, Druker BJ, Burgess WH, Samelson LE. VCP, the mammalian homolog of CDC48, is tyrosine phosphorylated in response to T cell antigen receptor activation. EMBO J. 1992;11:3533–3540. doi: 10.1002/j.1460-2075.1992.tb05436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frohlich KU, Fries HW, Rudiger M, Erdmann R, Botstein D, Mecke D. Yeast cell cycle protein Cdc48p shows full length homology to the mammalian protein VCP and is a member of a protein family involved in secretion, peroxisome formation and gene expression. J Cell Biol. 1991;114:443–453. doi: 10.1083/jcb.114.3.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghislain M, Dohmen RJ, Levy F, Varshavsky A. Cdc48p interacts with UFD3p, a WD repeat protein required for ubiquitin-mediated proteolysis in Saccharomyces cerevisiae. EMBO J. 1996;15:4884–4899. [PMC free article] [PubMed] [Google Scholar]
- Latterich M, Frohlich KU, Schekman R. Membrane fusion and the cell cycle: Cdc48p participates in the fusion of ER membranes. Cell. 1995;83:885–893. doi: 10.1016/0092-8674(95)90268-6. [DOI] [PubMed] [Google Scholar]
- Lecuit T, Cohen SM. Proximal-distal axis formation in the Drosophila leg. Nature. 1997;388:139–145. doi: 10.1038/40563. [DOI] [PubMed] [Google Scholar]
- Lin H, Yue L, Spradling AC. The Drosophila fusome, a germ line-specific organelle, contains membrane skeletal proteins and functions in cyst formation. Development. 1994;120:947–956. doi: 10.1242/dev.120.4.947. [DOI] [PubMed] [Google Scholar]
- Madeo F, Schlauer J, Zischa H, Mecke D, Frohlich KU. Tyrosine phosphorylation regulates cell cycle-dependent nuclear localization of Cdc48p. Mol Biol Cell. 1998;9:131–141. doi: 10.1091/mbc.9.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrail M, Hays T. The microtubule motor cytoplasmic dynein is required for spindle orientation during cell divisions and oocyte differentiation. Development. 1997;124:2409–2419. doi: 10.1242/dev.124.12.2409. [DOI] [PubMed] [Google Scholar]
- McKearin D. The Drosophila fusome, organelle biogenesis and germ cell differentiation: if you build it …. BioEssays. 1997;19:147–152. doi: 10.1002/bies.950190209. [DOI] [PubMed] [Google Scholar]
- McKearin D, Ohlstein B. A role for Drosophila Bag-of-marbles protein in the differentiation of cystoblasts from germ line stem cells. Development. 1995;121:2937–2947. doi: 10.1242/dev.121.9.2937. [DOI] [PubMed] [Google Scholar]
- McKearin D, Spradling AC. bag-of-marbles: a Drosophila gene required to initiate both male and female gametogenesis. Genes Dev. 1990;4:2242–2251. doi: 10.1101/gad.4.12b.2242. [DOI] [PubMed] [Google Scholar]
- Ohlstein B, McKearin D. Ectopic expression of the Drosophila Bam protein eliminates oogenic germline stem cells. Development. 1997;124:3651–3662. doi: 10.1242/dev.124.18.3651. [DOI] [PubMed] [Google Scholar]
- Oliver B, Pauli D, Mahowald AP. Genetic evidence that the ovo locus is involved in Drosophila germ line sex determination. Genetics. 1990;125:535–550. doi: 10.1093/genetics/125.3.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardue ML. In situ hybridization to DNA of chromosomes and nuclei. In: Roberts DB, editor. Drosophila: A Practical Approach. Oxford: IRL Press; 1986. pp. 111–137. [Google Scholar]
- Patel S, Latterich M. The AAA-team: related ATPases with diverse function. Trends Cell Biol. 1998;8:865–871. [PubMed] [Google Scholar]
- Patel SK, Indig FE, Olivieri N, Levine ND, Latterich M. Organelle membrane fusion: a novel function for the syntaxin homolog Ufe1p in ER membranes. Cell. 1998;92:611–620. doi: 10.1016/s0092-8674(00)81129-0. [DOI] [PubMed] [Google Scholar]
- Peters JM, Walsh MJ, Franke WW. An abundant and ubiquitous homo-oligomeric ring-shaped ATPase particle related to the putative vesicle fusion protein Sec 18 and NSF. EMBO J. 1990;9:1757–1767. doi: 10.1002/j.1460-2075.1990.tb08300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinter M, Jekely G, Szepesi RJ, Farkas A, Theopold U, Meyer HE, Lindholm D, Nassel DR, Hultmark D, Friedrich P. TER94, a Drosophila homolog of the membrane fusion protein CDC48/p97, is accumulated in nonproliferating cells: in the reproductive organs and in the brain of the imago. Insect Biochem Mol Biol. 1998;28:91–98. doi: 10.1016/s0965-1748(97)00095-7. [DOI] [PubMed] [Google Scholar]
- Rabouille C, Levine TP, Peters JM, Warren G. An NSF-like ATPase, p97, and NSF mediate cisternal regrowth from mitotic Golgi fragments. Cell. 1995;83:905–914. doi: 10.1016/0092-8674(95)90270-8. [DOI] [PubMed] [Google Scholar]
- Spradling AC. Developmental genetics of oogenesis. In: Bate M, Martinez-Arias A, editors. Drosophila Development. Cold Spring Harbor, NY: CSH Press; 1993. pp. 1–70. [Google Scholar]
- Storto PD, King RC. The role of polyfusomes in generating branched chains of cystocytes during Drosophila oogenesis. Dev Genet. 1989;10:70–86. doi: 10.1002/dvg.1020100203. [DOI] [PubMed] [Google Scholar]
- Stroumbakis ND, Li Z, Tolias PP. RNA- and single-stranded DNA-binding (SSB) proteins expressed during Drosophila melanogaster oogenesis: a homolog of bacterial and eukaryotic mitochondrial SSBs. Gene. 1994;143:171–177. doi: 10.1016/0378-1119(94)90093-0. [DOI] [PubMed] [Google Scholar]
- Tautz D, Pfeiffle C. A nonradioactive in situ hybridization method for localization of specific RNAs in Drosophila embryos reveals translational control of the segmentation gene hunchback. Chromosoma. 1989;98:81–85. doi: 10.1007/BF00291041. [DOI] [PubMed] [Google Scholar]
- Walker JE, Saraste MJ, Runswick JJ, Gray NJ. Distantly related sequences in the α- and β-subunits ATPase, myosin, kinases and other ATP-requiring enzymes and a common nucleotide binding fold. EMBO J. 1982;1:945–951. doi: 10.1002/j.1460-2075.1982.tb01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. Membrane partitioning during cell division. Annu Rev Biochem. 1993;62:323–348. doi: 10.1146/annurev.bi.62.070193.001543. [DOI] [PubMed] [Google Scholar]
- Warren G, Malhotra V. The organization of the Golgi apparatus. Curr Opin Cell Biol. 1998;10:493–498. doi: 10.1016/s0955-0674(98)80064-1. [DOI] [PubMed] [Google Scholar]
- Warren G, Wickner W. Organelle inheritance. Cell. 1996;84:395–400. doi: 10.1016/s0092-8674(00)81284-2. [DOI] [PubMed] [Google Scholar]
- Xie T, Spradling AC. decapentaplegic is essential for the maintenance and division of germline stem cells in the Drosophila ovary. Cell. 1998;94:251–260. doi: 10.1016/s0092-8674(00)81424-5. [DOI] [PubMed] [Google Scholar]
- Xu T, Rubin GM. Analysis of genetic mosaics in developing and adult Drosophila tissues. Development. 1993;117:1223–1237. doi: 10.1242/dev.117.4.1223. [DOI] [PubMed] [Google Scholar]
- Yue L, Spradling AC. hu-li tai shao, a gene required for ring canal formation during Drosophila oogenesis, encodes a homolog of adducin. Genes Dev. 1992;6:2443–2454. doi: 10.1101/gad.6.12b.2443. [DOI] [PubMed] [Google Scholar]
- Zaccai M, Lipshitz HD. Differential distributions of two adducin-like protein isoforms in the Drosophila ovary and early embryo. Zygote. 1996;4:159–166. doi: 10.1017/s096719940000304x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Ashendel CL, Becker GW, Morre J. Isolation and characterization of the principal ATPase associated with transitional endoplasmic reticulum. J Cell Biol. 1994;127:1871–1883. doi: 10.1083/jcb.127.6.1871. [DOI] [PMC free article] [PubMed] [Google Scholar]