Abstract
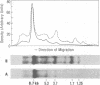
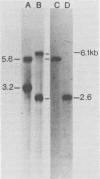
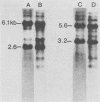
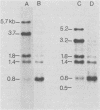
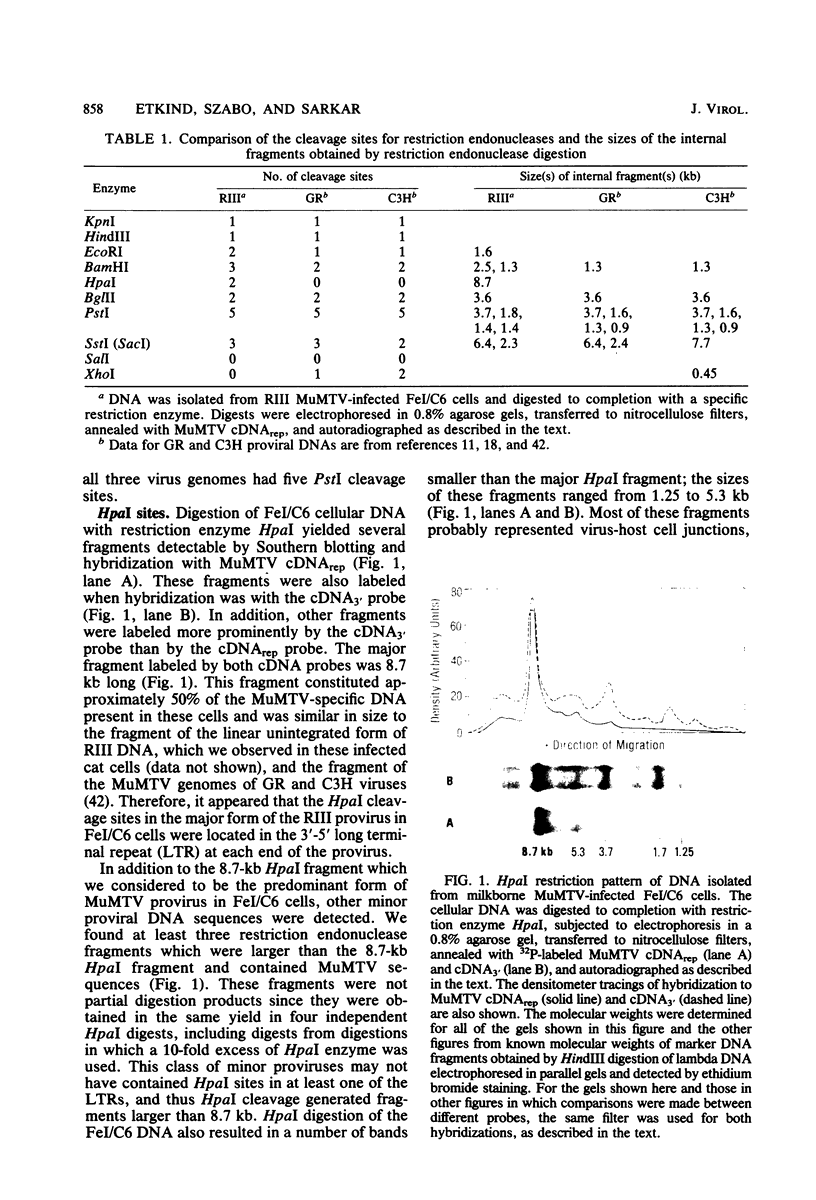
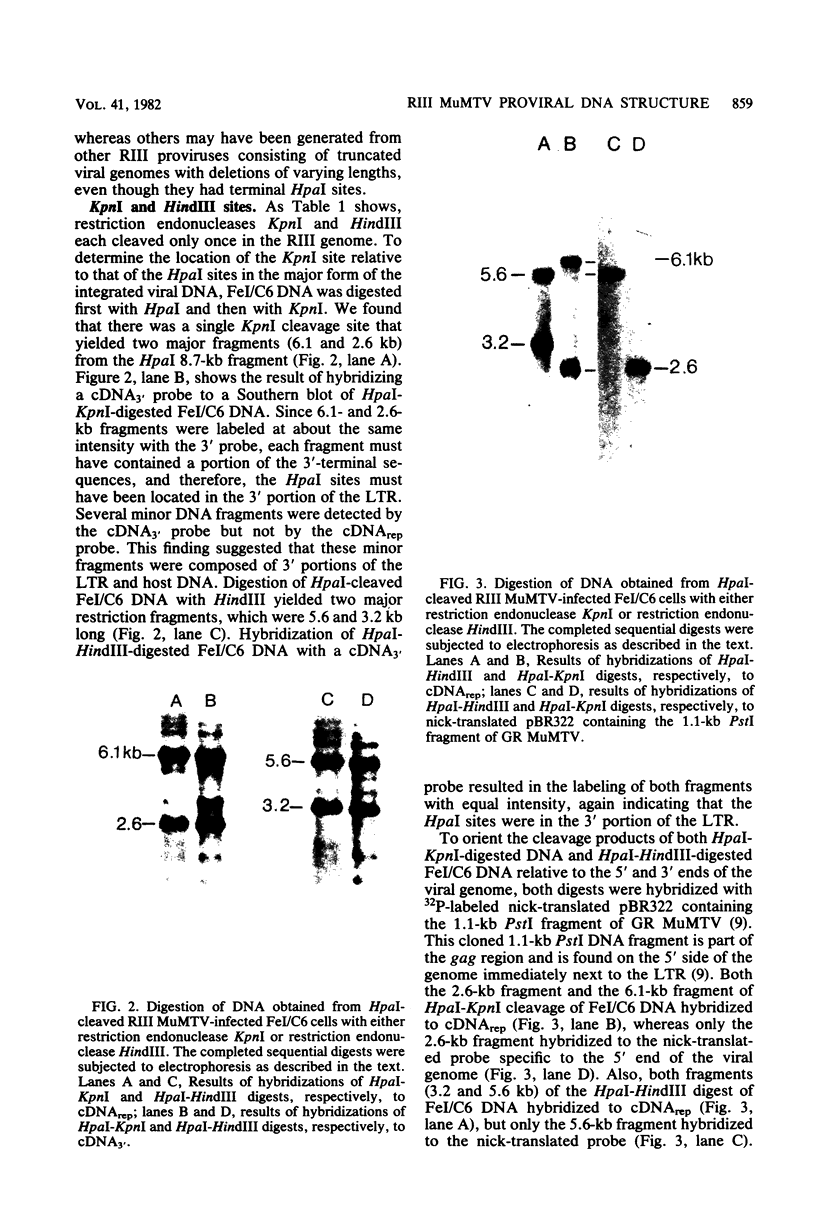
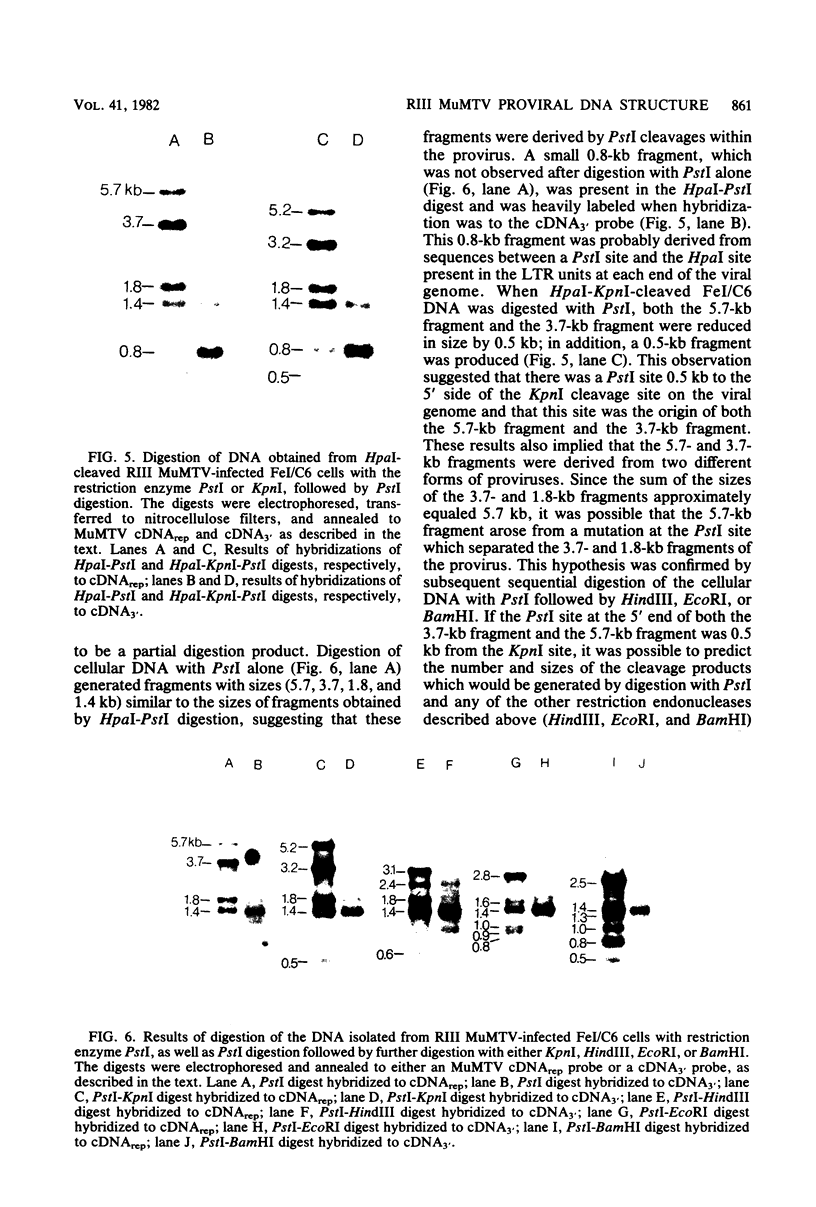
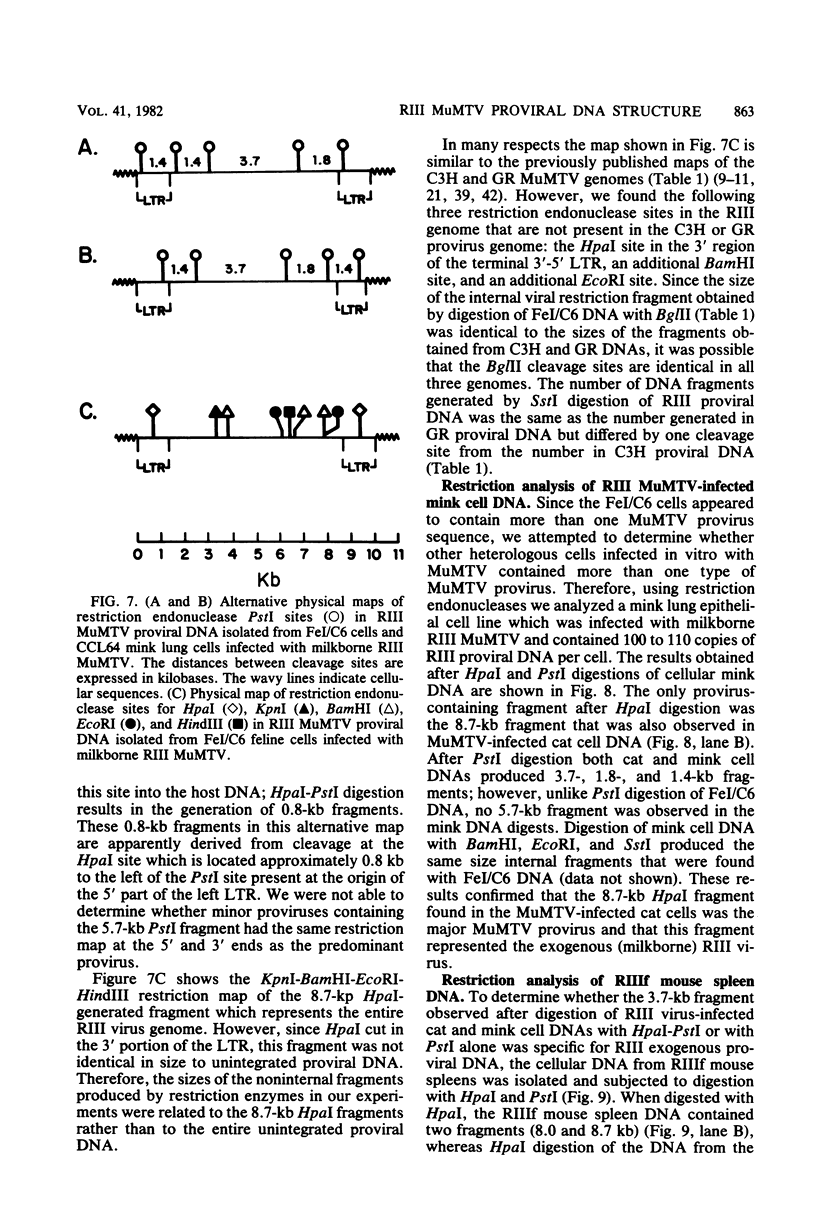
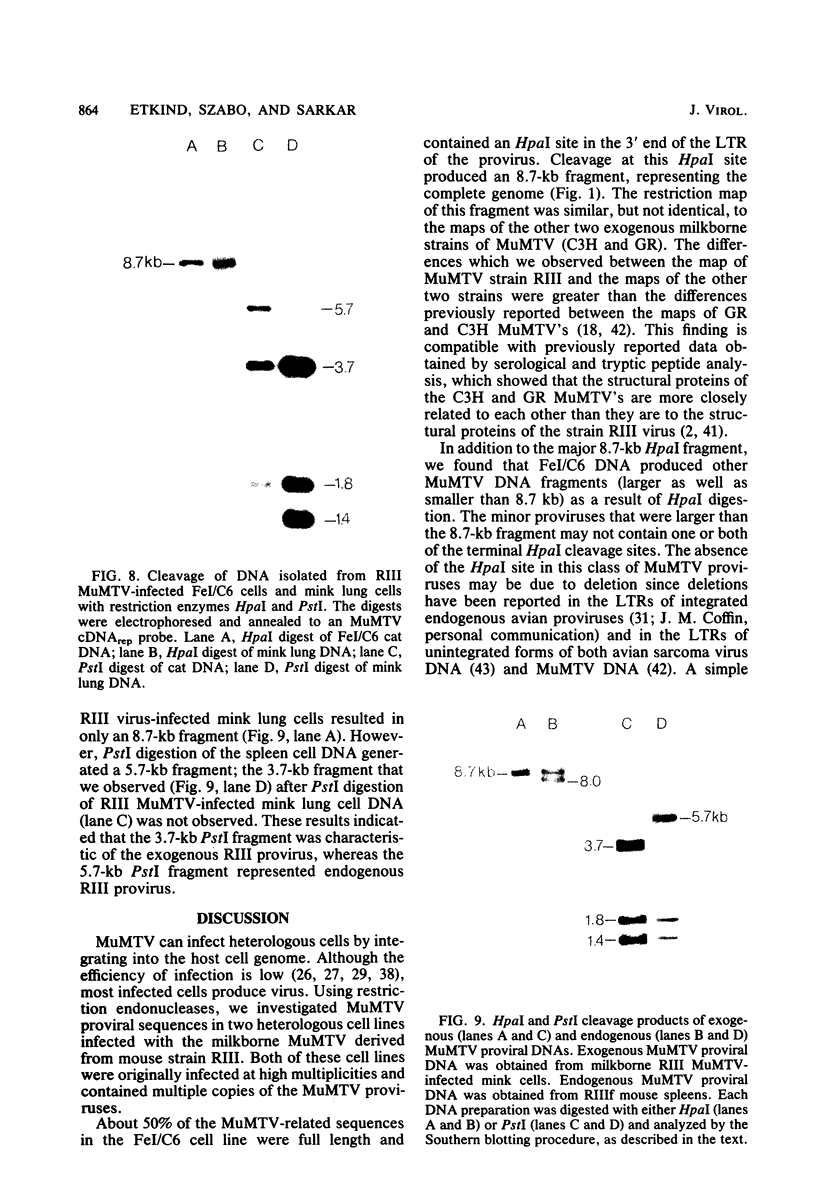
Cellular DNA containing integrated murine mammary tumor virus (MuMTV) was isolated from FeI/C6 feline kidney cells and CCL64 mink lung cells infected with milkborne RIII MuMTV. By using restriction enzyme HpaI, intact RIII MuMTV provirus (length, 8.7 kilobases [kb]) was excised from the cellular DNA. Subsequent restriction endonuclease analysis of this HpaI fragment with KpnI, HindIII, EcoRI, BamHI, BglII, PstI, SstI, SalI, and XhoI enabled us to construct a map of the RIII virus genome. A comparison of this map with the maps of the GR and C3H MuMTV's revealed that there are greater sequence differences between the RIII virus and the GR and C3H MuMTV proviruses than there are between the GR and C3H proviruses. The following are features of the restriction map unique to the RIII provirus: the presence of three BamHI and two EcoRI cleavage sites, a HpaI cleavage site in the terminal 3′-5′ repeat unit of the provirus, and the absence of an XhoI cleavage site. Another distinguishing feature of the RIII provirus is that the sizes of some of the restriction fragments produced by cleavage of the RIII provirus with PstI are different from the sizes of the fragments obtained by PstI cleavage of the GR and C3H proviruses. Like the GR proviral DNA, the RIII proviral DNA has three SstI (SacI) cleavage sites, whereas the C3H provirus has only two SstI sites. HpaI digestion of MuMTV-infected mink lung cell DNA revealed only one class of provirus (an 8.7-kb fragment); however, we observed several minor classes of RIII proviral DNA in addition to the major class of provirus DNA in infected cat kidney cells. PstI digestion of the HpaI 8.7-kb fragments from both feline and mink cells generated a 3.7-kb DNA fragment identical in size to a PstI-generated fragment that has been found in GR and C3H milkborne virus-infected cells. Although a fragment similar in size to the milkborne 3.7-kb PstI fragment has been found as an endogenous component in many C3H and GR mouse tissues, we did not observe such an endogenous fragment in the RIII mouse strain. Therefore, the 3.7-kb fragment may be useful as a marker for the milkborne RIII MuMTV provirus in RIII mice.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bentvelzen P., Daams J. H., Hageman P., Calafat J. Genetic transmission of viruses that incite mammary tumor in mice. Proc Natl Acad Sci U S A. 1970 Sep;67(1):377–384. doi: 10.1073/pnas.67.1.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentvelzen P. Host-virus interactions in murine mammary carcinogenesis. Biochim Biophys Acta. 1974 Dec 31;355(3-4):236–259. doi: 10.1016/0304-419x(74)90012-2. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Retroviruses. Annu Rev Biochem. 1978;47:35–88. doi: 10.1146/annurev.bi.47.070178.000343. [DOI] [PubMed] [Google Scholar]
- Bittner J. J. SOME POSSIBLE EFFECTS OF NURSING ON THE MAMMARY GLAND TUMOR INCIDENCE IN MICE. Science. 1936 Aug 14;84(2172):162–162. doi: 10.1126/science.84.2172.162. [DOI] [PubMed] [Google Scholar]
- Botchan M., Topp W., Sambrook J. The arrangement of simian virus 40 sequences in the DNA of transformed cells. Cell. 1976 Oct;9(2):269–287. doi: 10.1016/0092-8674(76)90118-5. [DOI] [PubMed] [Google Scholar]
- Buetti E., Diggelmann H. Cloned mouse mammary tumor virus DNA is biologically active in transfected mouse cells and its expression is stimulated by glucocorticoid hormones. Cell. 1981 Feb;23(2):335–345. doi: 10.1016/0092-8674(81)90129-x. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Majors J. E., Varmus H. E. Organization of mouse mammary tumor virus-specific DNA endogenous to BALB/c mice. J Virol. 1979 Nov;32(2):483–496. doi: 10.1128/jvi.32.2.483-496.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Endogenous mammary tumour virus DNA varies among wild mice and segregates during inbreeding. Nature. 1979 Mar 29;278(5703):418–423. doi: 10.1038/278418a0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crandell R. A., Fabricant C. G., Nelson-Rees W. A. Development, characterization, and viral susceptibility of a feline (Felis catus) renal cell line (CRFK). In Vitro. 1973 Nov-Dec;9(3):176–185. doi: 10.1007/BF02618435. [DOI] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Dudley J. P., Rosen J. M., Butel J. S. Differential expression of poly(A)-adjacent sequences of mammary tumor virus RNA in murine mammary cells. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5797–5801. doi: 10.1073/pnas.75.12.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duesberg P. H., Vogt P. K. RNA species obtained from clonal lines of avian sarcoma and from avian leukosis virus. Virology. 1973 Jul;54(1):207–219. doi: 10.1016/0042-6822(73)90130-x. [DOI] [PubMed] [Google Scholar]
- Fanning T. G., Puma J. P., Cardiff R. D. Selective amplification of mouse mammary tumor virus in mammary tumors of GR mice. J Virol. 1980 Oct;36(1):109–114. doi: 10.1128/jvi.36.1.109-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich R., Morris V. L., Goodman H. M., Bishop J. M., Varmus H. E. Differences between genomes of two strains of mouse mammary tumor virus as shown by partial RNA sequence analysis. Virology. 1976 Jul 15;72(2):330–340. doi: 10.1016/0042-6822(76)90162-8. [DOI] [PubMed] [Google Scholar]
- Gautsch J. W., Lerner R., Howard D., Teramoto Y. A., Schlom J. Strain-specific markers for the major structural proteins of highly oncogenic murine mammary tumor viruses by tryptic peptide analyses. J Virol. 1978 Sep;27(3):688–699. doi: 10.1128/jvi.27.3.688-699.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulian M. Incorporation of oligodeoxynucleotides into DNA. Proc Natl Acad Sci U S A. 1968 Sep;61(1):284–291. doi: 10.1073/pnas.61.1.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Buetti E., Diggelmann H., Hynes N. E. Characterization of endogenous and exogenous mouse mammary tumor virus proviral DNA with site-specific molecular clones. J Virol. 1980 Dec;36(3):734–745. doi: 10.1128/jvi.36.3.734-745.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helling R. B., Goodman H. M., Boyer H. W. Analysis of endonuclease R-EcoRI fragments of DNA from lambdoid bacteriophages and other viruses by agarose-gel electrophoresis. J Virol. 1974 Nov;14(5):1235–1244. doi: 10.1128/jvi.14.5.1235-1244.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heston W. E., Parks W. P. Mammary tumors and mammary tumor virus expression in hybrid mice of strains C57BL and GR. J Exp Med. 1977 Nov 1;146(5):1206–1220. doi: 10.1084/jem.146.5.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Howard D. K., Colcher D., Teramoto Y. A., Young J. M., Schlom J. Characterization of mouse mammary tumor viruses propagated in heterologous cells. Cancer Res. 1977 Aug;37(8 Pt 1):2696–2704. [PubMed] [Google Scholar]
- Howard D. K., Schlom J. Isolation of host-range variants of mouse mammary tumor viruses that efficiently infect cells in vitro. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5718–5722. doi: 10.1073/pnas.75.11.5718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes S. H., Shank P. R., Spector D. H., Kung H. J., Bishop J. M., Varmus H. E., Vogt P. K., Breitman M. L. Proviruses of avian sarcoma virus are terminally redundant, co-extensive with unintegrated linear DNA and integrated at many sites. Cell. 1978 Dec;15(4):1397–1410. doi: 10.1016/0092-8674(78)90064-8. [DOI] [PubMed] [Google Scholar]
- Lasfargues E. Y., Lasfargues J. C., Dion A. S., Greene A. E., Moore D. H. Experimental infection of a cat kidney cell line with the mouse mammary tumor virus. Cancer Res. 1976 Jan;36(1):67–72. [PubMed] [Google Scholar]
- Marcus S. L., Kopelman R., Sarkar N. H. Simultaneous purification of murine mammary tumor virus structural proteins: analysis of antigenic reactivities of native gp34 by radioimmunocompetition assays. J Virol. 1979 Aug;31(2):341–349. doi: 10.1128/jvi.31.2.341-349.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClements W., Hanafusa H., Tilghman S., Skalka A. Structural studies on oncornavirus-related sequences in chicken genomic DNA: two-step analyses of EcoRI and Bgl I restriction digests and tentative mapping of a ubiquitous endogenous provirus digests and tentative mapping of a ubiquitous endogenous provirus. Proc Natl Acad Sci U S A. 1979 May;76(5):2165–2169. doi: 10.1073/pnas.76.5.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Schlom J. Relationship in nucleic acid sequences between mouse mammary tumor virus variants. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4635–4639. doi: 10.1073/pnas.72.11.4635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Holben J. A., Charney J. Biologic characteristics of some mouse mammary tumor viruses. J Natl Cancer Inst. 1976 Oct;57(4):889–896. doi: 10.1093/jnci/57.4.889. [DOI] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Blair P. B., Bishop J. M., Varmus H. E. Nucleotide sequence homologies among mouse mammary tumor viruses. Virology. 1976 Apr;70(2):550–553. doi: 10.1016/0042-6822(76)90297-x. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Cardiff R. D., Varmus H. E., Yamamoto K. R. Infection of cultured rat hepatoma cells by mouse mammary tumor virus. Cell. 1977 Jan;10(1):11–18. doi: 10.1016/0092-8674(77)90134-9. [DOI] [PubMed] [Google Scholar]
- Ringold G. M., Shank P. R., Varmus H. E., Ring J., Yamamoto K. R. Integration and transcription of mouse mammary tumor virus DNA in rat hepatoma cells. Proc Natl Acad Sci U S A. 1979 Feb;76(2):665–669. doi: 10.1073/pnas.76.2.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schochetman G., Arthur L. O., Long C. W., Massey R. J. Mice with spontaneous mammary tumors develop type-specific neutralizing and cytotoxic antibodies against the mouse mammary tumor virus envelope protein gp52. J Virol. 1979 Oct;32(1):131–139. doi: 10.1128/jvi.32.1.131-139.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Cohen J. C., Varmus H. E., Yamamoto K. R., Ringold G. M. Mapping of linear and circular forms of mouse mammary tumor virus DNA with restriction endonucleases: evidence for a large specific deletion occurring at high frequency during circularization. Proc Natl Acad Sci U S A. 1978 May;75(5):2112–2116. doi: 10.1073/pnas.75.5.2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shank P. R., Hughes S. H., Kung H. J., Majors J. E., Quintrell N., Guntaka R. V., Bishop J. M., Varmus H. E. Mapping unintegrated avian sarcoma virus DNA: termini of linear DNA bear 300 nucleotides present once or twice in two species of circular DNA. Cell. 1978 Dec;15(4):1383–1395. doi: 10.1016/0092-8674(78)90063-6. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Swanstrom R., Shank P. R. X-Ray Intensifying Screens Greatly Enhance the Detection by Autoradiography of the Radioactive Isotopes 32P and 125I. Anal Biochem. 1978 May;86(1):184–192. doi: 10.1016/0003-2697(78)90333-0. [DOI] [PubMed] [Google Scholar]
- Teramoto Y. A., Kufe D., Schlom J. Multiple antigenic determinants on the major surface glycoprotein of murine mammary tumor viruses. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3564–3568. doi: 10.1073/pnas.74.8.3564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teramoto Y. A., Schlom J. Radioimmunoassays that demonstrate type-specific and group-specific antigenic reactivities for the major internal structural protein of murine mammary tumor viruses. Cancer Res. 1978 Jul;38(7):1990–1995. doi: 10.1203/00006450-199501000-00002. [DOI] [PubMed] [Google Scholar]
- Vaidya A. B., Lasfargues E. Y., Heubel G., Lasfargues J. C., Moore D. H. Murine mammary tumor virus: characterization of infection of nonmurine cells. J Virol. 1976 Jun;18(3):911–917. doi: 10.1128/jvi.18.3.911-917.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis G., Chopra H. C., Sawdon M. R. Induction of mammary tumors in C57BL/HAAG female mice by a low oncogenic mammary tumor virus. Int J Cancer. 1978 May 15;21(5):648–651. doi: 10.1002/ijc.2910210516. [DOI] [PubMed] [Google Scholar]
- Vogt P. K. Spontaneous segregation of nontransforming viruses from cloned sarcoma viruses. Virology. 1971 Dec;46(3):939–946. doi: 10.1016/0042-6822(71)90092-4. [DOI] [PubMed] [Google Scholar]