Abstract
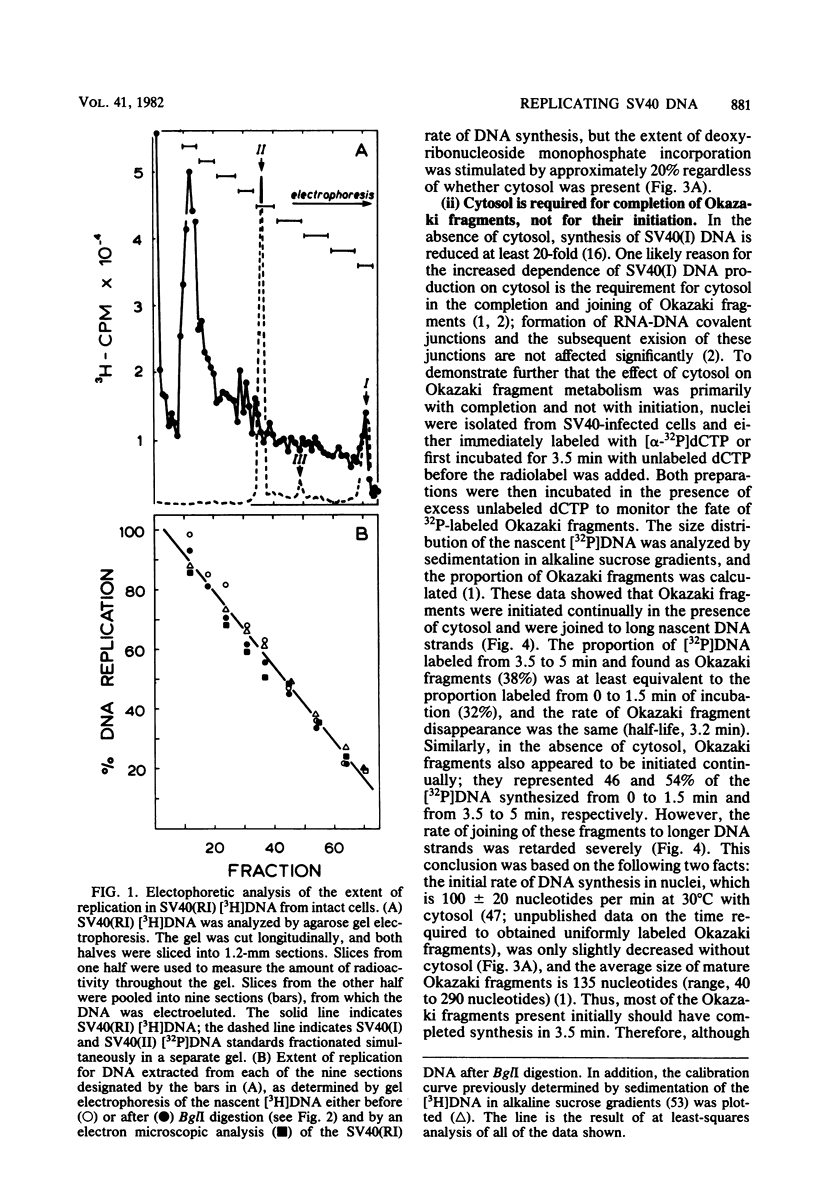
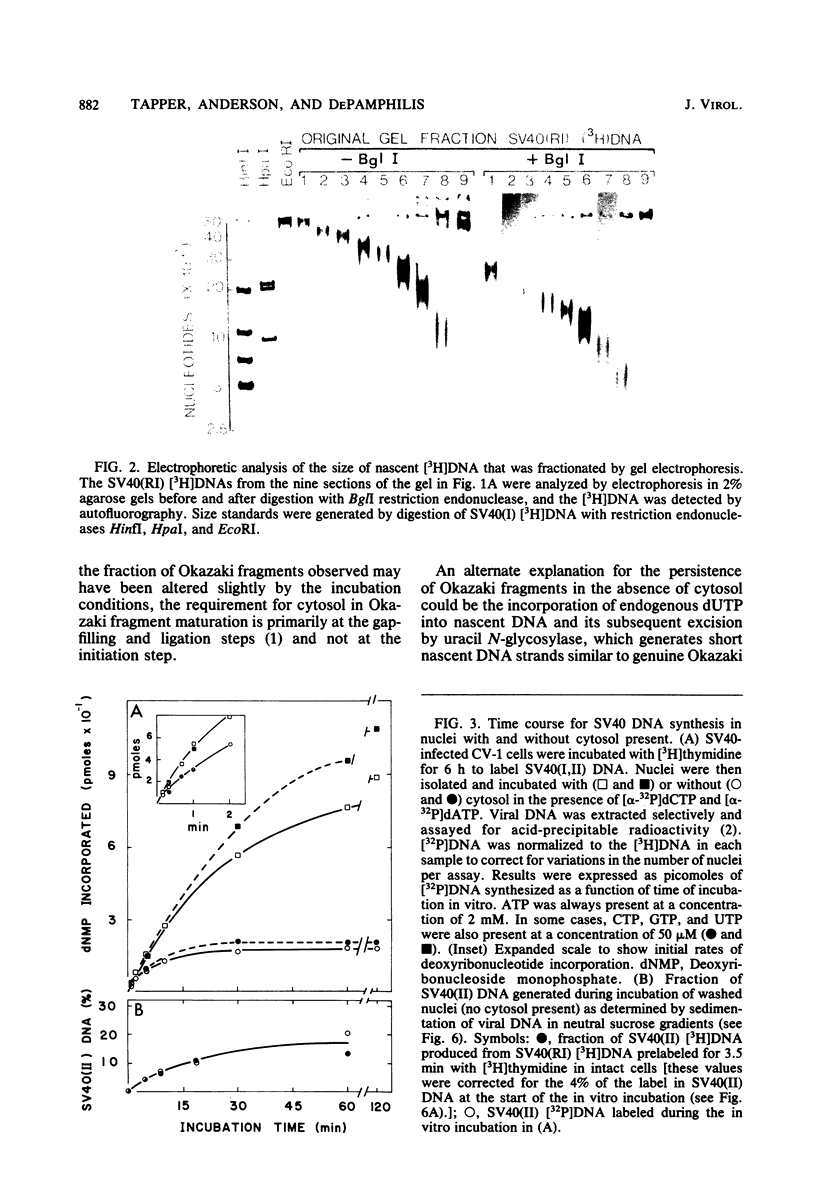
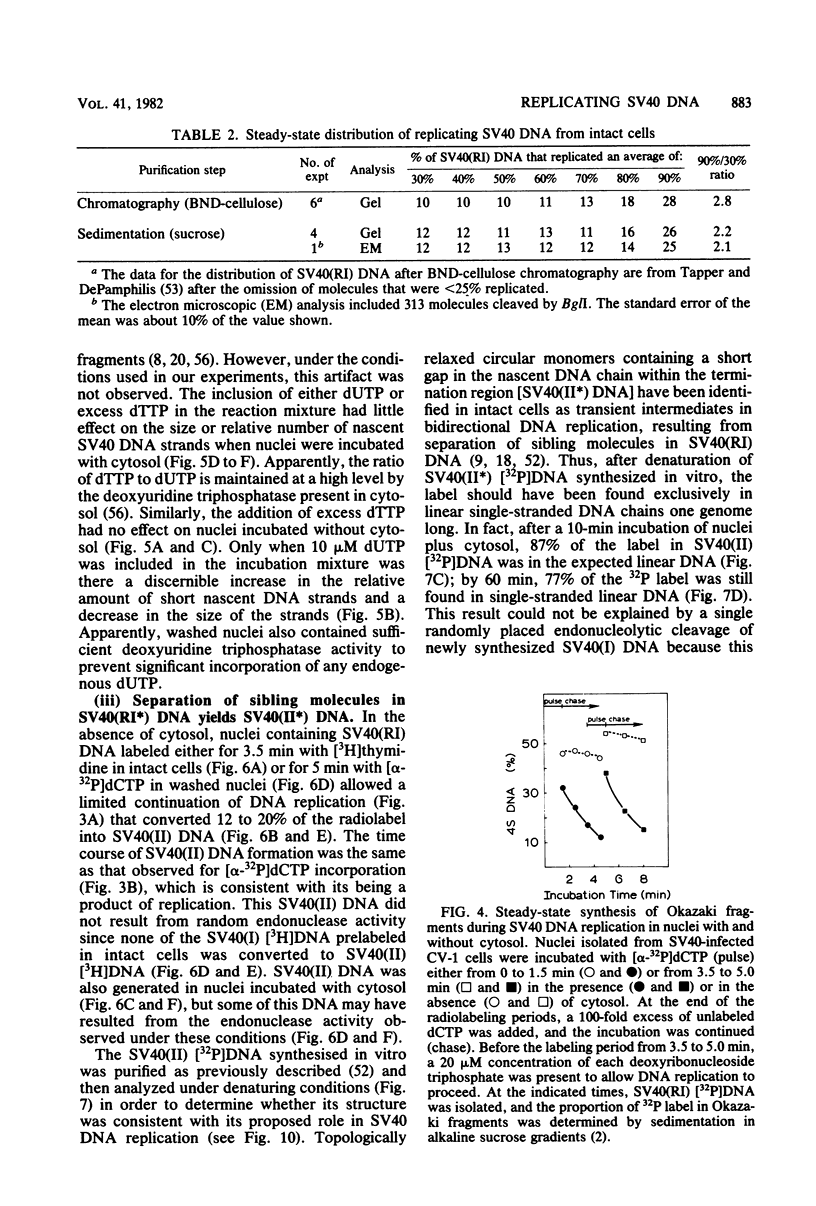
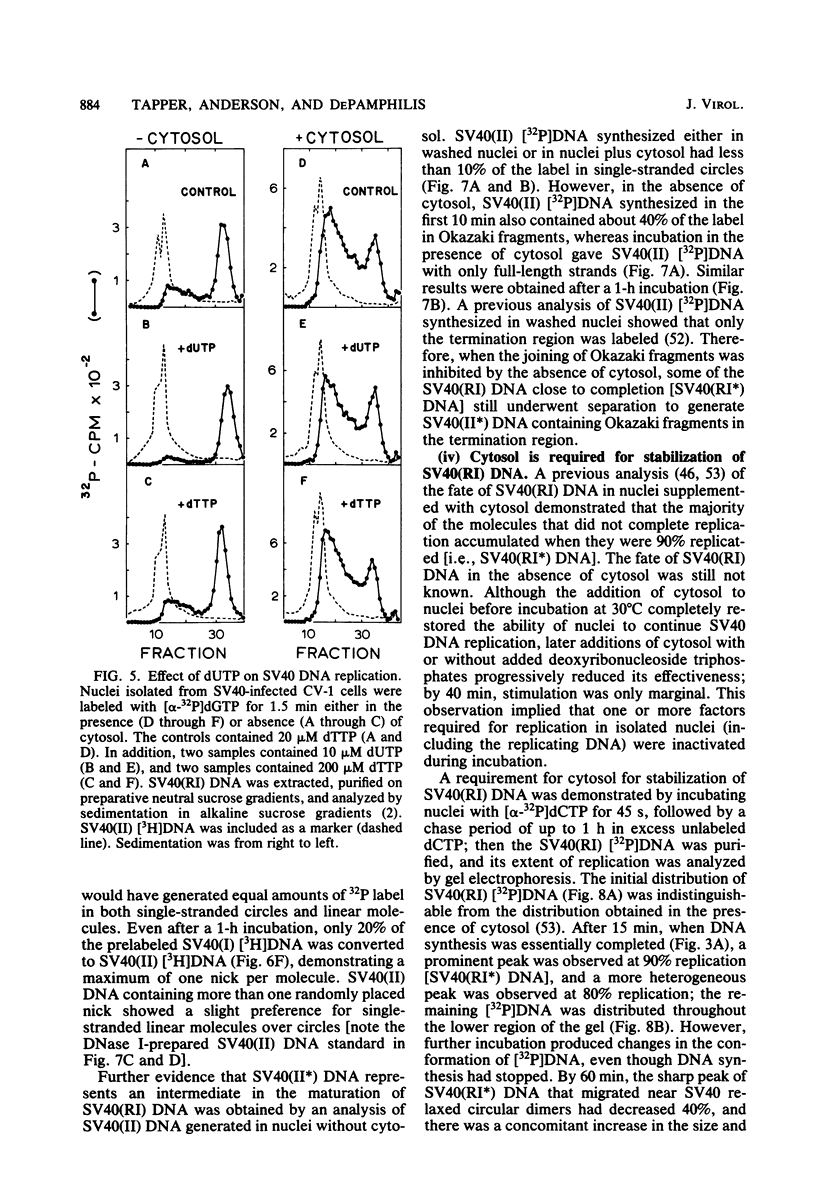
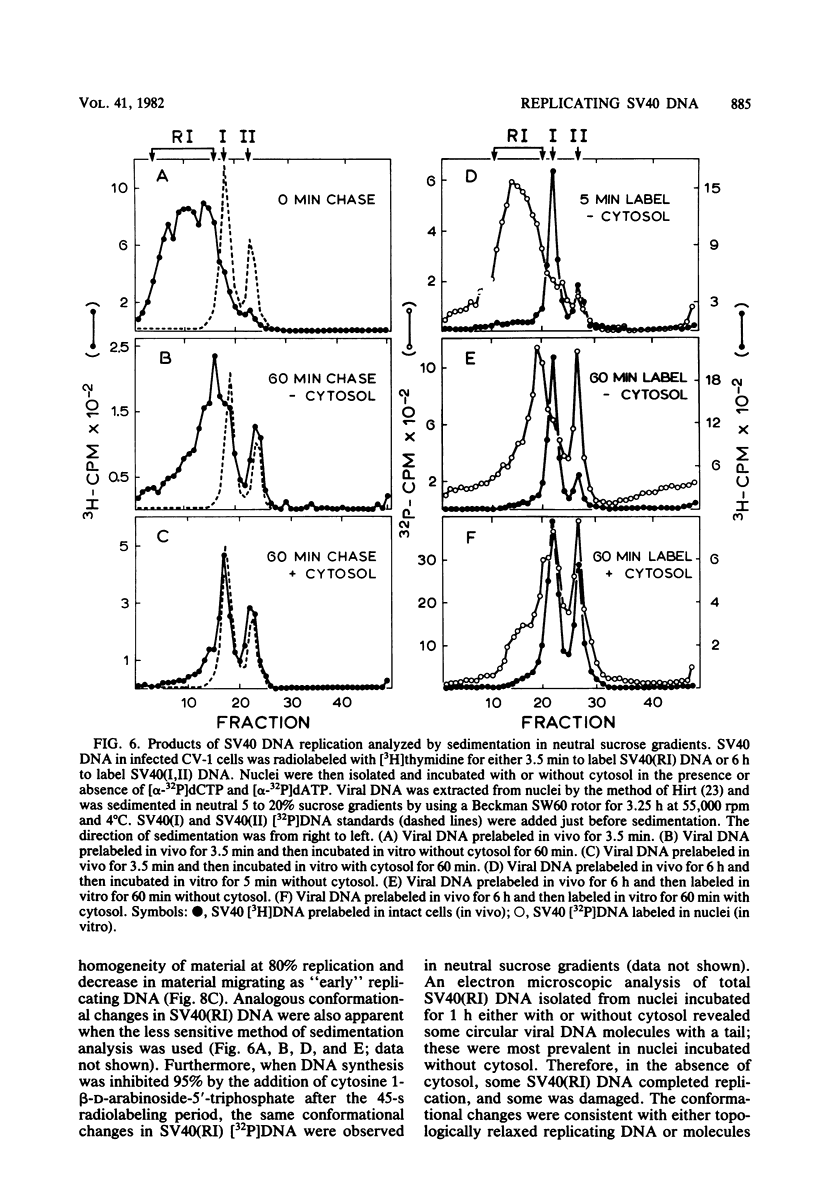
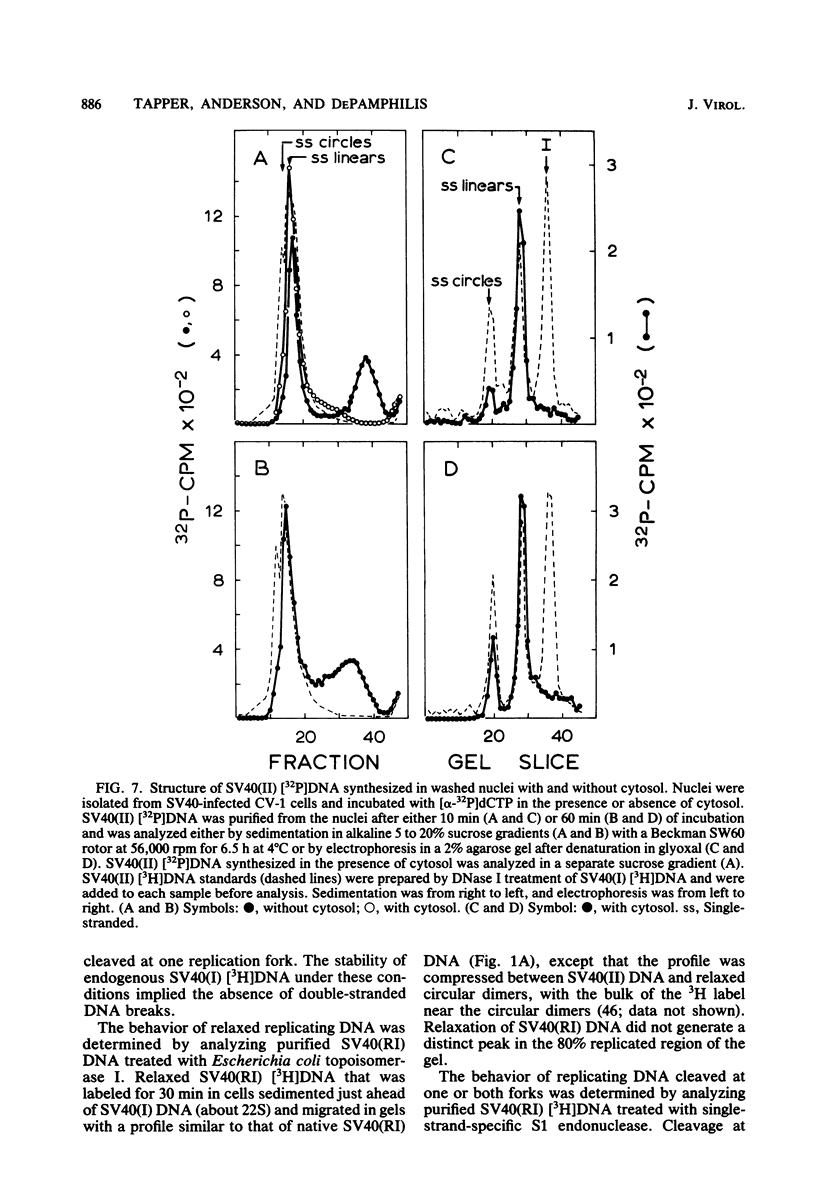
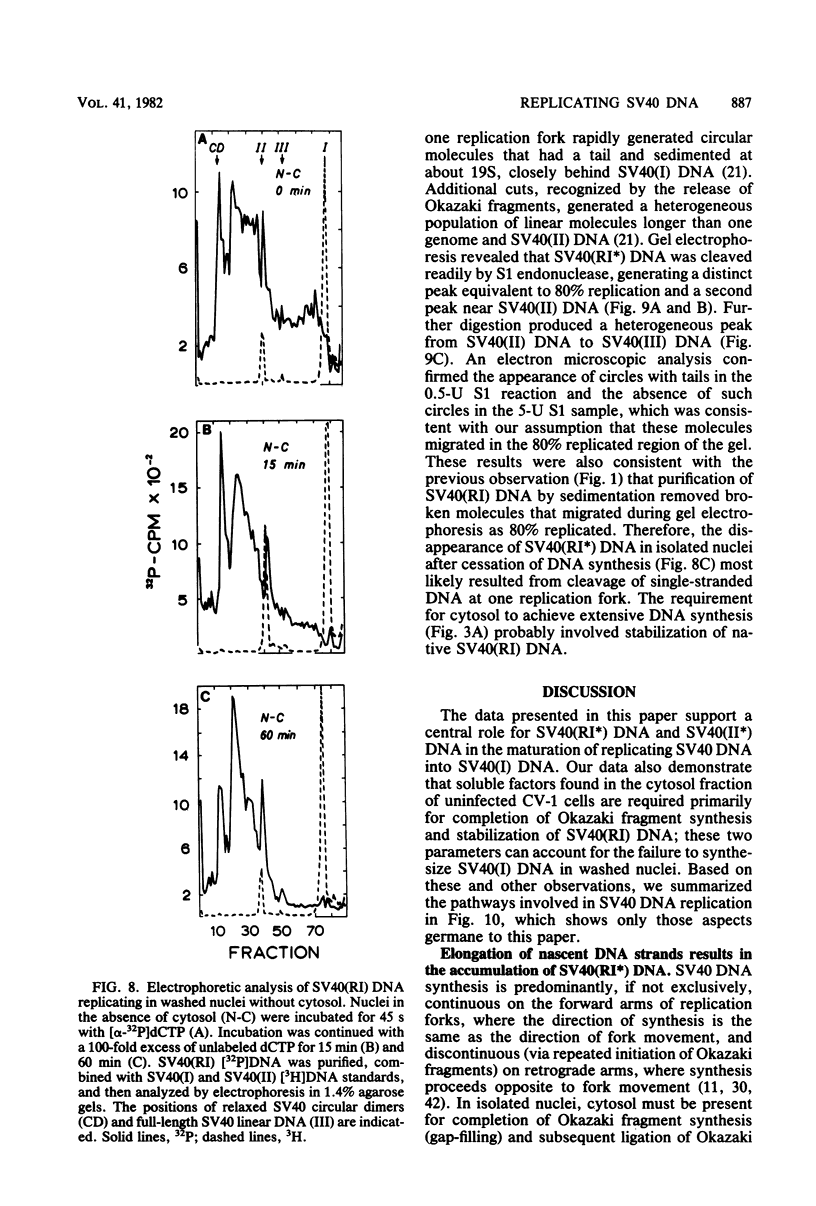
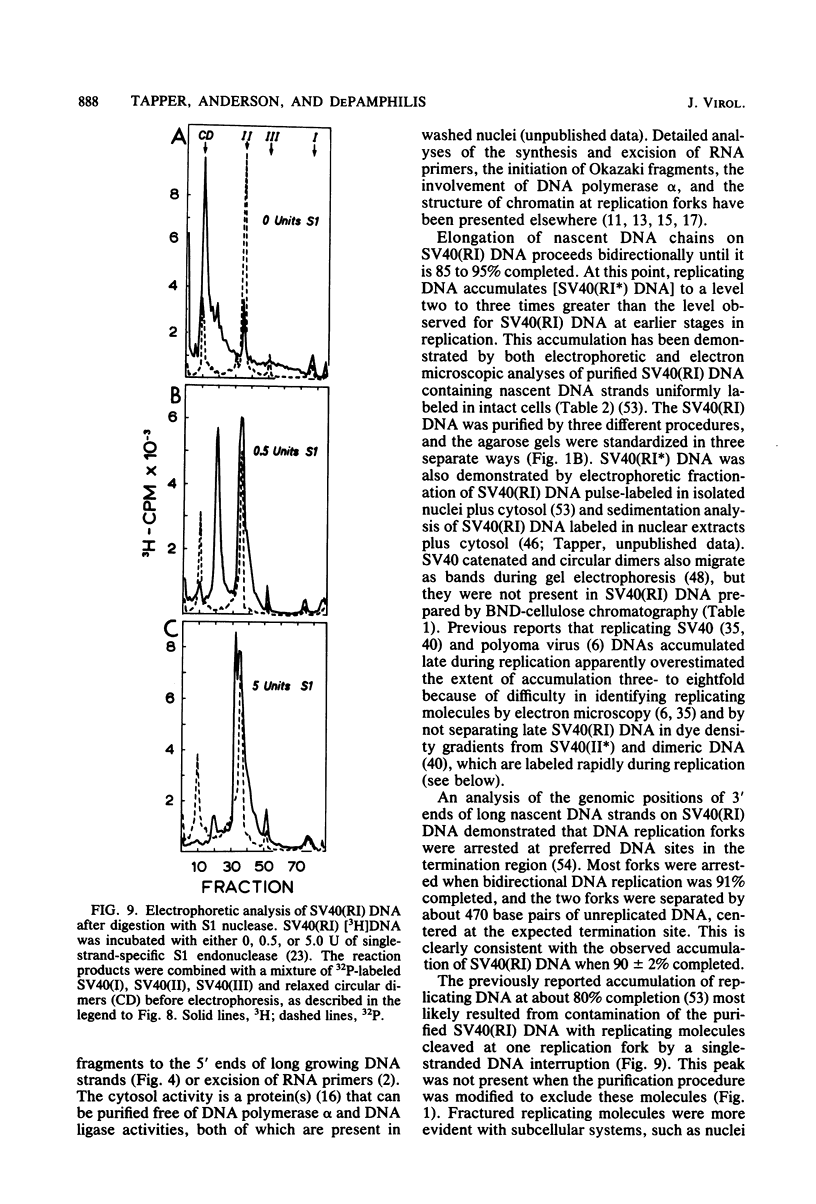
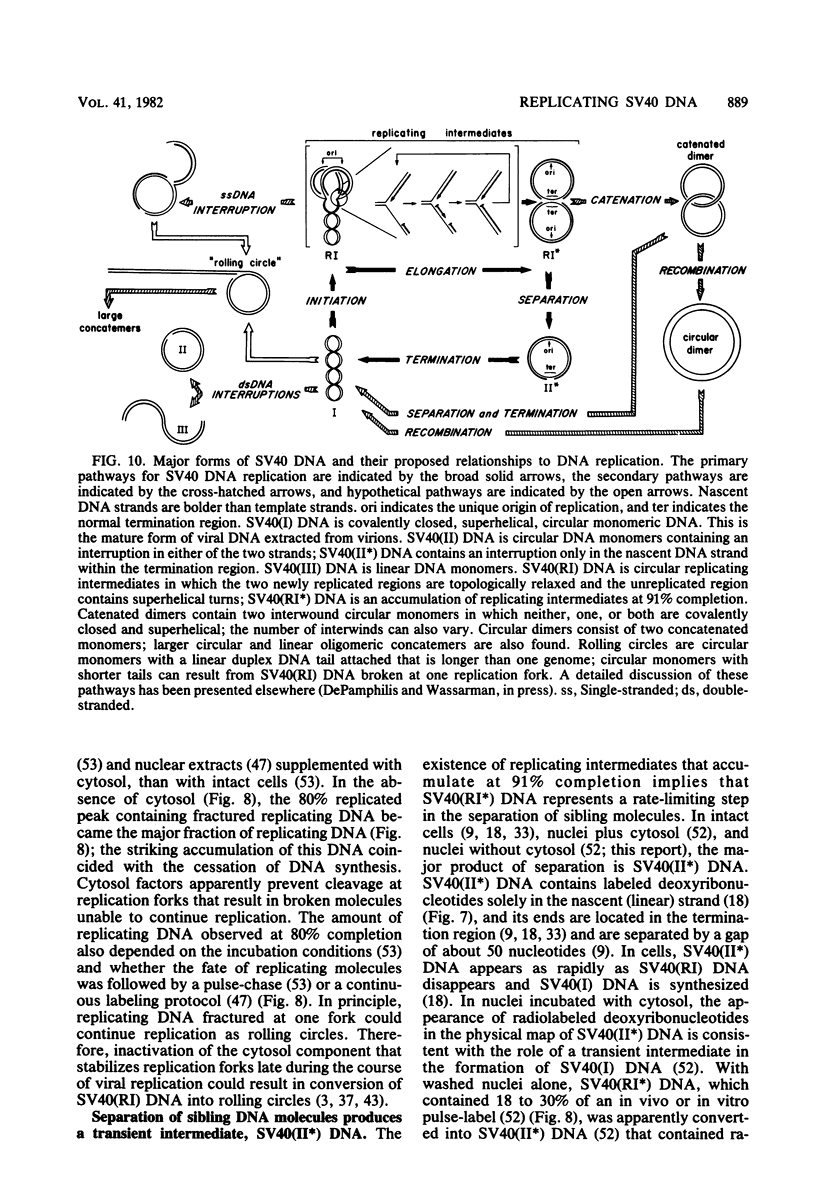
The maturation of replicating simian virus 40 (SV40) chromosomes into superhelical viral DNA monomers [SV40(I) DNA] was analyzed in both intact cells and isolated nuclei to investigate further the role of soluble cytosol factors in subcellular systems. Replicating intermediates [SV40(RI) DNA] were purified to avoid contamination by molecules broken at their replication forks, and the distribution of SV40(RI) DNA as a function of its extent of replication was analyzed by gel electrophoresis and electron microscopy. With virus-infected CV-1 cells, SV40(RI) DNA accumulated only when replication was 85 to 95% completed. These molecules [SV40(RI*) DNA] were two to three times more prevalent than an equivalent sample of early replicating DNA, consistent with a rate-limiting step in the separation of sibling chromosomes. Nuclei isolated from infected cells permitted normal maturation of SV40(RI) DNA into SV40(I) DNA when the preparation was supplemented with cytosol. However, in the absence of cytosol, the extent of DNA synthesis was diminished three- to fivefold (regardless of the addition of ribonucleotide triphosphates), with little change in the rate of synthesis during the first minute; also, the joining of Okazaki fragments to long nascent DNA was inhibited, and SV40(I) DNA was not formed. The fraction of short-nascent DNA chains that may have resulted from dUTP incorporation was insignificant in nuclei with or without cytosol. Pulse-chase experiments revealed that joining, but not initiation, of Okazaki fragments required cytosol. Cessation of DNA synthesis in nuclei without cytosol could be explained by an increased probability for cleavage of replication forks. These broken molecules masqueraded during gel electrophoresis of replicating DNA as a peak of 80% completed SV40(RI) DNA. Failure to convert SV40(RI*) DNA into SV40(I) DNA under these conditions could be explained by the requirement for cytosol to complete the gap-filling step in Okazaki fragment metabolism: circular monomers with their nascent DNA strands interrupted in the termination region [SV40(II*) DNA] accumulated with unjoined Okazaki fragments. Thus, separation of sibling chromosomes still occurred, but gaps remained in the terminal portions of their daughter DNA strands. These and other data support a central role for SV40(RI*) and SV40(II*) DNAs in the completion of viral DNA replication.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., DePamphilis M. L. Metabolism of Okazaki fragments during simian virus 40 DNA replication. J Biol Chem. 1979 Nov 25;254(22):11495–11504. [PubMed] [Google Scholar]
- Anderson S., Kaufman G., DePamphilis M. L. RNA primers in SV40 DNA replication: identification of transient RNA-DNA covalent linkages in replicating DNA. Biochemistry. 1977 Nov 15;16(23):4990–4998. doi: 10.1021/bi00642a009. [DOI] [PubMed] [Google Scholar]
- Bjursell G. Effects of 2'-deoxy-2'-azidocytidine on polyoma virus DNA replication: evidence for rolling circle-type mechanism. J Virol. 1978 Apr;26(1):136–142. doi: 10.1128/jvi.26.1.136-142.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjursell G., Magnusson G. Replication of polyoma DNA accumlation of early replicative intermediates during hydroxyurea inhibition. Virology. 1976 Oct 1;74(1):249–251. doi: 10.1016/0042-6822(76)90149-5. [DOI] [PubMed] [Google Scholar]
- Bjursell G., Munck V., Therkelsen A. J. Polyoma DNA synthesis in isolated nuclei: evidence for defective replication forks. J Virol. 1979 Jun;30(3):929–932. doi: 10.1128/jvi.30.3.929-932.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgaux P., Bourgaux-Ramoisy D., Seiler P. The replication of the ring-shaped DNA of polyoma virus. II. Identification of molecules at various stages of replication. J Mol Biol. 1971 Jul 14;59(1):195–206. doi: 10.1016/0022-2836(71)90421-9. [DOI] [PubMed] [Google Scholar]
- Brockman W. W., Gutai M. W., Nathans D. Evolutionary variants of simian virus 40: characterization of cloned complementing variants. Virology. 1975 Jul;66(1):36–52. doi: 10.1016/0042-6822(75)90177-4. [DOI] [PubMed] [Google Scholar]
- Brynolf K., Eliasson R., Reichard P. Formation of Okazaki fragments in polyoma DNA synthesis caused by misincorporation of uracil. Cell. 1978 Mar;13(3):573–580. doi: 10.1016/0092-8674(78)90330-6. [DOI] [PubMed] [Google Scholar]
- Chen M. C., Birkenmeier E., Salzman N. P. Simian virus 40 DNA replication: characterization of gaps in the termination region. J Virol. 1976 Feb;17(2):614–621. doi: 10.1128/jvi.17.2.614-621.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crémisi C. Chromatin replication revealed by studies of animal cells and papovaviruses (simian virus 40 and polyoma virus). Microbiol Rev. 1979 Sep;43(3):297–319. doi: 10.1128/mr.43.3.297-319.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick M. E., Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: prenucleosomal deoxyribonucleic acid is rapidly excised from replicating simian virus 40 chromosomes by micrococcal nuclease. Biochemistry. 1981 Nov 10;20(23):6648–6658. doi: 10.1021/bi00526a020. [DOI] [PubMed] [Google Scholar]
- DePamphilis M. L., Beard P., Berg P. Synthesis of Superhelical Simian Virus 40 Deoxyribonucleic Acid in Cell Lysates*. J Biol Chem. 1975 Jun 10;250(11):4340–4347. [PubMed] [Google Scholar]
- DePamphilis M. L., Berg P. Requirement of a Cytoplasmic Fraction for Synthesis of SV40 Deoxyribonucleic Acid in Isolated Nuclei*. J Biol Chem. 1975 Jun 10;250(11):4348–4354. [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Fareed G. C., McKerlie M. L., Salzman N. P. Characterization of Simian virus 40 DNA component II during viral DNA replication. J Mol Biol. 1973 Feb 25;74(2):95–111. doi: 10.1016/0022-2836(73)90101-0. [DOI] [PubMed] [Google Scholar]
- Goff S. P., Berg P. Structure and formation of circular dimers of simian virus 40 DNA. J Virol. 1977 Oct;24(1):295–302. doi: 10.1128/jvi.24.1.295-302.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafstrom R. H., Tseng B. Y., Goulian M. The incorporation of uracil into animal cell DNA in vitro. Cell. 1978 Sep;15(1):131–140. doi: 10.1016/0092-8674(78)90089-2. [DOI] [PubMed] [Google Scholar]
- Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: Okazaki fragments released from replicating SV40 chromosomes by single-strand specific endonucleases are not in nucleosomes. Biochemistry. 1979 Oct 16;18(21):4563–4571. doi: 10.1021/bi00588a017. [DOI] [PubMed] [Google Scholar]
- Herman T. M., DePamphilis M. L., Wassarman P. M. Structure of chromatin at deoxyribonucleic acid replication forks: location of the first nucleosomes on newly synthesized simian virus 40 deoxyribonucleic acid. Biochemistry. 1981 Feb 3;20(3):621–630. doi: 10.1021/bi00506a027. [DOI] [PubMed] [Google Scholar]
- Hsieh T., Brutlag D. ATP-dependent DNA topoisonmerase from D. melanogaster reversibly catenates duplex DNA rings. Cell. 1980 Aug;21(1):115–125. doi: 10.1016/0092-8674(80)90119-1. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. DNA replication in SV40-infected cells. VI. The effect of cycloheximide on the formation of SV40 oligomeric DNA. Virology. 1972 May;48(2):373–379. doi: 10.1016/0042-6822(72)90047-5. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. DNA replication of SV40-infected cells. VII. Formation of SV40 catenated and circular dimers. J Mol Biol. 1973 Jan 10;73(2):199–212. doi: 10.1016/0022-2836(73)90323-9. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. J. Infection of primary African green monkey cells with SV40 monomeric and dimeric DNA. J Mol Biol. 1971 Nov 14;61(3):735–738. doi: 10.1016/0022-2836(71)90076-3. [DOI] [PubMed] [Google Scholar]
- Jaenisch R., Levine A. DNA replication in SV40-infected cells. V. Circular and catenated oligomers of SV40 DNA. Virology. 1971 Jun;44(3):480–493. doi: 10.1016/0042-6822(71)90361-8. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Anderson S., DePamphilis M. L. RNA primers in Simian virus 40 DNA replication. II. Distribution of 5' terminal oligoribonucleotides in nascent DNA. J Mol Biol. 1977 Nov 5;116(3):549–567. doi: 10.1016/0022-2836(77)90083-3. [DOI] [PubMed] [Google Scholar]
- Kaufmann G., Bar-Shavit R., DePamphilis M. L. Okazaki pieces grow opposite to the replication fork direction during simian virus 40 DNA replication. Nucleic Acids Res. 1978 Jul;5(7):2535–2545. doi: 10.1093/nar/5.7.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly T. J., Jr, Nathans D. The genome of simian virus 40. Adv Virus Res. 1977;21:85–173. doi: 10.1016/s0065-3527(08)60762-9. [DOI] [PubMed] [Google Scholar]
- Lai C. J., Nathans D. Non-specific termination of simian virus 40 DNA replication. J Mol Biol. 1975 Sep 5;97(1):113–118. doi: 10.1016/s0022-2836(75)80026-x. [DOI] [PubMed] [Google Scholar]
- Laipis P. J., Sen A., Levine A. J., Mulder C. DNA replication in SV40 infected cells X. The structure of the 16 S gap circle intermediate in SV40 DNA synthesis. Virology. 1975 Nov;68(1):115–123. doi: 10.1016/0042-6822(75)90153-1. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Enhanced autoradiographic detection of 32P and 125I using intensifying screens and hypersensitized film. FEBS Lett. 1977 Oct 15;82(2):314–316. doi: 10.1016/0014-5793(77)80609-1. [DOI] [PubMed] [Google Scholar]
- Levine A. J., Kang H. S., Billheimer F. E. DNA replication in SV40 infected cells. I. Analysis of replicating SV40 DNA. J Mol Biol. 1970 Jun 14;50(2):549–568. doi: 10.1016/0022-2836(70)90211-1. [DOI] [PubMed] [Google Scholar]
- Magnusson G., Nilsson M. G. Replication of polyoma DNA in isolated nuclei: analysis of replication fork movement. J Virol. 1979 Nov;32(2):386–393. doi: 10.1128/jvi.32.2.386-393.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M. A., Howley P. M., Byrne J. C., Garon C. F. Characterization of supercoiled oligomeric SV40 DNA molecules in productively infected cells. Virology. 1976 May;71(1):28–40. doi: 10.1016/0042-6822(76)90091-x. [DOI] [PubMed] [Google Scholar]
- Martin R. F. Analysis of polyoma virus DNA replicative intermediates by agarose gel electrophoresis. J Virol. 1977 Sep;23(3):827–832. doi: 10.1128/jvi.23.3.827-832.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin R. G., Setlow V. P. The initiation of SV40 DNA synthesis is not unique to the replication origin. Cell. 1980 Jun;20(2):381–391. doi: 10.1016/0092-8674(80)90624-8. [DOI] [PubMed] [Google Scholar]
- Mayer A., Levine A. J. DNA replication in SV40-infected cells. 8. The distribution of replicating molecules at different stages of replication in SV40-infected cells. Virology. 1972 Nov;50(2):328–338. doi: 10.1016/0042-6822(72)90384-4. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlman D., Huberman J. A. Asymmetric Okazaki piece synthesis during replication of simian virus 40 DNA in vivo. Cell. 1977 Dec;12(4):1029–1043. doi: 10.1016/0092-8674(77)90167-2. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Berg P. Does simian virus 40 DNA integrate into cellular DNA during productive infection? J Virol. 1978 Nov;28(2):475–489. doi: 10.1128/jvi.28.2.475-489.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Sebring E. D., Garon C. F., Salzman N. P. Superhelix density of replicating simian virus 40 DNA molecules. J Mol Biol. 1974 Dec 5;90(2):371–379. doi: 10.1016/0022-2836(74)90380-5. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. Simian virus 40 DNA replication in isolated replicating viral chromosomes. J Virol. 1978 Oct;28(1):53–65. doi: 10.1128/jvi.28.1.53-65.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundin O., Varshavsky A. Terminal stages of SV40 DNA replication proceed via multiply intertwined catenated dimers. Cell. 1980 Aug;21(1):103–114. doi: 10.1016/0092-8674(80)90118-x. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Synthesis of (alpha-32P) ribo- and deoxyribonucleoside 5'-triphosphates. Methods Enzymol. 1974;29:102–115. [PubMed] [Google Scholar]
- Tabak H. F., Flavell R. A. A method for the recovery of DNA from agarose gels. Nucleic Acids Res. 1978 Jul;5(7):2321–2332. doi: 10.1093/nar/5.7.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tack L. C., Wassarman P. M., DePamphilis M. L. Chromatin assembly. Relationship of chromatin structure to DNA sequence during simian virus 40 replication. J Biol Chem. 1981 Aug 25;256(16):8821–8828. [PubMed] [Google Scholar]
- Tapper D. P., Anderson S., DePamphilis M. L. Maturation of replicating simian virus 40 DNA molecules in isolated nuclei by continued bidirectional replication to the normal termination region. Biochim Biophys Acta. 1979 Nov 22;565(1):84–97. doi: 10.1016/0005-2787(79)90084-4. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Discontinuous DNA replication: accumulation of Simian virus 40 DNA at specific stages in its replication. J Mol Biol. 1978 Apr 15;120(3):401–422. doi: 10.1016/0022-2836(78)90427-8. [DOI] [PubMed] [Google Scholar]
- Tapper D. P., DePamphilis M. L. Preferred DNA sites are involved in the arrest and initiation of DNA synthesis during replication of SV40 DNA. Cell. 1980 Nov;22(1 Pt 1):97–108. doi: 10.1016/0092-8674(80)90158-0. [DOI] [PubMed] [Google Scholar]
- Wake C. T., Wilson J. H. Defined oligomeric SV40 DNA: a sensitive probe of general recombination in somatic cells. Cell. 1980 Aug;21(1):141–148. doi: 10.1016/0092-8674(80)90121-x. [DOI] [PubMed] [Google Scholar]
- Wist E., Unhjem O., Krokan H. Accumulation of small fragments of DNA in isolated HeLa cell nuclei due to transient incorporation of dUMP. Biochim Biophys Acta. 1978 Sep 27;520(2):253–270. doi: 10.1016/0005-2787(78)90225-3. [DOI] [PubMed] [Google Scholar]



