Abstract
Endocytic uptake and intracellular transport of acidic FGF was studied in cells transfected with FGF receptor 4 (FGFR4). Acidification of the cytosol to block endocytic uptake from coated pits did not inhibit endocytosis of the growth factor in COS cells transfected with FGFR4, indicating that it is to a large extent taken up by an alternative endocytic pathway. Fractionation of the cells demonstrated that part of the growth factor receptor was present in a low-density, caveolin-containing fraction, but we were unable to demonstrate binding to caveolin in immunoprecipitation studies. Upon treatment of the cells with acidic FGF, the activated receptor, together with the growth factor, moved to a juxtanuclear compartment, which was identified as the recycling endosome compartment. When the cells were lysed with Triton X-100, 3-([3-chloramidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate, or 2-octyl glucoside, almost all surface-exposed and endocytosed FGFR4 was solubilized, but only a minor fraction of the total FGFR4 in the cells was found in the soluble fraction. The data indicate that the major part of FGFR4 is anchored to detergent-insoluble structures, presumably cytoskeletal elements associated with the recycling endosome compartment.
INTRODUCTION
Acidic FGF (aFGF or FGF-1) belongs to the large FGF family of growth factors that play important roles in cell proliferation and differentiation (Burgess and Maciag, 1989; Crumley et al., 1991; Basilico and Moscatelli, 1992). The growth factors bind to transmembrane receptors with a cytoplasmic split tyrosine kinase domain. There are four identified FGF receptors (FGFRs) and a number of splicing variants (Johnson et al., 1990, 1991; Hou et al., 1991; Partanen et al., 1991; Chellaiah et al., 1994). Different FGFs bind with different strengths to the different variants of the receptors. In addition, FGFs bind to cell surface heparans (Burgess and Maciag, 1989) as well as to a cysteine-rich binding protein of unknown function (Burrus et al., 1992; Gonatas et al., 1995).
aFGF induces a strong mitogenic response in some cells, whereas in other cells it induces differentiation (Burgess and Maciag, 1989). It is not known what mechanism determines which response will be induced in each case. Upon binding of the growth factor to receptors, the tyrosine kinase is activated, apparently as a result of dimerization of the receptors by the bound growth factor (DiGabriele et al., 1998). Subsequently, a number of downstream signaling molecules are activated, such as phospholipase Cγ and MAPK (Mason, 1994). There is increasing evidence that externally added aFGF also acts intracellularly and that upon binding to the receptor the growth factor is able to penetrate cellular membranes and enter the cytosol and the nucleus (Imamura et al., 1990, 1994; Zhan et al., 1992, 1993; Wiedlocha et al., 1994; Muñoz et al., 1997; Klingenberg et al., 1998).
After binding to FGFR, aFGF is internalized by endocytosis (Muñoz et al., 1997). It has so far not been determined whether this occurs from coated pits or from other structures of the surface membrane. Keratinocyte growth factor, which belongs to the FGF family, was reported to be present in clathrin-coated vesicles in NIH 3T3 cells that had been transfected with the keratinocyte growth factor receptor, a splicing variant of FGFR2 (Marchese et al., 1998), whereas basic FGF was found in caveolae in BHK cells (Gleizes et al., 1996). All four FGFRs contain a sequence that resembles the binding site for caveolin found in the epidermal growth factor receptor and other proteins (Couet et al., 1997).
In spite of the large amount of work done on the FGFs and their receptors, little is known about the intracellular trafficking of these proteins. In the present work, we studied endocytosis and intracellular transport of aFGF and FGFR4.
MATERIALS AND METHODS
Materials
PMSF, pronase, trypsin, cytochalasin D, 2-octyl glucoside, 3-([3-chloramidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate (CHAPS), wortmannin, nocodazole, and transferrin were obtained from Sigma Chemical (St. Louis, MO). Brefeldin A was from Epicentre Technologies (Madison, WI), disuccinimidyl suberate was from Pierce (Rockford, IL), and protein A–Sepharose and heparin-Sepharose were from Pharmacia (Uppsala, Sweden). Na125I was obtained from the Radiochemical Center, Amersham International (Buckinghamshire, United Kingdom). Anti-FGFR4, anti-FGFR1, anti-clathrin, and anti-phosphotyrosine antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-caveolin 1 antibodies were from Transduction Laboratories (Lexington, KY), anti-vimentin antibodies were from Sigma Chemical, and rabbit anti-EEA1 was obtained from Dr. Judy Callaghan at the Institute for Cancer Research in Oslo, Norway. Anti-human CI-M6PR was a gift from Dr. K. von Figura (Göttingen, Germany), anti-β-cop was obtained from Affinity BioReagents (Golden, CO), anti-calnexin was from Stressgen Biotechnologies (Victoria, British Columbia, Canada), and anti-transferrin receptor and Fugene-6 were from Boehringer Mannheim (Indianapolis, IN). Unlabeled aFGF was produced in bacteria, purified on a heparin-Sepharose column as described (Wiedlocha et al., 1996), and labeled chemically with 125I (Fraker and Speck, 1978). aFGF metabolically labeled with [35S]methionine was synthesized in a cell-free system as described previously (Wiedlocha et al., 1994). aFGF labeled with CY3 and transferrin labeled with alexa were obtained by incubating the proteins with CY3 (Amersham International) and alexa (Molecular Probes, Eugene, OR) according to the procedures given by the suppliers. Transferrin receptor cDNA in pcDNA3 was a gift from Dr. Toril Bremnes (Institute for Cell Biology, University of Oslo, Oslo, Norway).
Cells
COS-1, NIH 3T3, and CPAE cells were propagated in DMEM with 10% (vol/vol) FCS in a 5% CO2 atmosphere at 37°C
Transfections
Transient expression of the different FGF receptors and human transferrin receptor was performed by transfecting COS-1 cells with plasmid DNA (pcDNA3 with appropriate inserts as described by Muñoz et al. [1997]) with the use of Fugene-6 transfection reagent according to the procedure given by the supplier. Cells were used for experiments 48 h after transfection.
Cross-Linking of 125I-aFGF to Receptors and Subsequent Purification of Caveolin-enriched Membrane Fractions
Caveolin-enriched membrane fractions were prepared with a detergent-free method, as described previously (Song et al., 1996). The cells (in two 150-mm dishes) were washed twice with ice-cold binding buffer (DMEM with 50 mM HEPES, pH 7.4, and 10 U/ml heparin), and then the cells were kept at 4°C for 4 h in the same buffer containing 50 ng/ml 125I-aFGF. After washing once with cold binding buffer and once with PBS, the cells were treated for 20 min at 4°C with 0.3 mM disuccinimidyl suberate in PBS. After cross-linking, the cells were washed with cold 25 mM Tris buffer, pH 7.4, and twice with PBS. The cells were scraped into 2.2 ml of 0.5 M sodium carbonate, pH 11. The cell suspension was homogenized first with a syringe, then with a Dounce homogenizer (20 strokes), and finally with a sonicator (three 20-s bursts). The homogenate was then adjusted to 45% sucrose by the addition of 2.2 ml of 90% sucrose prepared in 25 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 0.15 M NaCl and placed in the bottom of an ultracentrifuge tube. A discontinuous sucrose gradient was formed by overlaying this solution with 4 ml of 35% sucrose and 4 ml of 5% sucrose [both in 25 mM 2-(N-morpholino)ethanesulfonic acid, pH 6.5, 0.15 M NaCl]). The tubes were centrifuged at 150,000 × g in a SW40 rotor for 19 h at 4°C, and then 12 or 13 1-ml fractions were collected manually from the top of the gradient. Aliquots (50 μl) of the fractions were subjected to SDS-PAGE on 7.5 or 10% gels. The cross-linked ligand-receptor complexes were detected by autoradiography, and caveolin and the receptors were visualized by Western blotting after transfer of the protein from the gel to a nitrocellulose membrane.
Measurement of Receptor-mediated Endocytosis of 125I-aFGF and 125I-Transferrin
To measure endocytic uptake of transferrin in cells with acidified cytosol, COS cells transfected with FGFR4 were incubated for 5 min at 37°C in HEPES medium, pH 5.5, with and without different concentrations of acetic acid. 125I-Transferrin was added and, after 5 min of incubation, the cells were washed three times with cold HEPES medium; subsequently, the cells were treated for 1 h at 0°C with HEPES medium containing 2 mg/ml pronase. Finally, the cells and the medium were transferred to Eppendorf tubes and centrifuged for 2 min, and the radioactivity in the pellet and the supernatant was measured.
Endocytosis of 125I-aFGF under similar conditions was measured by incubating cells for 5 min in HEPES, pH 5.5, with and without acetic acid as indicated; 50 ng/ml 125I-aFGF was added in the presence of 10 U/ml heparin, and the cells were incubated for 15 min. After this, the cells were washed three times with cold PBS and then kept for 6 min at 4°C in a solution containing 2 M NaCl, 20 mM Na-acetate, pH 4, to release surface-bound aFGF. The cells were then washed once in the same buffer and dissolved. The released surface-bound radioactivity and the remaining cell-associated radioactivity were measured.
For endocytosis experiments at neutral pH, confluent cultures of transfected COS cells grown on 35-mm plates were incubated with DMEM containing 50 mM HEPES, pH 7.4, and 10 U/ml heparin for 5 min at 37°C. After this, the cells were incubated at 37°C with 50 ng/ml 125I-aFGF for the time indicated. The cells were then treated as described above.
Fractionation of Cells
After lysis in lysis buffer (0.1 M NaCl, 10 mM Na2PO4, 1% Triton X-100, 1 mM EDTA, 1 mM PMSF, 4 μg/ml aprotinin, pH 7.4), cells were centrifuged for 15 min at 720 × g. The supernatant was designated the cytoplasmic fraction. The pellet was washed twice by resuspension in lysis buffer containing 0.3 M sucrose, layered onto lysis buffer containing 0.8 M sucrose, and centrifuged at 720 × g for 15 min at 4°C; then it was sonicated and centrifuged for 5 min at 15,800 × g. The supernatant after the last centrifugation was designated the nuclear fraction.
Surface Labeling of Cells with 125I by the Lactoperoxidase Method
COS cells transfected with FGFR4 were washed three times with HEPES-buffered saline (HBS; 10 mM HEPES, 0.15 M NaCl, pH 7) and incubated with Na125I in the presence of lactoperoxidase (20 μg/ml) and 0.00003% H2O2 for 5 min at 30°C. The labeling reaction was stopped by adding saturated tyrosine and 0.5 μM DTT. After washing with HBS, the cells were scraped in HBS containing protease inhibitors and lysed in lysis buffer (20 mM HEPES, pH 7, 50 mM NaCl, 1% Triton X-100, 10% glycerol, 1.5 mM MgCl2, 1 mM EDTA, 1 μg/ml PMSF, 1 μg/ml aprotinin, 1 μg/ml leupeptin) on ice for 30 min. Then the cells were fractionated by centrifugation into a cytosolic fraction and a nuclear fraction.
Immunofluorescence Staining
Cells grown on coverslips and incubated with CY3-aFGF or alexa-transferrin were fixed with 3% paraformaldehyde in PBS for 15 min at room temperature. For double-staining experiments, paraformaldehyde-fixed cells were permeabilized with 0.5% Triton X-100 in PBS for 4 min. Coverslips were then incubated in PBS containing 5% dry milk and 0.1% Tween-20 at room temperature for 20 min with the primary antibody, washed, and then incubated with the secondary antibody. After staining, the coverslips were mounted in Mowiol (Calbiochem, San Diego, CA). Confocal microscopy was performed with the use of a Leica (Wetzlar, Germany) confocal microscope. Images were taken at ×100 magnification and captured as images at 1024 × 1024 pixels. Montages of images were prepared with the use of Photoshop 4.0 (Adobe, Mountain View, CA).
RESULTS
Endocytosis of aFGF in COS Cells Transfected with FGFR4
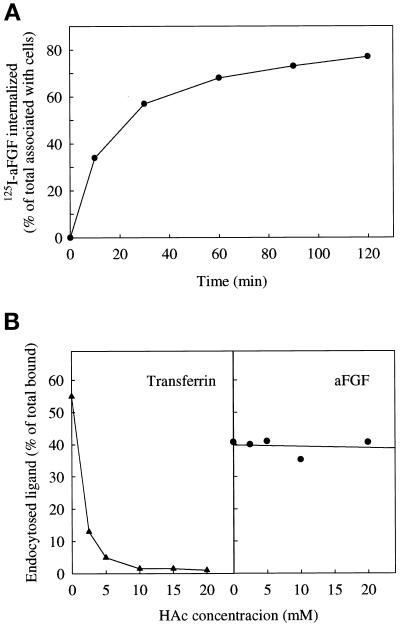
aFGF has been shown to be internalized upon binding to high-affinity FGFR (Sorokin et al., 1994; Muñoz et al., 1997). To study this process further, we transfected COS cells with FGFR4, incubated them with 125I-aFGF at 37°C for different periods of time, and measured endocytic uptake as radioactive material that could not be removed with 2 M NaCl, 20 mM Na-acetate, pH 4.0. Treatment with high salt/low pH removes surface-bound aFGF but not internalized growth factor (Muñoz et al., 1997). As shown in Figure 1A, the amount of internalized aFGF leveled off after 30 min, indicating that uptake and degradation or exocytosis had approached equilibrium at this time.
Figure 1.
Internalization of aFGF in cells transfected with FGFR4. (A) COS cells transiently transfected with FGFR4 were incubated at 37°C for the indicated periods of time in HEPES containing 10 U/ml heparin and 50 ng/ml 125I-aFGF. Then the cells were washed with PBS and treated for 6 min with 20 mM Na-acetate, pH 4, containing 2 M NaCl to remove surface-bound aFGF. The removed material was collected. The cells were subsequently dissolved, and the radioactivity in the medium and in the cells was measured. The data are expressed as the internalized radioactivity as a percentage of the total radioactivity associated with the cells. (B) Uptake of 125I-transferrin (▴) and 125I-aFGF (●) by COS cells transfected with FGFR4. The cells were incubated for 5 min at 37°C in HEPES medium, pH 5.5, with increasing concentrations of acetic acid. 125I-Transferrin or 125I-aFGF was then added as described above, and after 5 and 15 min of incubation, respectively, the amount of endocytosed proteins was measured as in A.
To determine if aFGF is internalized from clathrin-coated pits or from other membrane areas, we used acidification of the cytosol to inhibit internalization from clathrin-coated pits (Sandvig et al., 1987). Transferrin is endocytosed by this pathway (Hopkins, 1983). Figure 1B shows that acidification of the cytosol efficiently blocked endocytosis of transferrin, whereas the uptake of aFGF was not much reduced. This indicates that the major part of the uptake of aFGF occurs by clathrin-independent endocytosis in COS cells transfected with FGFR4.
FGFRs Partly Copurify with Caveolin-containing Structures
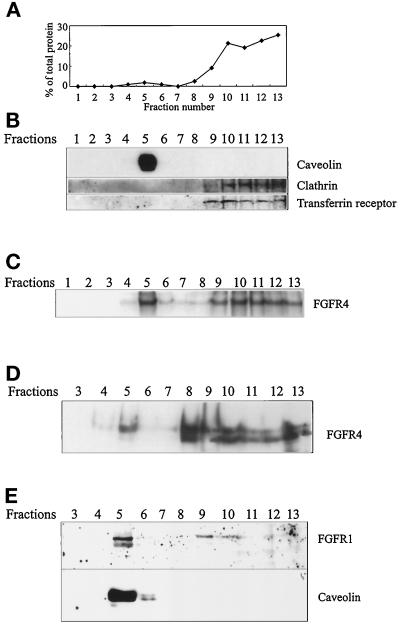
Caveolae have been implicated in vesicular transport and in signal transduction processes (Lisanti et al., 1994; Schnitzer et al., 1995a). Caveolin is a principal component of caveolae membranes. Because all four FGFRs contain a sequence resembling the sequence found to be involved in caveolin binding in a number of other proteins (Couet et al., 1997), we sought to determine if the growth factor receptor is present in caveolin-containing material. Caveolae have been shown to be separable from bulk cellular lipids and proteins by flotation in a discontinuous sucrose density gradient (Schnitzer et al., 1995a; Smart et al., 1995; Song et al., 1996). With the use of a detergent-free, carbonate-based fractionation method for the purification of caveolin-rich membrane domains, the vast majority of caveolin, the protein marker for caveolae, appeared in fraction 5, corresponding to the interface between the 5 and 35% sucrose layers (Figure 2, A and B). The bulk of the cellular protein was present in fractions 9–13 (Figure 2A). These fractions correspond to the position of the original lysate (mixed with sucrose) at the bottom of the gradient and would be expected to contain cytosolic proteins as well as membrane proteins. These fractions contained clathrin and the receptor for transferrin (Figure 2B).
Figure 2.
FGFRs partly comigrate with caveolin. (A) COS cells were homogenized in sodium carbonate, sonicated, and fractionated by floating in a sucrose density gradient. The protein content of each fraction is expressed as a percentage of the total amount of protein in the gradient. (B) Aliquots of the fractions in A were analyzed by SDS-PAGE and Western blotting with anti-caveolin, anti-clathrin, and anti-transferrin receptor antibodies. (C) COS cells transfected with FGFR4 were incubated with 125I-aFGF for 3 h at 4°C and treated for 20 min at 4°C with 0.3 mM disuccinimidyl suberate to cross-link the bound growth factor to the receptors. Then the cells were homogenized, sonicated, and fractionated as described above. The fractions were analyzed by SDS-PAGE and autoradiography. COS cells transfected with FGFR4 (D) and CPAE cells (E) were treated and fractionated as described above. The fractions were analyzed by Western blotting with anti-FGFR4 (D) or anti-FGFR1 and anti-caveolin (E).
To determine if part of the FGFRs cofractionated with caveolin-enriched membranes, COS cells transiently transfected with FGFR4 were allowed to bind 125I-aFGF in the cold and then treated with a chemical cross-linker. After cross-linking of 125I-aFGF to the receptors, the cells were fractionated to separate caveolin-enriched membranes from the bulk cellular material. Autoradiographic analysis demonstrated that a considerable part of the cross-linked material comigrated with caveolin, although most of the radioactivity was in the bottom fractions (Figure 2C).
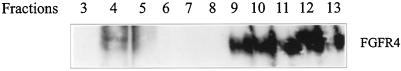
Analysis of the fractions of the gradient by Western blotting with antibodies to FGFR4 showed that only a small fraction of the total amount of FGFR4 was present in the fractions enriched in caveolin, whereas the bulk protein appeared in fractions 8–13 (Figure 2D).
Because COS cells do not express endogenous FGFR4, it was possible that the presence of FGFR4 in the caveolin-enriched fractions was an artifact of receptor overexpression. Therefore, we sought to determine if FGFR was present in caveolin-rich fractions in nontransfected CPAE cells, which express endogenous FGFR1. Fractionation of CPAE cells resulted in a similar distribution of FGFR1 and caveolin when the gradient fractions were analyzed by Western blotting (Figure 2E). We also carried out cross-linking experiments with 125I-aFGF in NIH 3T3 cells, which express endogenous FGFR1. Fractionation of NIH 3T3 cells under the same conditions as in Figure 2C resulted in a similar distribution of cross-linked material in the gradient (not shown). These data indicate that both FGFR4 and FGFR1 are partly present in a low-density, caveolin-enriched fraction.
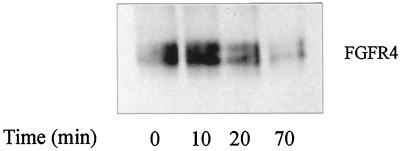
When COS cells transfected with FGFR4 were incubated with aFGF for different periods of time and the caveolin-containing fractions were isolated, submitted to SDS-PAGE, and immunoblotted with anti-FGFR4, the receptor was found to remain in the caveolin-containing fraction for ∼10 min after the addition of aFGF, and then the amount started to decline. After 70 min, the receptor was essentially absent from this fraction (Figure 3). This finding indicates that the addition of aFGF induces displacement of receptors from the caveolin-containing fraction, probably by endocytosis.
Figure 3.
Kinetics of aFGF-induced disappearance of FGFR4 from the caveolin-rich fraction. COS cells transfected with FGFR4 were grown for 12 h in the absence of serum and then incubated in the presence of 50 ng/ml aFGF for the indicated periods of time. Caveolin-rich fractions were prepared as described in Figure 2. Fractions 4 and 5 were pooled, dialyzed against distilled water, lyophilized, separated by SDS-PAGE, and visualized by immunoblotting with anti-FGFR4 antibodies.
To determine whether caveolae are involved in clathrin-independent endocytosis of aFGF, we tested different sterol-binding drugs that have been shown to disorganize caveolae by cholesterol chelation, because cholesterol is required for the integrity of caveolae (Bolard, 1986; Rothberg et al., 1990). We tested filipin, nystatin, and digitonin. None of these drugs was found to have an effect on aFGF endocytosis (our unpublished results), suggesting that aFGF is endocytosed by a pathway different from that used during endocytosis of clathrin-coated pits and caveolae.
Caveolin 1 has been shown to interact directly with a variety of cytoplasmic signal-transducing molecules and receptors (Song et al., 1996; Couet et al., 1997; Michel et al., 1997; Yamamoto et al., 1999). We tried to coimmunoprecipitate FGFR4 and caveolin with antibodies raised against either protein, as described previously (Couet et al., 1997), but we were unable to demonstrate any interaction (our unpublished results).
Localization of Tyrosine-phosphorylated Receptor
Caveolae and other low-density lipid domains concentrate protein kinases and their substrates (Liu et al., 1996, 1997; Mineo et al., 1996; Waugh et al., 1999). To determine if FGFR present in the low-density fractions is activated, FGFR4-transfected cells were starved for 12 h and then incubated in the presence of aFGF for 10 min, and subsequently the cells were lysed. After fractionation of the cells to obtain the caveolin-rich fractions, a Western blot was probed with anti-phosphotyrosine antibodies. Figure 4 shows that activated FGFR4 was present in caveolin-rich fractions 4 and 5 as expected, because we found FGFR in these domains for at least 20 min after stimulation with aFGF (Figure 3). The majority of the activated receptors, however, were found in the bottom fractions of the gradient.
Figure 4.
Stimulation of tyrosine kinase activity in caveolin-rich fractions of FGFR4-transfected COS cells. The cells were grown in the absence of serum for 12 h and then incubated in the presence of 50 ng/ml aFGF for 10 min. The cells were fractionated as in Figure 2B, and the fractions were analyzed by SDS-PAGE and immunoblotted with anti-phosphotyrosine antibodies.
To determine where in the cell the growth factor receptor is localized and where it is active, we transfected COS cells with wild-type FGFR4 or, as a control, with the kinase-negative FGFR4-K503R mutant receptor, and we carried out immunofluorescence studies with anti-FGFR4 and with anti-phosphotyrosine. In cells not treated with the growth factor, the receptor was found at the cell surface as well as inside the cell, presumably partly in the endoplasmic reticulum and the Golgi apparatus, as the synthesis of the receptor was ongoing (Figure 5A). A large amount of labeling was found in the juxtanuclear region. When the cells were labeled with antibodies against phosphotyrosine, the strongest labeling was found in the juxtanuclear region. Very little labeling was found at the cell surface. The presence of activated receptors in the absence of aFGF could be due to transphosphorylation of receptors because of the high number of receptors in transfected cells.
Figure 5.
Localization of activated FGFR4 in cells. COS cells transfected with FGFR4 (A and B) or the FGFR4-K503R mutant (C) were starved of serum for 5 h at 37°C and then kept for 2 h at 4°C in the absence (A) or in the presence (B and C) of aFGF. The cells were then either fixed (A) or incubated at 37°C for 15 min before fixation (B and C). Double staining was performed with anti-FGFR4 and anti-phosphotyrosine antibodies.
Upon incubation of the cells with aFGF at 37°C, the amount of receptors at the cell surface was reduced and the amount of phosphotyrosine in the perinuclear area was increased (Figure 5B). In the case of the kinase-negative mutant FGFR4-K503R, we did not see any labeling with anti-phosphotyrosine antibody (Figure 5C). This finding demonstrates that the phosphotyrosine visualized in cells transfected with the wild-type receptor is dependent on FGFR. Part of the labeling is probably due to tyrosine phosphorylation of additional proteins initiated by the activated FGFR4. A large part of the FGFR4 colocalized with the phosphotyrosine (Figure 5, A and B, merge).
Triton X-100 Insolubility of the Major Part of FGFR4
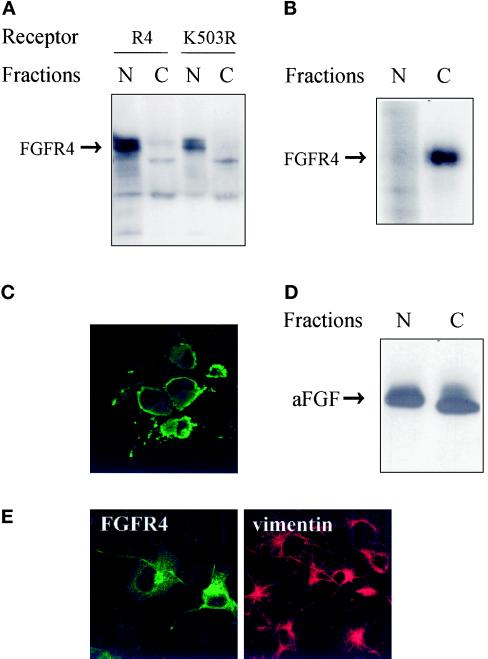
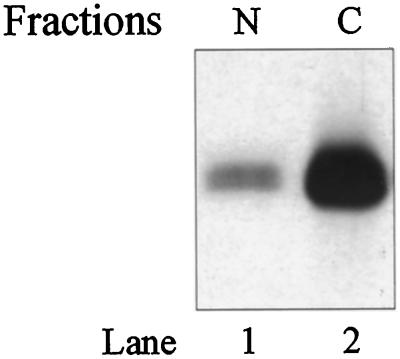
Many of the receptors were found in a juxtanuclear area by immunofluorescence, and we sought to determine if the receptors sedimented with the nuclei. COS cells transiently transfected with FGFR4 or with the kinase-negative mutant FGFR4-K503R were dissolved in buffer containing Triton X-100 and separated into a nuclear and a cytoplasmic fraction by centrifugation. The fractions were analyzed by Western blotting with anti-FGFR4 antibodies. Surprisingly, the receptors were found mainly in the Triton X-100–insoluble nuclear fraction (Figure 6A). Approximately 90% of the receptors were sedimented under these conditions. Treatment with either CHAPS (0.5%) or 2-octyl glucoside (60 mM), which dissolve cholesterol-rich membrane domains (Brown and Rose, 1992), did not alter this pattern (our unpublished results).
Figure 6.
Characterization of the expressed receptor. (A) Western blot analysis of FGFRs in subcellular fractions of COS cells. Transfected COS cells were lysed and fractionated into a nuclear (N) and a cytoplasmic (C) fraction. The fractions were precipitated with trichloroacetic acid and analyzed by SDS-PAGE. Blots were probed with anti-FGFR4 antibodies. (B) Localization of surface-labeled FGFR4. COS cells transfected with FGFR4 were surface labeled by incubating them with Na125I, lactoperoxidase, and H2O2 for 5 min, and then DTT and tyrosine were added to stop the reaction. The cells were subsequently lysed and fractionated into a nuclear (N) and a cytoplasmic (C) fraction. The fractions were sonicated and centrifuged, and the receptors in the supernatants were immunoprecipitated with anti-FGFR4 antibodies. The immunoprecipitates were analyzed by SDS-PAGE and autoradiography. (C) Localization of detergent-insoluble FGFR4. Transfected COS cells were grown on coverslips and extracted in buffer containing 1% Triton X-100 before fixation with 3% paraformaldehyde. Fixed cells were incubated with antibody to FGFR4 followed by FITC-conjugated secondary antibody. (D) Nuclear and cytosolic fractions contain functional FGFRs. COS cells transfected with FGFR4 were lysed and fractionated into a nuclear (N) and a cytoplasmic (C) fraction. Both fractions were carefully sonicated for 5 s, and insoluble material was removed by centrifugation. Then the supernatants were incubated with [35S]methionine-labeled aFGF and 10 U/ml heparin for 3 h at 4°C, immunoprecipitated with anti-FGFR4 antibodies, and analyzed by SDS-PAGE and autoradiography. (E) Effect of transfection on the distribution of vimentin. Transiently transfected COS cells were fixed and double stained with anti-FGFR4 and anti-vimentin antibodies followed by FITC- and CY3-conjugated secondary antibodies, respectively.
To determine if the presence of receptors in the nuclear fraction was a consequence of contamination with plasma membrane material, we labeled the cell surface of transiently transfected COS cells with 125I by the lactoperoxidase method and fractionated the cells into a nuclear and a cytoplasmic fraction. Both fractions were sonicated, and FGFR4 was immunoprecipitated from both fractions and analyzed by SDS-PAGE (Figure 6B). In this case, labeled receptors were found only in the cytosolic fraction. This finding excludes contamination of the nuclear fraction with plasma membrane receptors.
The data suggested that a large fraction of the FGFR4 is anchored to cytoskeletal material that sediments together with the nuclei. However, attempts to dissolve the receptors by treating the cells with cytochalasin D to disassemble actin filaments, with nocodazole to disassemble the microtubuli, or with both compounds at low temperature (4°C) were unsuccessful (our unpublished results). Also, in cells expressing the receptor mutant FGFRalan4 lacking a putative caveolin-binding sequence, as described in a forthcoming paper (Citores, Khnykin, Wesche, Klingenberg, Wiedlocha, and Olsnes, unpublished results), most of the receptors were found in the insoluble fraction.
To localize the Triton X-100–insoluble receptors, COS cells transfected with FGFR4 were extracted for 10 min in buffer containing 1% Triton X-100 before fixation and staining with anti-FGFR4 antibodies. The detergent-insoluble receptor was found mainly in the juxtanuclear and perinuclear regions (Figure 6C).
The possibility existed that the FGFR4 contained in the nuclear fraction was misfolded and inactive. To determine if these receptors were capable of interacting with aFGF, we examined the binding of [35S]methionine-labeled aFGF to sonicated cytoplasmic and nuclear fractions. The soluble material in each fraction was incubated with heparin and labeled aFGF for 3 h at 4°C and then immunoprecipitated with anti-FGFR4. High-affinity binding sites were detected in both the cytoplasmic and nuclear fractions (Figure 6D), indicating that at least part of the receptors sedimenting with the nuclei are functional.
It was recently demonstrated that aggresomes can be formed as a general cellular response to cytoplasmic accumulation of misfolded proteins. The aggregates are detergent insoluble and are localized in a juxtanuclear region, similar to the localization of FGFR. Aggresome formation is accompanied by a massive redistribution of vimentin intermediary filaments to a pericentriolar location (Johnston et al., 1998). After staining with anti-vimentin and anti-FGFR4 antibodies, there was no apparent difference between the labeling in transfected and untransfected cells (Figure 6E). This indicates that the presence of insoluble FGFR4 in the juxtanuclear region is not due to aggresome formation.
Accumulation of Endocytosed aFGF in a Juxtanuclear Area
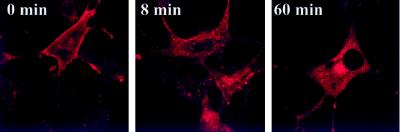
To determine the intracellular transport of aFGF in cells expressing high-affinity receptors, we labeled the growth factor with the fluorophore CY3 and incubated it with COS cells transfected with FGFR4. Heparin was present to avoid binding to cell surface heparans. The distribution of the growth factor in the cells was studied after incubation at 37°C for various periods of time. The data in Figure 7 demonstrate that when the cells were treated with the growth factor at 0°C, it was bound to the cell surface in a homogenous manner. When the cells were subsequently incubated for 8 min at 37°C, the amount of growth factor at the surface was reduced and the fluorescent growth factor appeared as intracellular dots, indicating uptake in vesicles. After longer incubation at 37°C (60 min), the growth factor started to accumulate in an area close to the nucleus. Similar localization of endocytosed aFGF was observed in BHK, U2OSDr1, and HeLa cells transfected with FGFR4 and in COS cells transfected with FGFR1 (our unpublished results). Therefore, the juxtanuclear localization is not unique to FGFR4 and COS cells.
Figure 7.
Transport of fluorescent aFGF in FGFR4-transfected COS cells. The cells were incubated at 4°C for 2 h with CY3-aFGF in the presence of heparin to allow binding of the growth factor and then either fixed immediately (0 min) or incubated at 37°C for 8 or 60 min before fixation.
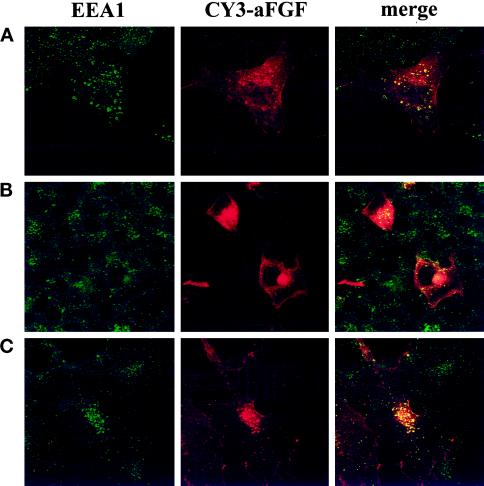
We next carried out double-labeling experiments with EEA1, a protein that is associated with early endosomes (Mu et al., 1995). As shown in Figure 8A, incubation for 8 min at 37°C resulted in good overlap of EEA1 and the fraction of aFGF that was observed as intracellular dots. This was demonstrated in the overlay experiments when spots labeled with both colors appeared yellow. After 2 h at 37°C, the growth factor appeared in a juxtanuclear area that did not stain for EEA1, indicating that it is different from early endosomes (Figure 8B). When the incubation for 2 h was at 16°C rather than 37°C, the growth factor remained in vesicles that stained positive for EEA1 (Figure 8C). At the lower temperature, therefore, the transfer to the juxtanuclear compartment is blocked. Incubation at 16°C has been shown to block transport of endocytosed material to late endosomes and to the recycling endosome compartment (Ren et al., 1998).
Figure 8.
Colocalization of aFGF with EEA1, a marker for early endosomes. FGFR4-transfected COS cells were incubated with CY3-aFGF for 8 min (A) or 2 h (B and C) at 37°C (A and B) or 16°C (C) and then fixed and stained with anti-EEA1 antibodies followed by FITC-conjugated secondary antibody.
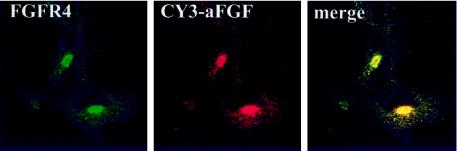
When the cells were allowed to take up CY3-labeled growth factor and then stained with a fluorescent antibody to FGFR4, there was a considerable overlap of the two fluorescent signals in the juxtanuclear region (Figure 9). This finding indicates that the major part of the receptor in the cells is localized to the compartment where growth factor accumulates after receptor-mediated endocytosis.
Figure 9.
Colocalization of FGFR4 and endocytosed aFGF. COS cells transfected with FGFR4 were incubated with CY3-aFGF for 2 h at 37°C, and then the cells were fixed and stained with anti-FGFR4.
Identification of the Organelle in Which Endocytosed Growth Factor and Its Receptor Accumulate
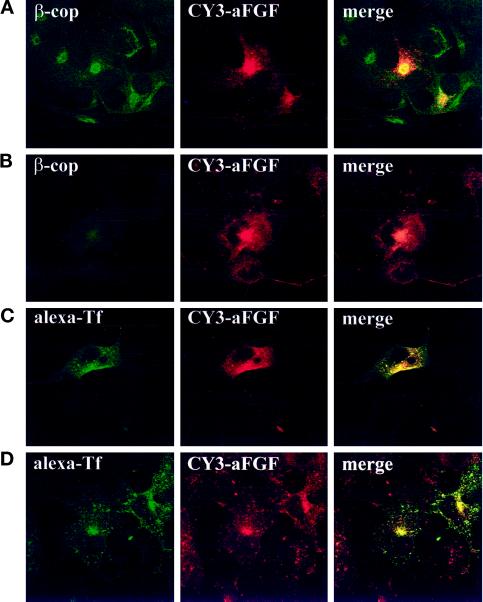
The area near the nucleus where the growth factor and FGFR accumulate is close to the Golgi apparatus. In attempts to identify the compartment, we first carried out fluorescence experiments with antibodies to β-cop, a marker for the Golgi apparatus (Glick and Malhotra, 1998), in cells that had been incubated with CY3-aFGF for 2 h at 37°C. As shown in Figure 10A, there was partial overlap with internalized growth factor at the fluorescence microscopy level of resolution.
Figure 10.
Colocalization of aFGF with markers for different intracellular organelles. (A and B) FGFR4-transfected COS cells were either untreated (A) or pretreated for 30 min with brefeldin A (B), incubated for 2 h at 37°C with CY3-aFGF, and then fixed and stained for the Golgi marker β-cop. (C and D) COS cells were cotransfected with the human transferrin receptor and FGFR4. The cells were then either left untreated (C) or pretreated for 30 min with 2 μg/ml brefeldin A (D) and incubated with CY3-aFGF for 2 h at 37°C. Subsequently, alexa-transferrin was bound to the cells at 4°C, and then the cells were washed and incubated for 20 min at 37°C and fixed.
Treatment with brefeldin A induces the disappearance of the Golgi apparatus in many cell types, including COS cells; therefore, we tested its effect on cells that internalized fluorescent growth factor. Under these conditions, β-cop was distributed over the whole cell area, consistent with the finding that in the presence of brefeldin A the Golgi components are transported back to the endoplasmic reticulum (Klausner et al., 1992). The localization of the internalized growth factor in the juxtanuclear area did not change as a result of brefeldin A treatment (Figure 10B). Clearly, therefore, the internalized growth factor is not transported to the Golgi apparatus.
With antibodies to mannose-6-phosphate receptor, a marker for late endosomes and the trans-Golgi network (Goda and Pfeffer, 1988), there was very little overlap with the growth factor or with FGFR4. The lysosomes, visualized with acridine orange, were partly localized in the juxtanuclear area, but they appeared as distinct vesicles, unlike in the compartment where aFGF and FGFR4 were found. With an antibody to calnexin, a marker for the endoplasmic reticulum (Helenius et al., 1992), we could not see any overlap with aFGF (our unpublished results).
When the cells were cotransfected with the transferrin receptor and treated with fluorescent transferrin for 20 min and aFGF for 2 h, there was almost complete overlap with the fluorescent growth factor (Figure 10C). (Note that not all transfected cells expressed both FGFR4 and transferrin receptors.) Transferrin endocytosed for 20 min is a marker for the recycling endosome compartment. The distribution of the internalized transferrin was not changed by treatment with brefeldin A (Figure 10D), in agreement with the finding that the recycling endosome compartment is not dispersed by treatment with the drug (Prekeris et al., 1998). Also, in experiments similar to those shown in Figure 5, A and B, the tyrosine phosphorylation pattern was unaffected by treatment with brefeldin A (our unpublished results).
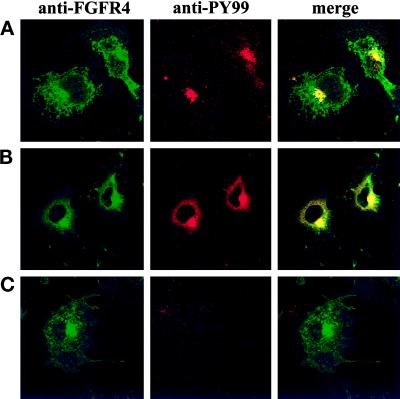
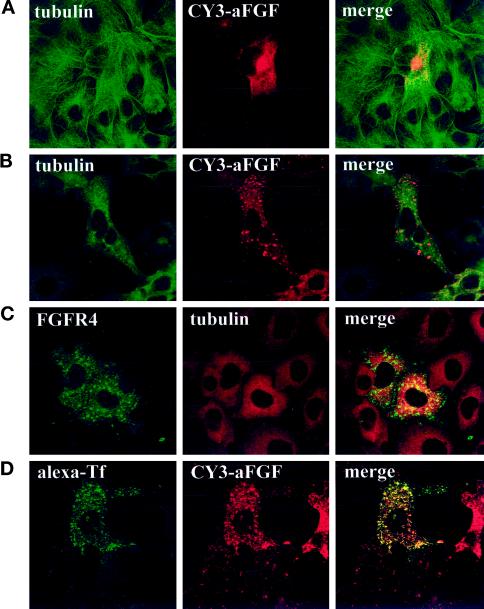
We also tested a number of other drugs for their ability to alter the localization of internalized growth factor in the cells. Inhibitors of PI3 kinase, such as wortmannin and LY294002, interfere with intracellular vesicular transport (Shepherd et al., 1996; Spiro et al., 1996) and conceivably could prevent the transport of the growth factor to the juxtanuclear region. Our data indicate that aFGF transport was not altered by these drugs (our unpublished results). Whereas cytochalasin D, which induces depolymerization of actin microfilaments, did not alter the distribution of the growth factor, treatment with nocodazole, which induces disassembly of microtubuli, prevented the appearance of the growth factor in the juxtanuclear region; instead, it was located in vesicular structures dispersed over the cytoplasm (Figure 11, A and B). Here it colocalized with transferrin (Figure 11D), in agreement with earlier observations that the recycling endosome compartment disaggregates in the presence of nocodazole (Ren et al., 1998). The same pattern was found for FGFR4 (Figure 11C) in nocodazole-treated cells. Staining with anti-phosphotyrosine demonstrated that the disaggregated recycling endosome compartment contained activated receptors (Figure 12).
Figure 11.
Effect of nocodazole on the intracellular localization of aFGF and FGFR4. COS cells transfected with FGFR4 (A–C) or cotransfected with FGFR4 and human transferrin receptor (D) were either left untreated (A) or pretreated for 30 min with 33 μM nocodazole (B–D). In some cases (A and B), the cells were then incubated with CY3-aFGF for 2.5 h at 37°C, fixed, and stained for tubulin. In C, the cells were stained with anti-FGFR4 and anti-tubulin. In D, the cells were treated with CY3-aFGF at 37°C for 2 h, alexa-transferrin was bound at 4°C, and then the cells were incubated for 20 min at 37°C.
Figure 12.
Effect of nocodazole on the localization of tyrosine-phosphorylated proteins in aFGF-treated cells transfected with FGFR4. COS cells transfected with FGFR4 were pretreated for 30 min with 33 μM nocodazole and then incubated for 2 h in the presence of 50 ng/ml aFGF. Then the cells were fixed and stained with anti-FGFR4 and anti-PY99 antibodies.
Triton X-100 Solubility of Endocytosed FGFR4
Because the major part of the expressed FGFR4 was present in a fraction not solubilized with Triton X-100 (Figure 6A), we sought to determine if the endocytosed aFGF was bound to free or anchored receptors. Cells were incubated with [35S]methionine-labeled aFGF for 10 h at 37°C and then fractionated into a cytosolic and a nuclear fraction, both of which were sonicated and immunoprecipitated with anti-FGFR4. The data in Figure 13 show that most of the growth factor taken up by the cells was present in the cytosolic fraction and only a small part was found in the nuclear fraction. Because the data in Figure 6A show that most of FGFR4 was in the Triton X-100–insoluble fraction, this indicates that most of the endocytosed receptors represent a subfraction that is not anchored.
Figure 13.
Triton X-100 solubility of internalized aFGF. COS cells transfected with FGFR4 were incubated with [35S]methionine-labeled aFGF for 10 h at 37°C and then washed with PBS and acid-salt buffer to remove surface-bound aFGF. The cells were lysed and fractionated into a nuclear (N) and a cytoplasmic (C) fraction, and then both fractions were sonicated. After removal of insoluble material by centrifugation, FGFR4 with bound labeled aFGF was immunoprecipitated with anti-FGFR4 antibodies and analyzed by SDS-PAGE and fluorography.
To test this possibility, we labeled surface-exposed receptors with 125I and lactoperoxidase in experiments similar to those shown in Figure 6B and then incubated the cells for 3 h at 37°C. Although part of the radioactive material disappeared during the incubation, probably as a result of degradation, the remaining labeled receptor was found mainly in the Triton X-100–soluble fraction (our unpublished results). Taken together, the data indicate that there is a large population of FGFR that is anchored in the recycling compartment and a much smaller population that is not anchored and that could be cycling between the surface and the juxtanuclear regions.
DISCUSSION
We have demonstrated that endocytic uptake of aFGF was not much inhibited by acidification of the cytosol to inhibit uptake from clathrin-coated pits. Although aFGF bound to the receptor was found to partially cofractionate with caveolin in a low-density fraction, we did not obtain evidence that the receptor interacts with caveolin. In a forthcoming paper (Citores, Khnykin, Wesche, Klingenberg, Wiedlocha, and Olsnes, unpublished results), we demonstrate that most of the endocytic uptake of the growth factor continues in cells expressing a mutant dynamin that is reported to block endocytosis from both clathrin-coated pits and caveolae. Apparently, therefore, the growth factor is endocytosed by an alternative pathway that involves neither clathrin nor caveolae. The internalized growth factor is first found associated with endosomes, but after 1 h at 37°C it is to a large extent found in a juxtanuclear organelle that was identified as the recycling endosome compartment.
When surface-bound proteins are taken up by endocytosis, they are first delivered to peripheral sorting endosomes irrespective of whether they are endocytosed from coated pits or from noncoated regions of the membrane (van Deurs et al., 1989). From the sorting endosomes, some material is rapidly recycled back to the cell surface and some is transported to late endosomes and lysosomes for degradation (van Deurs et al., 1989). Most of the receptors that are eventually returned to the cell surface are first transported to a distinct recycling compartment that is not accessible to material destined for the lysosomes. The recycling endosome compartment consists of a juxtanuclear pericentriolar collection of membranous tubular elements (Hopkins et al., 1994). The transferrin receptor is a marker for the recycling endosome compartment, and endocytosed transferrin is concentrated in this compartment. It has been estimated that ∼80% of the endocytosed transferrin is found in the recycling endosome compartment under steady-state conditions (Gruenberg and Maxfield, 1995).
The recycling endosome compartment partly overlaps with the Golgi apparatus at the resolution level of the fluorescence microscope. Also, the majority of FGFR4 was present at this location, but unlike in Golgi components, the FGFR was not relocated after treatment with brefeldin A. Most of the FGFR was found to be insoluble in Triton X-100, CHAPS, and 2-octyl glucoside, but it could be partly solubilized by sonication. This suggests that it is anchored to cytoskeletal elements. Because very little labeled growth factor taken up by the cells was found in the Triton X-100–insoluble fraction, we think that there are two populations of FGFR4 in the cells, one that is involved in endocytic uptake of the growth factor and another that is not. The last fraction could be anchored to the cytoskeleton, as was recently found for the anion antiporter AE1 (Ghosh et al., 1999) and the glucose transporter Glut1 (Bunn et al., 1999). It may also be the case with Glut4 (Pessin et al., 1999). Additionally, work by other authors has demonstrated the presence of FGFR in the Triton X-100–insoluble fraction, but at least in some cases it was found to be associated with the nuclei as such (Johnston et al., 1995; Maher, 1996; Stachowiak et al., 1996a,b, 1997).
The function of the immobilized receptors is not known. They could be released upon demand by a metabolic signal and then migrate to the surface, or they could play a role in the juxtanuclear region as such. Experiments attempting to elucidate this question are in progress.
There is ample evidence for the presence of cytoskeletal elements in the Golgi region. Spectrin and ankyrin have been found to be associated with the Golgi as well as with cytoplasmic vesicles in a variety of cells (De Matteis and Morrow, 1998). An isoform of β-spectrin was found to localize to the Golgi complex, and when pure erythroid β-spectrin was microinjected into cells, it was localized to the Golgi area (Beck et al., 1994). Ankyrin is important for linking the cytoskeleton to the membranes by binding to both β-spectrin and the cytoplasmic domain of integral membrane proteins (Bennett, 1992). Ankyrin also binds with high affinity to tubulin (Bennett and Davis, 1981). Golgi ankyrin and β-spectrin are found in the trans-Golgi network as large oligomeric and Triton X-100–insoluble complexes (Beck et al., 1997). The recycling endosome compartment was found to be particularly rich in cytoskeletal proteins (Pol et al., 1997).
At temperatures between 15 and 22°C, endocytosis still occurs, but the sorting in endosomes is considerably reduced. As a result, proteins such as the transferrin receptor and the glucose transporter Glut4 remain in peripheral structures that probably represent expanded vacuolar endosomes (Schmidt et al., 1997a,b; Wei et al., 1998). Also, aFGF was not transported from endosomes to the recycling compartment at 16°C.
The receptor was found to be tyrosine phosphorylated even in the absence of added aFGF. Possibly, the reason for this is that the high concentration of receptors is sufficient to ensure cross-phosphorylation even without growth factor–induced dimerization of the receptor. Interestingly, the vast majority of the phosphorylated receptors were found in the juxtanuclear compartment. Upon treatment with aFGF, there was an increase in phosphotyrosine at this location and in the perinuclear region. Therefore, we think that most of the receptor signaling occurs from this location. Also, when the sonicated cells were fractionated by floatation in a sucrose gradient, most of the tyrosine-phosphorylated receptor was in the heavy fraction and only a small amount was found in the fraction enriched in caveolin. Together, the data indicate that in the transfected cells most of the signaling takes place after the receptor is internalized. Interestingly, when the receptor was dispersed to more peripheral regions of the cell by treatment with nocodazole, the receptor remained activated, as determined by anti-phosphotyrosine immunofluorescence.
The presence of FGFR in the caveolin-containing fraction was found not only in cells overexpressing FGFRs but also in CPAE cells and NIH 3T3 cells that naturally express FGFR1. Therefore, the cofractionation with caveolin-containing structures is not an artifact of receptor overexpression. In this study, we used a carbonate-based method for the preparation of caveolin-containing membranes. The fraction thus isolated contained <5% of the total cell protein. There is some evidence that the caveolae fraction prepared in this way still includes at least two types of structures in addition to caveolae, i.e., detergent-insoluble glycolipid-rich domains (Schnitzer et al., 1995b) and low-density microdomains different from caveolae and glycolipid-rich domains (Waugh et al., 1999). We could not detect direct interaction of FGFR4 with caveolin by immunoprecipitation, but this does not exclude the possibility that a weak interaction could exist. Caveolin was recently shown to interact directly with EGF receptor and PDGF receptor and to inhibit autophosphorylation of the receptors (Couet et al., 1997; Yamamoto et al., 1999).
Externally added aFGF has been found by several laboratories, including our own, to be transported to the nuclear fraction (Imamura et al., 1990, 1994; Wiedlocha et al., 1994; Klingenberg et al., 1998). This fraction was defined in most cases as the material that sedimented with the nuclei after detergent lysis of the cells. The finding that most of the FGFR is found in this fraction raises the question of whether the growth factor believed to be transported to the nuclei was in fact bound to cytoskeleton-anchored FGFR present in the recycling compartment.
There are several reasons to assume that this is not the case. First, when the nuclear localization sequence present at the N terminus of aFGF was deleted, the growth factor was not found in the nuclear fraction (Imamura et al., 1990; Wiedlocha et al., 1994). Furthermore, aFGF is able to penetrate cellular membranes and enter the cytosol and nucleus in intact cells. When we added a farnesylation signal (a CAAX box) to the C terminus of the growth factor and incubated it with cells expressing FGFR, the growth factor became farnesylated (Wiedlocha et al., 1995). Part of the farnesylated growth factor was found in the nuclear fraction. Because farnesylation is known to occur only in the cytosol and in the nucleus (Lutz et al., 1992a,b; Sinensky et al., 1994), this finding demonstrated that the growth factor is indeed able to enter the nucleus. In the nucleus, the growth factor appears to be necessary for the induction of DNA synthesis, at least in some cells (Imamura et al., 1990; Wiedlocha et al., 1994, 1996).
Another piece of evidence that the growth factor is translocated into cells is the fact that externally added growth factor is phosphorylated by the cells in a unique PKC site (Klingenberg et al., 1998). PKC also has been found only inside cells.
In the present work, we were unable to demonstrate with certainty the presence of CY3-aFGF in the nucleus. There may be several reasons for this. First, it is difficult to exclude the possibility that a small amount of the CY3-aFGF is indeed in the nucleus in our experiments. The presence of basic FGF in the nucleus has been demonstrable only after prolonged incubation with the growth factor (more than 4 h), and it has been reported to take place only during the G1 phase of the cell cycle (Baldin et al., 1990). After prolonged incubation of cells with the CY3-labeled growth factor, most of the label disappears, probably as a result of degradation of the internalized fluorescent protein. Another possibility is that the CY3 label may interfere with translocation to the nucleus. We demonstrated previously that a fusion protein of aFGF with diphtheria toxin A fragment is unable to reach the nucleus when it is added as such to the cells, but it readily enters the nucleus when it is translocated into the cytosol by means of diphtheria toxin B fragment (Wiedlocha et al., 1994). It is possible that even minor modifications of the growth factor may interfere with translocation across cellular membranes. Further experiments are required to demonstrate where in the cell the membrane translocation of aFGF takes place.
ACKNOWLEDGMENTS
The skillful technical assistance of Mette Sværen is gratefully acknowledged. We thank Drs. Kirsten Sandvig, Pål Falnes, and Harald Stenmark for critical reading of the manuscript. L.C. is a Postdoctoral Fellow, and J.W. and E.K. are Predoctoral Fellows, of the Norwegian Cancer Society. This work was supported by the Novo Nordisk Foundation, the Norwegian Research Council, the Blix Fund for the Promotion of Medical Research, Rachel and Otto Kr. Bruun’s legate, and the Jahre Foundation.
Abbreviations used:
- aFGF
acidic FGF
- CHAPS
3-([3-chloramidopropyl]dimethylammonio)-2-hydroxy-1-propanesulfonate
- FGFR
FGF receptor
- HBS
HEPES-buffered saline
REFERENCES
- Baldin V, Roman AM, Bosc-Bierne I, Amalric F, Bouche G. Translocation of bFGF to the nucleus is G1 phase cell cycle specific in bovine aortic endothelial cells. EMBO J. 1990;9:1511–1517. doi: 10.1002/j.1460-2075.1990.tb08269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C, Moscatelli D. The FGF family of growth factors and oncogenes. Adv Cancer Res. 1992;59:115–165. doi: 10.1016/s0065-230x(08)60305-x. [DOI] [PubMed] [Google Scholar]
- Beck KA, Buchanan JA, Malhotra V, Nelson WJ. Golgi spectrin: identification of an erythroid beta-spectrin homolog associated with the Golgi complex. J Cell Biol. 1994;127:707–723. doi: 10.1083/jcb.127.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck KA, Buchanan JA, Nelson WJ. Golgi membrane skeleton: identification, localization and oligomerization of a 195 kDa ankyrin isoform associated with the Golgi complex. J Cell Sci. 1997;110:1239–1249. doi: 10.1242/jcs.110.10.1239. [DOI] [PubMed] [Google Scholar]
- Bennett V. Ankyrins: adaptors between diverse plasma membrane proteins and the cytoplasm. J Biol Chem. 1992;267:8703–8706. [PubMed] [Google Scholar]
- Bennett V, Davis J. Erythrocyte ankyrin: immunoreactive analogues are associated with mitotic structures in cultured cells and with microtubules in brain. Proc Natl Acad Sci USA. 1981;78:7550–7554. doi: 10.1073/pnas.78.12.7550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolard J. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim Biophys Acta. 1986;864:257–304. doi: 10.1016/0304-4157(86)90002-x. [DOI] [PubMed] [Google Scholar]
- Brown DA, Rose JK. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell. 1992;68:533–544. doi: 10.1016/0092-8674(92)90189-j. [DOI] [PubMed] [Google Scholar]
- Bunn RC, Jensen MA, Reed BC. Protein interactions with the glucose transporter binding protein GLUT1CBP that provide a link between GLUT1 and the cytoskeleton. Mol Biol Cell. 1999;10:819–832. doi: 10.1091/mbc.10.4.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess WH, Maciag T. The heparin-binding (fibroblast) growth factor family of proteins. Annu Rev Biochem. 1989;58:575–606. doi: 10.1146/annurev.bi.58.070189.003043. [DOI] [PubMed] [Google Scholar]
- Burrus LW, Zuber ME, Lueddecke BA, Olwin BB. Identification of a cysteine-rich receptor for fibroblast growth factors. Mol Cell Biol. 1992;12:5600–5609. doi: 10.1128/mcb.12.12.5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chellaiah AT, McEwen DG, Werner S, Xu J, Ornitz DM. Fibroblast growth factor receptor (FGFR) 3: alternative splicing in immunoglobulin-like domain III creates a receptor highly specific for acidic FGF/FGF-1. J Biol Chem. 1994;269:11620–11627. [PubMed] [Google Scholar]
- Couet J, Sargiacomo M, Lisanti MP. Interaction of a receptor tyrosine kinase, EGF-R, with caveolins: caveolin binding negatively regulates tyrosine and serine/threonine kinase activities. J Biol Chem. 1997;272:30429–30438. doi: 10.1074/jbc.272.48.30429. [DOI] [PubMed] [Google Scholar]
- Crumley G, Bellot F, Kaplow JM, Schlessinger J, Jaye M, Dionne CA. High-affinity binding and activation of a truncated FGF receptor by both aFGF and bFGF. Oncogene. 1991;6:2255–2262. [PubMed] [Google Scholar]
- De Matteis MA, Morrow JS. The role of ankyrin and spectrin in membrane transport and domain formation. Curr Opin Cell Biol. 1998;10:542–549. doi: 10.1016/s0955-0674(98)80071-9. [DOI] [PubMed] [Google Scholar]
- DiGabriele AD, Lax I, Chen DI, Svahn CM, Jaye M, Schlessinger J, Hendrickson WA. Structure of a heparin-linked biologically active dimer of fibroblast growth factor. Nature. 1998;393:812–817. doi: 10.1038/31741. [DOI] [PubMed] [Google Scholar]
- Fraker PJ, Speck JC., Jr Protein and cell membrane iodinations with a sparingly soluble chloramide, 1,3,4,6-tetrachloro-3a,6a-diphenylglycoluril. Biochem Biophys Res Commun. 1978;80:849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Cox KH, Cox JV. Chicken erythroid AE1 anion exchangers associate with the cytoskeleton during recycling to the Golgi. Mol Biol Cell. 1999;10:455–469. doi: 10.1091/mbc.10.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleizes PE, Noaillac-Depeyre J, Dupont MA, Gas N. Basic fibroblast growth factor (FGF-2) is addressed to caveolae after binding to the plasma membrane of BHK cells. Eur J Cell Biol. 1996;71:144–153. [PubMed] [Google Scholar]
- Glick BS, Malhotra V. The curious status of the Golgi apparatus. Cell. 1998;95:883–889. doi: 10.1016/s0092-8674(00)81713-4. [DOI] [PubMed] [Google Scholar]
- Goda Y, Pfeffer SR. Selective recycling of the mannose-6-phosphate/IGF-II receptor to the trans-Golgi network in vitro. Cell. 1988;55:309–320. doi: 10.1016/0092-8674(88)90054-2. [DOI] [PubMed] [Google Scholar]
- Gonatas JO, Mourelatos Z, Stieber A, Lane WS, Brosius J, Gonatas NK. MG-160, a membrane sialoglycoprotein of the medial cisternae of the rat Golgi apparatus, binds basic fibroblast growth factor and exhibits a high level of sequence identity to a chicken fibroblast growth factor receptor. J Cell Sci. 1995;108:457–467. doi: 10.1242/jcs.108.2.457. [DOI] [PubMed] [Google Scholar]
- Gruenberg J, Maxfield FR. Membrane transport in the endocytic pathway. Curr Opin Cell Biol. 1995;7:552–563. doi: 10.1016/0955-0674(95)80013-1. [DOI] [PubMed] [Google Scholar]
- Helenius A, Marquardt T, Braakman I. The endoplasmic reticulum as a protein-folding compartment. Trends Cell Biol. 1992;2:227–231. doi: 10.1016/0962-8924(92)90309-b. [DOI] [PubMed] [Google Scholar]
- Hopkins CR. Intracellular routing of transferrin and transferrin receptors in epidermoid carcinoma A431 cells. Cell. 1983;35:321–330. doi: 10.1016/0092-8674(83)90235-0. [DOI] [PubMed] [Google Scholar]
- Hopkins CR, Gibson A, Shipman M, Strickland DK, Trowbridge IS. In migrating fibroblasts, recycling receptors are concentrated in narrow tubules in the pericentriolar area, and then routed to the plasma membrane of the leading lamella. J Cell Biol. 1994;125:1265–1274. doi: 10.1083/jcb.125.6.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou JZ, Kan MK, McKeehan K, McBride G, Adams P, McKeehan WL. Fibroblast growth factor receptors from liver vary in three structural domains. Science. 1991;251:665–668. doi: 10.1126/science.1846977. [DOI] [PubMed] [Google Scholar]
- Imamura T, Engleka K, Zhan X, Tokita Y, Forough R, Roeder D, Jackson A, Maier JA, Hla T, Maciag T. Recovery of mitogenic activity of a growth factor mutant with a nuclear translocation sequence. Science. 1990;249:1567–1570. doi: 10.1126/science.1699274. [DOI] [PubMed] [Google Scholar]
- Imamura T, Oka S, Tanahashi T, Okita Y. Cell cycle-dependent nuclear localization of exogenously added fibroblast growth factor-1 in BALB/c 3T3 and human vascular endothelial cells. Exp Cell Res. 1994;215:363–372. doi: 10.1006/excr.1994.1353. [DOI] [PubMed] [Google Scholar]
- Johnson DE, Lee PL, Lu J, Williams LT. Diverse forms of a receptor for acidic and basic fibroblast growth factors. Mol Cell Biol. 1990;10:4728–4736. doi: 10.1128/mcb.10.9.4728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson DE, Lu J, Chen H, Werner S, Williams LT. The human fibroblast growth factor receptor genes: a common structural arrangement underlies the mechanisms for generating receptor forms that differ in their third immunoglobulin domain. Mol Cell Biol. 1991;11:4627–4634. doi: 10.1128/mcb.11.9.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston CL, Cox HC, Gomm JJ, Coombes RC. Fibroblast growth factor receptors (FGFRs) localize in different cellular compartments: a splice variant of FGFR-3 localizes to the nucleus. J Biol Chem. 1995;270:30643–30650. doi: 10.1074/jbc.270.51.30643. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Ward CL, Kopito RR. Aggresomes: a cellular response to misfolded proteins. J Cell Biol. 1998;143:1883–1898. doi: 10.1083/jcb.143.7.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner RD, Donaldson JG, Lippincott-Schwartz J. Brefeldin A: insights into the control of membrane traffic and organelle structure. J Cell Biol. 1992;116:1071–1080. doi: 10.1083/jcb.116.5.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg O, Wiedlocha A, Rapak A, Munoz R, Falnes PO, Olsnes S. Inability of the acidic fibroblast growth factor mutant K132E to stimulate DNA synthesis after translocation into cells. J Biol Chem. 1998;273:11164–11172. doi: 10.1074/jbc.273.18.11164. [DOI] [PubMed] [Google Scholar]
- Lisanti MP, Scherer PE, Tang Z-L, Sargicomo M. Caveolae, caveolin and caveolin-rich membrane domains: a signaling hypothesis. Trends Cell Biol. 1994;4:231–235. doi: 10.1016/0962-8924(94)90114-7. [DOI] [PubMed] [Google Scholar]
- Liu J, Oh P, Horner T, Rogers RA, Schnitzer JE. Organized endothelial cell surface signal transduction in caveolae distinct from glycosylphosphatidylinositol-anchored protein microdomains. J Biol Chem. 1997;272:7211–7222. doi: 10.1074/jbc.272.11.7211. [DOI] [PubMed] [Google Scholar]
- Liu P, Ying Y, Ko YG, Anderson RG. Localization of platelet-derived growth factor-stimulated phosphorylation cascade to caveolae. J Biol Chem. 1996;271:10299–10303. doi: 10.1074/jbc.271.17.10299. [DOI] [PubMed] [Google Scholar]
- Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M. Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci USA. 1992a;89:3000–3004. doi: 10.1073/pnas.89.7.3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz RJ, Trujillo MA, Denham KS, Wenger L, Sinensky M. Nucleoplasmic localization of prelamin A: implications for prenylation-dependent lamin A assembly into the nuclear lamina. Proc Natl Acad Sci USA. 1992b;89:5699. doi: 10.1073/pnas.89.7.3000. (errata). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maher PA. Nuclear translocation of fibroblast growth factor (FGF) receptors in response to FGF-2. J Cell Biol. 1996;134:529–536. doi: 10.1083/jcb.134.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchese C, Mancini P, Belleudi F, Felici A, Gradini R, Sansolini T, Frati L, Torrisi MR. Receptor-mediated endocytosis of keratinocyte growth factor. J Cell Sci. 1998;111:3517–3527. doi: 10.1242/jcs.111.23.3517. [DOI] [PubMed] [Google Scholar]
- Mason IJ. The ins and outs of fibroblast growth factors. Cell. 1994;78:547–552. doi: 10.1016/0092-8674(94)90520-7. (review). [DOI] [PubMed] [Google Scholar]
- Michel JB, Feron O, Sacks D, Michel T. Reciprocal regulation of endothelial nitric-oxide synthase by Ca2+-calmodulin and caveolin. J Biol Chem. 1997;272:15583–15586. doi: 10.1074/jbc.272.25.15583. [DOI] [PubMed] [Google Scholar]
- Mineo C, James GL, Smart EJ, Anderson RG. Localization of epidermal growth factor-stimulated Ras/Raf-1 interaction to caveolae membrane. J Biol Chem. 1996;271:11930–11935. doi: 10.1074/jbc.271.20.11930. [DOI] [PubMed] [Google Scholar]
- Mu FT, Callaghan JM, Steele-Mortimer O, Stenmark H, Parton RG, Campbell PL, McCluskey J, Yeo JP, Tock EP, Toh BH. EEA1, an early endosome-associated protein: EEA1 is a conserved alpha-helical peripheral membrane protein flanked by cysteine “fingers” and contains a calmodulin-binding IQ motif. J Biol Chem. 1995;270:13503–13511. doi: 10.1074/jbc.270.22.13503. [DOI] [PubMed] [Google Scholar]
- Muñoz R, Klingenberg O, Wiedlocha A, Rapak A, Falnes PO, Olsnes S. Effect of mutation of cytoplasmic receptor domain and of genistein on transport of acidic fibroblast growth factor into cells. Oncogene. 1997;15:525–536. doi: 10.1038/sj.onc.1201226. [DOI] [PubMed] [Google Scholar]
- Partanen J, Makela TP, Eerola E, Korhonen J, Hirvonen H, Claesson-Welsh L, Alitalo K. FGFR-4, a novel acidic fibroblast growth factor receptor with a distinct expression pattern. EMBO J. 1991;10:1347–1354. doi: 10.1002/j.1460-2075.1991.tb07654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pessin JE, Thurmond DC, Elmendorf JS, Coker KJ, Okada S. Molecular basis of insulin-stimulated GLUT4 vesicle trafficking. Location! Location! Location! J Biol Chem. 1999;274:2593–2596. doi: 10.1074/jbc.274.5.2593. [DOI] [PubMed] [Google Scholar]
- Pol A, Ortega D, Enrich C. Identification of cytoskeleton-associated proteins in isolated rat liver endosomes. Biochem J. 1997;327:741–746. doi: 10.1042/bj3270741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prekeris R, Klumperman J, Chen YA, Scheller RH. Syntaxin 13 mediates cycling of plasma membrane proteins via tubulovesicular recycling endosomes. J Cell Biol. 1998;143:957–971. doi: 10.1083/jcb.143.4.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren M, Xu G, Zeng J, De Lemos-Chiarandini C, Adesnik M, Sabatini DD. Hydrolysis of GTP on rab11 is required for the direct delivery of transferrin from the pericentriolar recycling compartment to the cell surface but not from sorting endosomes. Proc Natl Acad Sci USA. 1998;95:6187–6192. doi: 10.1073/pnas.95.11.6187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg KG, Ying YS, Kamen BA, Anderson RG. Cholesterol controls the clustering of the glycophospholipid-anchored membrane receptor for 5-methyltetrahydrofolate. J Cell Biol. 1990;111:2931–2938. doi: 10.1083/jcb.111.6.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig K, Olsnes S, Petersen OW, van Deurs B. Acidification of the cytosol inhibits endocytosis from coated pits. J Cell Biol. 1987;105:679–689. doi: 10.1083/jcb.105.2.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997a;137:445–458. doi: 10.1083/jcb.137.2.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt A, Hannah MJ, Huttner WB. Synaptic-like microvesicles of neuroendocrine cells originate from a novel compartment that is continuous with the plasma membrane and devoid of transferrin receptor. J Cell Biol. 1997b;137:1197. doi: 10.1083/jcb.137.2.445. (errata). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzer JE, Liu J, Oh P. Endothelial caveolae have the molecular transport machinery for vesicle budding, docking, and fusion including VAMP, NSF, SNAP, annexins, and GTPases. J Biol Chem. 1995a;270:14399–14404. doi: 10.1074/jbc.270.24.14399. [DOI] [PubMed] [Google Scholar]
- Schnitzer JE, McIntosh DP, Dvorak AM, Liu J, Oh P. Separation of caveolae from associated microdomains of GPI- anchored proteins. Science. 1995b;269:1435–1439. doi: 10.1126/science.7660128. [DOI] [PubMed] [Google Scholar]
- Shepherd PR, Reaves BJ, Davidson HW. Phosphoinositide 3-kinase and membrane traffic. Trends Cell Biol. 1996;6:92–97. doi: 10.1016/0962-8924(96)80998-6. [DOI] [PubMed] [Google Scholar]
- Sinensky M, Fantle K, Trujillo M, McLain T, Kupfer A, Dalton M. The processing pathway of prelamin A. J Cell Sci. 1994;107:61–67. doi: 10.1242/jcs.107.1.61. [DOI] [PubMed] [Google Scholar]
- Smart EJ, Ying YS, Mineo C, Anderson RG. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci USA. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song SK, Li S, Okamoto T, Quilliam LA, Sargiacomo M, Lisanti MP. Copurification and direct interaction of Ras with caveolin, an integral membrane protein of caveolae microdomains: detergent-free purification of caveolae microdomains. J Biol Chem. 1996;271:9690–9697. doi: 10.1074/jbc.271.16.9690. [DOI] [PubMed] [Google Scholar]
- Sorokin A, Mohammadi M, Huang J, Schlessinger J. Internalization of fibroblast growth factor receptor is inhibited by a point mutation at tyrosine 766. J Biol Chem. 1994;269:17056–17061. [PubMed] [Google Scholar]
- Spiro DJ, Boll W, Kirchhausen T, Wessling-Resnick M. Wortmannin alters the transferrin receptor endocytic pathway in vivo and in vitro. Mol Biol Cell. 1996;7:355–367. doi: 10.1091/mbc.7.3.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak EK, Maher PA, Tucholski J, Mordechai E, Joy A, Moffett J, Coons S, Stachowiak MK. Nuclear accumulation of fibroblast growth factor receptors in human glial cells: association with cell proliferation. Oncogene. 1997;14:2201–2211. doi: 10.1038/sj.onc.1201057. [DOI] [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Joy A, Mordechai E, Stachowiak EK. Nuclear accumulation of fibroblast growth factor receptors is regulated by multiple signals in adrenal medullary cells. Mol Biol Cell. 1996a;7:1299–1317. doi: 10.1091/mbc.7.8.1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stachowiak MK, Maher PA, Joy A, Mordechai E, Stachowiak EK. Nuclear localization of functional FGF receptor 1 in human astrocytes suggests a novel mechanism for growth factor action. Mol Brain Res. 1996b;38:161–165. doi: 10.1016/0169-328x(96)00010-1. [DOI] [PubMed] [Google Scholar]
- van Deurs B, Petersen OW, Olsnes S, Sandvig K. The ways of endocytosis. Int Rev Cytol. 1989;117:131–177. doi: 10.1016/s0074-7696(08)61336-4. [DOI] [PubMed] [Google Scholar]
- Waugh MG, Lawson D, Hsuan JJ. Epidermal growth factor receptor activation is localized within low-buoyant density, noncaveolar membrane domains. Biochem J. 1999;337:591–597. [PMC free article] [PubMed] [Google Scholar]
- Wei ML, Bonzelius F, Scully RM, Kelly RB, Herman GA. GLUT4 and transferrin receptor are differentially sorted along the endocytic pathway in CHO cells. J Cell Biol. 1998;140:565–575. doi: 10.1083/jcb.140.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedlocha A, Falnes PO, Madshus IH, Sandvig K, Olsnes S. Dual mode of signal transduction by externally added acidic fibroblast growth factor. Cell. 1994;76:1039–1051. doi: 10.1016/0092-8674(94)90381-6. [DOI] [PubMed] [Google Scholar]
- Wiedlocha A, Falnes PO, Rapak A, Klingenberg O, Muñoz R, Olsnes S. Translocation to cytosol of exogenous, CAAX-tagged acidic fibroblast growth factor. J Biol Chem. 1995;270:30680–30685. doi: 10.1074/jbc.270.51.30680. [DOI] [PubMed] [Google Scholar]
- Wiedlocha A, Falnes PO, Rapak A, Muñoz R, Klingenberg O, Olsnes S. Stimulation of proliferation of a human osteosarcoma cell line by exogenous acidic fibroblast growth factor requires both activation of receptor tyrosine kinase and growth factor internalization. Mol Cell Biol. 1996;16:270–280. doi: 10.1128/mcb.16.1.270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Toya Y, Jensen RA, Ishikawa Y. Caveolin is an inhibitor of platelet-derived growth factor receptor signaling. Exp Cell Res. 1999;247:380–388. doi: 10.1006/excr.1998.4379. [DOI] [PubMed] [Google Scholar]
- Zhan X, Hu X, Friedman S, Maciag T. Analysis of endogenous and exogenous nuclear translocation of fibroblast growth factor-1 in NIH 3T3 cells. Biochem Biophys Res Commun. 1992;188:982–991. doi: 10.1016/0006-291x(92)91328-n. [DOI] [PubMed] [Google Scholar]
- Zhan X, Hu X, Friesel R, Maciag T. Long term growth factor exposure and differential tyrosine phosphorylation are required for DNA synthesis in BALB/c 3T3 cells. J Biol Chem. 1993;268:9611–9620. [PubMed] [Google Scholar]