Abstract
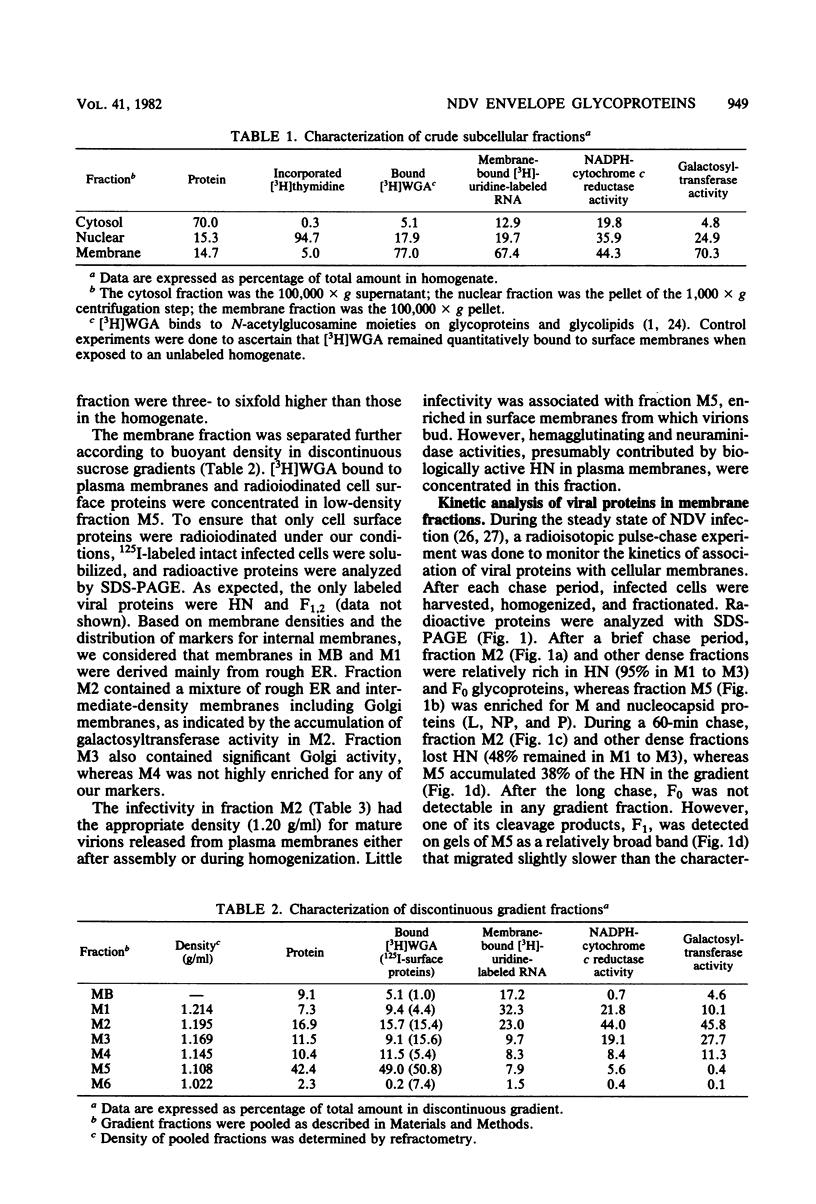
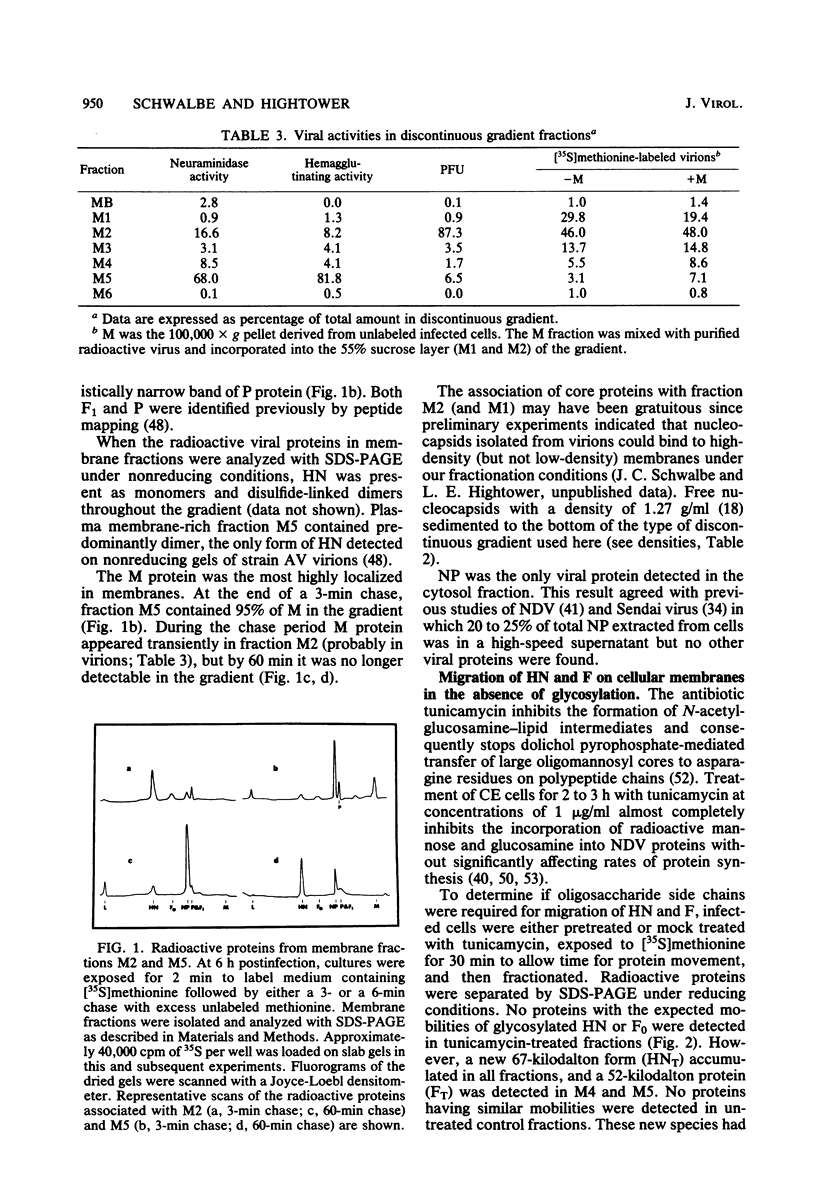
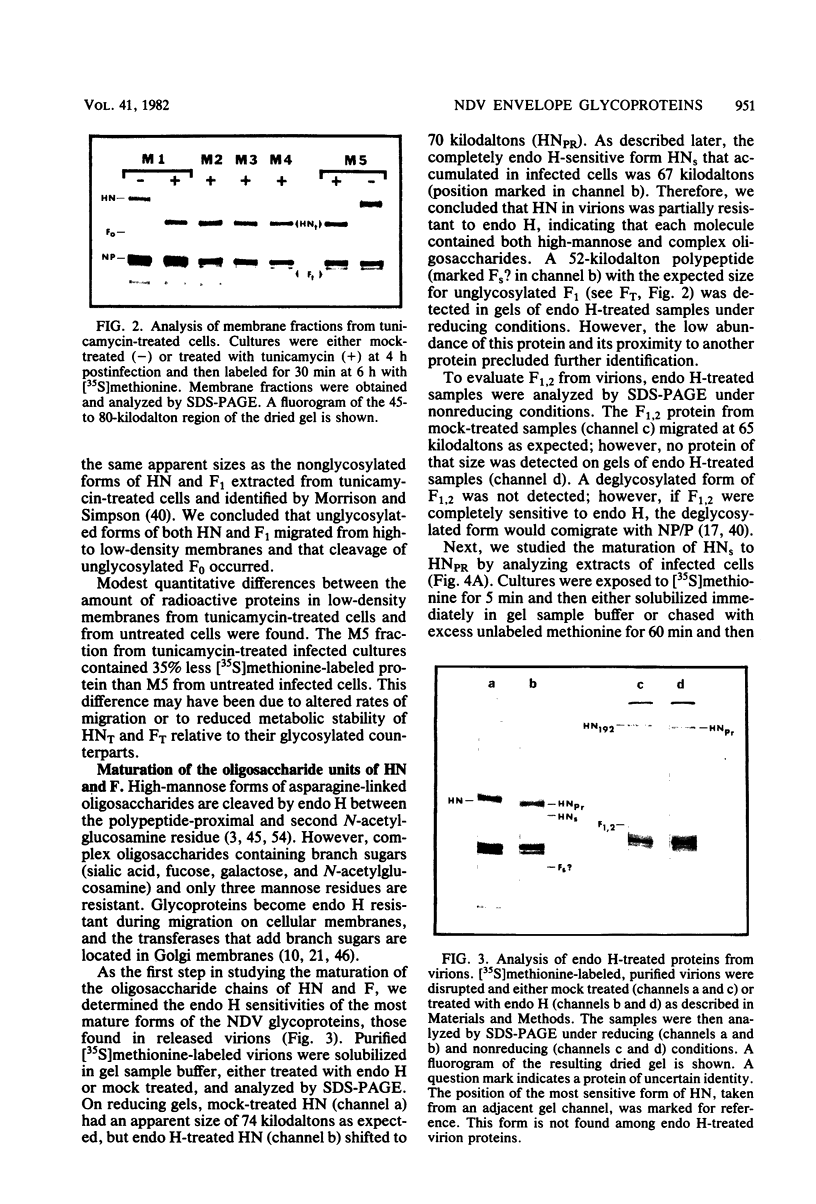
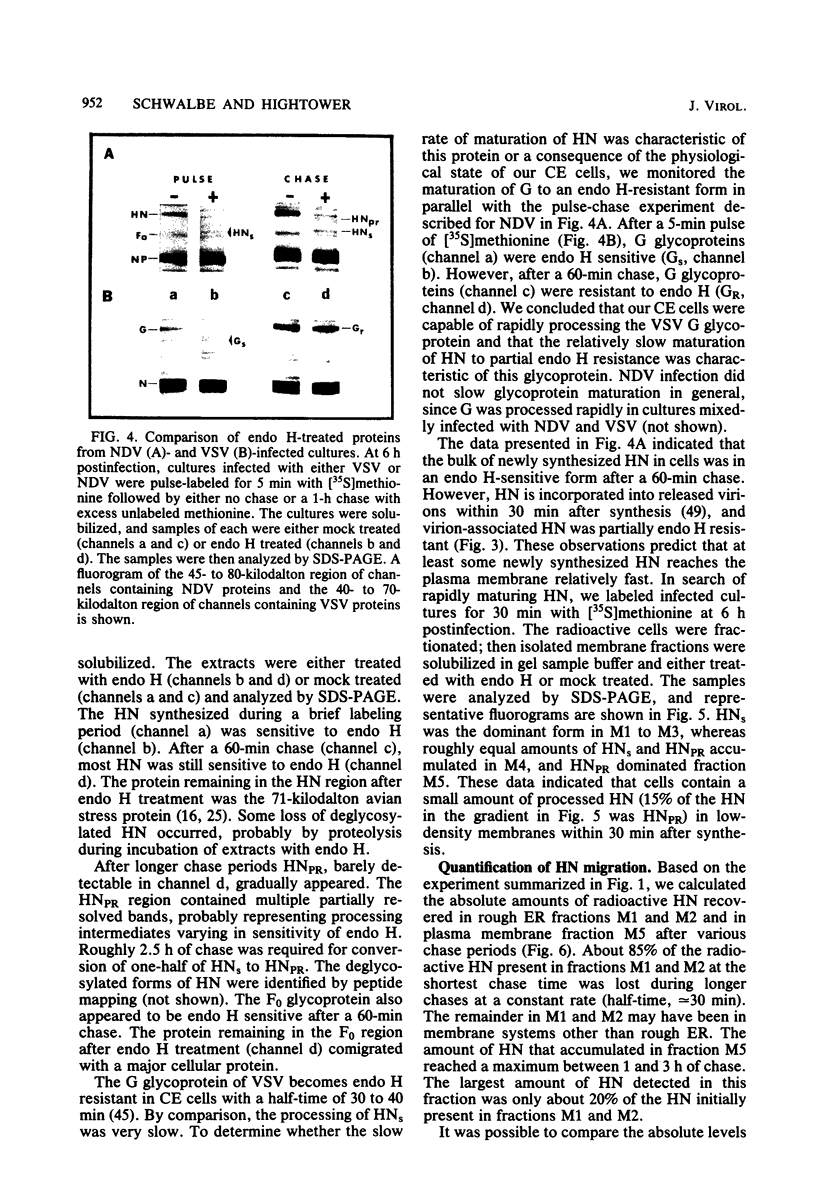
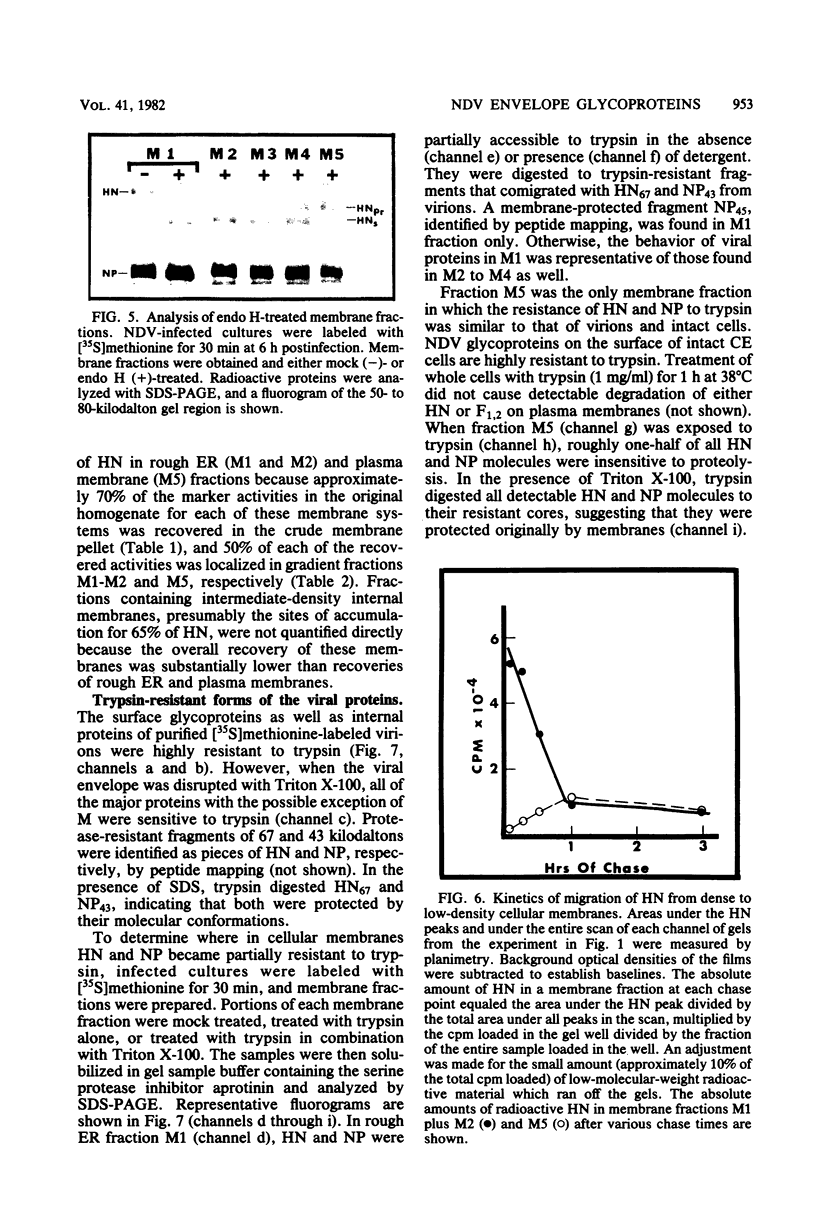
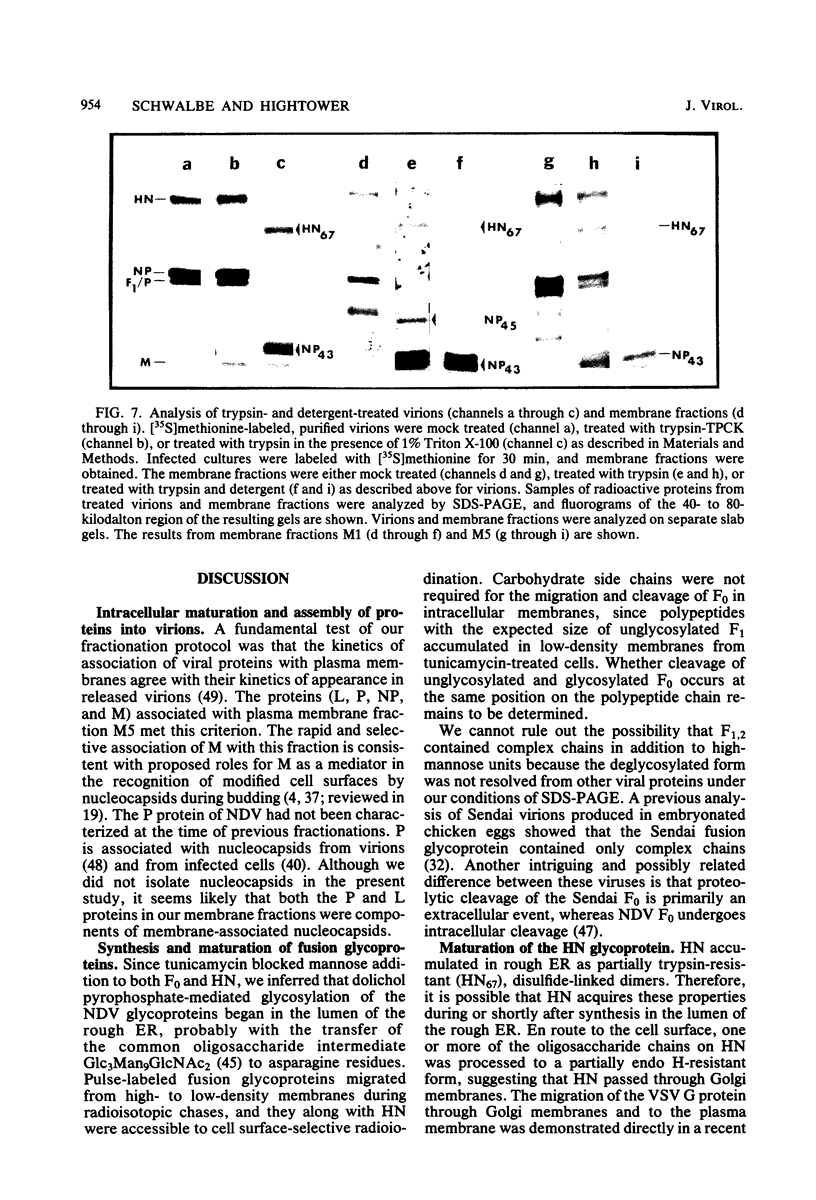
Based on subcellular fractionation data, the following maturation pathways were proposed for the Newcastle disease virus glycoproteins. During or shortly after synthesis in rough endoplasmic reticulum, hemagglutinin-neuraminidase (HN) and fusion (F0) glycoproteins underwent dolichol pyrophosphate-mediated glycosylation, and HN assumed a partially trypsin-resistant conformation. HN began to associate into disulfide-linked dimers in rough endoplasmic reticulum, and at least one of its oligosaccharide side chains was processed to a complex form en route to the cell surface. During migration in intracellular membranes, F0 was proteolytically cleaved to F1.2. Neither HN nor F1,2 required oligosaccharide side chains for migration to plasma membranes, and cleavage of F0 also occurred without glycosylation. Virion- and plasma membrane-associated HN contained both complex and high-mannose oligosaccharide chains on the same molecule, and F1,2 contained at least high-mannose forms. Several of the properties of HN were notable for a viral glycoprotein. The oligosaccharide side chains of HN were modified very slowly in chick cells, whereas those of the G glycoprotein of vesicular stomatitis virus were rapidly processed to a complex form. Therefore, their different rates of migration and carbohydrate processing were intrinsic properties of these glycoproteins. Consistent with its slow maturation, the HN glycopolypeptide accumulated to high levels in intracellular membranes as well as in plasma membranes. Intracellular HN contained immature oligosaccharide side chains, suggesting that it accumulated in the pre-Golgi/Golgi segment of the maturation pathway. The major site of accumulation of mature HN with neuraminidase activity was the plasma membrane.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMINOFF D. Methods for the quantitative estimation of N-acetylneuraminic acid and their application to hydrolysates of sialomucoids. Biochem J. 1961 Nov;81:384–392. doi: 10.1042/bj0810384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A. K., Neuberger A., Sharon N. The purification, composition and specificity of wheat-germ agglutinin. Biochem J. 1973 Jan;131(1):155–162. doi: 10.1042/bj1310155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa M., Muramatsu T. Endo-beta-N-acetylglucosaminidases acting on the carbohydrate moieties of glycoproteins. The differential specificities of the enzymes from Streptomyces griseus and Diplococcus pneumoniae. J Biochem. 1974 Aug;76(2):307–317. doi: 10.1093/oxfordjournals.jbchem.a130572. [DOI] [PubMed] [Google Scholar]
- BANG F. B. The development of the virus of Newcastle disease in epithelial and fibroblast cells in tissue culture. Bull Johns Hopkins Hosp. 1953 Apr;92(4):291–307. [PubMed] [Google Scholar]
- Ball L. A., White C. N. Order of transcription of genes of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1976 Feb;73(2):442–446. doi: 10.1073/pnas.73.2.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann J. E., Tokuyasu K. T., Singer S. J. Passage of an integral membrane protein, the vesicular stomatitis virus glycoprotein, through the Golgi apparatus en route to the plasma membrane. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1746–1750. doi: 10.1073/pnas.78.3.1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen H. A., Lyles D. S. Structure of Sendai viral proteins in plasma membranes of virus-infected cells. J Virol. 1981 Mar;37(3):1079–1082. doi: 10.1128/jvi.37.3.1079-1082.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Bretz R., Bretz H., Palade G. E. Distribution of terminal glycosyltransferases in hepatic Golgi fractions. J Cell Biol. 1980 Jan;84(1):87–101. doi: 10.1083/jcb.84.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bächi T. Intramembrane structural differentiation in Sendai virus maturation. Virology. 1980 Oct 15;106(1):41–49. doi: 10.1016/0042-6822(80)90219-6. [DOI] [PubMed] [Google Scholar]
- Chambers P., Samson A. C. A new structural protein for Newcastle disease virus. J Gen Virol. 1980 Sep;50(1):155–166. doi: 10.1099/0022-1317-50-1-155. [DOI] [PubMed] [Google Scholar]
- Chatis P. A., Morrison T. G. Mutational changes in the vesicular stomatitis virus glycoprotein affect the requirement of carbohydrate in morphogenesis. J Virol. 1981 Jan;37(1):307–316. doi: 10.1128/jvi.37.1.307-316.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcription and translation of Newcastle disease virus mRNA's in vitro. J Virol. 1978 Oct;28(1):324–336. doi: 10.1128/jvi.28.1.324-336.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins P. L., Hightower L. E., Ball L. A. Transcriptional map for Newcastle disease virus. J Virol. 1980 Sep;35(3):682–693. doi: 10.1128/jvi.35.3.682-693.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonno R. J., Stone H. O. Isolation of a transcriptive complex from Newcastle disease virions. J Virol. 1976 Sep;19(3):1080–1089. doi: 10.1128/jvi.19.3.1080-1089.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePierre J. W., Ernster L. Enzyme topology of intracellular membranes. Annu Rev Biochem. 1977;46:201–262. doi: 10.1146/annurev.bi.46.070177.001221. [DOI] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S., Ozawa H. Isolation and characterization of Golgi membranes from bovine liver. J Cell Biol. 1969 Oct;43(1):59–79. doi: 10.1083/jcb.43.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer B., Fleischer S. Preparation and characterization of golgi membranes from rat liver. Biochim Biophys Acta. 1970 Dec 1;219(2):301–319. doi: 10.1016/0005-2736(70)90209-9. [DOI] [PubMed] [Google Scholar]
- Gibson R., Schlesinger S., Kornfeld S. The nonglycosylated glycoprotein of vesicular stomatitis virus is temperature-sensitive and undergoes intracellular aggregation at elevated temperatures. J Biol Chem. 1979 May 10;254(9):3600–3607. [PubMed] [Google Scholar]
- Hakomori S. I., Murakami W. T. Glycolipids of hamster fibroblasts and derived malignant-transformed cell lines. Proc Natl Acad Sci U S A. 1968 Jan;59(1):254–261. doi: 10.1073/pnas.59.1.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein metabolism during the steady state of Newcastle disease virus infection. I. Kinetics of amino acid and protein accumulation. J Virol. 1975 Apr;15(4):696–706. doi: 10.1128/jvi.15.4.696-706.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E., Bratt M. A. Protein synthesis in Newcastle disease virus-infected chicken embryo cells. J Virol. 1974 Apr;13(4):788–800. doi: 10.1128/jvi.13.4.788-800.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hightower L. E. Cultured animal cells exposed to amino acid analogues or puromycin rapidly synthesize several polypeptides. J Cell Physiol. 1980 Mar;102(3):407–427. doi: 10.1002/jcp.1041020315. [DOI] [PubMed] [Google Scholar]
- Hubbard A. L., Cohn Z. A. The enzymatic iodination of the red cell membrane. J Cell Biol. 1972 Nov;55(2):390–405. doi: 10.1083/jcb.55.2.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Summers D. F. Association of vesicular stomatitis virus proteins with HeLa cell membranes and released virus. J Virol. 1976 Dec;20(3):637–645. doi: 10.1128/jvi.20.3.637-645.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KILHAM L., MORGAN C., WYCKOFF R. W. G. The electron microscopy of chick embryo membranes infected with Newcastle disease. J Immunol. 1951 Dec;67(6):523–528. [PubMed] [Google Scholar]
- Knipe D. M., Baltimore D., Lodish H. F. Separate pathways of maturation of the major structural proteins of vesicular stomatitis virus. J Virol. 1977 Mar;21(3):1128–1139. doi: 10.1128/jvi.21.3.1128-1139.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohama T., Shimizu K., Ishida N. Carbohydrate composition of the envelope glycoproteins of Sendai virus. Virology. 1978 Oct 15;90(2):226–234. doi: 10.1016/0042-6822(78)90306-9. [DOI] [PubMed] [Google Scholar]
- Lamb R. A., Choppin P. W. The synthesis of Sendai virus polypeptides in infected cells. II. Intracellular distribution of polypeptides. Virology. 1977 Sep;81(2):371–381. doi: 10.1016/0042-6822(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Lyles D. S. Glycoproteins of Sendai virus are transmembrane proteins. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5621–5625. doi: 10.1073/pnas.76.11.5621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwell M. A., Fox C. F. Protein-protein interactions within paramyxoviruses identified by native disulfide bonding or reversible chemical cross-linking. J Virol. 1980 Jan;33(1):152–166. doi: 10.1128/jvi.33.1.152-166.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merz D. C., Prehm P., Scheid A., Choppin P. W. Inhibition of the neuraminidase of paramyxoviruses by halide ions: a possible means of modulating the two activities of the HN protein. Virology. 1981 Jul 15;112(1):296–305. doi: 10.1016/0042-6822(81)90635-8. [DOI] [PubMed] [Google Scholar]
- Morrison T. G., Simpson D. Synthesis, stability, and cleavage of Newcastle disease virus glycoproteins in the absence of glycosylation. J Virol. 1980 Oct;36(1):171–180. doi: 10.1128/jvi.36.1.171-180.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai Y., Ogura H., Klenk H. Studies on the assembly of the envelope of Newcastle disease virus. Virology. 1976 Feb;69(2):523–538. doi: 10.1016/0042-6822(76)90482-7. [DOI] [PubMed] [Google Scholar]
- Nakamura K., Compans R. W. Effects of glucosamine, 2-deoxyglucose, and tunicamycin on glycosylation, sulfation, and assembly of influenza viral proteins. Virology. 1978 Feb;84(2):303–319. doi: 10.1016/0042-6822(78)90250-7. [DOI] [PubMed] [Google Scholar]
- Prehm P., Scheid A., Choppin P. W. The carbohydrate structure of the glycoproteins of the paramyxovirus SV5 grown in bovine kidney cells. J Biol Chem. 1979 Oct 10;254(19):9669–9677. [PubMed] [Google Scholar]
- Ragnotti G., Lawford G. R., Campbell P. N. Biosynthesis of microsomal nicotinamide-adenine dinucleotide phosphate-cytochrome c reductase by membrane-bound and free polysomes from rat liver. Biochem J. 1969 Apr;112(2):139–147. doi: 10.1042/bj1120139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins P. W., Hubbard S. C., Turco S. J., Wirth D. F. Proposal for a common oligosaccharide intermediate in the synthesis of membrane glycoproteins. Cell. 1977 Dec;12(4):893–900. doi: 10.1016/0092-8674(77)90153-2. [DOI] [PubMed] [Google Scholar]
- Schachter H., Jabbal I., Hudgin R. L., Pinteric L., McGuire E. J., Roseman S. Intracellular localization of liver sugar nucleotide glycoprotein glycosyltransferases in a Golgi-rich fraction. J Biol Chem. 1970 Mar 10;245(5):1090–1100. [PubMed] [Google Scholar]
- Seto J. T., Garten W., Rott R. The site of cleavage in infected cells and polypeptides of representative paramyxoviruses grown in cultured cells of the chorioallantoic membrane. Arch Virol. 1981;67(1):19–30. doi: 10.1007/BF01314598. [DOI] [PubMed] [Google Scholar]
- Smith G. W., Hightower L. E. Identification of the P proteins and other disulfide-linked and phosphorylated proteins of Newcastle disease virus. J Virol. 1981 Jan;37(1):256–267. doi: 10.1128/jvi.37.1.256-267.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith G. W., Schwalbe J. C., Hightower L. E. Maturation of the hemagglutinin-neuraminidase and fusion glycoproteins of two biologically distinct strains of Newcastle disease virus. Prog Clin Biol Res. 1980;40:275–291. [PubMed] [Google Scholar]
- Strous G. J., Lodish H. F. Intracellular transport of secretory and membrane proteins in hepatoma cells infected by vesicular stomatitis virus. Cell. 1980 Dec;22(3):709–717. doi: 10.1016/0092-8674(80)90547-4. [DOI] [PubMed] [Google Scholar]
- Takatsuki A., Tamura G. Effect of tunicamycin on the synthesis of macromolecules in cultures of chick embryo fibroblasts infected with Newcastle disease virus. J Antibiot (Tokyo) 1971 Nov;24(11):785–794. doi: 10.7164/antibiotics.24.785. [DOI] [PubMed] [Google Scholar]
- Tarentino A. L., Maley F. Purification and properties of an endo-beta-N-acetylglucosaminidase from Streptomyces griseus. J Biol Chem. 1974 Feb 10;249(3):811–817. [PubMed] [Google Scholar]
- WALLACH D. F., KAMAT V. B. PLASMA AND CYTOPLASMIC MEMBRANE FRAGMENTS FROM EHRLICH ASCITES CARCINOMA. Proc Natl Acad Sci U S A. 1964 Sep;52:721–728. doi: 10.1073/pnas.52.3.721. [DOI] [PMC free article] [PubMed] [Google Scholar]