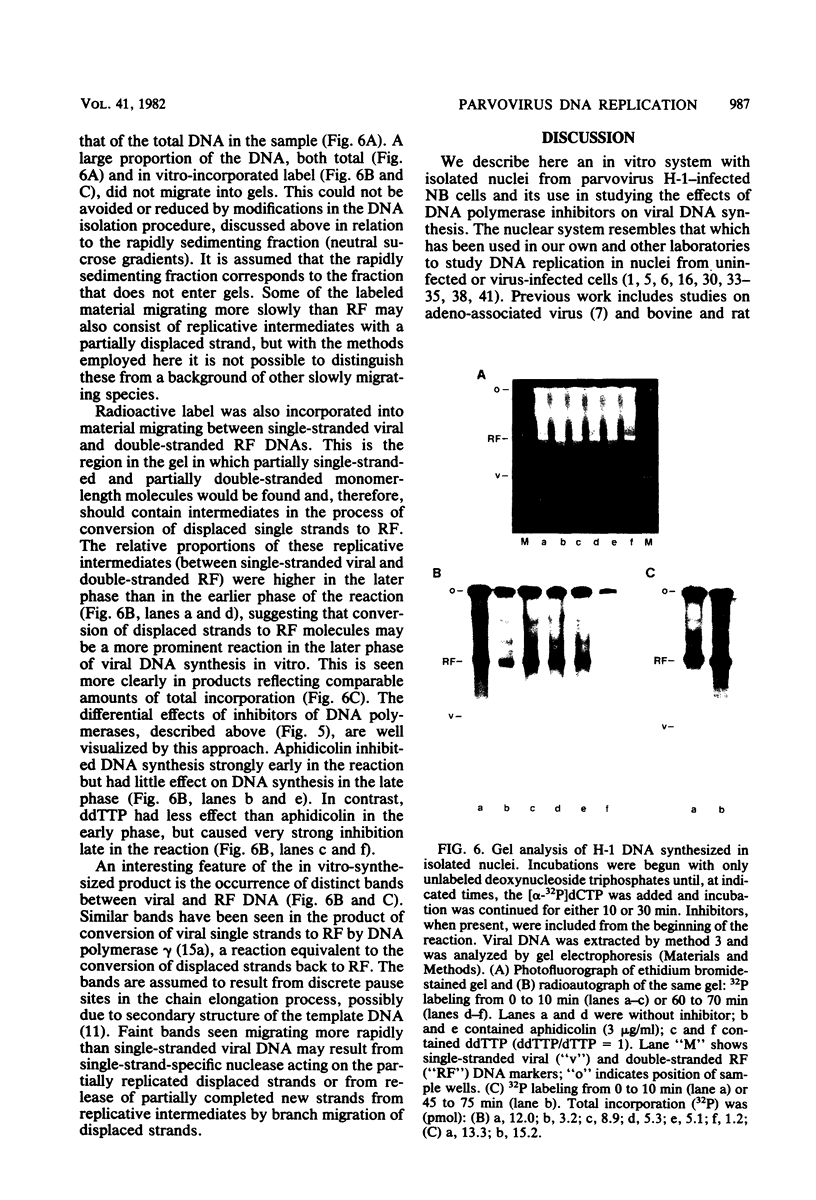
Abstract
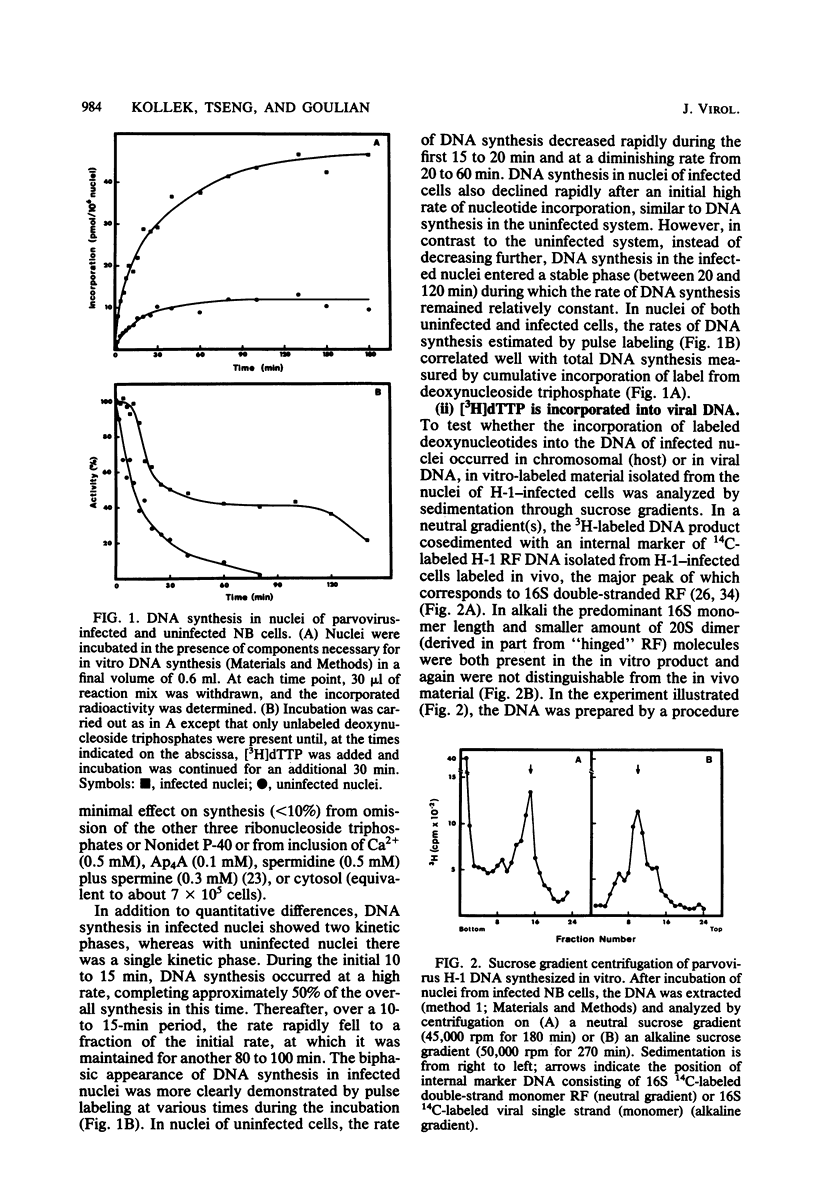
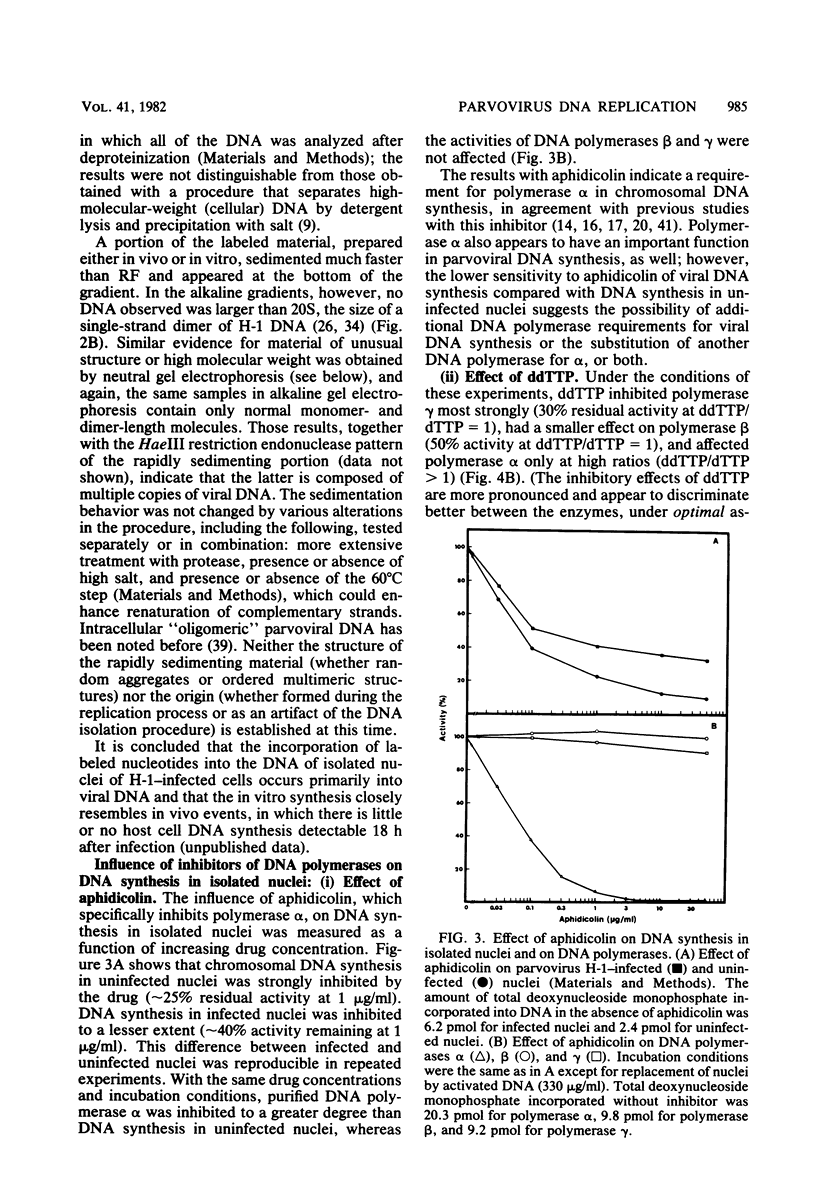
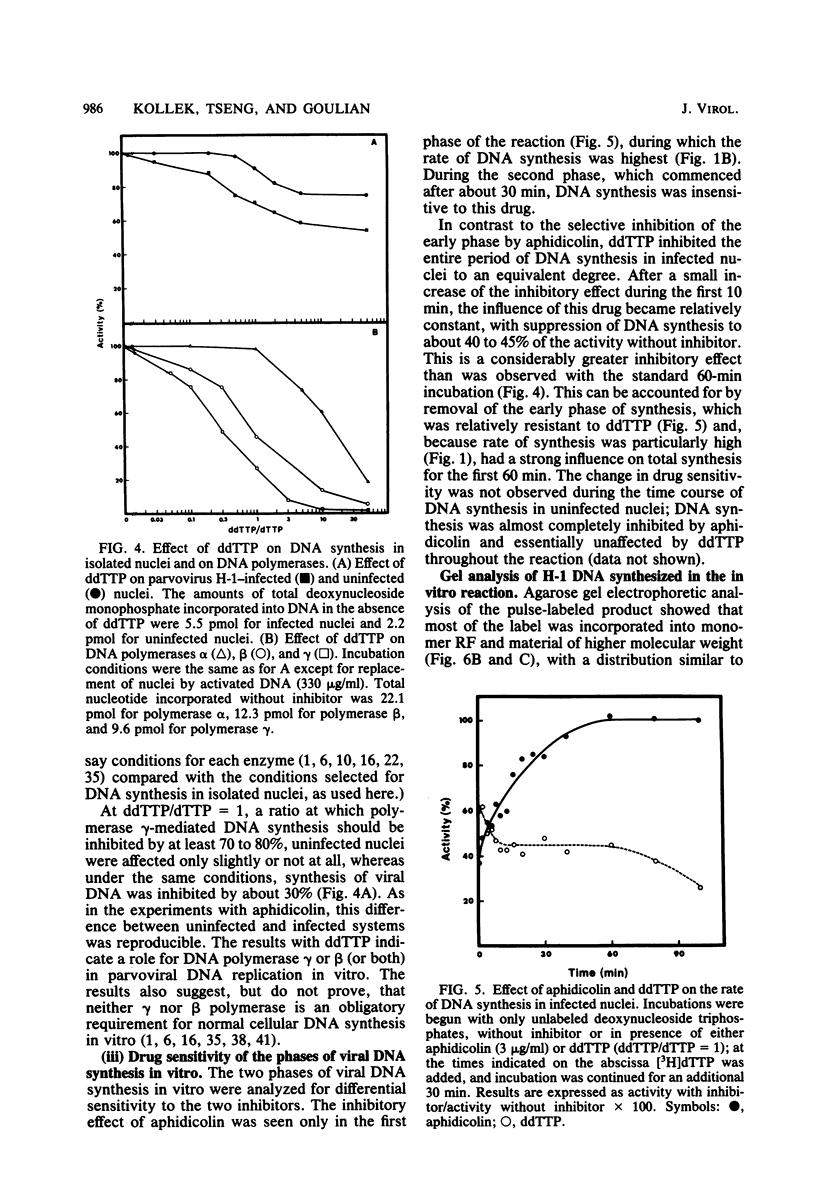
An in vitro system using nuclei from parvovirus H-1-infected cells was used to characterize the influence of inhibitors of mammalian DNA polymerases on viral DNA synthesis. The experiments tested the effects of aphidicolin, which is highly specific for DNA polymerase alpha, and 2',3'-dideoxythymidine-5'-triphosphate (ddTTP), which inhibits cellular DNA polymerases in the order gamma greater than beta greater than alpha. Both aphidicolin and ddTTP were inhibitory, indicating that both polymerase alpha and a ddttp-sensitive enzyme are required for viral DNA synthesis. This was seen more clearly in kinetic measurements, which indicated an initial period of rapid DNA synthesis with the participation of polymerase alpha, followed by a period of less rapid, but more sustained, rate of DNA synthesis carried out by a ddTTP-sensitive enzyme, probably polymerase gamma. One interpretation of the results is that polymerase alpha functions in a strand displacement stage of the viral DNA replication mechanism, whereas polymerase gamma serves to convert the displaced single strands back to double-strand replicative form.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abboud M. M., Horwitz M. S. The DNA polymerases associated with the adenovirus type 2 replication complex: effect of 2'-3'-dideoxythymidine-5'-triphosphate on viral DNA synthesis. Nucleic Acids Res. 1979 Mar;6(3):1025–1039. doi: 10.1093/nar/6.3.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brison O., Kedinger C., Wilhelm J. Enzymatic properties of viral replication complexes isolated from adenovirus type 2-infected HeLa cell nuclei. J Virol. 1977 Nov;24(2):423–435. doi: 10.1128/jvi.24.2.423-435.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Wassarman P. M. Replication of eukaryotic chromosomes: a close-up of the replication fork. Annu Rev Biochem. 1980;49:627–666. doi: 10.1146/annurev.bi.49.070180.003211. [DOI] [PubMed] [Google Scholar]
- Edenberg H. J., Anderson S., DePamphilis M. L. Involvement of DNA polymerase alpha in simian virus 40 DNA replication. J Biol Chem. 1978 May 10;253(9):3273–3280. [PubMed] [Google Scholar]
- Handa H., Carter B. J. Adeno-associated virus DNA replication complexes in herpes simplex virus or adenovirus-infected cells. J Biol Chem. 1979 Jul 25;254(14):6603–6610. [PubMed] [Google Scholar]
- Hauswirth W. W., Berns K. I. Origin and termination of adeno-associated virus DNA replication. Virology. 1977 May 15;78(2):488–499. doi: 10.1016/0042-6822(77)90125-8. [DOI] [PubMed] [Google Scholar]
- Holmes A. M. Studies on the inhibition of highly purified calf thymus 8S and 7.3S DNA polymerase alpha by aphidicolin. Nucleic Acids Res. 1981 Jan 10;9(1):161–168. doi: 10.1093/nar/9.1.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C. C., Hearst J. E. Pauses at positions of secondary structure during in vitro replication of single-stranded fd bacteriophage DNA by T4 DNA polymerase. Anal Biochem. 1980 Mar 15;103(1):127–139. doi: 10.1016/0003-2697(80)90246-8. [DOI] [PubMed] [Google Scholar]
- Ikeda J. E., Enomoto T., Hurwitz J. Replication of adenovirus DNA-protein complex with purified proteins. Proc Natl Acad Sci U S A. 1981 Feb;78(2):884–888. doi: 10.1073/pnas.78.2.884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda J. E., Longiaru M., Horwitz M. S., Hurwitz J. Elongation of primed DNA templates by eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1980 Oct;77(10):5827–5831. doi: 10.1073/pnas.77.10.5827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami S., Taguchi T., Ohashi M., Oguro M., Nagano H., Mano Y. Aphidicolin prevents mitotic cell division by interfering with the activity of DNA polymerase-alpha. Nature. 1978 Oct 5;275(5679):458–460. doi: 10.1038/275458a0. [DOI] [PubMed] [Google Scholar]
- Kaplan L. M., Kelinman R. E., Horwitz M. S. Replication of adenovirus type 2 DNA in vitro. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4425–4429. doi: 10.1073/pnas.74.10.4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollek R., Goulian M. Synthesis of parvovirus H-1 replicative form from viral DNA by DNA polymerase gamma. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6206–6210. doi: 10.1073/pnas.78.10.6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krokan H., Schaffer P., DePamphilis M. L. Involvement of eucaryotic deoxyribonucleic acid polymerases alpha and gamma in the replication of cellular and viral deoxyribonucleic acid. Biochemistry. 1979 Oct 2;18(20):4431–4443. doi: 10.1021/bi00587a025. [DOI] [PubMed] [Google Scholar]
- Kwant M. M., van der Vliet P. C. Differential effect of aphidicolin on adenovirus DNA synthesis and cellular DNA synthesis. Nucleic Acids Res. 1980 Sep 11;8(17):3993–4007. doi: 10.1093/nar/8.17.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner R. L., Kelly T. J., Jr The structure of replicating adenovirus 2 DNA molecules. Cell. 1977 Dec;12(4):1007–1020. doi: 10.1016/0092-8674(77)90165-9. [DOI] [PubMed] [Google Scholar]
- Longiaru M., Ikeda J. E., Jarkovsky Z., Horwitz S. B., Horwitz M. S. The effect of aphidicolin on adenovirus DNA synthesis. Nucleic Acids Res. 1979 Jul 25;6(10):3369–3386. doi: 10.1093/nar/6.10.3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak B., Baril E. F. HeLa DNA polymerase alpha activity in vitro: specific stimulation by a non-enzymic protein factor. Nucleic Acids Res. 1978 Jan;5(1):221–239. doi: 10.1093/nar/5.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Ogasawara M., Matsukage A. Inhibition of the activity of DNA polymerase alpha by 2',3'-dideoxythymidine 5'-triphosphate. Biochem Biophys Res Commun. 1979 Jun 27;88(4):1255–1262. doi: 10.1016/0006-291x(79)91115-x. [DOI] [PubMed] [Google Scholar]
- Rekosh D. M., Russell W. C., Bellet A. J., Robinson A. J. Identification of a protein linked to the ends of adenovirus DNA. Cell. 1977 Jun;11(2):283–295. doi: 10.1016/0092-8674(77)90045-9. [DOI] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salzman L. A. Evidence for terminal S1-nuclease-resistant regions on single-stranded linear DNA. Virology. 1977 Jan;76(1):454–458. doi: 10.1016/0042-6822(77)90322-1. [DOI] [PubMed] [Google Scholar]
- Schlabach A., Fridlender B., Bolden A., Weissbach A. DNA-dependent DNA polymerases from HeLa cell nuclei. II. Template and substrate utilization. Biochem Biophys Res Commun. 1971 Aug 20;44(4):879–885. doi: 10.1016/0006-291x(71)90793-5. [DOI] [PubMed] [Google Scholar]
- Sheinin R., Humbert J. Some aspects of eukaryotic DNA replication. Annu Rev Biochem. 1978;47:277–316. doi: 10.1146/annurev.bi.47.070178.001425. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Goulian M. DNA synthesis in human lymphocyts: intermediates in DNA synthesis, in vitro and in vivo. J Mol Biol. 1975 Dec 5;99(2):317–337. doi: 10.1016/s0022-2836(75)80149-5. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Grafstrom R. H., Revie D., Oertel W., Goulian M. Studies on early intermediates in the synthesis of DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):263–270. doi: 10.1101/sqb.1979.043.01.032. [DOI] [PubMed] [Google Scholar]
- Waqar M. A., Evans M. J., Huberman J. A. Effect of 2',3'-dideoxythymidine-5'-triphosphate on HeLa cell in vitro DNA synthesis: evidence that DNA polymerase alpha is the only polymerase required for cellular DNA replication. Nucleic Acids Res. 1978 Jun;5(6):1933–1946. doi: 10.1093/nar/5.6.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winnacker E. L. Adenovirus DNA: structure and function of a novel replicon. Cell. 1978 Aug;14(4):761–773. doi: 10.1016/0092-8674(78)90332-x. [DOI] [PubMed] [Google Scholar]
- Wist E., Prydz H. The effect of aphidicolin on DNA synthesis in isolated HeLa cell nuclei. Nucleic Acids Res. 1979 Apr;6(4):1583–1590. doi: 10.1093/nar/6.4.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Arens M., Green M. Adenovirus deoxyribonucleic acid replication. Isolation of a soluble replication system and analysis of the in vitro DNA product. J Biol Chem. 1977 Nov 25;252(22):7940–7946. [PubMed] [Google Scholar]
- van der Vliet P. C., Kwant M. M. Role of DNA polymerase gamma in adenovirus DNA replication. Mechanism of inhibition by 2',3'-dideoxynucleoside 5'-triphosphates. Biochemistry. 1981 Apr 28;20(9):2628–2632. doi: 10.1021/bi00512a041. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Kwant M. M. Role of DNA polymerase gamma in adenovirus DNA replication. Nature. 1978 Nov 30;276(5687):532–534. doi: 10.1038/276532a0. [DOI] [PubMed] [Google Scholar]
- van der Werf S., Bouché J. P., Méchali M., Girard M. Involvement of both DNA polymerases alpha and gamma in the replication of adenovirus deoxyribonucleic acid in vitro. Virology. 1980 Jul 15;104(1):56–72. doi: 10.1016/0042-6822(80)90365-7. [DOI] [PubMed] [Google Scholar]