Abstract
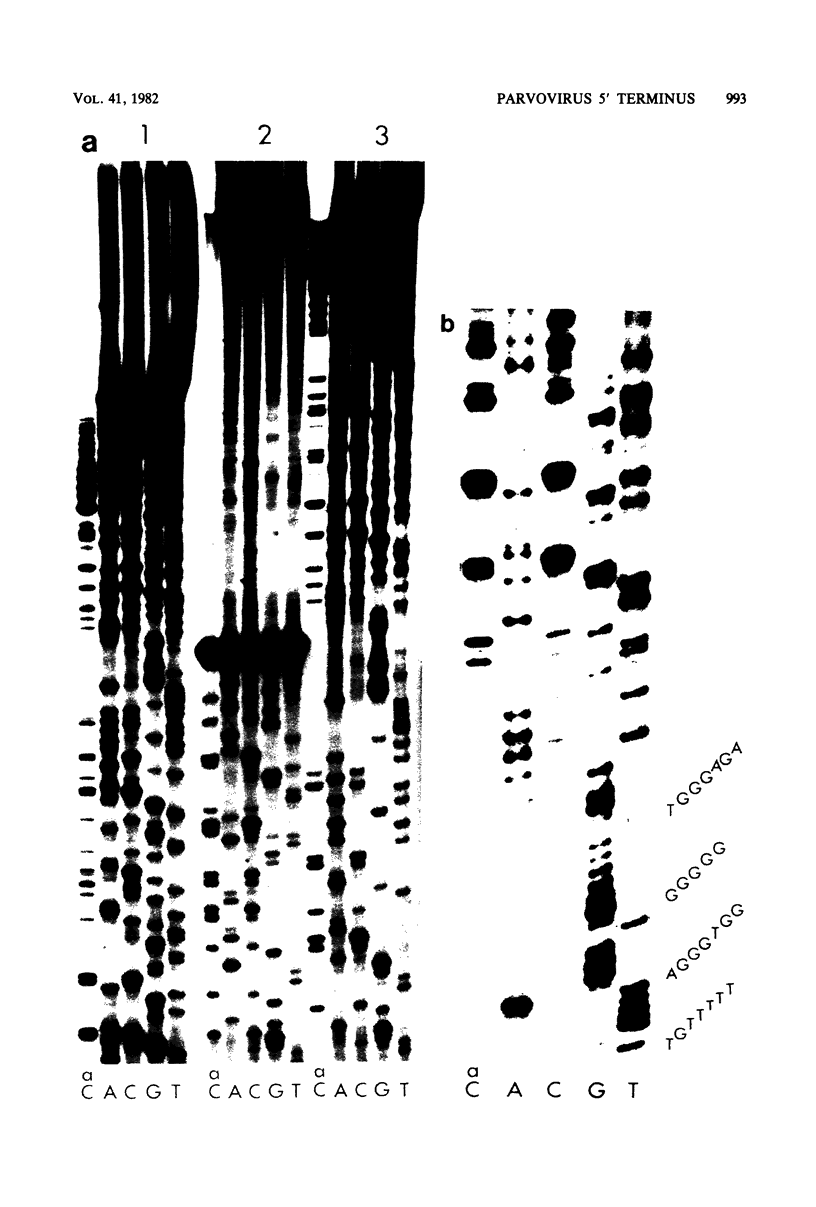
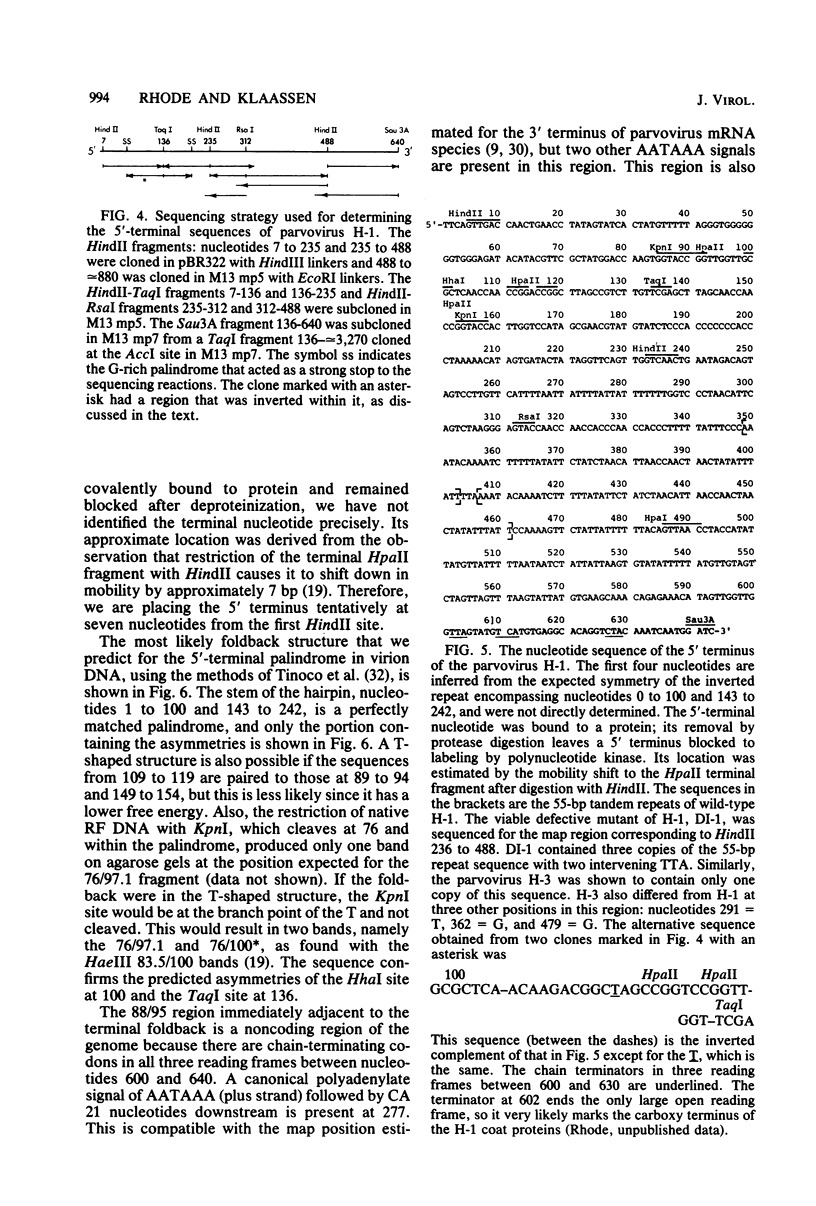
The nucleotide sequence of the 5' terminus of the parvovirus H-1 was determined. There are two orientations of the 242-base-pair terminal palindrome in native replicative form DNA, one inverted with respect to the other. Adjacent to the terminal palindrome is an AT-rich region that is noncoding and contains a 55-base-pair tandem repeat. The addition mutant of H-1, DI-1, was also sequenced in this region and shown to have three copies of the tandem repeat sequence. Similarly, the related parvovirus H-3 contains only one copy of this repeat sequence. This region contains the replication origin for parvovirus replicative form DNA replication. Some of the implications of these results are discussed.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astell C. R., Smith M., Chow M. B., Ward D. C. Sequence of the 3' terminus of the genome from Kilham rat virus, a nondefective parvovirus. Virology. 1979 Jul 30;96(2):669–674. doi: 10.1016/0042-6822(79)90127-2. [DOI] [PubMed] [Google Scholar]
- Astell C. R., Smith M., Chow M. B., Ward D. C. Structure of the 3' hairpin termini of four rodent parvovirus genomes: nucleotide sequence homology at origins of DNA replication. Cell. 1979 Jul;17(3):691–703. doi: 10.1016/0092-8674(79)90276-9. [DOI] [PubMed] [Google Scholar]
- Bourguignon G. J., Tattersall P. J., Ward D. C. DNA of minute virus of mice: self-priming, nonpermuted, single-stranded genome with a 5'-terminal hairpin duplex. J Virol. 1976 Oct;20(1):290–306. doi: 10.1128/jvi.20.1.290-306.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier-Smith T. Palindromic base sequences and replication of eukaryote chromosome ends. Nature. 1974 Aug 9;250(5466):467–470. doi: 10.1038/250467a0. [DOI] [PubMed] [Google Scholar]
- Dagert M., Ehrlich S. D. Prolonged incubation in calcium chloride improves the competence of Escherichia coli cells. Gene. 1979 May;6(1):23–28. doi: 10.1016/0378-1119(79)90082-9. [DOI] [PubMed] [Google Scholar]
- Gautschi M., Reinhard P. Synthesis of a mammalian parvovirus in Brij-58-lysed cells. J Virol. 1978 Aug;27(2):453–456. doi: 10.1128/jvi.27.2.453-456.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Lebovitz R. M., Roeder R. G. Expression of the autonomous parvovirus H1 genome: evidence for a single transcriptional unit and multiple spliced polyadenylated transcripts. Cell. 1979 Aug;17(4):967–977. doi: 10.1016/0092-8674(79)90336-2. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidecker G., Messing J., Gronenborn B. A versatile primer for DNA sequencing in the M13mp2 cloning system. Gene. 1980 Jun;10(1):69–73. doi: 10.1016/0378-1119(80)90145-6. [DOI] [PubMed] [Google Scholar]
- Lusby E., Fife K. H., Berns K. I. Nucleotide sequence of the inverted terminal repetition in adeno-associated virus DNA. J Virol. 1980 May;34(2):402–409. doi: 10.1128/jvi.34.2.402-409.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeoch D. J., Crawford L. V., Follett E. A. The DNAs of three parvoviruses. J Gen Virol. 1970 Jan;6(1):33–40. doi: 10.1099/0022-1317-6-1-33. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revie D., Tseng B. Y., Grafstrom R. H., Goulian M. Covalent association of protein with replicative form DNA of parvovirus H-1. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5539–5543. doi: 10.1073/pnas.76.11.5539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Defective interfering particles of parvovirus H-1. J Virol. 1978 Aug;27(2):347–356. doi: 10.1128/jvi.27.2.347-356.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. II. Isolation and characterization of H-1 replicative form DNA. J Virol. 1974 Feb;13(2):400–410. doi: 10.1128/jvi.13.2.400-410.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. III. Factors affecting H-1 RF DNA synthesis. J Virol. 1974 Oct;14(4):791–801. doi: 10.1128/jvi.14.4.791-801.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. IX. Physical mapping studies of the H-1 genome. J Virol. 1977 May;22(2):446–458. doi: 10.1128/jvi.22.2.446-458.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. VI. Characterization of a replication terminus of H-1 replicative-form DNA. J Virol. 1977 Feb;21(2):694–712. doi: 10.1128/jvi.21.2.694-712.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd Replication process of the parvovirus H-1. X. Isolation of a mutant defective in replicative-form DNA replication. J Virol. 1978 Jan;25(1):215–223. doi: 10.1128/jvi.25.1.215-223.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekiya T., Kobayashi M., Seki T., Koike K. Nucleotide sequence of a cloned fragment of rat mitochondrial DNA containing the replication origin. Gene. 1980 Oct;11(1-2):53–62. doi: 10.1016/0378-1119(80)90086-4. [DOI] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VII. Electron microscopy of replicative-form DNA synthesis. J Virol. 1977 Feb;21(2):713–723. doi: 10.1128/jvi.21.2.713-723.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Replication process of the parvovirus H-1. VIII. Partial denaturation mapping and localization of the replication origin of H-1 replicative-form DNA with electron microscopy. J Virol. 1977 Feb;21(2):724–731. doi: 10.1128/jvi.21.2.724-731.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer I. I., Rhode S. L., 3rd Ultrastructural studies of H-1 parvovirus replication. VI. simultaneous autoradiographic and immunochemical intranuclear localization of viral DNA synthesis and protein accumulation. J Virol. 1978 Jan;25(1):349–360. doi: 10.1128/jvi.25.1.349-360.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith A. J. DNA sequence analysis by primed synthesis. Methods Enzymol. 1980;65(1):560–580. doi: 10.1016/s0076-6879(80)65060-5. [DOI] [PubMed] [Google Scholar]
- Tal J., Ron D., Tattersall P., Bratosin S., Aloni Y. About 30% of minute virus of mice RNA is spliced out following polyadenylation. Nature. 1979 Jun 14;279(5714):649–651. doi: 10.1038/279649a0. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Tseng B. Y., Grafstrom R. H., Revie D., Oertel W., Goulian M. Studies on early intermediates in the synthesis of DNA in animal cells. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):263–270. doi: 10.1101/sqb.1979.043.01.032. [DOI] [PubMed] [Google Scholar]
- Usategui-Gomez M., Toolan H. W., Ledinko N., al-Lami F., Hopkins M. S. Single-stranded DNA from the Parvovirus, H-1. Virology. 1969 Nov;39(3):617–621. doi: 10.1016/0042-6822(69)90117-2. [DOI] [PubMed] [Google Scholar]
- Wolter S., Richards R., Armentrout R. W. Cell cycle-dependent replication of the DNA of minute virus of mice, a parvovirus. Biochim Biophys Acta. 1980 May 30;607(3):420–431. doi: 10.1016/0005-2787(80)90152-5. [DOI] [PubMed] [Google Scholar]