Abstract
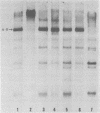
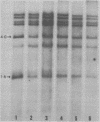
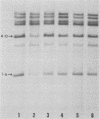
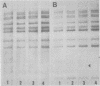
The methylation and amplification of mouse mammary tumor virus (MuMTV) proviral DNA was investigated in normal, premalignant, and malignant tissues of GR/A mice. The proviral methylation pattern was examined with the restriction enzyme HhaI, which fails to cleave methylated DNA. MuMTV proviral DNA from liver, kidney, and heart was highly methylated. Proviral DNA was somewhat undermethylated in mammary gland cells from virgin and lactating mice and extensively undermethylated in cells from premalignant outgrowths, pregnancy-dependent tumors, and pregnancy-independent tumors. The restriction enzyme SacI was used to detect additional proviruses in the same cells. No additional proviral copies of MuMTV were detected in liver, kidney, or heart cells or in mammary gland cells from virgin mice. Some mammary gland cells from lactating mice appeared to contain additional copies of the endogenous, highly oncogenic GT-MTV-2 provirus. Premalignant outgrowth, pregnancy-dependent tumor, and pregnancy-independent tumor cells contained an average of two to three additional copies per cell of the GT-MTV-2 provirus. Thus, neoplasia in GR/A mice was directly associated with quantized increases in MuMTV proviral DNA undermethylation and GR-MTV-2 proviral DNA amplification. Restriction enzyme analysis suggested that premalignant outgrowths and pregnancy-dependent tumors both consisted largely of heterogenous cell populations, although some evidence of clonal dominance was detected.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley R. L., Cardiff R. D., Mitchell D. J., Faulkin L. J., Lund J. K. Development and characterization of mouse hyperplastic mammary outgrowth lines from BALB/cfC3H hyperplastic alveolar nodules. Cancer Res. 1980 Nov;40(11):4232–4242. [PubMed] [Google Scholar]
- Bird A. P., Southern E. M. Use of restriction enzymes to study eukaryotic DNA methylation: I. The methylation pattern in ribosomal DNA from Xenopus laevis. J Mol Biol. 1978 Jan 5;118(1):27–47. doi: 10.1016/0022-2836(78)90242-5. [DOI] [PubMed] [Google Scholar]
- Brown D. D. Gene expression in eukaryotes. Science. 1981 Feb 13;211(4483):667–674. doi: 10.1126/science.6256857. [DOI] [PubMed] [Google Scholar]
- Cardiff R. D., Fanning T. G., Morris D. W., Ashley R. L., Faulkin L. J. Restriction endonuclease studies of hyperplastic outgrowth lines from BALB/cfC3H mouse hyperplastic mammary nodules. Cancer Res. 1981 Aug;41(8):3024–3029. [PubMed] [Google Scholar]
- Cardiff R. D., Wellings S. R., Faulkin L. J. Biology of breast preneoplasia. Cancer. 1977 Jun;39(6 Suppl):2734–2746. doi: 10.1002/1097-0142(197706)39:6<2734::aid-cncr2820390661>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Cohen J. C. Methylation of milk-borne and genetically transmitted mouse mammary tumor virus proviral DNA. Cell. 1980 Mar;19(3):653–662. doi: 10.1016/s0092-8674(80)80042-0. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Shank P. R., Morris V. L., Cardiff R., Varmus H. E. Integration of the DNA of mouse mammary tumor virus in virus-infected normal and neoplastic tissue of the mouse. Cell. 1979 Feb;16(2):333–345. doi: 10.1016/0092-8674(79)90010-2. [DOI] [PubMed] [Google Scholar]
- Cohen J. C., Varmus H. E. Proviruses of mouse mammary tumor virus in normal and neoplastic tissues from GR and C3Hf mouse strains. J Virol. 1980 Aug;35(2):298–305. doi: 10.1128/jvi.35.2.298-305.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEOME K. B., FAULKIN L. J., Jr, BERN H. A., BLAIR P. B. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959 Jun;19(5):515–520. [PubMed] [Google Scholar]
- Fanning T. G., Puma J. P., Cardiff R. D. Identification and partial characterization of an endogenous form of mouse mammary tumor virus that is transcribed into the virion-associated RNA genome. Nucleic Acids Res. 1980 Dec 11;8(23):5715–5723. doi: 10.1093/nar/8.23.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning T. G., Puma J. P., Cardiff R. D. Selective amplification of mouse mammary tumor virus in mammary tumors of GR mice. J Virol. 1980 Oct;36(1):109–114. doi: 10.1128/jvi.36.1.109-114.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guntaka R. V., Rao P. Y., Mitsialis S. A., Katz R. Modification of avian sarcoma proviral DNA sequences in nonpermissive XC cells but not in permissive chicken cells. J Virol. 1980 May;34(2):569–572. doi: 10.1128/jvi.34.2.569-572.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holliday R., Pugh J. E. DNA modification mechanisms and gene activity during development. Science. 1975 Jan 24;187(4173):226–232. [PubMed] [Google Scholar]
- Hynes N. E., Groner B., Diggelmann H., Van Nie R., Michalides R. Genomic location of mouse mammary tumor virus proviral DNA in normal mouse tissue and in mammary tumors. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 2):1161–1168. doi: 10.1101/sqb.1980.044.01.125. [DOI] [PubMed] [Google Scholar]
- Kuo M. T., Mandel J. L., Chambon P. DNA methylation: correlation with DNase I sensitivity of chicken ovalbumin and conalbumin chromatin. Nucleic Acids Res. 1979 Dec 20;7(8):2105–2113. doi: 10.1093/nar/7.8.2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majors J. E., Varmus H. E. Nucleotide sequences at host-proviral junctions for mouse mammary tumour virus. Nature. 1981 Jan 22;289(5795):253–258. doi: 10.1038/289253a0. [DOI] [PubMed] [Google Scholar]
- Mandel J. L., Chambon P. DNA methylation: organ specific variations in the methylation pattern within and around ovalbumin and other chicken genes. Nucleic Acids Res. 1979 Dec 20;7(8):2081–2103. doi: 10.1093/nar/7.8.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalides R., Vlahakis G., Schlom J. A biochemical approach to the study of the transmission of mouse mammary tumor viruses in mouse strains RIII and C3H. Int J Cancer. 1976 Jul 15;18(1):105–115. doi: 10.1002/ijc.2910180114. [DOI] [PubMed] [Google Scholar]
- Michalides R., van Deemter L., Nuss R. R., van Nie R. Identification of the Mtv-2 gene responsible for the early appearance of mammary tumors in the GR mouse by nucleic acid hybridization. Proc Natl Acad Sci U S A. 1978 May;75(5):2368–2372. doi: 10.1073/pnas.75.5.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore D. H., Long C. A., Vaidya A. B., Sheffield J. B., Dion A. S., Lasfargues E. Y. Mammary tumor viruses. Adv Cancer Res. 1979;29:347–418. doi: 10.1016/s0065-230x(08)60850-7. [DOI] [PubMed] [Google Scholar]
- Morris V. L., Medeiros E., Ringold G. M., Bishop J. M., Varmus H. E. Comparison of mouse mammary tumor virus-specific DNA in inbred, wild and Asian mice, and in tumors and normal organs from inbred mice. J Mol Biol. 1977 Jul;114(1):73–91. doi: 10.1016/0022-2836(77)90284-4. [DOI] [PubMed] [Google Scholar]
- Nusse R., de Moes J., Hilkens J., van Nie R. Localization of a gene for expression of mouse mammary tumor virus antigens in the GR/Mtv-2- mouse strain. J Exp Med. 1980 Sep 1;152(3):712–719. doi: 10.1084/jem.152.3.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Riggs A. D. X inactivation, differentiation, and DNA methylation. Cytogenet Cell Genet. 1975;14(1):9–25. doi: 10.1159/000130315. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Waalwijk C., Flavell R. A. DNA methylation at a CCGG sequence in the large intron of the rabbit beta-globin gene: tissue-specific variations. Nucleic Acids Res. 1978 Dec;5(12):4631–4634. doi: 10.1093/nar/5.12.4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Nie R., Dux A. Biological and morphological characteristics of mammary tumors in GR mice. J Natl Cancer Inst. 1971 Apr;46(4):885–897. [PubMed] [Google Scholar]
- van der Ploeg L. H., Flavell R. A. DNA methylation in the human gamma delta beta-globin locus in erythroid and nonerythroid tissues. Cell. 1980 Apr;19(4):947–958. doi: 10.1016/0092-8674(80)90086-0. [DOI] [PubMed] [Google Scholar]