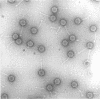
Abstract
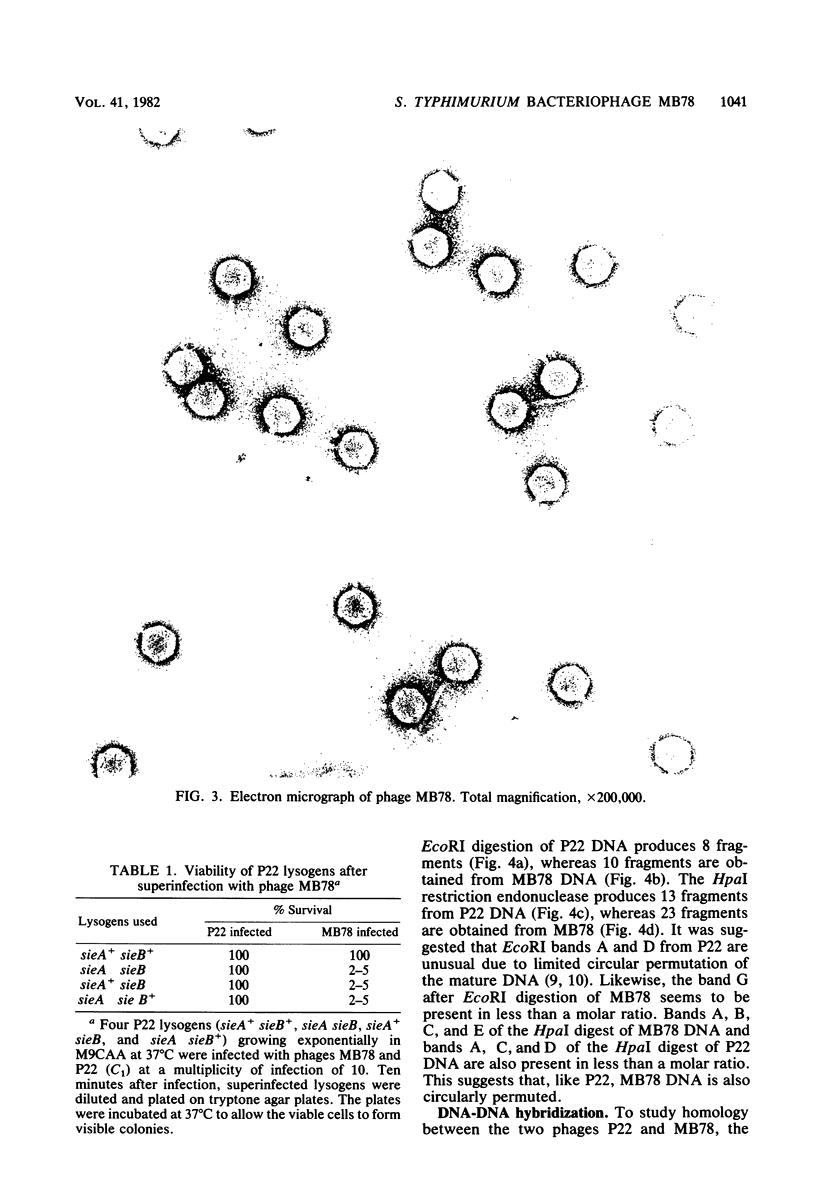
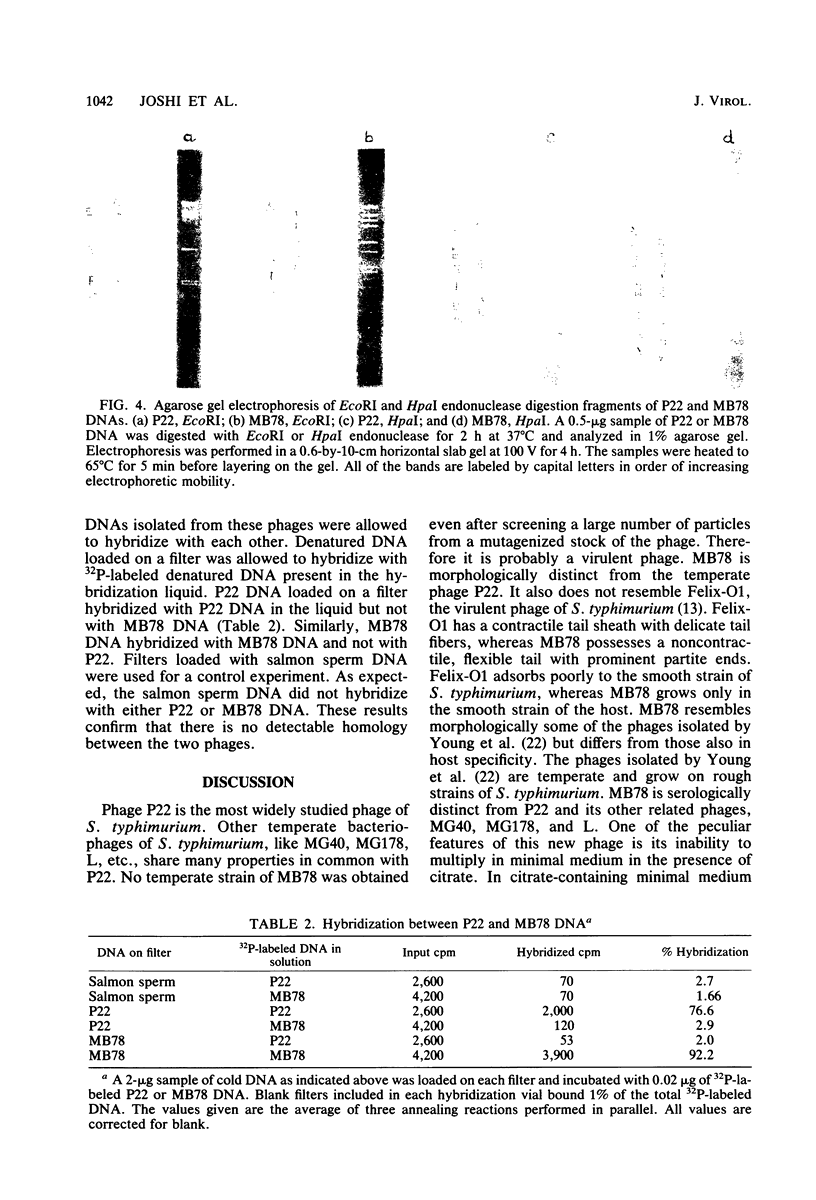
The isolation and some properties of a virulent bacteriophage of Salmonella typhimurium, MB78, which is morphologically, serologically, and physiologically unrelated to P22, are reported. The phage has a noncontractile long tail with partite ends. It cannot multiply in minimal medium in the presence of citrate. MB78-infected cells are, however, killed in such medium. This phage cannot grow in rifampin-resistant mutants of the host. The latent period of growth of this phage is much shorter than that of P22. Both sieA and sieB genes of the resident P22 prophage are required to exclude the superinfecting MB78 phage, whereas all temperate phages related to P22 are excluded by either one or both of the genes individually. Restriction endonuclease cleavage patterns of P22 and MB78 are distinctly different. The absence of homology between the two phages P22 and MB78 suggests that MB78 is not related to phage P22.
Full text
PDF
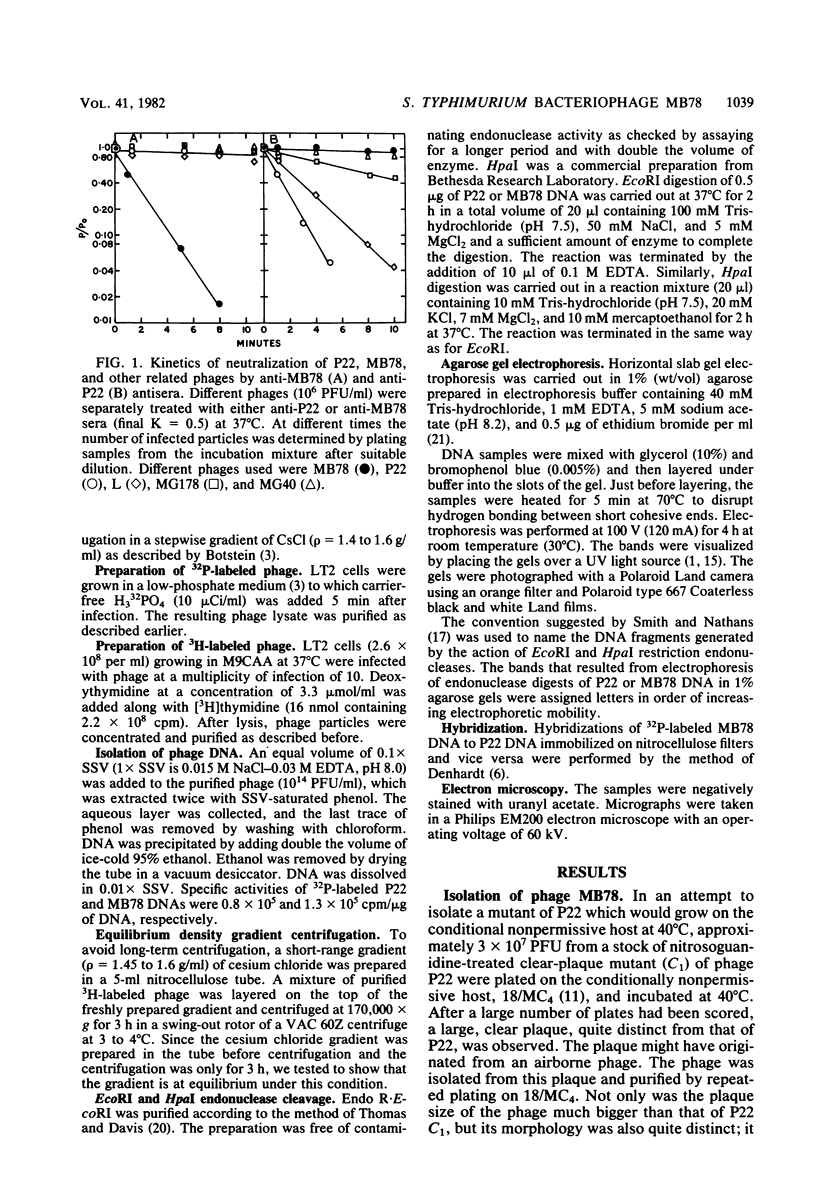
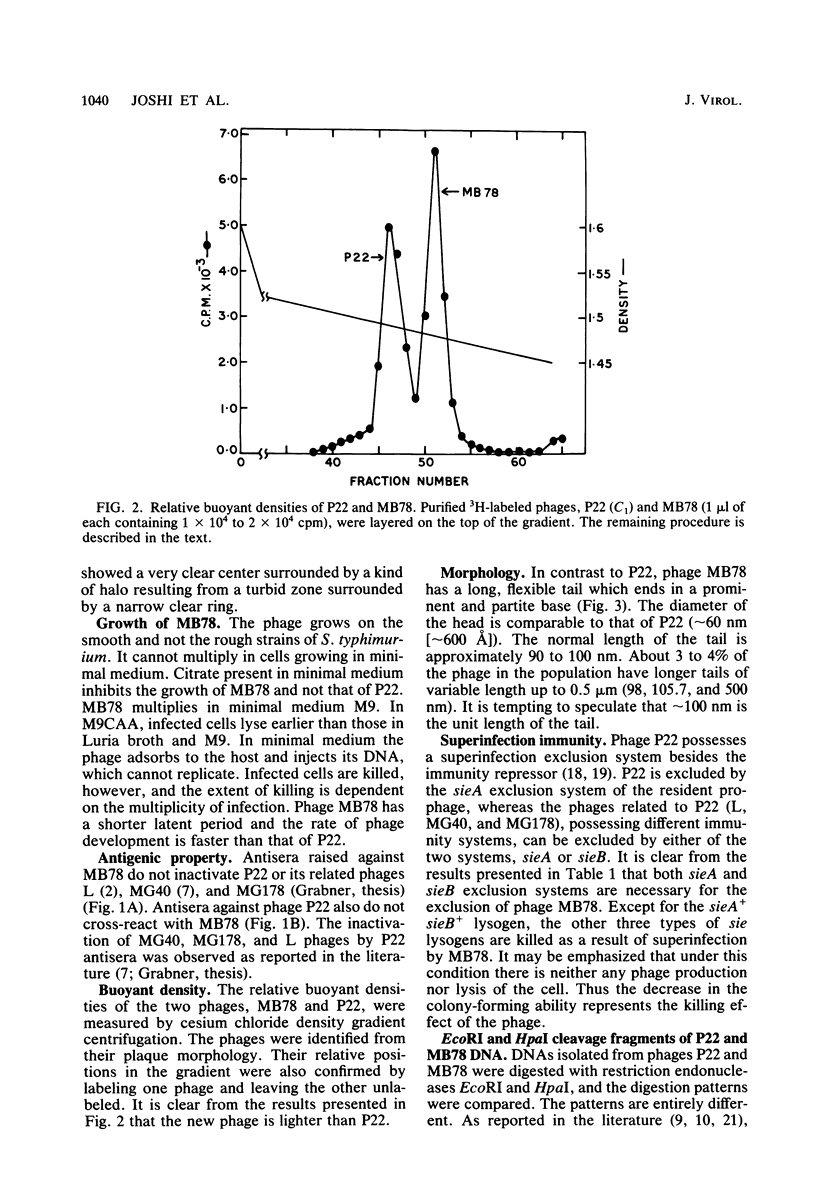

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aaij C., Borst P. The gel electrophoresis of DNA. Biochim Biophys Acta. 1972 May 10;269(2):192–200. doi: 10.1016/0005-2787(72)90426-1. [DOI] [PubMed] [Google Scholar]
- Bezdek M., Amati P. Properties of P22 and A related Salmonella typhimurium phage. I. General features and host specificity. Virology. 1967 Feb;31(2):272–278. doi: 10.1016/0042-6822(67)90171-7. [DOI] [PubMed] [Google Scholar]
- Botstein D., Herskowitz I. Properties of hybrids between Salmonella phage P22 and coliphage lambda. Nature. 1974 Oct 18;251(5476):584–589. doi: 10.1038/251584a0. [DOI] [PubMed] [Google Scholar]
- Botstein D. Synthesis and maturation of phage P22 DNA. I. Identification of intermediates. J Mol Biol. 1968 Jun 28;34(3):621–641. doi: 10.1016/0022-2836(68)90185-x. [DOI] [PubMed] [Google Scholar]
- Chakravorty M. Induction and repression of L-arabinose isomerase in bacteriophage-infected Salmonella typhimurium. J Virol. 1970 May;5(5):541–547. doi: 10.1128/jvi.5.5.541-547.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Grabner M., Hartman P. E. MG40 phage, a transducing phage related to P22. Virology. 1968 Mar;34(3):521–530. doi: 10.1016/0042-6822(68)90071-8. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Smith G. R., Ames B. N. Adenosine 3':5'-cyclic monophosphate concentration in the bacterial host regulates the viral decision between lysogeny and lysis. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2258–2262. doi: 10.1073/pnas.68.9.2258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson E. N., Jackson D. A., Deans R. J. EcoRI analysis of bacteriophage P22 DNA packaging. J Mol Biol. 1978 Jan 25;118(3):365–388. doi: 10.1016/0022-2836(78)90234-6. [DOI] [PubMed] [Google Scholar]
- Jackson E. N., Miller H. I., Adams M. L. EcoRI restriction endonuclease cleavage site map of bacteriophage P22DNA. J Mol Biol. 1978 Jan 25;118(3):347–363. doi: 10.1016/0022-2836(78)90233-4. [DOI] [PubMed] [Google Scholar]
- Joshi A. R., Chakravorty M. Bacteriophage P22 development is temperature sensitive in thiolutin resistant mutants of Salmonella typhimurium. Biochem Biophys Res Commun. 1979 Jul 12;89(1):1–6. doi: 10.1016/0006-291x(79)90935-5. [DOI] [PubMed] [Google Scholar]
- LEVINE M. Mutations in the temperate phage P22 and lysogeny in Salmonella. Virology. 1957 Feb;3(1):22–41. doi: 10.1016/0042-6822(57)90021-1. [DOI] [PubMed] [Google Scholar]
- Lindberg A. A., Holme T. Influence of O side chains on the attachment of the Felix O-1 bacteriophage to Salmonella bacteria. J Bacteriol. 1969 Aug;99(2):513–519. doi: 10.1128/jb.99.2.513-519.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PHILIPSON L., ALBERTSSON P. A., FRICK G. The purification and concentration of viruses by aqueous polymerphase systems. Virology. 1960 Jul;11:553–571. doi: 10.1016/0042-6822(60)90100-8. [DOI] [PubMed] [Google Scholar]
- SMITH H. O., LEVINE M. THE SYNTHESIS OF PHAGE AND HOST DNA IN THE ESTABLISHMENT OF LYSOGENY. Virology. 1965 Apr;25:585–590. doi: 10.1016/0042-6822(65)90086-3. [DOI] [PubMed] [Google Scholar]
- Smith H. O., Nathans D. Letter: A suggested nomenclature for bacterial host modification and restriction systems and their enzymes. J Mol Biol. 1973 Dec 15;81(3):419–423. doi: 10.1016/0022-2836(73)90152-6. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D., Wright A. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. III. Failure of superinfecting phage DNA to enter sieA+ lysogens. Virology. 1974 Dec;62(2):350–366. doi: 10.1016/0042-6822(74)90398-5. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Wright A., Botstein D. Superinfection exclusion by P22 prophage in lysogens of Salmonella typhimurium. IV. Genetics and physiology of sieB exclusion. Virology. 1974 Dec;62(2):367–384. doi: 10.1016/0042-6822(74)90399-7. [DOI] [PubMed] [Google Scholar]
- Thomas M., Davis R. W. Studies on the cleavage of bacteriophage lambda DNA with EcoRI Restriction endonuclease. J Mol Biol. 1975 Jan 25;91(3):315–328. doi: 10.1016/0022-2836(75)90383-6. [DOI] [PubMed] [Google Scholar]
- Weaver S., Levine M. Replication in situ and DNA encapsulation following induction of an excision-defective lysogen of Salmonella bacteriophage P22. J Mol Biol. 1978 Jan 25;118(3):389–411. doi: 10.1016/0022-2836(78)90235-8. [DOI] [PubMed] [Google Scholar]
- Young B. G. Some phages released from P22-infected salmonella. Virology. 1966 Feb;28(2):249–264. doi: 10.1016/0042-6822(66)90149-8. [DOI] [PubMed] [Google Scholar]