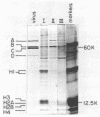
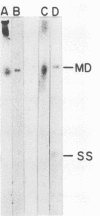
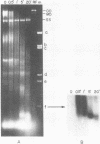
Abstract
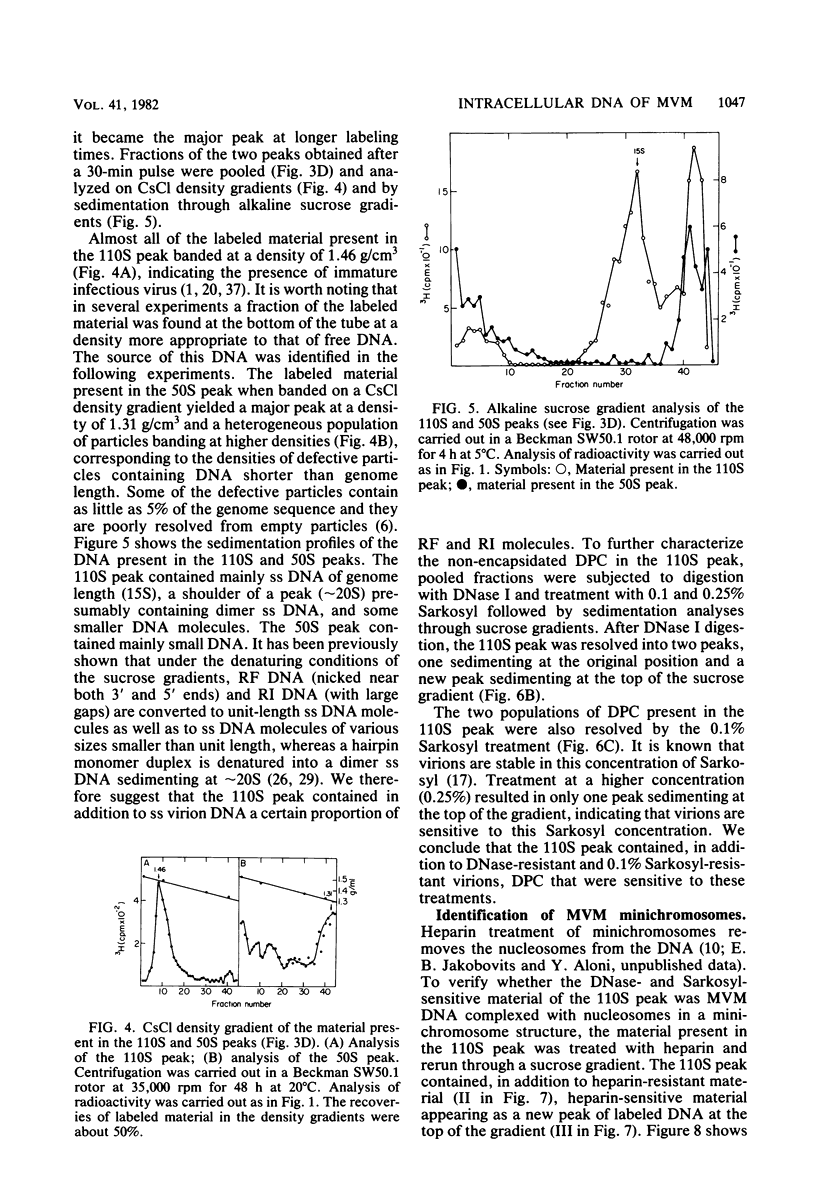
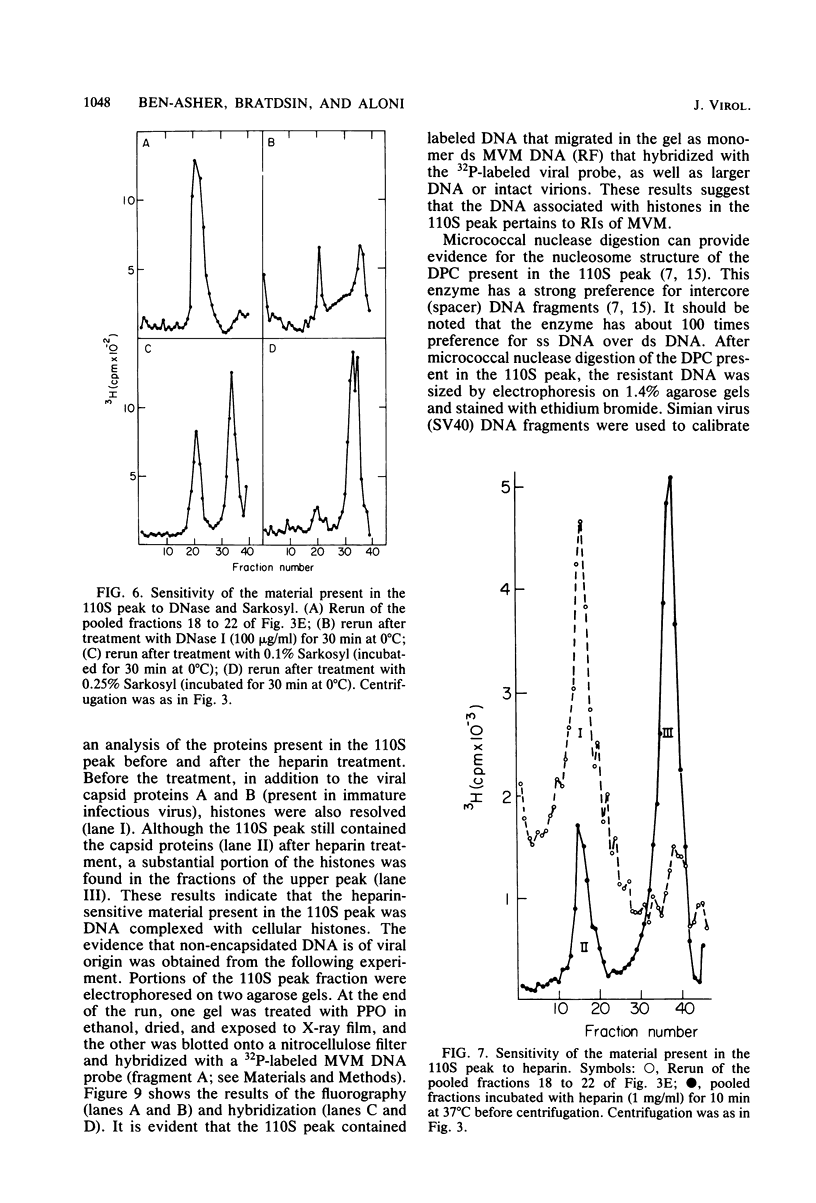
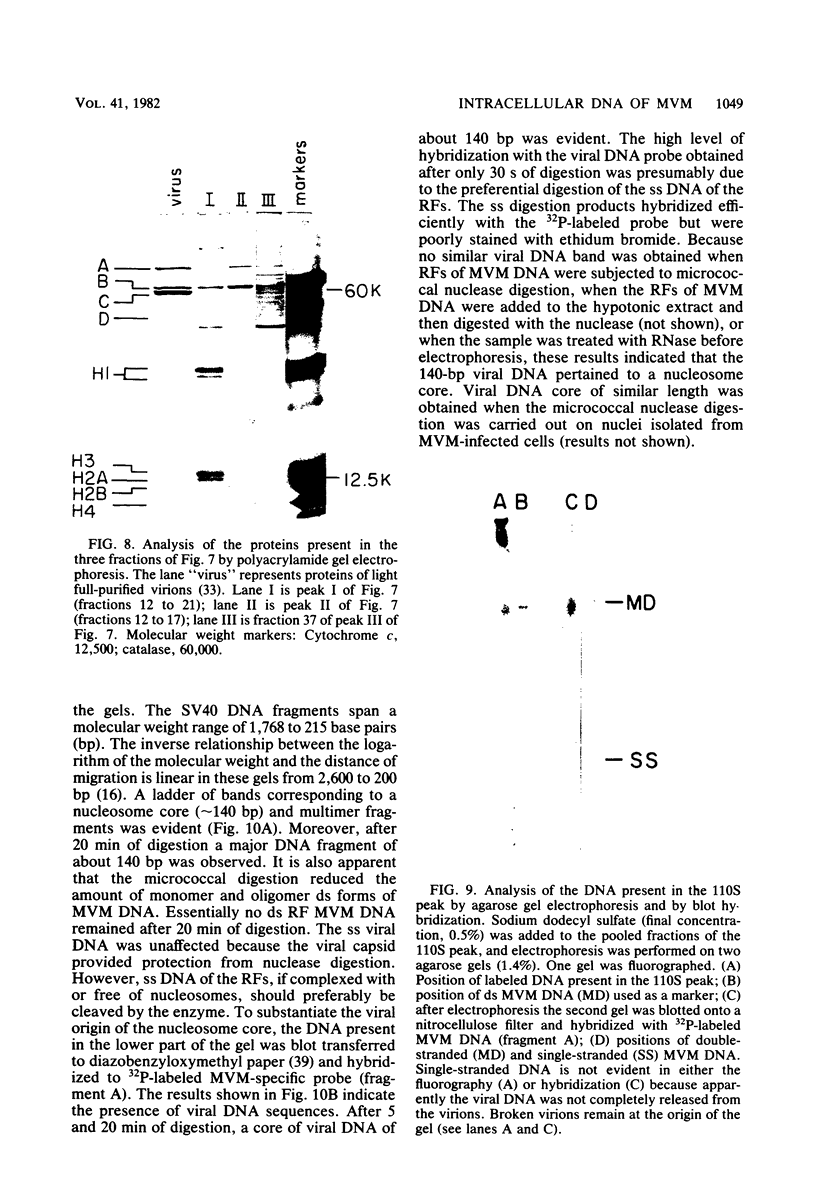
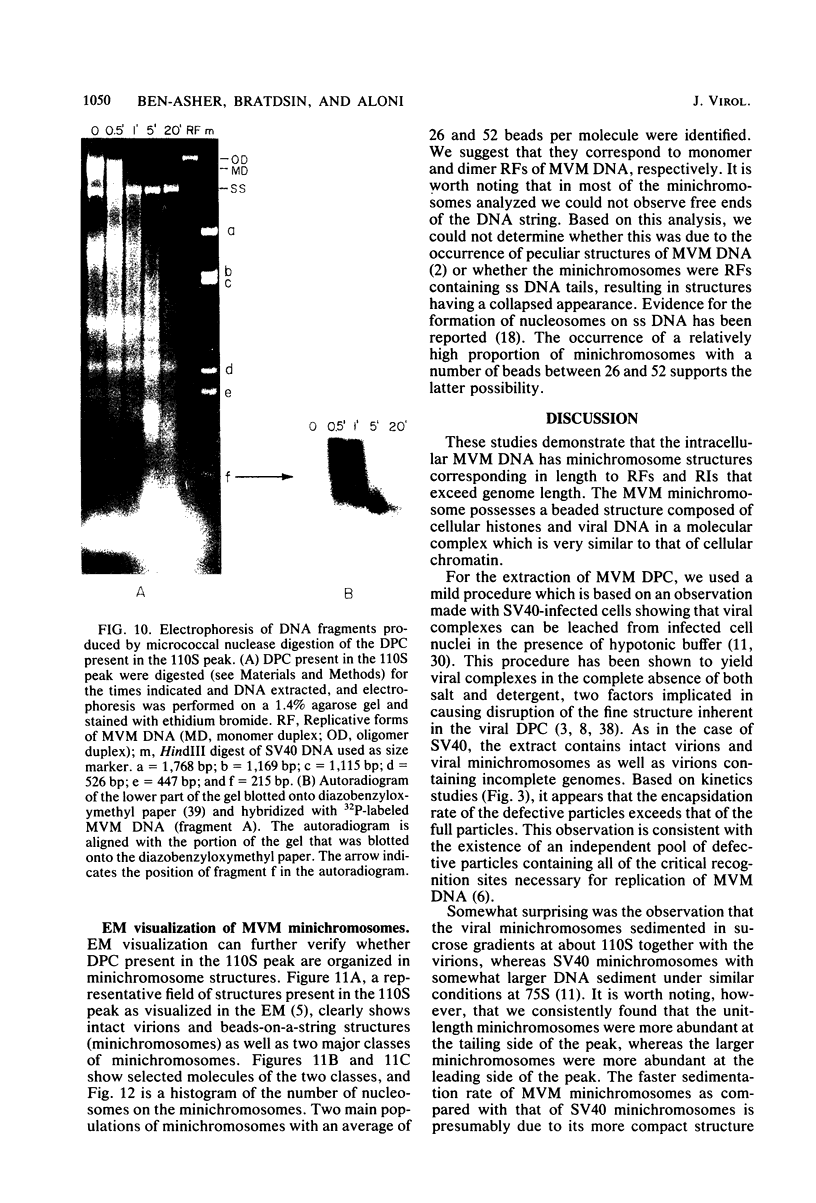
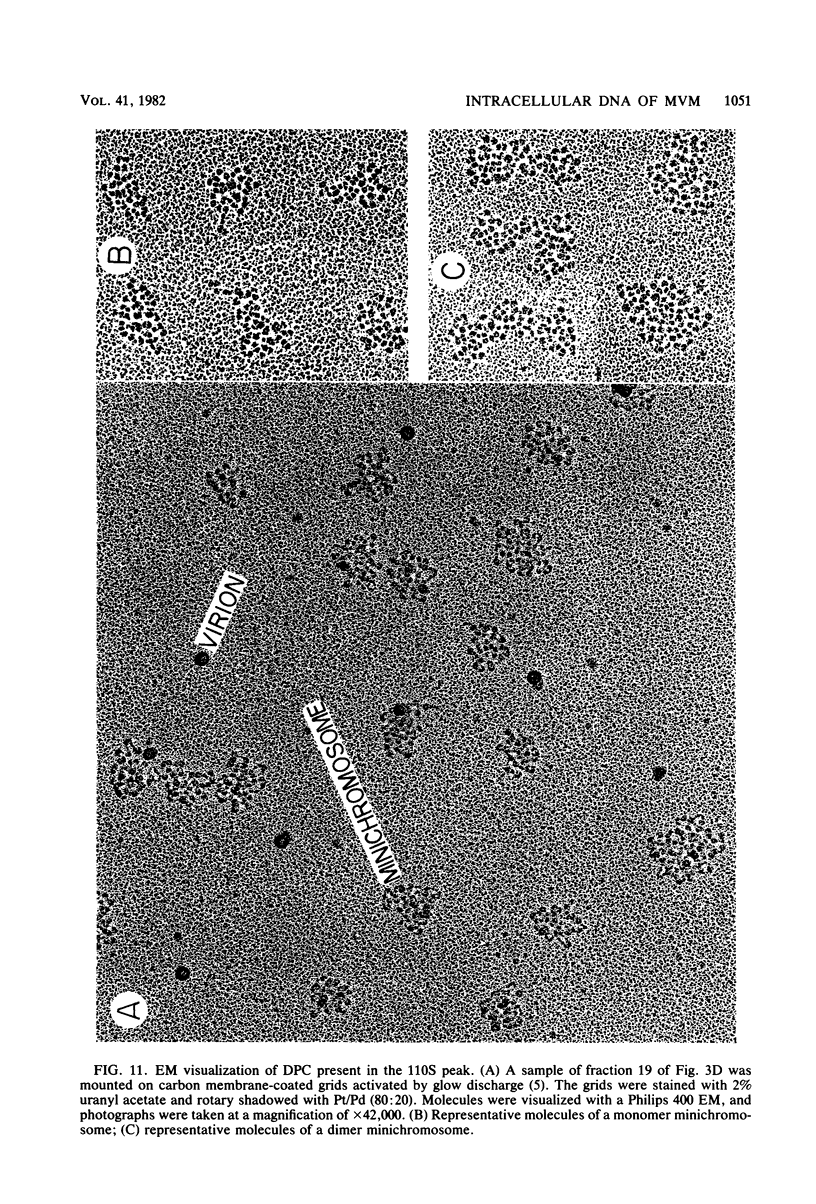
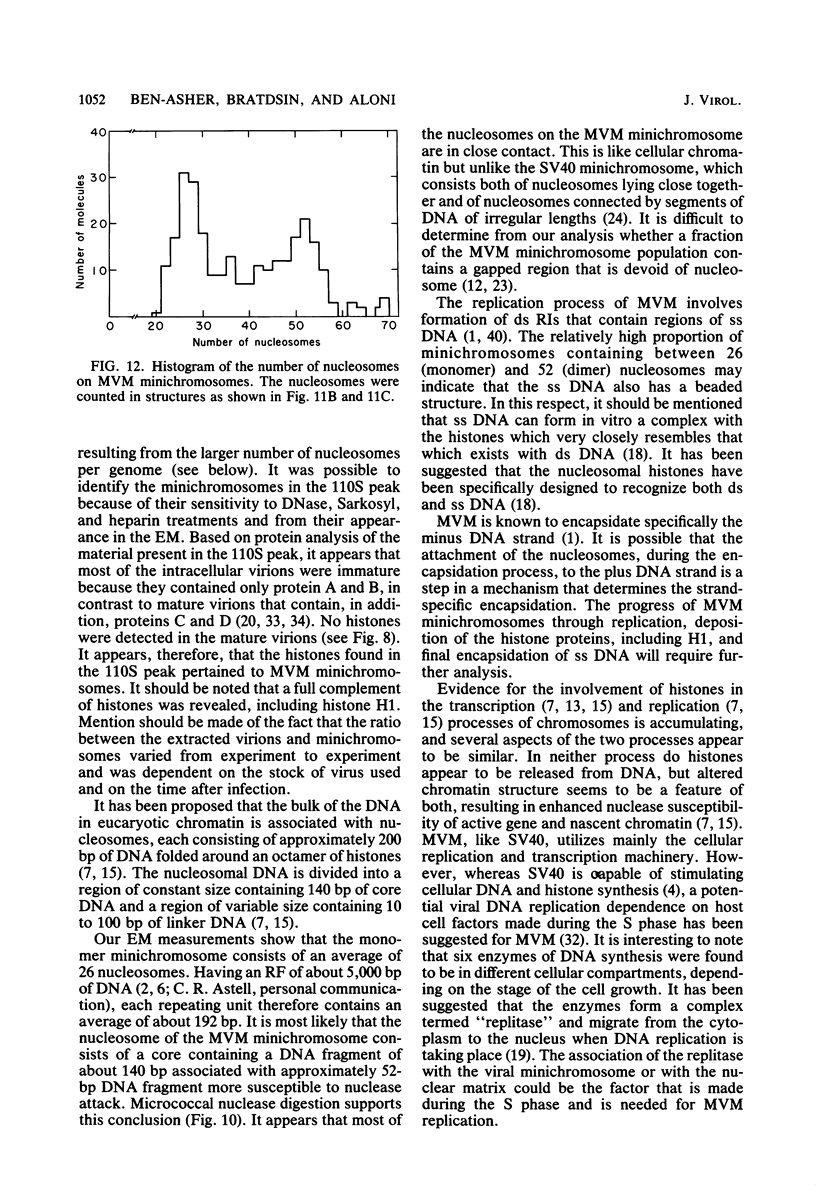
Minute virus of mice (MVM) nucleoprotein complexes were leached from infected cell nuclei in the presence of a hypotonic buffer. Detailed biochemical analyses performed on the extracted complexes revealed nucleoprotein complexes sedimenting together with virions at 110S and defective particles sedimenting at 50S. In contrast to the virions, the nucleoprotein complexes were found to be sensitive to treatment with DNase, Sarkosyl, and heparin. They were found to be composed of replicative forms of MVM DNA and cellular histones. After extensive micrococcal nuclease digestion performed on purified nucleoprotein complexes, a viral nucleosomes core containing a DNA segment of about 140 base pairs in length was identified. These complexes when visualized by electron microscopy revealed the existence of beaded structures (minichromosomes) having 26 and 52 beads per monomer and dimer molecules, respectively. We suggest that the organization of the intracellular viral DNA in a minichromosome structure is an essential step in the virus growth cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bratosin S., Laub O., Tal J., Aloni Y. Mechanism for circularization of linear DNAs: circular parvovirus MVM DNA is formed by a "noose" sliding in a "lasso"-like DNA structure. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4289–4293. doi: 10.1073/pnas.76.9.4289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen G., Griffith J. Salt and divalent cations affect the flexible nature of the natural beaded chromatin structure. Nucleic Acids Res. 1977 Jun;4(6):1837–1851. doi: 10.1093/nar/4.6.1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das G. C., Niyogi S. K. Structure, replication, and transcription of the SV40 genome. Prog Nucleic Acid Res Mol Biol. 1981;25:187–241. doi: 10.1016/s0079-6603(08)60485-7. [DOI] [PubMed] [Google Scholar]
- Dubochet J., Ducommun M., Zollinger M., Kellenberger E. A new preparation method for dark-field electron microscopy of biomacromolecules. J Ultrastruct Res. 1971 Apr;35(1):147–167. doi: 10.1016/s0022-5320(71)80148-x. [DOI] [PubMed] [Google Scholar]
- Faust E. A., Ward D. C. Incomplete genomes of the parvovirus minute virus of mice: selective conservation of genome termini, including the origin for DNA replication. J Virol. 1979 Oct;32(1):276–292. doi: 10.1128/jvi.32.1.276-292.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenfeld G. Chromatin. Nature. 1978 Jan 12;271(5641):115–122. doi: 10.1038/271115a0. [DOI] [PubMed] [Google Scholar]
- Fernandez-Munoz R., Coca-Prados M., Hsu M. T. Intracellular forms of simian virus 40 nucleoprotein complexes. I. Methods of isolation and characterization in CV-1 cells. J Virol. 1979 Feb;29(2):612–623. doi: 10.1128/jvi.29.2.612-623.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R., Lebovitz R. M., Roeder R. G. Expression of the autonomous parvovirus H1 genome: evidence for a single transcriptional unit and multiple spliced polyadenylated transcripts. Cell. 1979 Aug;17(4):967–977. doi: 10.1016/0092-8674(79)90336-2. [DOI] [PubMed] [Google Scholar]
- Groner Y., Hurwitz J. Synthesis of RNA containing a methylated blocked 5' terminus by HeLa nuclear homogenates. Proc Natl Acad Sci U S A. 1975 Aug;72(8):2930–2934. doi: 10.1073/pnas.72.8.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits E. B., Aloni Y. Isolation and characterization of various forms of simian virus 40 DNA-protein complexes. Virology. 1980 Apr 15;102(1):107–118. doi: 10.1016/0042-6822(80)90074-4. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Bratosin S., Aloni Y. A nucleosome-free region in SV40 minichromosomes. Nature. 1980 May 22;285(5762):263–265. doi: 10.1038/285263a0. [DOI] [PubMed] [Google Scholar]
- Jakobovits E. B., Saragosti S., Yaniv M., Aloni Y. Escherichia coli RNA polymerase in vitro mimics simian virus 40 in vivo transcription when the template is viral nucleoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6556–6560. doi: 10.1073/pnas.77.11.6556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lilley D. M., Pardon J. F. Structure and function of chromatin. Annu Rev Genet. 1979;13:197–233. doi: 10.1146/annurev.ge.13.120179.001213. [DOI] [PubMed] [Google Scholar]
- McDonell M. W., Simon M. N., Studier F. W. Analysis of restriction fragments of T7 DNA and determination of molecular weights by electrophoresis in neutral and alkaline gels. J Mol Biol. 1977 Feb 15;110(1):119–146. doi: 10.1016/s0022-2836(77)80102-2. [DOI] [PubMed] [Google Scholar]
- Palter K. B., Foe V. E., Alberts B. M. Evidence for the formation of nucleosome-like histone complexes on single-stranded DNA. Cell. 1979 Oct;18(2):451–467. doi: 10.1016/0092-8674(79)90064-3. [DOI] [PubMed] [Google Scholar]
- Prem veer Reddy G., Pardee A. B. Multienzyme complex for metabolic channeling in mammalian DNA replication. Proc Natl Acad Sci U S A. 1980 Jun;77(6):3312–3316. doi: 10.1073/pnas.77.6.3312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Saragosti S., Moyne G., Yaniv M. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell. 1980 May;20(1):65–73. doi: 10.1016/0092-8674(80)90235-4. [DOI] [PubMed] [Google Scholar]
- Shelton E. R., Wassarman P. M., DePamphilis M. L. Structure of Simian virus 40 chromosomes in nuclei from infected monkey cells. J Mol Biol. 1978 Nov 15;125(4):491–510. doi: 10.1016/0022-2836(78)90312-1. [DOI] [PubMed] [Google Scholar]
- Siegl G. The parvoviruses. Virol Monogr. 1976;15:1–109. [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stein G. S., Stein J. L., Park W. D., Detke S., Lichtler A. C., Shephard E. A., Jansing R. L., Phillips I. R. Regulation of histone gene expression in HeLa S3 cells. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 2):1107–1120. doi: 10.1101/sqb.1978.042.01.112. [DOI] [PubMed] [Google Scholar]
- Su R. T., DePamphilis M. L. In vitro replication of simian virus 40 DNA in a nucleoprotein complex. Proc Natl Acad Sci U S A. 1976 Oct;73(10):3466–3470. doi: 10.1073/pnas.73.10.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tal J., Ron D., Tattersall P., Bratosin S., Aloni Y. About 30% of minute virus of mice RNA is spliced out following polyadenylation. Nature. 1979 Jun 14;279(5714):649–651. doi: 10.1038/279649a0. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Cawte P. J., Shatkin A. J., Ward D. C. Three structural polypeptides coded for by minite virus of mice, a parvovirus. J Virol. 1976 Oct;20(1):273–289. doi: 10.1128/jvi.20.1.273-289.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Crawford L. V., Shatkin A. J. Replication of the parvovirus MVM. II. Isolation and characterization of intermediates in the replication of the viral deoxyribonucleic acid. J Virol. 1973 Dec;12(6):1446–1456. doi: 10.1128/jvi.12.6.1446-1456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P. Replication of the parvovirus MVM. I. Dependence of virus multiplication and plaque formation on cell growth. J Virol. 1972 Oct;10(4):586–590. doi: 10.1128/jvi.10.4.586-590.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Ward D. C. Rolling hairpin model for replication of parvovirus and linear chromosomal DNA. Nature. 1976 Sep 9;263(5573):106–109. doi: 10.1038/263106a0. [DOI] [PubMed] [Google Scholar]
- Varshavsky A. J., Bakayev V. V., Chumackov P. M., Georgiev G. P. Minichromosome of simian virus 40: presence of histone HI. Nucleic Acids Res. 1976 Aug;3(8):2101–2113. doi: 10.1093/nar/3.8.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]