Abstract
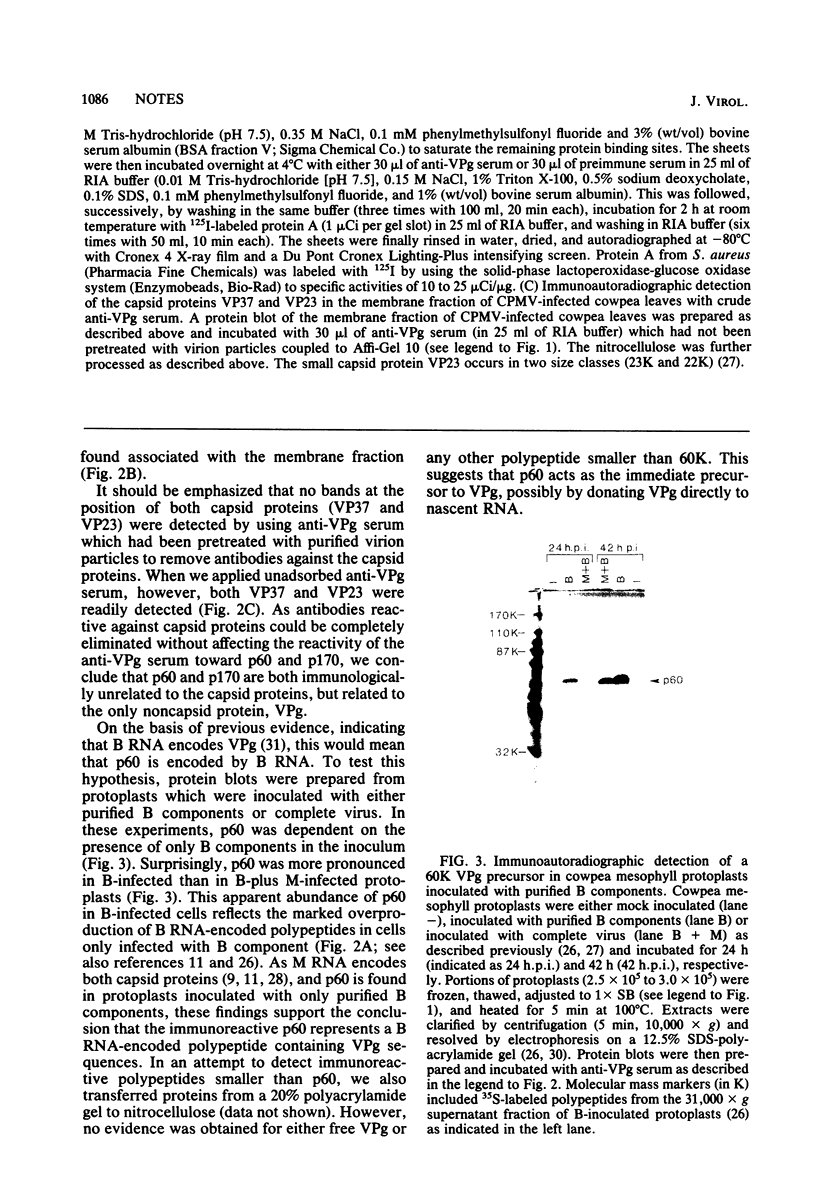
We have prepared a rabbit antiserum specifically directed against the genome-linked protein (VPg) of cowpea mosaic virus by injecting an hydrolysate of purified virion RNA. Using this antiserum as a probe in combination with “Western” (protein) blots of subcellular fractions of cowpea mosaic virus-infected cowpea (Vigna unguiculata) cells, we have detected a bottom component RNA-encoded, 60,000-dalton polypeptide which is membrane bound and presumably represents the immediate precursor of VPg.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bittner M., Kupferer P., Morris C. F. Electrophoretic transfer of proteins and nucleic acids from slab gels to diazobenzyloxymethyl cellulose or nitrocellulose sheets. Anal Biochem. 1980 Mar 1;102(2):459–471. doi: 10.1016/0003-2697(80)90182-7. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnette W. N. "Western blotting": electrophoretic transfer of proteins from sodium dodecyl sulfate--polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem. 1981 Apr;112(2):195–203. doi: 10.1016/0003-2697(81)90281-5. [DOI] [PubMed] [Google Scholar]
- Burroughs J. N., Brown F. Presence of a covalently linked protein on calicivirus RNA. J Gen Virol. 1978 Nov;41(2):443–446. doi: 10.1099/0022-1317-41-2-443. [DOI] [PubMed] [Google Scholar]
- Daubert S. D., Bruening G., Najarian R. C. Protein bound to the genome RNAs of cowpea mosaic virus. Eur J Biochem. 1978 Dec 1;92(1):45–51. doi: 10.1111/j.1432-1033.1978.tb12721.x. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franssen H., Goldbach R., Broekhuijsen M., Moerman M., van Kammen A. Expression of Middle-Component RNA of Cowpea Mosaic Virus: In Vitro Generation of a Precursor to Both Capsid Proteins by a Bottom-Component RNA-Encoded Protease from Infected Cells. J Virol. 1982 Jan;41(1):8–17. doi: 10.1128/jvi.41.1.8-17.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh A., Dasgupta R., Salerno-Rife T., Rutgers T., Kaesberg P. Southern bean mosaic viral RNA has a 5'-linked protein but lacks 3' terminal poly(A). Nucleic Acids Res. 1979 Dec 20;7(8):2137–2146. doi: 10.1093/nar/7.8.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldbach R. W., Schilthuis J. G., Rezelman G. Comparison of in vivo and in vitro translation of cowpea mosaic virus RNAs. Biochem Biophys Res Commun. 1981 Mar 16;99(1):89–94. doi: 10.1016/0006-291x(81)91716-2. [DOI] [PubMed] [Google Scholar]
- Golini F., Nomoto A., Wimmer E. The genome-linked protein of picornaviruses. IV. Difference in the VPg's of encephalomyocarditis virus and poliovirus as evidence that the genome-linked proteins are virus-coded. Virology. 1978 Aug;89(1):112–118. doi: 10.1016/0042-6822(78)90045-4. [DOI] [PubMed] [Google Scholar]
- Hruby D. E., Roberts W. K. Encephalomyocarditis virus RNA. III. Presence of a genome-associated protein. J Virol. 1978 Jan;25(1):413–415. doi: 10.1128/jvi.25.1.413-415.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Adler C. J., Rothberg P. G., Martinko J., Nathenson S. G., Wimmer E. The genome-linked protein of picornaviruses. VII. Genetic mapping of poliovirus VPg by protein and RNA sequence studies. Cell. 1980 Aug;21(1):295–302. doi: 10.1016/0092-8674(80)90137-3. [DOI] [PubMed] [Google Scholar]
- Klootwijk J., Klein I., Zabel P., van Kammen A. Cowpea mosaic virus RNAs have neither m7GpppN ... nor mono-, di- or triphosphates at their 5' ends. Cell. 1977 May;11(1):73–82. doi: 10.1016/0092-8674(77)90318-x. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Pallansch M. A., Kew O. M., Palmenberg A. C., Golini F., Wimmer E., Rueckert R. R. Picornaviral VPg sequences are contained in the replicase precursor. J Virol. 1980 Aug;35(2):414–419. doi: 10.1128/jvi.35.2.414-419.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmenberg A. C., Pallansch M. A., Rueckert R. R. Protease required for processing picornaviral coat protein resides in the viral replicase gene. J Virol. 1979 Dec;32(3):770–778. doi: 10.1128/jvi.32.3.770-778.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R. Synthesis and proteolytic processing of cowpea mosaic virus proteins in reticulocyte lysates. Virology. 1979 Jul 30;96(2):463–477. doi: 10.1016/0042-6822(79)90104-1. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renart J., Reiser J., Stark G. R. Transfer of proteins from gels to diazobenzyloxymethyl-paper and detection with antisera: a method for studying antibody specificity and antigen structure. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3116–3120. doi: 10.1073/pnas.76.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezelman G., Goldbach R., Van Kammen A. Expression of bottom component RNA of cowpea mosaic virus in cowpea protoplasts. J Virol. 1980 Nov;36(2):366–373. doi: 10.1128/jvi.36.2.366-373.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Rezelman G., van Kammen A. The inhibition of cowpea mosaic virus replication by actinomycin D. Virology. 1979 Jan 30;92(2):299–309. doi: 10.1016/0042-6822(79)90135-1. [DOI] [PubMed] [Google Scholar]
- Sangar D. V., Rowlands D. J., Harris T. J., Brown F. Protein covalently linked to foot-and-mouth disease virus RNA. Nature. 1977 Aug 18;268(5621):648–650. doi: 10.1038/268648a0. [DOI] [PubMed] [Google Scholar]
- Sefton B. M., Beemon K., Hunter T. Comparison of the expression of the src gene of Rous sarcoma virus in vitro and in vivo. J Virol. 1978 Dec;28(3):957–971. doi: 10.1128/jvi.28.3.957-971.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Rottier P., Davies J. W., Zabel P., Van Kammen A. A protein linked to the 5' termini of both RNA components of the cowpea mosaic virus genome. Nucleic Acids Res. 1978 Dec;5(12):4505–4522. doi: 10.1093/nar/5.12.4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley J., Van Kammen A. Nucleotide sequences adjacent to the proteins covalently linked to the cowpea mosaic virus genome. Sequence determination after labelling in vitro using RNA ligase. Eur J Biochem. 1979 Nov 1;101(1):45–49. doi: 10.1111/j.1432-1033.1979.tb04214.x. [DOI] [PubMed] [Google Scholar]
- Stellwag E. J., Dahlberg A. E. Electrophoretic transfer of DNA, RNA and protein onto diazobenzyloxymethyl (DBM) - paper. Nucleic Acids Res. 1980 Jan 25;8(2):299–317. doi: 10.1093/nar/8.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symington J., Green M., Brackmann K. Immunoautoradiographic detection of proteins after electrophoretic transfer from gels to diazo-paper: analysis of adenovirus encoded proteins. Proc Natl Acad Sci U S A. 1981 Jan;78(1):177–181. doi: 10.1073/pnas.78.1.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Jongen-Neven I., van Kammen A. In Vitro Replication of Cowpea Mosaic Virus RNA III. Template Recognition by Cowpea Mosaic Virus RNA Replicase. J Virol. 1979 Jan;29(1):21–33. doi: 10.1128/jvi.29.1.21-33.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel P., Weenen-Swaans H., van Kammen A. In vitro replication of cowpea mosaic virus RNA: I. Isolation and properties of the membrane-bound replicase. J Virol. 1974 Nov;14(5):1049–1055. doi: 10.1128/jvi.14.5.1049-1055.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kammen A. The relationship between the components of cowpea mosaic virus. I. Two ribonucleoprotein particles necessary for the infectivity of CPMV. Virology. 1968 Feb;34(2):312–318. doi: 10.1016/0042-6822(68)90241-9. [DOI] [PubMed] [Google Scholar]





