Abstract
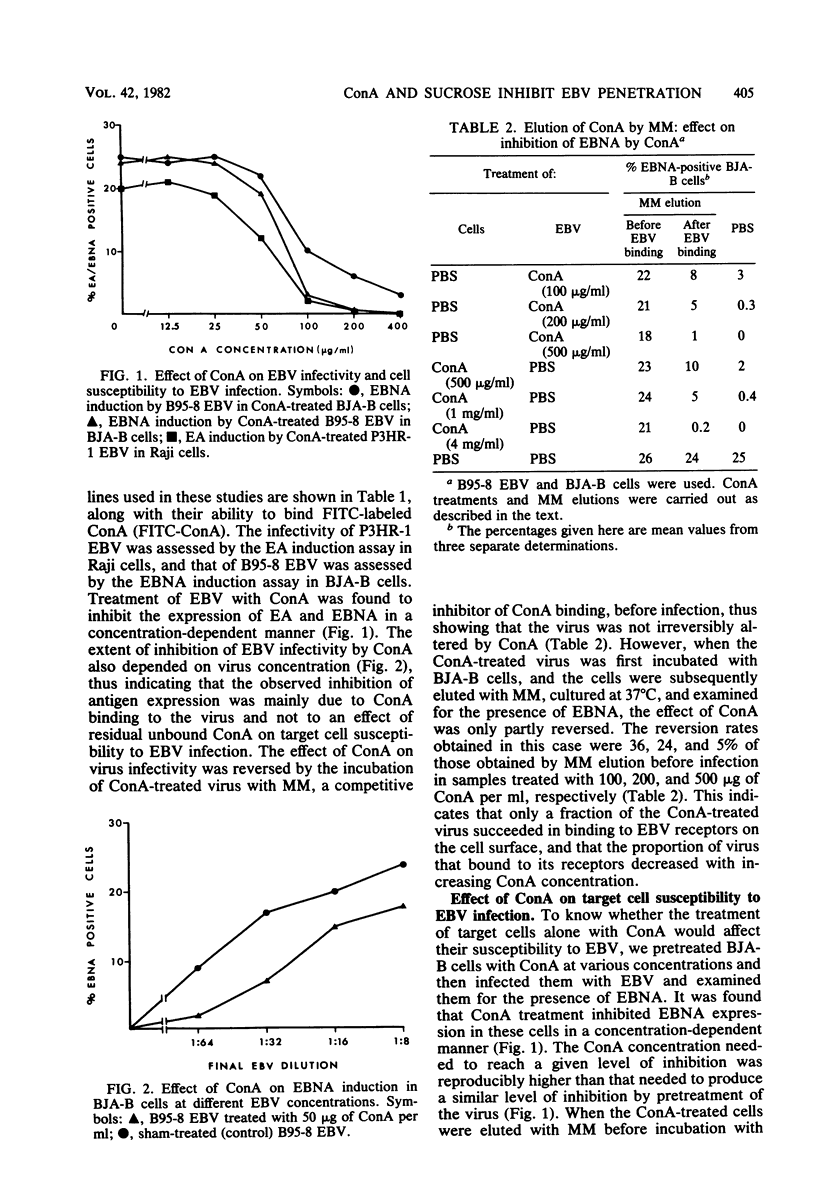
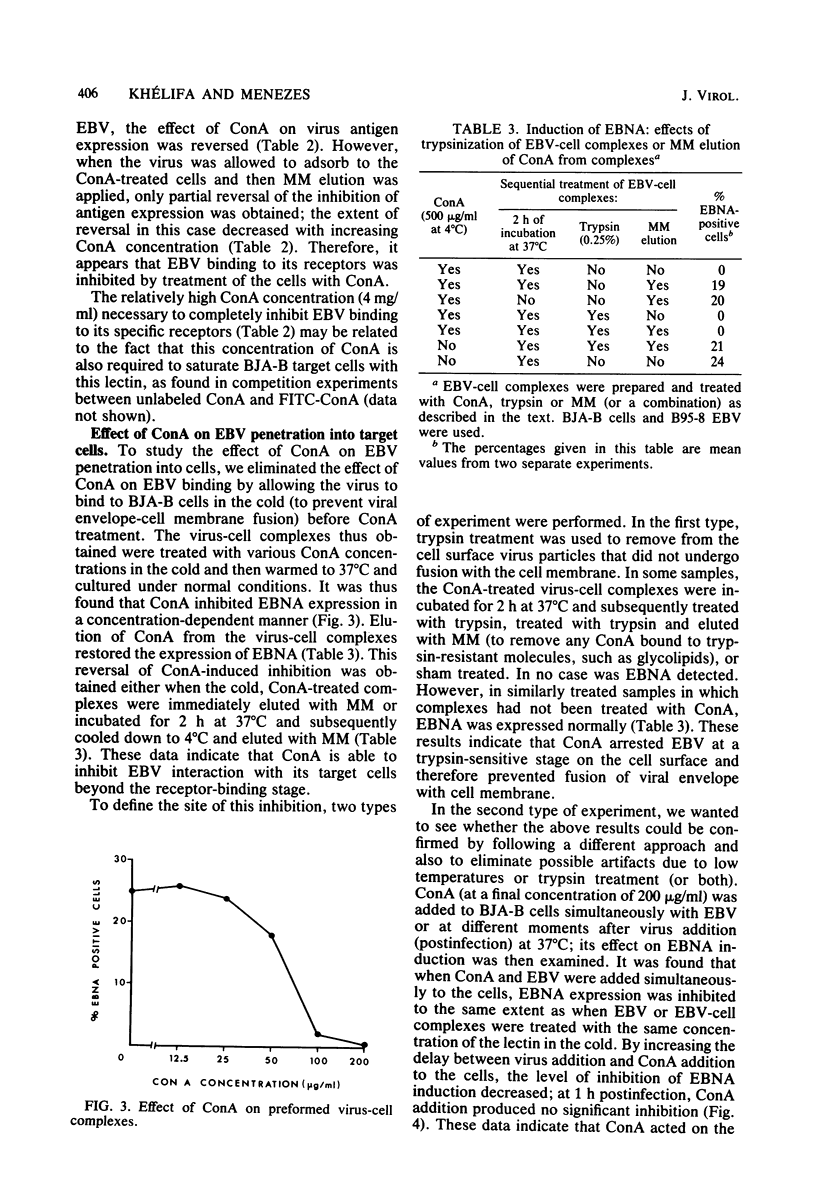
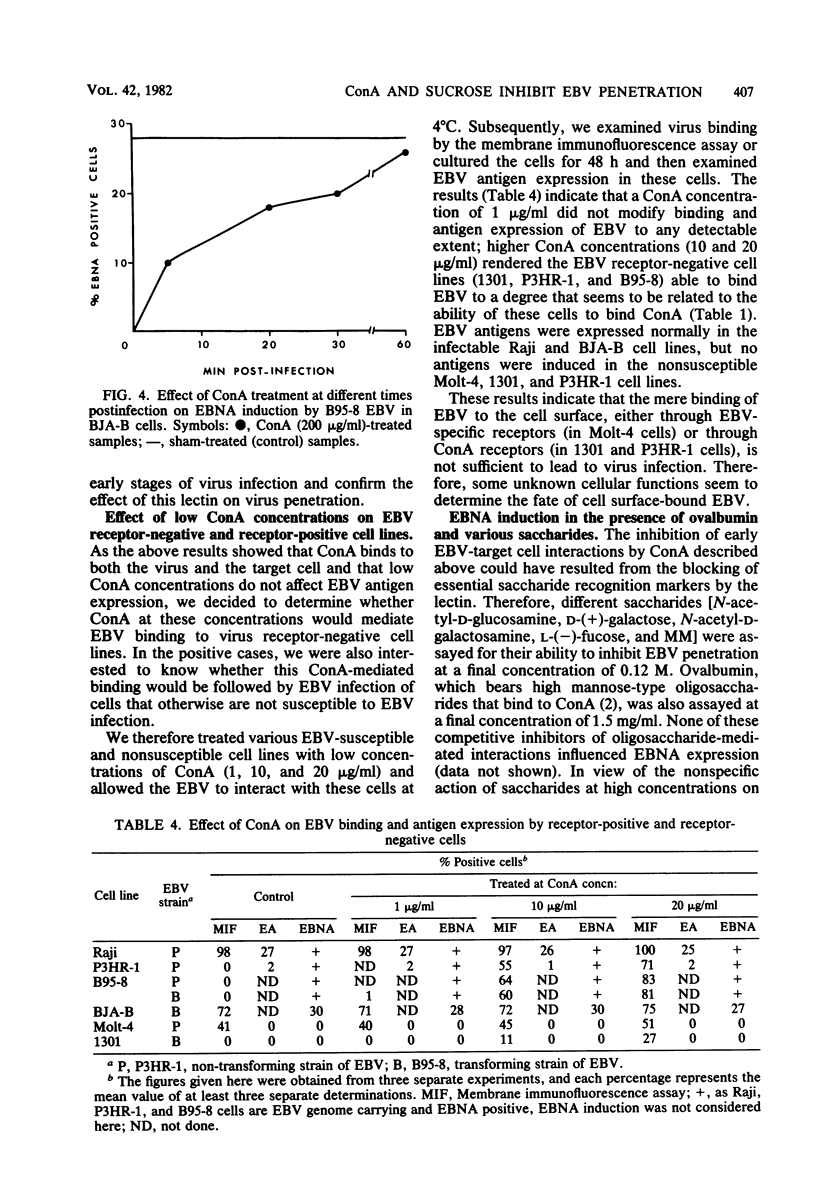
To study some aspects of Epstein-Barr virus (EBV) penetration into target cells, the effect of concanavalin A (ConA) and various saccharides on virus infectivity and cell susceptibility to EBV infection was examined. ConA treatment of the target cells, EBV, or EBV-cell complexes was found to inhibit virus antigen expression. Several control experiments with α-d-methyl-mannoside elution of ConA, removal of nonfused EBV particles from the cell surface by trypsin treatment, and addition of ConA at different times postinfection were performed to define the site of ConA action on EBV infection. ConA appeared to have a dual action: (i) it inhibited EBV binding to virus receptors, and (ii) it blocked the penetration of receptor-bound virus into target cells at a trypsin-sensitive stage, thus indicating that ConA prevented the fusion of viral envelope with the target cell membrane. A high sucrose concentration (0.25 M), known to inhibit cell membrane movements, was also found to block EBV penetration at a trypsinsensitive stage, thus suggesting the implication of cell membrane movements and underlying activities (or both) in viral envelope fusion. Lower concentrations of various monosaccharides (0.12 M) did not influence EBV infection. Under conditions of ConA treatment that did not influence EBV infectivity and target cells susceptibility, ConA was able to mediate virus binding to EBV receptornegative cell lines, but no virus antigens were expressed in these cells. These observations reinforced the idea that the mere attachment of EBV to lymphoid cells is not sufficient to lead to infection. In light of the present and previously published data, we postulate the existence of a specific cellular mechanism that allows the penetration of EBV into the target (B) lymphocyte.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahkong Q. F., Fisher D., Tampion W., Lucy J. A. Mechanisms of cell fusion. Nature. 1975 Jan 17;253(5488):194–195. doi: 10.1038/253194a0. [DOI] [PubMed] [Google Scholar]
- Baenziger J. U., Fiete D. Structural determinants of concanavalin A specificity for oligosaccharides. J Biol Chem. 1979 Apr 10;254(7):2400–2407. [PubMed] [Google Scholar]
- Day J. F., Thornburg R. W., Thorpe S. R., Baynes J. W. Carbohydrate-mediated clearance of antibody . antigen complexes from the circulation. The role of high mannose oligosaccharides in the hepatic uptake of IgM . antigen complexes. J Biol Chem. 1980 Mar 25;255(6):2360–2365. [PubMed] [Google Scholar]
- Dolyniuk M., Pritchett R., Kieff E. Proteins of Epstein-Barr virus. I. Analysis of the polypeptides of purified enveloped Epstein-Barr virus. J Virol. 1976 Mar;17(3):935–949. doi: 10.1128/jvi.17.3.935-949.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elie Metchnikoff (1845-1916), advocate of phagocytosis. JAMA. 1968 Jan 8;203(2):139–141. [PubMed] [Google Scholar]
- GOLDSTEIN I. J., HOLLERMAN C. E., SMITH E. E. PROTEIN-CARBOHYDRATE INTERACTION. II. INHIBITION STUDIES ON THE INTERACTION OF CONCANAVALIN A WITH POLYSACCHARIDES. Biochemistry. 1965 May;4:876–883. doi: 10.1021/bi00881a013. [DOI] [PubMed] [Google Scholar]
- Glaser R., de Thé G., Lenoir G., Ho J. H. Superinfection epithelial nasopharyngeal carcinoma cells with Epstein-Barr virus. Proc Natl Acad Sci U S A. 1976 Mar;73(3):960–963. doi: 10.1073/pnas.73.3.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunther G. R., Wang J. L., Yahara I., Cunningham B. A., Edelman G. M. Concanavalin A derivatives with altered biological activities. Proc Natl Acad Sci U S A. 1973 Apr;70(4):1012–1016. doi: 10.1073/pnas.70.4.1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W., Diehl V. Relation of Burkitt's tumor-associated herpes-ytpe virus to infectious mononucleosis. Proc Natl Acad Sci U S A. 1968 Jan;59(1):94–101. doi: 10.1073/pnas.59.1.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henle G., Henle W. Immunofluorescence in cells derived from Burkitt's lymphoma. J Bacteriol. 1966 Mar;91(3):1248–1256. doi: 10.1128/jb.91.3.1248-1256.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Grace J. T., Jr Cloning of immunoglobulin-producing human leukemic and lymphoma cells in long-term cultures. Proc Soc Exp Biol Med. 1967 Jan;124(1):107–111. doi: 10.3181/00379727-124-31677. [DOI] [PubMed] [Google Scholar]
- Hsu M. C., Scheid A., Choppin P. W. Reconstitution of membranes with individual paramyxovirus glycoproteins and phospholipid in cholate solution. Virology. 1979 Jun;95(2):476–491. doi: 10.1016/0042-6822(79)90502-6. [DOI] [PubMed] [Google Scholar]
- Jondal M., Klein G., Oldstone M. B., Bokish V., Yefenof E. Surface markers on human B and T lymphocytes. VIII. Association between complement and Epstein-Barr virus receptors on human lymphoid cells. Scand J Immunol. 1976;5(4):401–410. doi: 10.1111/j.1365-3083.1976.tb00294.x. [DOI] [PubMed] [Google Scholar]
- Lyles D. S., Landsberger F. R. Kinetics of Sendai virus envelope fusion with erythrocyte membranes and virus-induced hemolysis. Biochemistry. 1979 Nov 13;18(23):5088–5095. doi: 10.1021/bi00590a011. [DOI] [PubMed] [Google Scholar]
- Maeda Y., Kim J., Koseki I., Mekada E., Shiokawa Y., Okada Y. Modification of cell membranes with viral envelopes during fusion of cells with HVJ (Sendai virus). III. Effects of mono- and di-saccharides on cell fusion and membrane movement of fused cells. Exp Cell Res. 1977 Aug;108(1):95–106. doi: 10.1016/s0014-4827(77)80014-1. [DOI] [PubMed] [Google Scholar]
- Menezes J., Jondal M., Leibold W., Dorval G. Epstein-Barr virus interactions with human lymphocyte subpopulations: virus adsorption, kinetics of expression of Epstein-Barr virus-associated nuclear antigen, and lymphocyte transformation. Infect Immun. 1976 Feb;13(2):303–310. doi: 10.1128/iai.13.2.303-310.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G. Biological differences between Epstein-Barr virus (EBV) strains with regard to lymphocyte transforming ability, superinfection and antigen induction. Exp Cell Res. 1975 May;92(2):478–484. doi: 10.1016/0014-4827(75)90404-8. [DOI] [PubMed] [Google Scholar]
- Menezes J., Leibold W., Klein G., Clements G. Establishment and characterization of an Epstein-Barr virus (EBC)-negative lymphoblastoid B cell line (BJA-B) from an exceptional, EBV-genome-negative African Burkitt's lymphoma. Biomedicine. 1975 Jul;22(4):276–284. [PubMed] [Google Scholar]
- Menezes J., Seigneurin J. M., Patel P., Bourkas A., Lenoir G. Presence of Epstein-Barr virus receptors, but absence of virus penetration, in cells of an Epstein-Barr virus genome-negative human lymphoblastoid T line (Molt 4). J Virol. 1977 Jun;22(3):816–821. doi: 10.1128/jvi.22.3.816-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller G., Lipman M. Release of infectious Epstein-Barr virus by transformed marmoset leukocytes. Proc Natl Acad Sci U S A. 1973 Jan;70(1):190–194. doi: 10.1073/pnas.70.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minowada J., Onuma T., Moore G. E. Rosette-forming human lymphoid cell lines. I. Establishment and evidence for origin of thymus-derived lymphocytes. J Natl Cancer Inst. 1972 Sep;49(3):891–895. [PubMed] [Google Scholar]
- Miyake Y., Kim J., Okada Y. Effects of cytochalasin D on fusion of cells by HVJ (Sendai virus). Exp Cell Res. 1978 Oct 1;116(1):167–178. doi: 10.1016/0014-4827(78)90073-3. [DOI] [PubMed] [Google Scholar]
- Okada Y., Kim J. Interaction of concanavalin A with enveloped viruses and host cells. Virology. 1972 Nov;50(2):507–515. doi: 10.1016/0042-6822(72)90401-1. [DOI] [PubMed] [Google Scholar]
- Ozawa M., Asano A., Okada Y. Biological activities of glycoproteins of HVJ (Sendai virus) studied by reconstitution of hybrid envelope and by concanavalin A-mediated binding: a new function of HANA protein and structural requirement of F protein in hemolysis. Virology. 1979 Nov;99(1):197–202. doi: 10.1016/0042-6822(79)90055-2. [DOI] [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel P., Menezes J. Epstein-Barr virus (EBV)-lymphoid cell interactions. I. Quantification of EBV particles required for the membrane immunofluorescence assay and the comparative expression of EBV receptors on different human B, T and null cell lines. J Gen Virol. 1981 Mar;53(Pt 1):1–11. doi: 10.1099/0022-1317-53-1-1. [DOI] [PubMed] [Google Scholar]
- Pizer L. I., Cohen G. H., Eisenberg R. J. Effect of tunicamycin on herpes simplex virus glycoproteins and infectious virus production. J Virol. 1980 Apr;34(1):142–153. doi: 10.1128/jvi.34.1.142-153.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal K. S., Yanovich S., Inbar M., Strominger J. L. Translocation of a hydrocarbon fluorescent probe between Epstein-Barr virus and lymphoid cells: an assay for early events in viral infection. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5076–5080. doi: 10.1073/pnas.75.10.5076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seigneurin J. M., Vuillaume M., Lenoir G., De-Thé G. Replication of Epstein-Barr virus: ultrastructural and immunofluorescent studies of P3HR1-superinfected Raji cells. J Virol. 1977 Dec;24(3):836–845. doi: 10.1128/jvi.24.3.836-845.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stahl P. D., Rodman J. S., Miller M. J., Schlesinger P. H. Evidence for receptor-mediated binding of glycoproteins, glycoconjugates, and lysosomal glycosidases by alveolar macrophages. Proc Natl Acad Sci U S A. 1978 Mar;75(3):1399–1403. doi: 10.1073/pnas.75.3.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara M. Effect of concanavalin A on viral infectivity, maturation and cytopathogenicity in vesicular stomatitis virus-infected cells. Kobe J Med Sci. 1979 Dec;25(4):205–216. [PubMed] [Google Scholar]
- Volsky D. J., Shapiro I. M., Klein G. Transfer of Epstein-Barr virus receptors to receptor-negative cells permits virus penetration and antigen expression. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5453–5457. doi: 10.1073/pnas.77.9.5453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. L., Edelman G. M. Binding and functional properties of concanavalin A and its derivatives. I. Monovalent, divalent, and tetravalent derivatives stable at physiological pH. J Biol Chem. 1978 May 10;253(9):3000–3007. [PubMed] [Google Scholar]
- Yamamoto K., Inoue K. Interaction of paramyxoviruses with concanavalin A-modified erythrocyte membranes. Virology. 1978 Jan;84(1):203–206. doi: 10.1016/0042-6822(78)90234-9. [DOI] [PubMed] [Google Scholar]
- de-Thé G., Geser A., Day N. E., Tukei P. M., Williams E. H., Beri D. P., Smith P. G., Dean A. G., Bronkamm G. W., Feorino P. Epidemiological evidence for causal relationship between Epstein-Barr virus and Burkitt's lymphoma from Ugandan prospective study. Nature. 1978 Aug 24;274(5673):756–761. doi: 10.1038/274756a0. [DOI] [PubMed] [Google Scholar]


