Abstract
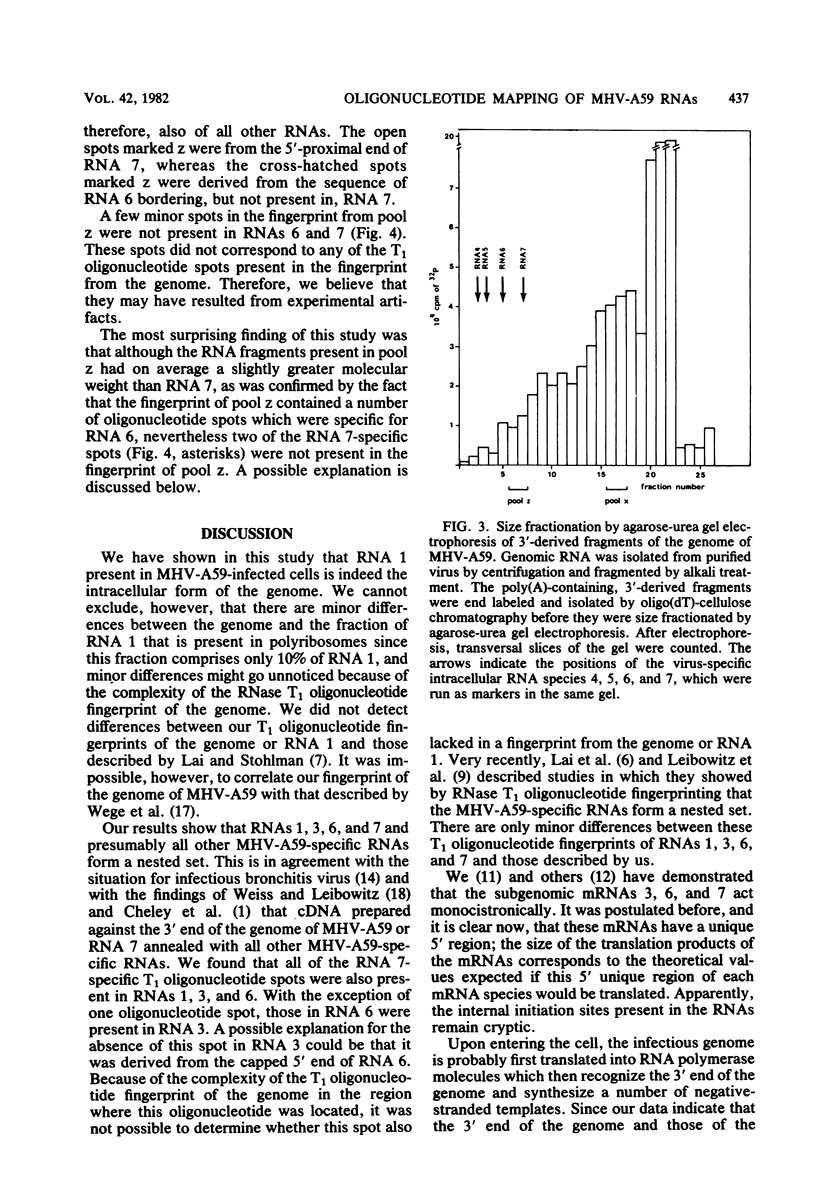
We have shown by T1 oligonucleotide fingerprinting that the genome of mouse hepatitis virus strain A59 and its intracellular RNA 1 have identical fingerprints and that RNA 1 and the subgenomic RNAs 3, 6, and 7 contain common sequences. To localize the homologous region between the RNAs, we compared fingerprints of the 3′ terminus of the genome with those of RNA 7. The genome was partially degraded with alkali, and polyadenylate-containing fragments were purified by oligodeoxythymidylate-cellulose chromatography. The fragments were size fractionated by agarose-urea gel electrophoresis, and two pools, x and z, containing 3′-derived fragments of the genome with apparent molecular weights of 0.1 × 106 to 0.14 × 106 and 0.6 × 106 to 0.8 × 106, respectively, were further analyzed by RNase T1 oligonucleotide fingerprinting. Comparison of the fingerprints of RNAs 6 and 7 with those of pools x and z showed that these subgenomic RNAs extend inwards from the 3′ terminus of the genome. The RNA fragments present in pool z were on average slightly larger than RNA 7 as confirmed by the presence in pool z of T1 oligonucleotide spots specific for RNA 6 but not present in RNA 7. However, two large oligonucleotide spots derived from RNA 7, which were also present in RNAs 1, 3, and 6 and in the virion RNA, were not found in the T1 oligonucleotide map of pool z. A possible explanation is that the two spots were derived from a leader sequence. The results of UV transcription mapping experiments (L. Jacobs, W. J. M. Spaan, M. C. Horzinek, and B. A. M. van der Zeijst, J. Virol. 39:401-406, 1981) excluded the possibility that such a leader sequence arises by splicing from a larger precursor molecule, but either a virus-specific RNA primer molecule for the synthesis of mRNAs or an RNA polymerase jumping mechanism could explain the presence of a leader sequence.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheley S., Anderson R., Cupples M. J., Chan E. C., Morris V. L. Intracellular murine hepatitis virus-specific RNAs contain common sequences. Virology. 1981 Jul 30;112(2):596–604. doi: 10.1016/0042-6822(81)90305-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewley J., Gentsch J., Bishop D. H. Three unique viral RNA species of snowshoe hare and La Crosse bunyaviruses. J Virol. 1977 May;22(2):459–468. doi: 10.1128/jvi.22.2.459-468.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Uhlenbeck O. C. 3'-terminal labelling of RNA with T4 RNA ligase. Nature. 1978 Oct 12;275(5680):560–561. doi: 10.1038/275560a0. [DOI] [PubMed] [Google Scholar]
- Jacobs L., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Synthesis of subgenomic mRNA's of mouse hepatitis virus is initiated independently: evidence from UV transcription mapping. J Virol. 1981 Aug;39(2):401–406. doi: 10.1128/jvi.39.2.401-406.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Brayton P. R., Armen R. C., Patton C. D., Pugh C., Stohlman S. A. Mouse hepatitis virus A59: mRNA structure and genetic localization of the sequence divergence from hepatotropic strain MHV-3. J Virol. 1981 Sep;39(3):823–834. doi: 10.1128/jvi.39.3.823-834.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai M. M., Stohlman S. A. Comparative analysis of RNA genomes of mouse hepatitis viruses. J Virol. 1981 May;38(2):661–670. doi: 10.1128/jvi.38.2.661-670.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langridge J., Langridge P., Bergquist P. L. Extraction of nucleic acids from agarose gels. Anal Biochem. 1980 Apr;103(2):264–271. doi: 10.1016/0003-2697(80)90266-3. [DOI] [PubMed] [Google Scholar]
- Leibowitz J. L., Wilhelmsen K. C., Bond C. W. The virus-specific intracellular RNA species of two murine coronaviruses: MHV-a59 and MHV-JHM. Virology. 1981 Oct 15;114(1):39–51. doi: 10.1016/0042-6822(81)90250-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. Analysis of the genome of an endogenous, ecotropic retrovirus of the AKR strain of mice: micromethod for detailed characterization of high-molecular-weight RNA. J Virol. 1980 Jan;33(1):349–365. doi: 10.1128/jvi.33.1.349-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottier P. J., Spaan W. J., Horzinek M. C., van der Zeijst B. A. Translation of three mouse hepatitis virus strain A59 subgenomic RNAs in Xenopus laevis oocytes. J Virol. 1981 Apr;38(1):20–26. doi: 10.1128/jvi.38.1.20-26.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Wege H., Barthel A., ter Meulen V. Coronavirus JHM: cell-free synthesis of structural protein p60. J Virol. 1980 Jan;33(1):10–17. doi: 10.1128/jvi.33.1.10-17.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaan W. J., Rottier P. J., Horzinek M. C., van der Zeijst B. A. Isolation and identification of virus-specific mRNAs in cells infected with mouse hepatitis virus (MHV-A59). Virology. 1981 Jan 30;108(2):424–434. doi: 10.1016/0042-6822(81)90449-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. I. Identification and characterization of virus-specified RNA. J Virol. 1980 Jun;34(3):665–674. doi: 10.1128/jvi.34.3.665-674.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern D. F., Kennedy S. I. Coronavirus multiplication strategy. II. Mapping the avian infectious bronchitis virus intracellular RNA species to the genome. J Virol. 1980 Nov;36(2):440–449. doi: 10.1128/jvi.36.2.440-449.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wege H., Siddell S., Sturm M., Ter Meulen V. Coronavirus JHM: characterization of intracellular viral RNA. J Gen Virol. 1981 May;54(Pt 1):213–217. doi: 10.1099/0022-1317-54-1-213. [DOI] [PubMed] [Google Scholar]
- Wege H., Stephenson J. R., Koga M., Wege H., ter Meulen V. Genetic variation of neurotropic and non-neurotropic murine coronaviruses. J Gen Virol. 1981 May;54(Pt 1):67–74. doi: 10.1099/0022-1317-54-1-67. [DOI] [PubMed] [Google Scholar]
- Weiss S. R., Leibowitz J. L. Comparison of the RNAs of murine and human coronaviruses. Adv Exp Med Biol. 1981;142:245–259. doi: 10.1007/978-1-4757-0456-3_20. [DOI] [PubMed] [Google Scholar]
- de Wachter R., Fiers W. Preparative two-dimensional polyacrylamide gel electrophoresis of 32 P-labeled RNA. Anal Biochem. 1972 Sep;49(1):184–197. doi: 10.1016/0003-2697(72)90257-6. [DOI] [PubMed] [Google Scholar]





