Abstract
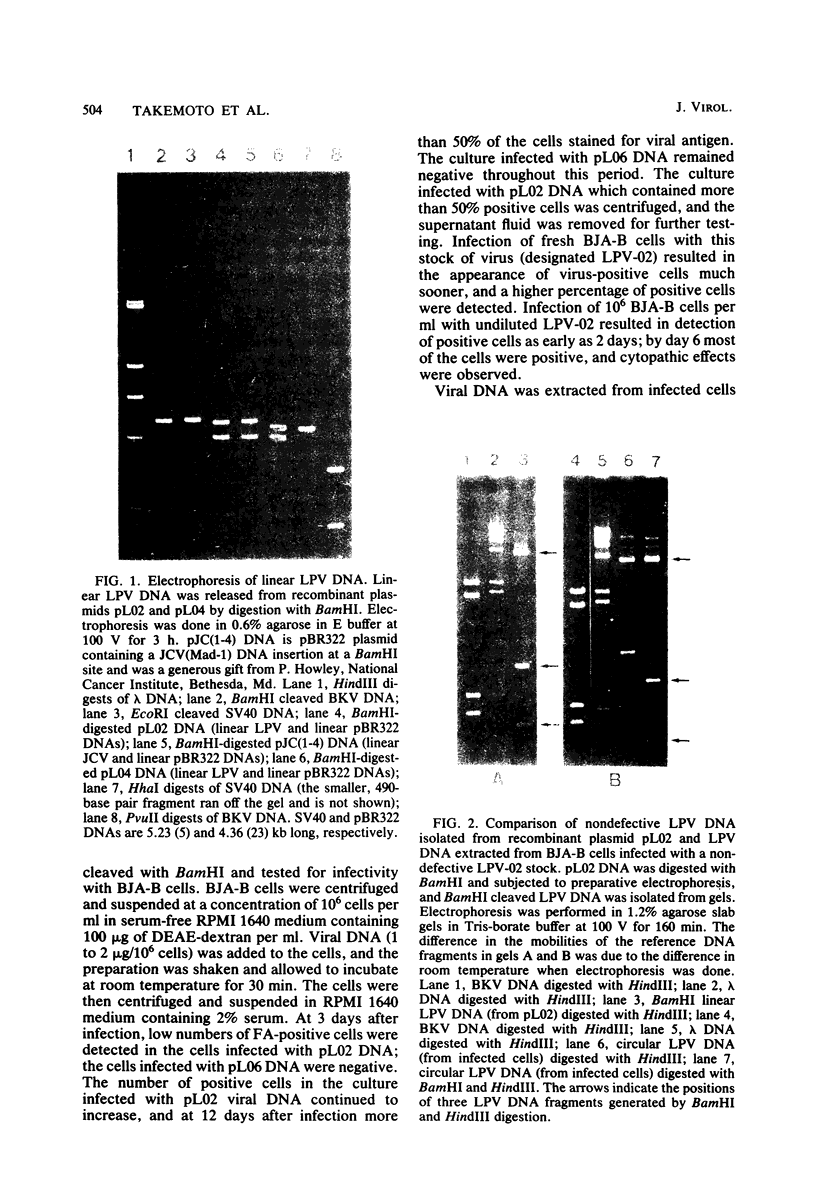
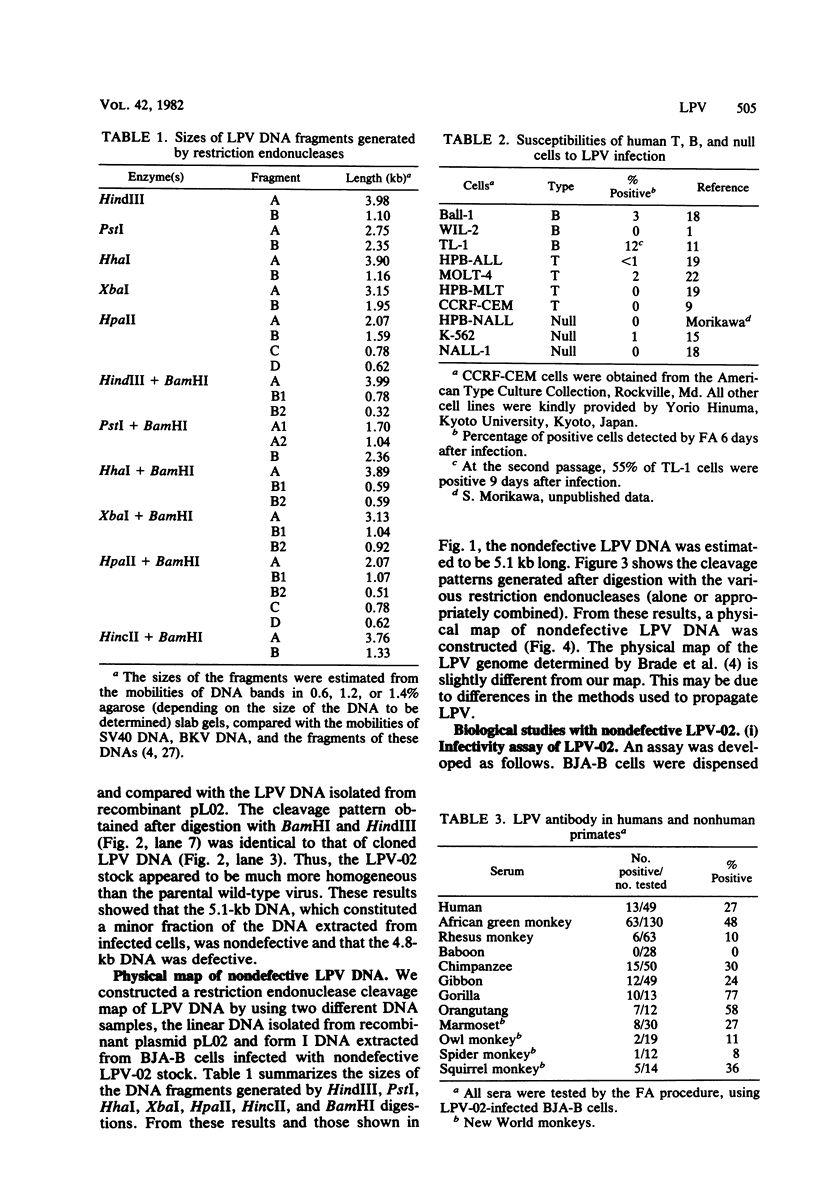
The growth of African green monkey lymphotropic papovavirus (LPV) in human lymphoblastoid cell line BJA-B was found to be slow and inefficient due to the accumulation of defective particles. An analysis of molecularly cloned LPV DNAs showed that 3 of 19 clones had DNAs that were longer (5.1 kilobases) than the DNAs of the other clones. The 5.1-kilobase DNA was infectious for BJA-B cells, whereas the shorter (4.8-kilobase) molecules were defective. Unlike the wild-type virus, stocks of LPV made from cloned, infectious DNAs were homogeneous and had higher titers. Using stocks of nondefective LPV, we investigated other biological properties. LPV replication in another human B-lymphoblastoid cell line was observed. The virus did not cause tumors when it was inoculated into newborn hamsters. Serological surveys of human and nonhuman primate sera indicated that virtually all primates, including humans, show evidence of infection by viruses antigenically related to LPV.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alspaugh M. A., Jensen F. C., Rabin H., Tan E. M. Lymphocytes transformed by Epstein-Barr virus. Induction of nuclear antigen reactive with antibody in rheumatoid arthritis. J Exp Med. 1978 Apr 1;147(4):1018–1027. doi: 10.1084/jem.147.4.1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolivar F., Rodriguez R. L., Greene P. J., Betlach M. C., Heyneker H. L., Boyer H. W., Crosa J. H., Falkow S. Construction and characterization of new cloning vehicles. II. A multipurpose cloning system. Gene. 1977;2(2):95–113. [PubMed] [Google Scholar]
- Brade L., Müller-Lantzsch N., zur Hausen H. B-lymphotropic papovavirus and possibility of infections in humans. J Med Virol. 1981;6(4):301–308. doi: 10.1002/jmv.1890060405. [DOI] [PubMed] [Google Scholar]
- Brade L., Vogl W., Gissman L., zur Hausen H. Propagation of B-lymphotropic papovavirus (LPV) in human B-lymphoma cells and characterization of its DNA. Virology. 1981 Oct 15;114(1):228–235. doi: 10.1016/0042-6822(81)90268-3. [DOI] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen S. N., Chang A. C., Hsu L. Nonchromosomal antibiotic resistance in bacteria: genetic transformation of Escherichia coli by R-factor DNA. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2110–2114. doi: 10.1073/pnas.69.8.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. W., Thomas M., Cameron J., St John T. P., Scherer S., Padgett R. A. Rapid DNA isolations for enzymatic and hybridization analysis. Methods Enzymol. 1980;65(1):404–411. doi: 10.1016/s0076-6879(80)65051-4. [DOI] [PubMed] [Google Scholar]
- FOLEY G. E., LAZARUS H., FARBER S., UZMAN B. G., BOONE B. A., MCCARTHY R. E. CONTINUOUS CULTURE OF HUMAN LYMPHOBLASTS FROM PERIPHERAL BLOOD OF A CHILD WITH ACUTE LEUKEMIA. Cancer. 1965 Apr;18:522–529. doi: 10.1002/1097-0142(196504)18:4<522::aid-cncr2820180418>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Guerry P., LeBlanc D. J., Falkow S. General method for the isolation of plasmid deoxyribonucleic acid. J Bacteriol. 1973 Nov;116(2):1064–1066. doi: 10.1128/jb.116.2.1064-1066.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y., Matsumura Y., Nishihira T., Watanabe I., Ohi R., Kasai M., Kumagai K., Kamada N. Burkitt's lymphoma cell line bearing surface IgA and negative for nuclear antigen of Epstein-Barr virus (EBNA). Jpn J Exp Med. 1980 Dec;50(6):423–434. [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Rowe W. P., Martin M. A. Molecular cloning of polyoma virus DNA in Escherichia coli: plasmid vector system. Science. 1979 Mar 2;203(4383):883–887. doi: 10.1126/science.217087. [DOI] [PubMed] [Google Scholar]
- Klein G., Lindahl T., Jondal M., Leibold W., Menézes J., Nilsson K., Sundström C. Continuous lymphoid cell lines with characteristics of B cells (bone-marrow-derived), lacking the Epstein-Barr virus genome and derived from three human lymphomas. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3283–3286. doi: 10.1073/pnas.71.8.3283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein G., Zeuthen J., Eriksson I., Terasaki P., Bernoco M., Rosén A., Masucci G., Povey S., Ber R. Hybridization of a myeloid leukemia-derived human cell line (K562) with a human Burkitt's lymphoma line (P3HR-1). J Natl Cancer Inst. 1980 Apr;64(4):725–738. [PubMed] [Google Scholar]
- McCutchan J. H., Pagano J. S. Enchancement of the infectivity of simian virus 40 deoxyribonucleic acid with diethylaminoethyl-dextran. J Natl Cancer Inst. 1968 Aug;41(2):351–357. [PubMed] [Google Scholar]
- Miyoshi I., Hiraki S., Tsubota T., Kubonishi I., Matsuda Y., Nakayama T., Kishimoto H., Kimura I., Masuji H. Human B cell, T cell and null cell leukaemic cell lines derived from acute lymphoblastic leukaemias. Nature. 1977 Jun 30;267(5614):843–844. doi: 10.1038/267843a0. [DOI] [PubMed] [Google Scholar]
- Morikawa S., Tatsumi E., Baba M., Harada T., Yasuhira K. Two E-rosette-forming lymphoid cell lines. Int J Cancer. 1978 Feb 15;21(2):166–170. doi: 10.1002/ijc.2910210207. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reissig M., Kelly T. J., Jr, Daniel R. W., Rangan S. R., Shah K. V. Identification of the stumptailed macaque virus as a new papovavirus. Infect Immun. 1976 Jul;14(1):225–231. doi: 10.1128/iai.14.1.225-231.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahai Srivastava B. I., Minowada J. Terminal deoxynucleotidyl transferase activity in a cell line (molt-4) derived from the peripheral blood of a patient with acute lymphoblastic leukemia. Biochem Biophys Res Commun. 1973 Apr 2;51(3):529–535. doi: 10.1016/0006-291x(73)91346-6. [DOI] [PubMed] [Google Scholar]
- Sutcliffe J. G. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harb Symp Quant Biol. 1979;43(Pt 1):77–90. doi: 10.1101/sqb.1979.043.01.013. [DOI] [PubMed] [Google Scholar]
- Takemoto K. K., Mullarkey M. F. Human papovavirus, BK strain: biological studies including antigenic relationship to simian virus 40. J Virol. 1973 Sep;12(3):625–631. doi: 10.1128/jvi.12.3.625-631.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valis J. D., Newell N., Reissig M., Malherbe H., Kaschula V. R., Shah K. V. Characterization of SA12 as a simian virus 40-related papovavirus of chacma baboons. Infect Immun. 1977 Oct;18(1):247–252. doi: 10.1128/iai.18.1.247-252.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker D. L., Padgett B. L., ZuRhein G. M., Albert A. E., Marsh R. F. Human papovavirus (JC): induction of brain tumors in hamsters. Science. 1973 Aug 17;181(4100):674–676. doi: 10.1126/science.181.4100.674. [DOI] [PubMed] [Google Scholar]
- Yang R. C., Wu R. BK virus DNA: complete nucleotide sequence of a human tumor virus. Science. 1979 Oct 26;206(4417):456–462. doi: 10.1126/science.228391. [DOI] [PubMed] [Google Scholar]
- zur Hausen H., Gissmann L. Lymphotropic papovaviruses isolated from African green monkey and human cells. Med Microbiol Immunol. 1979 Aug;167(3):137–153. doi: 10.1007/BF02121180. [DOI] [PubMed] [Google Scholar]