Abstract
A variety of physiological and pathological factors induce cellular swelling in the brain. Changes in cell volume activate several types of ion channels, which mediate the release of inorganic and organic osmolytes and allow for compensatory cell volume decrease. Volume-regulated anion channels (VRAC) are thought to be responsible for the release of some of organic osmolytes, including the excitatory neurotransmitters glutamate and aspartate. In the present study, we compared the in vivo properties of the swelling-activated release of glutamate, aspartate, and another major brain osmolyte taurine. Cell swelling was induced by perfusion of hypoosmotic (low [NaCl]) medium via a microdialysis probe placed in the rat cortex. The hypoosmotic medium produced several-fold increases in the extracellular levels of glutamate, aspartate and taurine. However, the release of the excitatory amino acids differed from the release of taurine in several respects including: (i) kinetic properties, (ii) sensitivity to isoosmotic changes in [NaCl], and (iii) sensitivity to hydrogen peroxide, which is known to modulate VRAC. Consistent with the involvement of VRAC, hypoosmotic medium-induced release of the excitatory amino acids was inhibited by the anion channel blocker DNDS, but not by the glutamate transporter inhibitor TBOA or Cd2+, which inhibits exocytosis. In order to elucidate the mechanisms contributing to taurine release, we studied its release properties in cultured astrocytes and cortical synaptosomes. Similarities between the results obtained in vivo and in synaptosomes suggest that the swelling-activated release of taurine in vivo may be of neuronal origin. Taken together, our findings indicate that different transport mechanisms and/or distinct cellular sources mediate hypoosmotic medium-induced release of the excitatory amino acids and taurine in vivo.
Introduction
The release of organic osmolytes in response to cellular swelling is mediated by one or more volume-sensitive permeability pathways [1]–[3]. Although this phenomenon occurs in all tissues, it has special significance in the brain since two major swelling-sensitive organic osmolytes, glutamate and taurine, also mediate or modulate neuronal communication [4]–[6]. Several neural pathologies, most notably cerebral ischemia, hyponatremia, hepatic encephalopathy and traumatic brain injury, are associated with pronounced cell swelling, which is largely restricted to astrocytes [7]–[9]. Pathological cell swelling is likely related to tissue damage since pharmacological inhibitors that block volume-sensitive anion permeability pathway(s) suppress the pathological release of the excitatory amino acids, glutamate and aspartate, and reduce infarct size in animal models of stroke and ischemia [10]–[15]. These findings have led to the proposal that the swelling-activated release of excitatory amino acids may play a critical role in promoting ischemic tissue damage [7], [9], [16].
The swelling-induced release of the excitatory amino acids glutamate and aspartate and the sulfonic acid taurine is thought to be mediated by Volume Regulated Anion Channels (VRACs), which are also termed in the literature as Volume Sensitive Outward Rectifying (VSOR) Cl− channels or Volume-Sensitive Organic osmolyte/Anion Channels (VSOAC) [17]–[19]. VRACs are traditionally identified as volume-sensitive Cl−/anion channels that are activated in response to cell swelling in nearly all cell types studied. However, in spite of extensive research efforts, the molecular identity of these channels remains unknown [19], [20]. The major physiological role of VRAC is cell volume regulation. Upon activation in swollen cells, VRACs mediate the release of inorganic and organic anions and, in conjunction with swelling-activated K+ channels, facilitate reductions in intracellular osmolarity and subsequent regulatory volume decrease. In addition to Cl−, VRACs are also permeable to bicarbonate (HCO3 −), several other inorganic anions, as well as small organic osmolytes such as amino acids, polyols, and methylamines [2], [21], [22].
The evidence that taurine release is mediated by VRAC, or a very similar permeability pathway, largely stems from studies in cultured neuronal and glial cells, which show that both swelling-activated [3H]taurine and 125I− (Cl−) fluxes are inhibited by a variety of VRAC blockers, including the selective VRAC inhibitor DCPIB [23]–[27]. Several electrophysiological studies have confirmed that VRACs are permeable to taurine, at least under conditions when its molecule is negatively charged [21], [22], [28]. Nevertheless, there is continuous debate as to whether taurine shares the same permeability pathway with Cl− and other anionic amino acids [29]–[31]. In particular, in several cell types and in brain slices, swelling-activated [3H]taurine efflux shows different kinetics and pharmacological properties, when compared to Cl− (125I−) or D-[3H]aspartate release [32]–[37].
Although VRAC and VRAC-mediated amino acid fluxes have been extensively studied in cultured cells, there is limited information regarding their properties in intact brain tissue. Several studies, which used perfusion of hypoosmotic medium via microdialysis probes to induce cell swelling in the brain, found increased extracellular levels of taurine and several amino acids, which are known to permeate through VRAC [38]–[41]. Likewise, Phillis and co-workers found hypoosmotic medium-stimulated release of taurine and the VRAC-permeable amino acids using a cortical cup perfusion technique, and such a release was strongly inhibited by the putative VRAC-blockers DNDS, NPPB, niflumic acid and tamoxifen [42].
In the present work, we used a microdialysis approach in anesthetized animals to compare properties of swelling-activated fluxes of the excitatory amino acids glutamate and aspartate to those of taurine in the rat cortex in vivo.
Materials and Methods
Animal surgery and microdialysis procedures
All animal procedures performed in this work were approved by the institution's animal care and use committee and adhered to the NIH guidelines for care and use of laboratory animals. Male Sprague-Dawley rats (Taconic Farms), weighing between 325 and 425 g, were allowed free access to food and water. Rats were given atropine sulfate (0.5 mg/kg, i.m.) to reduce respiratory tract fluid secretion, and anesthetized with isoflurane prior to intubation. Intubated rats were mechanically ventilated with a gas mixture of 2.25% isoflurane in 30% O2/balance N2. A saline drip (0.9% NaCl) was administered intraperitoneally throughout the experimental procedure to prevent dehydration. Body temperature was monitored throughout the experiment with a rectal probe and was maintained between 36°C and 36.5°C with a heating pad.
Animals were placed in a stereotaxic frame and microdialysis probes (2 mm tip, 20 kD cutoff, CMA Microdialysis, North Chelmsford, MA, U.S.A.) were slowly lowered through burr holes into the frontoparietal cortex (from bregma, 1 mm anterior; ±4 mm lateral; 2.6 mm down from the dura). Artificial cerebral spinal fluid (aCSF; in mM: 120 NaCl, 2.7 KCl, 1 MgSO4, 1.2 CaCl2, 25 NaHCO3, 0.05 ascorbic acid; pH = 7.3) was perfused at 2 µl/min through the microdialysis probes. After two hours of probe stabilization at least two 20 minute perfusate samples were collected by a CMA-170 refrigerated fraction collector (CMA Microdialysis) to determine baseline amino acid levels before the application of drug or hypoosmotic medium. Hypoosmotic aCSF (in mM: 25 NaCl, 2.7 KCl, 1 MgSO4, 1.2 CaCl2, 25 NaHCO3, 0.05 ascorbic acid; pH = 7.3) was perfused at 2 µl/min for one hour and perfusate samples were collected every 5 minutes. Each rat was implanted with two microdialysis probes placed bilaterally in the cortex, with one probe serving as a control (hypoosmotic solution only) and the probe on the other side (chosen at random) serving as the experimental condition (hypoosmotic solution plus drug). All drugs were delivered through the microdialysis probes.
Amino acid analysis in microdialysate samples
Dialysate concentrations of the amino acids were determined by reverse-phase high performance liquid chromatography (HPLC) using a Hewlett-Packard Series 1100 HPLC system. Pre-column derivatization of the amino acids was done with o-phthaldialdehyde/2-mercaptoethanol. The derivatives were separated using a C18 Varian column (4.6×100 mm, 3 µm particle diameter). The fluorescence signal was detected by a Hewlett-Packard 1046A programmable fluorescence detector. Amino acid standards were used to calculate the concentrations of the amino acids in the perfusate.
Preparation of primary astrocyte cultures
Confluent primary astrocyte cultures were prepared from the cerebral cortex of newborn Sprague-Dawley rats as described elsewhere [43], with minor modifications summarized below. Newborn Sprague-Dawley rats were euthanized by rapid decapitation, the cerebral cortices were separated from the meninges and basal ganglia, and tissue was dissociated using the neutral protease Dispase II (Roche Applied Science, Indianapolis, IN, U.S.A.). Dissociated cells were seeded on poly-D-lysine coated 18×18 mm glass coverslips (Caroline Biological Supply Co, Burlington, NC, U.S.A.) for efflux experiments, or 12-well tissue culture plates for uptake experiments. Cell cultures were grown for 3–4 weeks in Minimal Essential Medium (MEM) supplemented with 10% heat inactivated horse serum (HIHS), 50 U/ml penicillin and 50 µg/ml streptomycin at 37°C in a humidified atmosphere of 5% CO2/95% air. Culture medium was replaced twice a week. After two weeks of cultivation, penicillin and streptomycin were removed from the culture medium. Immunocytochemistry showed ≥95% of the cells stained positively for the astrocytic marker glial fibrillary acid protein.
Preparation of rat cortical synaptosomes
Rat cortical synaptosomes were isolated from the cortical tissue of male Sprague-Dawley rats (Taconic Farms) weighing between 180 and 230 g according to [44] with modifications described elsewhere [45]. Final synaptosomal pellets were resuspended in HEPES-buffered medium containing (in mM): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 D-glucose, 10 HEPES; pH = 7.4. Synaptosomes were incubated for 30–40 min at 37°C in order to allow them to restore transmembrane ion gradients before further use in amino acid release experiments.
[3H]Taurine and D-[3H]aspartate efflux assays
[3H]Taurine or D-[3H]aspartate efflux measurements were performed in astrocyte cultures as follows. Astrocytes grown on glass coverslips were loaded overnight with either [3H]taurine (4 µCi/ml) or D-[3H]aspartate (4 µCi/ml) in 2.5 ml of MEM containing 10% HIHS in a CO2 incubator set for 5% CO2/95% air at 37°C. Before the start of the efflux measurements, the cells were washed free of extracellular isotope and residual serum-containing medium in HEPES-buffered solution. The basal HEPES-buffered medium contained (in mM): 135 NaCl, 3.8 KCl, 1.2 MgSO4, 1.3 CaCl2, 1.2 KH2PO4, 10 D-glucose, 10 HEPES; pH = 7.4. The coverslips were inserted into a Lucite perfusion chamber which had a depression precisely cut in the bottom to accommodate the coverslip and a Teflon screw top leaving a space above the cells of around 100–150 µm in height. The cells were superfused at a flow rate of 1.2 ml/min in an incubator set at 37°C with isoosmotic or hypoosmotic HEPES-buffered media. To prepare hypoosmotic medium, the concentration of NaCl was reduced to 85 mM. The osmolarities of all buffers were checked using a freezing point osmometer (µOsmette, Precision Systems, Natick, MA, U.S.A.) and were 287–290 and 197–200 mOsm for isoosmotic and hypoosmotic media, respectively. Superfusate fractions were collected at one minute intervals. At the end of each experiment, the isotope remaining in the cells was extracted with a solution containing 2% sodium dodecyl sulfate (SDS) plus 8 mM EDTA. Four ml Ecoscint scintillation cocktail (National Diagnostics, Atlanta, GA, U.S.A.) was added and each fraction was counted for [3H] in a Tri-Carb 1900TR Liquid Scintillation Analyzer (PerkinElmer, Boston, MA, U.S.A.). Percent fractional isotope release for each time point was calculated by dividing radioactivity released in each 1-min interval by the radioactivity left in the cells (the sum of all the radioactive counts in the remaining fractions up to the beginning of the fraction being measured, plus the radioactivity left in the cell digest).
In a few experiments, astrocytes were simultaneously loaded with D-[3H]aspartate (2 µCi/ml) and [14C]taurine (1 µCi/ml) to compare properties of swelling-activated fluxes of excitatory amino acids and taurine in one cell preparation. In these instances [3H] and [14C] radioactivity was determined in the same perfusate samples using a Tri-Carb 1900TR Liquid Scintillation Analyzer and double-label DPM software.
To measure taurine release in synaptosomal preparations, synaptosomal suspensions were loaded with [3H]taurine (0.5 µCi/ml) for 1 hour at 37°C in basal HEPES-buffered solution. The extracellular isotope was washed by adding 9 volumes of ice-cold medium containing (in mM): 243 sucrose, 5 KCl, 1.2 MgSO4, 10 HEPES, 10 glucose; pH = 7.4. Synaptosomes were sedimented (10,000 g, 2 min at 2°C) and resuspended in the same sucrose medium, which prevents spontaneous synaptosome depolarization at low temperatures. Aliquots of [3H]taurine-loaded synaptosomes (∼0.2–0.3 mg protein) were injected in glass tubes containing 4.5 mL of HEPES-buffered basal, low [NaCl] hypoosmotic, or low [NaCl] isoosmotic media, as specified in the Results section. After 5-min incubation at 37°C, taurine efflux was terminated by rapid vacuum filtration through GF/C glass microfiber filters (Whatman-GE Healthcare, Florham Park, NJ, U.S.A.). Filters were placed in scintillation vials containing a 4 ml Ecoscint scintillation cocktail and counted for radioactivity remaining in the synaptosomes. Relative taurine efflux values (% loaded/5 min) were calculated by comparing the radioactivity in experimental samples to isotope content in samples filtered through GF/C without incubation at 37°C (“0 time”).
[3H]Taurine and D-[3H]aspartate uptake assay
Cultured astrocytes for these experiments were grown in 12-well tissue culture plates according to the cell culture method described in the previous section. Serum-containing medium was washed out, and the cells were incubated for 30 minutes at 37°C with basal HEPES-buffered medium containing 0.5 µCi/mL of [3H]taurine or D-[3H]aspartate plus 10 µM of unlabeled taurine or L-glutamate, respectively. Following the 30-min incubation period cells were washed four times with ice-cold physiological phosphate buffered solution. Cells were then lysed with 2% SDS plus 8 mM EDTA. The isotope content in the lysate was used as a measure of taurine ([3H]taurine) or L-glutamate (D-[3H]aspartate) uptake. Four ml Ecoscint scintillation cocktail was added to each lysates and [3H] was counted in a Liquid Scintillation Analyzer.
Statistical analysis
The statistical significance of the differences in the amino acid release and uptake were determined with ANOVA or repeated measures ANOVA, as specified throughout the text and in figure legends. For the in vivo experiments, planned comparisons were performed with repeated measures ANOVA to determine differences in amino acid release only during hypoosmotic medium exposure. Origin 7.5 (OriginLab, Northampton, MA) and Statistica 6.1 (StatSoft, Tulsa, OH) were used for statistical analysis.
Chemicals
Cadmium chloride (CdCl2), hydrogen peroxide (H2O2), mannitol and ouabain were purchased from Sigma (St. Louis, MI, U.S.A). [3H]Taurine or D-[3H]aspartate were from GE Healthcare-Amersham (Buckinghamshire, U.K.). DL-Threo-β-benzyloxyaspartic acid (DL-TBOA) was obtained from Tocris (Ellisville, MI, U.S.A.). 4,4′-dinitrostilbene-2,2′-disulfonic acid, disodium salt (DNDS) and all cell culture reagents were from Invitrogen (Carlsbad, CA, U.S.A.). All other chemicals including amino acid standards for the HPLC experiments were purchased from Sigma or Aldrich (Milwaukee, WI, U.S.A.) and were the highest purity available.
Results
Differences in kinetics of cortical amino acid and taurine release in response to perfusion of hypoosmotic medium or low NaCl isoosmotic medium
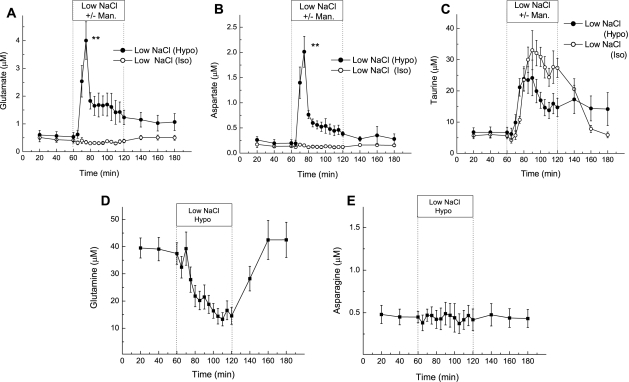
In order to examine volume-sensitive amino acid release in vivo, rat cortices were perfused via a microdialysis probe with hypoosmotic medium in which [NaCl] was reduced from 120 to 25 mM NaCl (65% reduction in osmolarity; medium also contained 25 mM NaHCO3 and other salts as specified in the Methods section). We used a larger reduction in medium osmolarity compared to that typically employed in vitro to account for the fact hypoosmotic media perfused via microdialysis probes are gradually diluted with the extracellular fluids upon their diffusion in the brain. Hypoosmotic medium initiated substantial increases in the levels of VRAC-permeable glutamate, aspartate and taurine (Fig. 1a–c). In the same experiments the extracellular levels of the VRAC-impermeable amino acids, asparagine and glutamine, were either downregulated (glutamine) or not altered (asparagine) by the hypoosmotic medium (Fig. 1d–e). Increases in the extracellular levels of glutamate and aspartate had similar kinetics. Dialysate levels of both amino acids peaked at 15 minutes (∼6.5- and ∼5-fold increases over baseline, for glutamate and aspartate, respectively), then quickly decreased to levels which were only 2-3-fold higher than the basal release, with additional recovery observed after switching to isoosmotic medium (Fig. 1a, b). In contrast, in the same samples, the swelling-activated release of taurine was consistently delayed by 5 minutes versus excitatory amino acids, had a substantially slower inactivation, and never recovered after returning to isoosmotic conditions (Fig. 1c).
Figure 1. Effect of hypoosmotic or isoosmotic low [NaCl] medium on amino acid levels measured in the rat cortex in vivo.
(a–c) Microdialysis probes, implanted in the rat frontoparietal cortex, were perfused with hypoosmotic medium (−95 mM NaCl, −65% osmolarity) or isoosmotic low NaCl medium (−95 mM NaCl +167 mM mannitol) for one hour. In these experiments, the rat brain was perfused with both the hypoosmotic and isoosmotic medium on opposite sides of the cortex. The data represent average dialysate levels of glutamate (a), aspartate (b), and taurine (c) ±SEM from 5 rats. ** p<0.01, hypoosmotic vs. isoosmotic low [NaCl], repeated measures ANOVA. (d–e) In several experiments dialysate levels of glutamine (d, N = 5), and asparagine (e, n = 3) were additionally measured on the “hypoosmotic” side of the brain.
To determine if the increases in glutamate, aspartate and taurine levels were due to changes in osmolarity or a consequence of reduced [NaCl]e, the same rats were simultaneously perfused, via microdialysis probes placed in the contralateral cortex, with low [NaCl] (25 mM) medium that was made isoosmotic by the addition of 167 mM mannitol. The isoosmotic low [NaCl] medium failed to induce an increase in dialysate concentrations of glutamate and aspartate (Fig 1a, b). In the same samples, however, we found a large increase in the extracellular levels of taurine in response to isoosmotic [NaCl]e reduction, which was not statistically different from the hypoosmotic-stimulated augmentation (Fig 1c).
In order to understand the nature of the low [NaCl]-induced taurine release, we exposed cultured astrocytes and isolated nerve endings (synaptosomes) prepared from rat cortical tissue to low [NaCl] media made isoosmotic with mannitol. In striking contrast to the in vivo microdialysis data, cultured astrocytes preloaded with [3H]taurine failed to show any increase in taurine release levels when perfused with the same low [NaCl] isoosmotic medium (Fig 2a). In cortical synaptosomes, we found modest (∼3-fold) increases in [3H]taurine release under isoosmotic low [NaCI] conditions (2b). However, such increases were much smaller when compared to the releases induced by the hypoosmotic reduction in [NaCI] (∼15 fold, Fig. 2b). This was in contrast to our in vivo data which showed very similar increases in taurine levels with both hypoosmotic and isoosmotic low [NaCl] medium (compare Figs 2b and 1c).
Figure 2. Isoosmotic low [NaCl] medium does not induce taurine release from cultured rat astrocytes but modestly enhances taurine release from rat cortical synaptosomes.
(a) Effect of hypoosmotic or isoosmotic reductions in [NaCl] on [3H]taurine release from astrocytes. The data represent mean values ±SEM of integral 10-min releases under isoosmotic (Basal), hypoosmotic (Hypo), or isoosmotic low [NaCl] solutions. n = 4 for each group. *p<0.05, ***p<0.001, vs. basal. (b) Integral 5-min releases of [3H]taurine from synaptosomes exposed to isoosmotic (Basal), hypoosmotic (Hypo) or isoosmotic media with lowered [NaCl]. Means ±SEM of 3 experiments. ** p<0.01, vs. basal.
Since taurine transporter function is dependent on the transmembrane Na+ gradient, we speculated that the increased levels of taurine seen in vivo upon application of low extracellular [NaCl] hypoosmotic or isoosmotic media may in part be due to inhibition of the taurine transporter. To address this issue, we evaluated how decreases in [Na+]e affect [3H]taurine uptake in cultured astrocytes. Given that glutamate and aspartate levels in vivo are insensitive to the isoosmotic decrease of [Na+]e (see Fig. 1a, b), we additionally compared the effect of low [Na+]e on [3H]taurine uptake to the uptake of D-[3H]aspartate. As seen in Fig. 3, decreases in [Na+]e to 50 mM (equivalent to the low [Na+]e used in the in vivo experiments) significantly inhibited both [3H]taurine and D-[3H]aspartate (L-glutamate) uptake, with taurine uptake inhibited to a greater extent. However, the observed in vitro difference in the transporters' sensitivities to [Na+]e may not in itself be sufficient to explain the drastic sensitivity of taurine release to isoosmotic modulation of the [NaCl] observed in vivo.
Figure 3. Dependence of taurine and glutamate uptake on extracellular [Na+] in cultured astrocytes.
Taurine and glutamate transport rates were measured in primary astrocyte cultures using [3H]taurine and d-[3H]aspartate. Extracellular concentrations of amino acids were adjusted to 10 µM using unlabeled taurine or l-glutamate. To compare glutamate versus taurine uptake, the values were normalized to uptake levels under basal conditions ([Na+]o = 135 mM). Note that under basal conditions absolute d-[3H]aspartate uptake rate (nmols/mg protein) was ∼5-fold higher compared to taurine. Data are the mean values ±SEM of three experiments from each group.
Effects of the anion channel blocker DNDS on hypoosmotic medium-stimulated amino acid and taurine release in vivo and in vitro
Given that our data show that taurine and excitatory amino acid levels are differentially regulated in vivo by low [NaCl], we further investigated the potential mechanisms responsible for the elevated amino acid levels in response to hypoosmotic medium by using different amino acid transport inhibitors. Our first aim was to determine if the increases in extracellular excitatory amino acid levels is mediated by a VRAC-like pathway, as has been extensively shown in vitro. Therefore, we used the anion channel blocker DNDS, which has an IC50 for VRACs of ∼1–2 mM [46]. Although DNDS has a low potency for inhibiting VRACs, it is one of the few anion channel inhibitors that can be used in microdialysis studies because it does not produce toxic effects or changes in the basal amino acid release levels. The more potent VRAC blockers NPPB or phloretin caused strong and progressive increases in microdialysate glutamate and aspartate levels, which likely reflect cytotoxicity (Y. Jin, R.E. Haskew-Layton, P.J. Feustel, H.K. Kimelberg, A.A. Mongin, unpublished observations). Ten mM DNDS, when added one hour prior to and during hypoosmotic medium exposure, significantly inhibited the hypoosmotic-stimulated release of the excitatory amino acids glutamate and aspartate, but did not alter basal excitatory amino acid levels (Fig 4a, b). DNDS also potently inhibited hypoosmotic taurine release, and in addition, also reduced taurine levels under basal conditions (Fig. 4c).
Figure 4. Effect of the anion channel blocker DNDS on hypoosmotic medium-induced amino acid release in the rat cortex.
(a–c) Microdialysis probes were perfused on opposite sides of the cortex with hypoosmotic medium (HYPO, −65% osmolarity) in the presence or absence of 10 mM DNDS, given one hour prior to and during one-hour hypoosmotic medium perfusion. The data represent average dialysate levels of glutamate (a), aspartate (b) and taurine (c) +/−SEM from 5 rats. * p<0.05, HYPO vs. HYPO+DNDS (glutamate); ** p<0.01, HYPO vs. HYPO+DNDS (aspartate); *** p<0.001 HYPO vs. HYPO+DNDS (taurine). Significance was determined by repeated measures ANOVA.
To verify the efficacy of DNDS as a VRAC blocker we tested the effect of DNDS on swelling-activated D-[3H]aspartate release in cultured astrocytes, which is entirely mediated by VRACs [47]. Two mM DNDS was sufficient to suppress D-[3H]aspartate release by ∼70% (Fig. 5a), was similarly effective against astrocytic [3H]taurine release (∼75%, data not shown), and nearly completely suppressed swelling-activated [3H]taurine release from cortical synaptosomes (Fig. 5b). To further explore whether DNDS may inhibit taurine transporters and in this way affects extracellular taurine levels in vivo, we tested the effects of DNDS on taurine uptake in cultured astrocytes. DNDS did not affect astrocytic [3H]taurine uptake up to the concentration of 32 mM, suggesting that this compound does not alter taurine transporter function (data not shown).
Figure 5. Effect of DNDS on swelling-activated D-[3H]aspartate release from cultured astrocytes and swelling-activated [3H]taurine uptake in cortical synaptosomes.
(a) Cultured astrocytes preloaded with D-[3H]aspartate were superfused with hypoosmotic medium in the presence or absence of 2 mM DNDS. The data are the mean values of five experiments for each group ±SEM. *** p<0.001 hypo, vs. DNDS. (b) Release of preloaded [3H]taurine from cortical synaptosomes was measured under isoosmotic (BASAL) and hypoosmotic (HYPO) conditions in the presence or absence of 2 mM DNDS. The data are the mean values of integral 10-min [3H]taurine release ±SEM of three experiments performed in quadruplicate. ***p<0.001 vs. isoosmotic control (BASAL), ###p<0.001 vs. hypoosmotic control (HYPO).
Effects of the Ca2+ channel blocker Cd2+ on hypoosmotic medium-stimulated amino acid and taurine release in vivo
To additionally explore the mechanisms responsible for the excitatory amino acid release in vivo, we tested for the contribution of alternative release mechanisms. One such mechanism is synaptic Ca2+-dependent release from a vesicular pool. Several reports suggest that hypoosmotic medium induces membrane depolarization and promotes increases in [Ca2+]i in synaptosomes and brain slices, which may trigger exocytotic neurotransmitter release [48]–[50]. To exclude the possible involvement of exocytosis in mediating hypoosmotic-induced amino acid release we used a broad spectrum blocker of voltage-sensitive Ca2+ channels, cadmium (Cd2+). Ca2+-free artificial cerebral spinal fluid containing 300 µM Cd2+, given 20 minutes prior to and during Ca2+-free hypoosmotic medium perfusion, did not alter glutamate, aspartate or taurine release (Fig. 6a–c). Previous studies have found that 300 µM Cd2+, administered through microdialysis probes, is effective in blocking electrically stimulated serotonin release and membrane depolarization-induced norepinephrine release [51], [52], and 30 µM Cd2+ is sufficient to reduce tonic glutamate release in the amygdala [53]. Thus, our data showing that amino acid release in vivo is insensitive to 300 µM Cd2+ suggests that hypoosmotic-stimulated excitatory amino acid release is not due to the stimulation of exocytosis.
Figure 6. Effect of the Ca2+ channel blocker Cd2+ on hypoosmotic medium-induced amino acid release in the cortex.
(a–c) Microdialysis probes were perfused with hypoosmotic medium (HYPO) in the presence or absence of 300 µM Cd2+ given 20 minutes prior to and during one-hour hypoosmotic medium perfusion. Each rat had two microdialysis probes implanted on opposite sides of the cortex (one perfused with HYPO alone and the other with HYPO+Cd2+). The data represent the average dialysate levels ±SEM of glutamate (a), aspartate (b), and taurine (c) from 5 rats.
Effects of the glutamate transporter blocker DL-TBOA on hypoosmotic medium-stimulated excitatory amino acid release in vivo
Low [Na+]e may potentially elevate dialysate glutamate and aspartate levels by inducing the reversal of glutamate transporters, which are dependent on normal Na+ and K+ gradients [54]. Therefore to rule out the involvement of glutamate transport reversal in mediating hypoosmotic-stimulated excitatory amino acid release we used the broad-spectrum non-transportable glutamate transporter blocker DL-TBOA that blocks all neuronal and glial transporters [55], [54]. 500 µM DL-TBOA given 20 minutes prior to and during hypoosmotic medium perfusion, significantly increased rather than decreased excitatory amino acid release and did not affect taurine levels (Fig. 7a, b), suggesting that normal transport operation is maintained under hypoosmotic conditions and transport reversal is not responsible for the hypoosmotic medium-stimulated excitatory amino acid release. As expected, the in vivo extracellular levels of taurine were not affected by DL-TBOA (data not shown).
Figure 7. Effect of the glutamate transporter inhibitor dl-TBOA on hypoosmotic medium induced amino acid release in the cortex and glutamate transporter reversal in cultured astrocytes.
(a–b) Microdialysis probes implanted on opposite sides of the cortex were perfused with hypoosmotic medium in the presence or absence of 500 µM dl-TBOA, given 20 minutes prior to and during one hour hypoosmotic medium perfusion. The data represent average dialysate levels of glutamate (a), aspartate (b) ±SEM from 4 rats. ** p<0.01 HYPO vs. HYPO+TBOA. (c) DL-TBOA effectively prevented reversal of glutamate transporter in cultured astrocytes. Cultured astrocytes were superfused for one hour with 1 mM ouabain and additionally for 20 min high [KCl] (100 mM) plus ouabain to induce glutamate transporter reversal. 300 µM dl-TBOA was given 10 minutes prior to and during the high [KCl] perfusion in the presence of ouabain. The data are the average values ±SEM for three experiments in each group. ** p<0.01 KCl vs. KCl+TBOA.
Although DL-TBOA has been well characterized as an inhibitor of normal glutamate transporter function [55], we wanted to verify DL-TBOA's effectiveness in blocking glutamate transporters working in the reverse mode. Cultured astrocytes, preloaded with D-[3H]aspartate, were treated for 40 minutes with 1.0 mM ouabain to increase [Na+]i, prior to and during 20 minutes perfusion with an isoosmotic 100 mM [K+]e medium. These treatment conditions have previously been shown to induce the reversal of glutamate transporters in cultured astrocytes [56]. In vitro, 300 µM DL-TBOA given 10 minutes prior to and during 10 mM [K+] perfusion completely blocked glutamate transport reversal-induced D-[3H]aspartate release, verifying that DL-TBOA is an effective inhibitor of glutamate transport reversal (Fig. 7c).
Effects of H2O2 on hypoosmotic-stimulated release of excitatory amino acid and taurine in vivo and in vitro
We further investigated if reactive oxygen species modulate swelling-sensitive excitatory amino acid release in the brain, as seen in cultured astrocytes (Haskew-Layton et al., 2005). One mM H2O2, administered 20 minutes prior to and during hypoosmotic medium perfusion, did not affect basal levels of glutamate or aspartate but significantly enhanced the swelling-evoked release of both excitatory amino acids (Fig. 8a,b). In contrast, H2O2 did not alter dialysate levels of taurine under hypoosmotic conditions (Fig. 8c), suggesting that excitatory amino acids and taurine release are differentially regulated. To verify that H2O2 does not upregulate glutamate and aspartate release via a VRAC-independent mechanism, we tested the effects of 1 mM H2O2 on amino acid levels in the absence of hypoosmotic medium in a separate set of experiments. As seen in Fig. 8a, b, when superfused under isoosmotic conditions, 1 mM H2O2 did not produce a substantial increase in excitatory amino acid levels but did cause a small gradual upward shift in the baseline.
Figure 8. Effect of H2O2 on hypoosmotic medium induced amino acid release in the cortex.
(a–c) Two microdialysis probes implanted on opposite sides of the cortex were perfused with hypoosmotic medium in the presence or absence of 1 mM H2O2 given 20 minutes prior to and during one-hour hypoosmotic medium perfusion. The data represent the average dialysate levels ±SEM of glutamate (a), aspartate (b) and taurine (c) from 9 rats. ** p<0.01 HYPO vs. HYPO+H2O2. In separate experiments, rats were perfused with 1 mM H2O2 alone (N = 5).
Since H2O2 has been reported to alter glutamate uptake [57], we tested the effects of 1–1,000 µM H2O2 on the excitatory amino acid uptake in cultured astrocytes. H2O2 did not alter the excitatory amino acid uptake in vitro up to the highest concentration tested (data not shown). These data, in conjunction with the lack of a H2O2 effect on the basal levels of excitatory amino acid in vivo, suggest that the effects of H2O2 on hypoosmotic glutamate and aspartate levels are unlikely due to uptake inhibition.
In order to model the effects of H2O2 on taurine release in astroglial and neuronal cells, we tested in vitro the effect of 300 µM H2O2 on swelling-activated amino acid release in cultured rat astrocytes and cortical synaptosomes. Astrocytes were simultaneously preloaded with D-[3H]aspartate and [14C]taurine to reveal any potential differences between release properties of the excitatory amino acids and taurine in the same cells. As in our previous study (Haskew-Layton et al., 2005), H2O2 strongly potentiated the swelling-induced release of D-[3H]aspartate (data not shown), as well as the release of taurine (Fig. 9a) by 2-3-fold. In striking contrast, the same concentration of H2O2 was completely ineffective in potentiating [3H]taurine release in synaptosomes, whether H2O2 added 10 minutes before and during application of hypoosmotic medium (Fig. 9b) or acutely (data not shown). These results suggest that the H2O2-insensitive, hypoosmotic-medium stimulated taurine release observed in vivo may originate from a neuronal compartment.
Figure 9. Effect of H2O2 on swelling-activated taurine release from cultured astrocytes and cortical synaptosomes.
(a) The effect of H2O2 on swelling-activated [14C]taurine release from cultured astrocytes. Astrocytes were preloaded overnight with [14C]taurine and D-[3H]aspartate. 300 µM H2O2 was added to the media 10 min before and during exposure to hypoosmotic medium. For clarity, only [14C]taurine release is shown. The data are the mean values ±SEM of three experiments. **p<0.01 vs. hypotonic control. (b) The effect of H2O2 on swelling activated [3H]taurine release from rat cortical synaptosomes. Integral [3H]taurine release was measured for 5 minutes as described in Material and methods. 300 µM H2O2 was present in media 10 min before and during measurements of taurine release. Data are the mean values ±SEM of three independent experiments performed in quadruplicate. ***p<0.001 vs. basal release.
Discussion
In the present in vivo study we employed a microdialysis approach to compare properties of the swelling-activated release of major organic osmolytes, the excitatory amino acids glutamate and aspartate and the sulfonic acid taurine, in the rat cortex. Swelling-activated amino acid release may mimic pathological processes that occur in ischemia and several other neuropathologies, where extensive astroglial cell swelling has been detected [7], [9]. To model the effects of cell swelling without the contribution of numerous volume-insensitive glutamate release pathways, we utilized perfusion of hypoosmotic medium via a microdialysis probe, rather than performing the studies in the ischemic brain. The major finding of our work is that hypoosmotic-medium induced release of excitatory amino acids and taurine exhibit significant differences in their kinetic properties, sensitivity to isoosmotic changes in extracellular [NaCl] and response to perfusion of the reactive oxygen species H2O2. Such differences indicate that these organic osmolytes are released from different cellular pools and/or via different release pathways.
Properties of hypoosmotic medium-induced excitatory amino acid release are consistent with the involvement of VRAC
Previous findings from the literature suggest that there is substantial similarity between swelling-activated amino acid release in vitro and in situ. In cultured astrocytes and neuronal cells, hypoosmotic medium promotes cell swelling and triggers the release of several uncharged or negatively charged amino acids such as glycine, alanine, taurine, glutamate, and aspartate [23], [24], [26], [47], [58], [59]. Several in situ studies performed in brain slices have found that the release properties of isotope-labeled and endogenous excitatory amino acids and taurine are similar to those observed in vitro [36], [60]–[62]. Such organic osmolyte release occurs via a non-saturable pathway, which is inhibited by a variety of Cl− channel blockers, and therefore likely mediated by an anion channel. In vitro electrophysiological studies found that volume-regulated anion channels (VRAC) are permeable to glutamate, aspartate, taurine, and glycine, but not to the majority of other amino acids [21], [22], [63]–[65]. However, it is currently debated whether one or more permeability pathways contribute to the release of organic osmolytes [29]–[31]. Furthermore, some reports additionally proposed that hypoosmotic swelling may promote the release of excitatory amino acids via a Ca2+-independent mode of exocytosis [66], [67].
In vivo, our present work and several previous studies found that hypoosmotic medium stimulates the release of VRAC-permeable amino acids (glutamate, aspartate, and taurine), while the levels of VRAC-impermeable amino acids (e.g., asparagine and glutamine) remain unaffected or decreased [38], [39], [42]. The broad spectrum Cl− channel blocker DNDS inhibited the release of excitatory amino acids and taurine, at concentrations that block VRAC activity in vitro. We have found that DNDS is well tolerated in vivo, unlike other commonly used and more potent VRAC blockers, such as NPPB and phloretin. Consistent with the involvement of VRAC, increases in the extracellular levels of the excitatory amino acids were seen upon application of hypoosmotic medium (low [NaCl]e) but not in response to isoosmotic changes in [NaCl]e (NaCl replaced with mannitol). Furthermore, swelling-induced excitatory amino acid release was not blocked by inhibitors of two alternative glutamate and aspartate release pathways, i.e. reversal of glutamate transporters and exocytotic release.
Although cell swelling is thought to be the primary stimulus responsible for initiating VRAC opening in the pathological brain, little else is known about the activation or modulation of VRACs in the intact tissue. Our recent in vitro work demonstrated that swelling activated excitatory amino acid release via VRAC is potently modulated by reactive nitrogen species and reactive oxygen species [68], [69]. In the present microdialysis experiments, the reactive oxygen species H2O2 strongly increased hypoosmotic levels of glutamate and aspartate, but had little effect when administered under isoosmotic conditions. Taken together with the pharmacological data, these findings are in line with the idea that VRAC is the primary source of the excitatory amino acid release in response to hypoosmotic medium-induced (and pathological) cell swelling.
Hypoosmotic medium-induced release of taurine differs from the excitatory amino acid release
Unexpectedly, we found marked differences in taurine and excitatory amino acid release. Taurine is widely regarded as an important osmoregulatory molecule in the brain and in other tissues because it is one of the most abundant organic osmolytes and effectively permeates a putative VRAC-like pathway [23], [22], [28], [43], [70]. Consistent with its osmoregulatory role, taurine release in cultured astrocytes, hippocampal slices, and in vivo microdialysis experiments has been found to be potently upregulated under hypoosmotic conditions (reductions in [NaCl]e), but is insensitive to isoosmotic changes in [NaCl]e [23], [41], [66]. Therefore, our observation, that cortical taurine levels are strongly elevated by either hypoosmotic or isoosmotic low [NaCl] media, was rather surprising. Nevertheless, such a finding is not unique. A similar sensitivity of taurine to changes in [NaCl]e independent of changes in osmolarity has been found in two microdialysis studies measuring taurine levels in the rat hippocampus and in slices prepared from the mouse brain stem [71], [72]. Using an alternative approach, in which extracellular amino acids were sampled via a cortical cup, Phillis et al. also observed that replacement of extracellular NaCl with choline-Cl or MMDG-Cl strongly elevated superfusate levels of taurine but not those of glutamate or aspartate [73].
Besides the differences in sensitivity to isoosmotic [NaCl]e decreases, taurine release was (i) consistently delayed by ∼5 minutes, compared to the swelling-activate release of the excitatory amino acids, (ii) showed much slower inactivation, and (iii) was completely insensitive to the application of H2O2. Since the amino acid measurements were performed in the same samples, these data unequivocally point to different release mechanisms or different cellular sources. The idea of diverse transport pathways for taurine and other osmolytes has been suggested in the past, based on dissimilar properties of taurine and excitatory amino acid release in cultured astrocytes, hippocampal brain slices, and in a mammary cell line [35], [66], [74]. However, because the molecular identity of the volume-sensitive organic osmolyte release pathway is unknown, this hypothesis is difficult to address.
An alternative hypothesis that would explain the atypical behavior of taurine is that in the cortex taurine and excitatory amino acids are released from different cellular pools. Glutamate is uniformly distributed in the brain, however it is somewhat more concentrated in neurons since its concentration in astrocytes is lowered by the activity of glutamine synthase [75]. In contrast, the cellular localization of taurine is highly heterogeneous. Depending on the brain region, taurine is concentrated within either glial cells or neurons. For instance, in the cerebellum and the putamen taurine is primarily localized to neurons, while in the thalamus, hypothalamus and brain stem it is concentrated in glial cells [76]–[78]. In the cortex there have been conflicting reports suggesting that taurine is preferentially localized to either neurons or glial cells [79], [80].
In order to model properties of astroglial and neuronal taurine release we performed experiments in primary rat astrocytes and cortical synaptosomes. In cultured astrocytes, taurine release was completely dissimilar to the release in vivo: it was absolutely insensitive to isoosmotic decreases in [NaCl]e, and was strongly potentiated by H2O2 in swollen cells. Since we were unable to mimic the astrocytic profile of taurine release in vivo, it suggests that astrocytes may contribute to only a negligible portion of taurine release in the cortex. On the other hand, similar to our in vivo data, swelling-activated taurine release in synaptosomes mimicked the in vivo release in that it was completely insensitive to H2O2 and sensitive to isoosmotic reductions in [NaCl]e (albeit to a much weaker degree than the in vivo response). Although these synaptosomal data do not perfectly match the in vivo results, they suggest a possibility that taurine release in the cerebral cortex originates from a neuronal pool. Ineffective uptake of extracellular taurine in vivo under hypoosmotic conditions and in response to isoosmotic reductions in [NaCl] may be determined by a lower density of taurine transporters and their high dependence on extracellular [Na+] and [Cl−] (see Fig. 3). In contrast, glutamate transporters are expressed at a very high density in the brain and driven by the transmembrane gradients of K+, Na+, and Cl−, and are therefore less sensitive to changes in [NaCl]e [54], [75]. An integrated model, providing an explanation for our in vivo and in vitro data, is presented in Fig. 10.
Figure 10. Hypothetical explanation of the experimental data showing differences in taurine and glutamate release in vivo.
A reduction in medium osmolarity (↓[osm]e) in the rat cortex causes an increase in the extracellular levels of the excitatory amino acids glutamate and aspartate and the sulfonic acid taurine via a mechanism sensitive to the anion channel blocker DNDS. Despite these similarities, the excitatory amino acid and taurine release demonstrate different kinetics and are likely mediated by different transport pathways (1 and 2) and/or originate from different cellular sources. The taurine pathway (1) but not the excitatory amino acid pathway (2) is activated by isoosmotic lowering of [NaCl]e. Conversely, the swelling-activated excitatory amino acid release pathway (2) but not the taurine pathway (1) is potentiated by H2O2. Alternative transport pathways that were considered in this study include: [Na+]e-dependent taurine transporters (3), [Na+]e/[K+]i-dependent glutamate transporters in neurons (4), and in astrocytes (5), which are sensitive to TBOA; and vesicular glutamate release (6), which is sensitive to the voltage-gated Ca2+ channel blocker Cd2+. Based on the similarities of excitatory amino acid release in vivo and in cultured astrocytes, we speculate that glutamate release in vivo largely originates from glial cells. Similarities between taurine release in vivo and in synaptosomes suggest that taurine release may be of a neuronal origin.
Relevance to pathophysiological amino acid and taurine release in ischemia
There is ample evidence that VRAC contributes to pathological excitatory amino acid release in a number of neurological conditions associated with cell swelling, including ischemia, hyponatremia, hepatic encephalopathy, and traumatic brain injury (reviewed in [7], [9]). In animal ischemia models, VRAC blockers reduce pathological elevations in the extracellular levels of excitatory amino acids [10], [11], [14], [81], and potently protect the animal brain against ischemic damage [12], [82], [83]. Most recently, the selective VRAC inhibitor DCPIB has been found to reduce intra-ischemic glutamate release and potently reduce ischemic infarction in a rat focal reversible ischemia model [15]. Consistent with in vivo findings, in a slice model of cerebral spreading depression, VRAC inhibitors delay the onset of glutamate-dependent depolarizations and reduce glutamate release, which is at least partially associated with cell swelling [84]. Interestingly, characteristics of intra-ischemic taurine release also show strong deviation from those of glutamate and aspartate. For instance, in a rat global ischemia model ischemic striatal taurine release was weekly sensitive to 1 and 10 mM DNDS, as compared to the strong inhibition of pathological excitatory amino acid release [11]. Although in the present experiments, both the hypoosmotic release of taurine and the excitatory amino acids were potently suppressed by DNDS, the global ischemia data are consistent with the idea that taurine and excitatory amino acids may be released from different cellular pools and/or via different transport mechanisms. Properties of swelling activated excitatory amino acid release in vivo strongly resembled the positive modulation of the excitatory amino acid release via a putative VRAC pathway, and in particular were strongly upregulated by H2O2, as seen in cultured astrocytes [69]. Since H2O2 levels are strongly upregulated in ischemia and reperfusion [85], [86], the additive effects of cell swelling and reactive oxygen species may contribute to excitotoxic tissue damage in stroke and perhaps other neuropathologies.
In summary, we found that cellular swelling in the rat cortex in vivo triggers release of glutamate and aspartate with properties strongly resembling swelling-activated excitatory amino acid release in cultured astrocytes, which is thought to be mediated by VRAC [47]. In contrast, hypoosmotic medium-induced taurine release seemingly is derived from a different cellular source or mediated by different transporter mechanism(s). Since taurine release properties could be mimicked in synaptosomal preparations, we speculate that such release may originate from a neuronal compartment.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported in part by NIH grants F31 NS046961 (to REH-L), R01 NS035205 (to HKK), R21 NS052516 (to AAM), and AMC grant 201-311053 (to AAM). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Lang F, Busch GL, Ritter M, Volkl H, Waldegger S, et al. Functional significance of cell volume regulatory mechanisms. Physiol Rev. 1998;78:247–306. doi: 10.1152/physrev.1998.78.1.247. [DOI] [PubMed] [Google Scholar]
- 2.Kirk K, Strange K. Functional properties and physiological roles of organic solute channels. Annu Rev Physiol. 1998;60:719–739. doi: 10.1146/annurev.physiol.60.1.719. [DOI] [PubMed] [Google Scholar]
- 3.Wehner F, Olsen H, Tinel H, Kinne-Saffran E, Kinne RK. Cell volume regulation: osmolytes, osmolyte transport, and signal transduction. Rev Physiol Biochem Pharmacol. 2003;148:1–80. doi: 10.1007/s10254-003-0009-x. [DOI] [PubMed] [Google Scholar]
- 4.Fonnum F. Glutamate: a neurotransmitter in mammalian brain. J Neurochem. 1984;42:1–11. doi: 10.1111/j.1471-4159.1984.tb09689.x. [DOI] [PubMed] [Google Scholar]
- 5.Huxtable RJ. Physiological actions of taurine. Physiol Rev. 1992;72:101–163. doi: 10.1152/physrev.1992.72.1.101. [DOI] [PubMed] [Google Scholar]
- 6.Cartmell J, Schoepp DD. Regulation of neurotransmitter release by metabotropic glutamate receptors. J Neurochem. 2000;75:889–907. doi: 10.1046/j.1471-4159.2000.0750889.x. [DOI] [PubMed] [Google Scholar]
- 7.Kimelberg HK. Current concepts of brain edema. Review of laboratory investigations. J Neurosurg. 1995;83:1051–1059. doi: 10.3171/jns.1995.83.6.1051. [DOI] [PubMed] [Google Scholar]
- 8.Sykova E, Mazel T, Vargova L, Vorisek I, Prokopova-Kubinova S. Extracellular space diffusion and pathological states. Prog Brain Res. 2000;125:155–178. doi: 10.1016/S0079-6123(00)25008-5. [DOI] [PubMed] [Google Scholar]
- 9.Mongin AA, Kimelberg HK. Astrocytic swelling in neuropathology. In: Kettenmann H, Ransom B, editors. Neuroglia (2nd edition) Oxford-New York: Oxford University Press; 2005. pp. 550–562. [Google Scholar]
- 10.Phillis JW, Song D, O'Regan MH. Inhibition by anion channel blockers of ischemia-evoked release of excitotoxic and other amino acids from rat cerebral cortex. Brain Res. 1997;758:9–16. doi: 10.1016/s0006-8993(97)00155-8. [DOI] [PubMed] [Google Scholar]
- 11.Seki Y, Feustel PJ, Keller RW, Jr, Tranmer BI, Kimelberg HK. Inhibition of ischemia-induced glutamate release in rat striatum by dihydrokinate and an anion channel blocker. Stroke. 1999;30:433–440. doi: 10.1161/01.str.30.2.433. [DOI] [PubMed] [Google Scholar]
- 12.Kimelberg HK, Feustel PJ, Jin Y, Paquette J, Boulos A, et al. Acute treatment with tamoxifen reduces ischemic damage following middle cerebral artery occlusion. Neuroreport. 2000;11:2675–2679. doi: 10.1097/00001756-200008210-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kimelberg HK, Jin Y, Charniga C, Feustel PJ. Neuroprotective activity of tamoxifen in permanent focal ischemia. J Neurosurg. 2003;99:138–142. doi: 10.3171/jns.2003.99.1.0138. [DOI] [PubMed] [Google Scholar]
- 14.Feustel PJ, Jin Y, Kimelberg HK. Volume-regulated anion channels are the predominant contributors to release of excitatory amino acids in the ischemic cortical penumbra. Stroke. 2004;35:1164–1168. doi: 10.1161/01.STR.0000124127.57946.a1. [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Zhang H, Feustel PJ, Kimelberg HK. DCPIB, a specific inhibitor of volume regulated anion channels (VRACs), reduces infarct size in MCAo and the release of glutamate in the ischemic cortical penumbra. Exp Neurol. 2008;210:514–520. doi: 10.1016/j.expneurol.2007.11.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mongin AA. Disruption of ionic and cell volume homeostasis in cerebral ischemia: The perfect storm. Pathophysiology. 2007;14:183–193. doi: 10.1016/j.pathophys.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Strange K, Emma F, Jackson PS. Cellular and molecular physiology of volume-sensitive anion channels. Am J Physiol. 1996;270:C711–C730. doi: 10.1152/ajpcell.1996.270.3.C711. [DOI] [PubMed] [Google Scholar]
- 18.Okada Y. Volume expansion-sensing outward-rectifier Cl− channel: fresh start to the molecular identity and volume sensor. Am J Physiol Cell Physiol. 1997;273:C755–C789. doi: 10.1152/ajpcell.1997.273.3.C755. [DOI] [PubMed] [Google Scholar]
- 19.Nilius B, Droogmans G. Amazing chloride channels: an overview. Acta Physiol Scand. 2003;177:119–147. doi: 10.1046/j.1365-201X.2003.01060.x. [DOI] [PubMed] [Google Scholar]
- 20.Okada Y. Cell volume-sensitive chloride channels: phenotypic properties and molecular identity. Contrib Nephrol. 2006;152:9–24. doi: 10.1159/000096285. [DOI] [PubMed] [Google Scholar]
- 21.Banderali U, Roy G. Anion channels for amino-acids in Mdck cells. Am J Physiol. 1992;263:C1200–C1207. doi: 10.1152/ajpcell.1992.263.6.C1200. [DOI] [PubMed] [Google Scholar]
- 22.Jackson PS, Strange K. Volume-sensitive anion channels mediate swelling-activated inositol and taurine efflux. Am J Physiol. 1993;265:C1489–C1500. doi: 10.1152/ajpcell.1993.265.6.C1489. [DOI] [PubMed] [Google Scholar]
- 23.Pasantes-Morales H, Moran J, Schousboe A. Volume-sensitive release of taurine from cultured astrocytes: properties and mechanism. Glia. 1990;3:427–432. doi: 10.1002/glia.440030514. [DOI] [PubMed] [Google Scholar]
- 24.Schousboe A, Sanchez OR, Moran J, Pasantes-Morales H. Hyposmolarity-induced taurine release in cerebellar granule cells is associated with diffusion and not with high-affinity transport. J Neurosci Res. 1991;30:661–665. doi: 10.1002/jnr.490300409. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Olea R, Pena C, Moran J, Pasantes-Morales H. Inhibition of volume regulation and efflux of osmoregulatory amino acids by blockers of Cl− transport in cultured astrocytes. Neurosci Lett. 1993;156:141–144. doi: 10.1016/0304-3940(93)90458-w. [DOI] [PubMed] [Google Scholar]
- 26.Heacock AM, Kerley D, Gurda GT, Vantroostenberghe AT, Fisher SK. Potentiation of the osmosensitive release of taurine and d-aspartate from SH-SY5Y neuroblastoma cells after activation of M3 muscarinic cholinergic receptors. J Pharmacol Exp Ther. 2004;311:1097–1104. doi: 10.1124/jpet.104.072553. [DOI] [PubMed] [Google Scholar]
- 27.Cheema TA, Pettigrew VA, Fisher SK. Receptor regulation of the volume-sensitive efflux of taurine and iodide from human SH-SY5Y neuroblastoma cells: Differential requirements for Ca2+ and protein kinase C. J Pharmacol Exp Ther. 2007;320:1068–1077. doi: 10.1124/jpet.106.115741. [DOI] [PubMed] [Google Scholar]
- 28.Olson JE, Li GZ. Increased potassium, chloride, and taurine conductances in astrocytes during hypoosmotic swelling. Glia. 1997;20:254–261. doi: 10.1002/(sici)1098-1136(199707)20:3<254::aid-glia9>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 29.Junankar PR, Kirk K. Organic osmolyte channels: A comparative view. Cell Physiol Biochem. 2000;10:355–360. doi: 10.1159/000016368. [DOI] [PubMed] [Google Scholar]
- 30.Franco R. Osmosensitive taurine release: does taurine share the same efflux pathway with chloride and other amino acid osmolytes? Adv Exp Med Biol. 2003;526:189–196. [PubMed] [Google Scholar]
- 31.Shennan DB. Swelling-induced taurine transport: relationship with chloride channels, anion-exchangers and other swelling-activated transport pathways. Cell Physiol Biochem. 2008;21:15–28. doi: 10.1159/000113743. [DOI] [PubMed] [Google Scholar]
- 32.Lambert IH, Hoffmann EK. Cell swelling activates separate taurine and chloride channels in Ehrlich mouse ascites tumor cells. J Membr Biol. 1994;142:289–298. doi: 10.1007/BF00233436. [DOI] [PubMed] [Google Scholar]
- 33.Shennan DB, McNeillie SA, Curran DE. The effect of a hyposmotic shock on amino acid efflux from lactating rat mammary tissue: stimulation of taurine and glycine efflux via a pathway distinct from anion exchange and volume-activated anion channels. Exp Physiol. 1994;79:797–808. doi: 10.1113/expphysiol.1994.sp003808. [DOI] [PubMed] [Google Scholar]
- 34.Stutzin A, Torres R, Oporto M, Pacheco P, Eguiguren AL, et al. Separate taurine and chloride efflux pathways activated during regulatory volume decrease. Am J Physiol Cell Physiol. 1999;277:C392–C402. doi: 10.1152/ajpcell.1999.277.3.C392. [DOI] [PubMed] [Google Scholar]
- 35.Mongin AA, Reddi JM, Charniga C, Kimelberg HK. [3H]Taurine and d-[3H]aspartate release from astrocyte cultures are differently regulated by tyrosine kinases. Am J Physiol Cell Physiol. 1999;276:C1226–C1230. doi: 10.1152/ajpcell.1999.276.5.C1226. [DOI] [PubMed] [Google Scholar]
- 36.Franco R, Quesada O, Pasantes-Morales H. Efflux of osmolyte amino acids during isovolumic regulation in hippocampal slices. J Neurosci Res. 2000;61:701–711. doi: 10.1002/1097-4547(20000915)61:6<701::AID-JNR14>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 37.Tomassen SF, Fekkes D, De Jonge HR, Tilly BC. Osmotic swelling-provoked release of organic osmolytes in human intestinal epithelial cells. Am J Physiol Cell Physiol. 2004;286:C1417–C1422. doi: 10.1152/ajpcell.00468.2003. [DOI] [PubMed] [Google Scholar]
- 38.Wade JV, Olson JP, Samson FE, Nelson SR, Pazdernik TL. A possible role for taurine in osmoregulation within the brain. J Neurochem. 1988;51:740–745. doi: 10.1111/j.1471-4159.1988.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 39.Lehmann A. Effects of microdialysis-perfusion with anisoosmotic media on extracellular amino acids in the rat hippocampus and skeletal muscle. J Neurochem. 1989;53:525–535. doi: 10.1111/j.1471-4159.1989.tb07365.x. [DOI] [PubMed] [Google Scholar]
- 40.Taylor DL, Davies SE, Obrenovitch TP, Doheny MH, Patsalos PN, et al. Investigation into the role of N-acetylaspartate in cerebral osmoregulation. J Neurochem. 1995;65:275–281. doi: 10.1046/j.1471-4159.1995.65010275.x. [DOI] [PubMed] [Google Scholar]
- 41.Morales I, Dopico JG, Sabate M, Gonzalez-Hernandez T, Rodriguez M. Substantia nigra osmoregulation: taurine and ATP involvement. Am J Physiol Cell Physiol. 2007;292:C1934–C1941. doi: 10.1152/ajpcell.00593.2006. [DOI] [PubMed] [Google Scholar]
- 42.Estevez AY, O'Regan MH, Song D, Phillis JW. Effects of anion channel blockers on hyposmotically induced amino acid release from the in vivo rat cerebral cortex. Neurochem Res. 1999;24:447–452. doi: 10.1023/a:1020902104056. [DOI] [PubMed] [Google Scholar]
- 43.Mongin AA, Cai ZH, Kimelberg HK. Volume-dependent taurine release from cultured astrocytes requires permissive [Ca2+]i and calmodulin. Am J Physiol Cell Physiol. 1999;277:C823–C832. doi: 10.1152/ajpcell.1999.277.4.C823. [DOI] [PubMed] [Google Scholar]
- 44.Hajos F. An improved method for the preparation of synaptosomal fractions in high purity. Brain Res. 1975;93:485–489. doi: 10.1016/0006-8993(75)90186-9. [DOI] [PubMed] [Google Scholar]
- 45.Nedvetsky PI, Konev SV, Rakovich AA, Petrenko SV, Mongin AA. Effects of nitric oxide donors on Ca2+-dependent [14C]GABA release from brain synaptosomes: The role of SH-groups. Biochemistry (Mosc) 2000;65:1027–1035. [PubMed] [Google Scholar]
- 46.Jentsch TJ, Stein V, Weinreich F, Zdebik AA. Molecular structure and physiological function of chloride channels. Physiol Rev. 2002;82:503–568. doi: 10.1152/physrev.00029.2001. [DOI] [PubMed] [Google Scholar]
- 47.Abdullaev IF, Rudkouskaya A, Schools GP, Kimelberg HK, Mongin AA. Pharmacological comparison of swelling-activated excitatory amino acid release and Cl− currents in rat cultured astrocytes. J Physiol. 2006;572:677–689. doi: 10.1113/jphysiol.2005.103820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chebabo SR, Hester MA, Jing J, Aitken PG, Somjen GG. Interstitial space, electrical resistance and ion concentrations during hypotonia of rat hippocampal slices. J Physiol. 1995;487:685–697. doi: 10.1113/jphysiol.1995.sp020910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mongin AA, Aksentsev SL, Orlov SN, Konev SV. Hypoosmotic shock activates Ca2+ channels in isolated nerve terminals. Neurochem Int. 1997;31:835–843. doi: 10.1016/s0197-0186(97)00033-8. [DOI] [PubMed] [Google Scholar]
- 50.Tuz K, Pena-Segura C, Franco R, Pasantes-Morales H. Depolarization, exocytosis and amino acid release evoked by hyposmolarity from cortical synaptosomes. Eur J Neurosci. 2004;19:916–924. doi: 10.1111/j.0953-816x.2004.03209.x. [DOI] [PubMed] [Google Scholar]
- 51.Sharp T, Bramwell SR, Grahame-Smith DG. Release of endogenous 5-hydroxytryptamine in rat ventral hippocampus evoked by electrical stimulation of the dorsal raphe nucleus as detected by microdialysis: sensitivity to tetrodotoxin, calcium and calcium antagonists. Neuroscience. 1990;39:629–637. doi: 10.1016/0306-4522(90)90247-2. [DOI] [PubMed] [Google Scholar]
- 52.Liu X, Onaka T, Yagi K. Facilitation of Ca2+ store-dependent noradrenaline release after an N-methyl-D-aspartate receptor antagonist in the rat supraoptic nucleus. J Neuroendocrinol. 2001;13:894–904. doi: 10.1046/j.1365-2826.2001.00711.x. [DOI] [PubMed] [Google Scholar]
- 53.Minami A, Takeda A, Nishibaba D, Takefuta S, Oku N. Cadmium toxicity in synaptic neurotransmission in the brain. Brain Res. 2001;894:336–339. doi: 10.1016/s0006-8993(01)02022-4. [DOI] [PubMed] [Google Scholar]
- 54.Anderson CM, Swanson RA. Astrocyte glutamate transport: review of properties, regulation, and physiological functions. Glia. 2000;32:1–14. [PubMed] [Google Scholar]
- 55.Shimamoto K, Lebrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, et al. dl-threo-beta-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 56.Rutledge EM, Kimelberg HK. Release of [3H]-D-aspartate from primary astrocyte cultures in response to raised external potassium. J Neurosci. 1996;16:7803–7811. doi: 10.1523/JNEUROSCI.16-24-07803.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Trotti D, Danbolt NC, Volterra A. Glutamate transporters are oxidant-vulnerable: a molecular link between oxidative and excitotoxic neurodegeneration? Trends Pharmacol Sci. 1998;19:328–334. doi: 10.1016/s0165-6147(98)01230-9. [DOI] [PubMed] [Google Scholar]
- 58.Kimelberg HK, Goderie SK, Higman S, Pang S, Waniewski RA. Swelling-induced release of glutamate, aspartate, and taurine from astrocyte cultures. J Neurosci. 1990;10:1583–1591. doi: 10.1523/JNEUROSCI.10-05-01583.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Pasantes-Morales H, Murray RA, Sanchez-Olea R, Moran J. Regulatory volume decrease in cultured astrocytes. II. Permeability pathway to amino acids and polyols. Am J Physiol. 1994;266:C172–C178. doi: 10.1152/ajpcell.1994.266.1.C172. [DOI] [PubMed] [Google Scholar]
- 60.Law RO. Taurine efflux and the regulation of cell volume in incubated slices of rat cerebral cortex. Biochim Biophys Acta. 1994;1221:21–28. doi: 10.1016/0167-4889(94)90211-9. [DOI] [PubMed] [Google Scholar]
- 61.Law RO. Volume regulation and the efflux of amino acids from cells in incubated slices of rat cerebral cortex. I. Characteristics of transport mechanisms. Biochim Biophys Acta. 1996;1314:34–42. doi: 10.1016/s0167-4889(96)00070-5. [DOI] [PubMed] [Google Scholar]
- 62.Bothwell JH, Rae C, Dixon RM, Styles P, Bhakoo KK. Hypo-osmotic swelling-activated release of organic osmolytes in brain slices: implications for brain oedema in vivo. J Neurochem. 2001;77:1632–1640. doi: 10.1046/j.1471-4159.2001.00403.x. [DOI] [PubMed] [Google Scholar]
- 63.Jackson PS, Morrison R, Strange K. The volume-sensitive organic osmolyte-anion channel VSOAC is regulated by nonhydrolytic ATP binding. Am J Physiol. 1994;267:C1203–C1209. doi: 10.1152/ajpcell.1994.267.5.C1203. [DOI] [PubMed] [Google Scholar]
- 64.Roy G. Amino-acid current through anion channels in cultured human glial-cells. J Membr Biol. 1995;147:35–44. doi: 10.1007/BF00235396. [DOI] [PubMed] [Google Scholar]
- 65.Boese SH, Wehner F, Kinne RK. Taurine permeation through swelling-activated anion conductance in rat IMCD cells in primary culture. Am J Physiol. 1996;271:F498–F507. doi: 10.1152/ajprenal.1996.271.3.F498. [DOI] [PubMed] [Google Scholar]
- 66.Franco R, Torres-Marquez ME, Pasantes-Morales H. Evidence for two mechanisms of amino acid osmolyte release from hippocampal slices. Pflugers Arch. 2001;442:791–800. doi: 10.1007/s004240100604. [DOI] [PubMed] [Google Scholar]
- 67.Waseem TV, Rakovich AA, Lavrukevich TV, Konev SV, Fedorovich SV. Calcium regulates the mode of exocytosis induced by hypotonic shock in isolated neuronal presynaptic endings. Neurochem Int. 2005;46:235–242. doi: 10.1016/j.neuint.2004.09.002. [DOI] [PubMed] [Google Scholar]
- 68.Haskew RE, Mongin AA, Kimelberg HK. Peroxynitrite enhances astrocytic volume-sensitive excitatory amino acid release via a src tyrosine kinase-dependent mechanism. J Neurochem. 2002;82:903–912. doi: 10.1046/j.1471-4159.2002.01037.x. [DOI] [PubMed] [Google Scholar]
- 69.Haskew-Layton RE, Mongin AA, Kimelberg HK. Hydrogen peroxide potentiates volume-sensitive excitatory amino acid release via a mechanism involving Ca2+/calmodulin-dependent protein kinase II. J Biol Chem. 2005;280:3548–3554. doi: 10.1074/jbc.M409803200. [DOI] [PubMed] [Google Scholar]
- 70.Moran J, Maar T, Pasantes-Morales H. Cell volume regulation in taurine deficient cultured astrocytes. Adv Exp Med Biol. 1994;359:361–367. doi: 10.1007/978-1-4899-1471-2_37. [DOI] [PubMed] [Google Scholar]
- 71.Lehmann A, Sandberg M. In vivo substitution of choline for sodium evokes a selective osmoinsensitive increase of extracellular taurine in the rat hippocampus. J Neurochem. 1990;54:126–129. doi: 10.1111/j.1471-4159.1990.tb13291.x. [DOI] [PubMed] [Google Scholar]
- 72.Saransaari P, Oja SS. Characteristics of taurine release in slices from adult and developing mouse brain stem. Amino Acids. 2006;31:35–43. doi: 10.1007/s00726-006-0290-5. [DOI] [PubMed] [Google Scholar]
- 73.Phillis JW, Song D, O'Regan MH. Effects of hyperosmolarity and ion substitutions on amino acid efflux from the ischemic rat cerebral cortex. Brain Res. 1999;828:1–11. doi: 10.1016/s0006-8993(99)01235-4. [DOI] [PubMed] [Google Scholar]
- 74.Calvert DT, Shennan DB. Volume-activated taurine efflux from the in situ perfused lactating rat mammary gland. Acta Physiol Scand. 1998;162:97–105. doi: 10.1046/j.1365-201X.1998.0267f.x. [DOI] [PubMed] [Google Scholar]
- 75.Danbolt NC. Glutamate uptake. Prog Neurobiol. 2001;65:1–105. doi: 10.1016/s0301-0082(00)00067-8. [DOI] [PubMed] [Google Scholar]
- 76.Madsen S, Ottersen OP, Storm-Mathisen J. Immunocytochemical visualization of taurine: neuronal localization in the rat cerebellum. Neurosci Lett. 1985;60:255–260. doi: 10.1016/0304-3940(85)90586-5. [DOI] [PubMed] [Google Scholar]
- 77.Storm-Mathisen J, Ottersen OP, Fu-Long T, Gundersen V, Laake JH, et al. Metabolism and transport of amino acids studied by immunocytochemistry. Med Biol. 1986;64:127–132. [PubMed] [Google Scholar]
- 78.Decavel C, Hatton GI. Taurine immunoreactivity in the rat supraoptic nucleus: prominent localization in glial cells. J Comp Neurol. 1995;354:13–26. doi: 10.1002/cne.903540103. [DOI] [PubMed] [Google Scholar]
- 79.Hussy N, Deleuze C, Desarmenien MG, Moos FC. Osmotic regulation of neuronal activity: a new role for taurine and glial cells in a hypothalamic neuroendocrine structure. Prog Neurobiol. 2000;62:113–134. doi: 10.1016/s0301-0082(99)00071-4. [DOI] [PubMed] [Google Scholar]
- 80.Pow DV, Sullivan R, Reye P, Hermanussen S. Localization of taurine transporters, taurine, and (3)H taurine accumulation in the rat retina, pituitary, and brain. Glia. 2002;37:153–168. doi: 10.1002/glia.10026. [DOI] [PubMed] [Google Scholar]
- 81.Phillis JW, Song D, O'Regan MH. Tamoxifen, a chloride channel blocker, reduces glutamate and aspartate release from the ischemic cerebral cortex. Brain Res. 1998;780:352–355. doi: 10.1016/s0006-8993(97)01352-8. [DOI] [PubMed] [Google Scholar]
- 82.Kohut JJ, Bednar MM, Kimelberg HK, McAuliffe TL, Gross CE. Reduction in ischemic brain injury in rabbits by the anion transport inhibitor L-644,711. Stroke. 1992;23:93–97. doi: 10.1161/01.str.23.1.93. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Jin Y, Behr MJ, Feustel PJ, Morrison JP, et al. Behavioral and histological neuroprotection by tamoxifen after reversible focal cerebral ischemia. Exp Neurol. 2005;196:41–46. doi: 10.1016/j.expneurol.2005.07.002. [DOI] [PubMed] [Google Scholar]
- 84.Basarsky TA, Feighan D, MacVicar BA. Glutamate release through volume-activated channels during spreading depression. J Neurosci. 1999;19:6439–6445. doi: 10.1523/JNEUROSCI.19-15-06439.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hyslop PA, Zhang Z, Pearson DV, Phebus LA. Measurement of striatal H2O2 by microdialysis following global forebrain ischemia and reperfusion in the rat: correlation with the cytotoxic potential of H2O2 in vitro. Brain Res. 1995;671:181–186. doi: 10.1016/0006-8993(94)01291-o. [DOI] [PubMed] [Google Scholar]
- 86.Chan PH. Reactive oxygen radicals in signaling and damage in the ischemic brain. J Cereb Blood Flow Metab. 2001;21:2–14. doi: 10.1097/00004647-200101000-00002. [DOI] [PubMed] [Google Scholar]