Abstract
Creatine plays an important role in energy metabolism in the heart. Cardiomyocytes accumulate creatine via a specific creatine transporter (CrT), the capacity of which is reduced in the failing heart, resulting in lower myocardial creatine concentration. Therefore, to gain insight into how the CrT is regulated, we studied two mouse models of severely altered myocardial creatine levels. Cardiac creatine uptake levels were measured in isolated hearts from creatine-free guanidinoacetate-N-methyl transferase knock out (GAMT−/−) mice and from mice overexpressing the myocardial CrT (CrT-OE) using 14C-radiolabeled creatine. CrT mRNA levels were measured using real time RT-PCR and creatine levels with HPLC. Hearts from GAMT−/− mice showed a 7-fold increase in Vmax of creatine uptake and a 1.4-fold increase in CrT mRNA levels. The increase in Cr uptake and in CrT mRNA levels, however, was almost completely prevented when mice were fed a creatine supplemented diet, indicating that creatine uptake is subject to negative feedback regulation. Cardiac creatine uptake levels in CrT-OE mice were increased on average 2.7-fold, showing a considerable variation, in line with a similar variation in creatine content. Total CrT mRNA levels correlated well with myocardial creatine content (r = 0.67; p < 0.0001) but endogenous CrT mRNA levels did not correlate at all with myocardial creatine content (r = 0.01; p = 0.96). This study shows that creatine uptake can be massively upregulated in the heart, by almost an order of magnitude and that this upregulation is subject to feedback inhibition. In addition, our results strongly suggest that CrT activity is predominantly regulated by mechanisms other than alterations in gene expression.
Keywords: Creatine transport, Creatine, Energy metabolism, GAMT, Transgenic mouse model
1. Introduction
In tissues with a high energy demand, such as myocardium, creatine plays an important role in energy metabolism. ATP synthesized by the mitochondria reacts with creatine in the creatine kinase (CK) reaction to form ADP and phosphocreatine (PCr). At the ATP consuming site the reverse reaction takes place replenishing ATP levels and maintaining low ADP concentrations.
Creatine originates from both dietary intake and from biosynthesis, predominantly in the liver and kidneys. Cardiomyocytes cannot synthesize creatine and so must accumulate it from the plasma against a large concentration gradient via a specific Na+–creatine cotransporter (CrT) [1]. Creatine is lost from the cardiomyocyte due to spontaneous, nonenzymatic conversion into creatinine, which is membrane permeable and diffuses out of the cell, and, possibly, via efflux of creatine itself [1,2]. Such uptake and the resulting intracellular creatine concentration are tightly controlled. For example, oral creatine supplementation in rats results in elevated plasma creatine concentration, but due to downregulation of the cardiac creatine uptake capacity, myocardial creatine levels remain unchanged [2].
Heart failure is characterized by reduced myocardial PCr and total creatine (Cr) levels [3,4]. We have previously shown that, in both rat and mouse models of heart failure due to chronic coronary ligation, the reduced myocardial creatine content can at least in part be explained by reduced myocardial creatine uptake capacity [5] and CrT gene expression [6].
However, little is known about how and to what extent CrT expression and activity can be regulated and, thus on how and why CrT function is downregulated in heart failure. Upregulation of uptake capacity can be achieved via administration of the creatine analogue β-guanidinoproprionic acid (β-GPA), which acts as a competitive inhibitor of the CrT [2], suggesting that intracellular creatine concentration is an important regulating factor. A recent report demonstrated that CrT activity can be upregulated by the serum and glucocorticoid kinase 1 (SGK1) in Xenopus oocytes expressing the CrT [7], but it is unknown whether cardiac CrT is regulated in the same way.
We recently reported the first two genetically manipulated mouse models of altered myocardial creatine concentration. Guanidinoacetate-N-methyl transferase knock out (GAMT−/−) mice are unable to synthesize creatine [8], such that when fed a creatine-free diet they are entirely creatine-free. Instead, these mice accumulate the creatine precursor guanidinoacetate (GA), which is phosphorylated in the CK reaction instead of creatine and partially takes over the role of creatine [9]. However, the CK reaction velocity is reduced by two orders of magnitude with GA as substrate [10]. In contrast, mice overexpressing the cardiac CrT (CrT-OE) have elevated myocardial creatine levels [11]. The cardiac creatine uptake capacity of both these mouse models is currently unknown. An understanding of the creatine uptake capacity under these extreme conditions (i.e. no creatine at all and forced increase of creatine levels) could provide important new insights into the regulation of cardiac creatine transport.
We hypothesized that absence of creatine would trigger upregulation of the cardiac creatine uptake capacity, and that overexpression of the myocardial CrT would result in increased creatine uptake levels. To test this, and to quantify the degree of potential CrT upregulation, we measured the creatine uptake capacity in isolated hearts from GAMT−/− and CrT overexpressing mice. In addition, we measured CrT mRNA levels in hearts of these mice as well as protein levels of SGK1, a candidate CrT regulator, in the hearts of GAMT−/− mice.
2. Methods
2.1. Mice
GAMT−/− and wildtype (WT) littermates used for experiments were created by intercrossing heterozygous GAMT+/− mice, as previously described [8]. From 6 weeks of age, mice were housed per genotype to prevent ingestion of creatine in GAMT−/− mice through coprophagia. CrT overexpressing mice were created as described before [11]. These mice express the rabbit CrT with a human c-myc epitope tag (CrT-myc) under control of the murine ventricular myosin light chain 2 (MLC2v) promoter. All transgenic mice (CrT-OE) and wildtype (WT) littermates used in experiments were generated by mating male transgenics with C57BL/6 females. All animals were genotyped by PCR using standard conditions.
Mice were kept in cages with a 12 h light–dark cycle and controlled temperature (20–22 °C), fed creatine-free chow and water ad libitum. All studies were performed in 5–8 month old mice, and conform with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996) and with the UK Home Office Animals (Scientific Procedures) Act 1986.
2.2. Creatine uptake measurements in perfused heart
Mice were heparinised and anaesthetised (pentobarbitone ∼ 140 mg/kg body weight i.p.). Hearts were excised, cannulated and perfused in Langendorff mode at 80 mmHg and 37 °C with Krebs-Henseleit buffer gassed with 95% O2/5% CO2 (pH 7.4) containing (in mM): 149 Na+, 5.9 K+, 1.2 Mg2+, 2.25 Ca2+, 1.2 SO42−, 126.2 Cl−, 0.5 EDTA2−, 25 HCO3−, 1.2 H2PO4−, 11 d-glucose, 1.8 pyruvate and 0.2 lactate. Contractility was assessed using a fluid-filled intraventricular balloon connected to a pressure transducer (AD Instruments Ltd, UK). The end-diastolic pressure (EDP) was set to ∼10 mmHg.
To determine Michaelis–Menten kinetics of creatine uptake in hearts from GAMT−/− and WT mice, buffer was supplemented with either one of three different concentrations of creatine: 100 (n = 4/group), 500 (n = 5/group) and 1000 μM (n = 4/group); as previously described [5], this had no effect on cardiac function and hearts were stable for the duration of the protocol (up to 1 h). After stabilization, hearts were perfused with trace amounts of [14C]creatine (Apin Chemicals Ltd, UK) added to the buffer for 30 min followed by a 15min 14C-label free washout. At the end of each of the perfusion protocols, hearts were divided into left ventricle, right ventricle and atria. Tissue samples were weighed and minced with scissors in glass scintillation vials containing 0.5 mL of a 9:1 v/v solution of hyamine hydroxide (MP Biomedicals, UK) and water. The vials were incubated shaking at 60 °C until clear (∼ 3–4 h). To minimize colour quenching of samples, 100 μL of 30% (w/w) hydrogen peroxide was added followed by heating at 60 °C for another 30min. After the samples were cooled to room temperature, 10 mL of Cytoscint ES (MP Biomedicals, UK) was added to each sample followed by vigorous mixing. The vials were then allowed to equilibrate in the dark for at least 60 min before scintillation counting.
In a separate set of experiments hearts were used from WT and GAMT−/− mice that were fed a creatine supplemented diet (5 g/kg) to test if any differences between the two genotypes were specific for the absence of creatine. Creatine uptake in hearts from these mice was measured in the presence of 1 mM extracellular creatine (n = 3/group).
We previously described that our CrT-OE hearts showed a large variation in CrT-myc mRNA and creatine levels [11]. We expected a similarly large variation in creatine uptake capacity, which would make determination of Michaelis–Menten kinetics impossible, as creatine uptake can only be measured for one extracellular creatine concentration per heart. Therefore, creatine uptake was only measured at 1000 μmol/L extracellular creatine in these hearts from CrT-OE (n = 9) and WT (n = 5) mice to estimate Vmax.
2.3. mRNA measurements
The relative quantities of endogenous, transgenic, and total CrT, were measured using the primers shown below. Results were normalized to the expression levels of the housekeeping gene 36B4 [12], using the double-standard curve method, in which standard curves spanning five log dilutions of left ventricular RNA were constructed for both the 36B4 and CrT primers.
| Primer | Sequence |
|---|---|
| Endogenous CrT F | 5′-ACTGGGAGGTGACCTTGTGC-3′ |
| Endogenous CrT R | 5′-CGATCTTTCCTGTTGACTTG-3′ |
| CrT c-myc F | 5′-TTGGAGTACAGAGCTCAGGA-3′ |
| CrT c-myc R | 5′-TCACAGATCCTCTTCTGAGATGAG-3′ |
| Total CrT F | 5′-ACTGTGTGGAGATCTTCCGC-3′ |
| Total CrT R | 5′-CAGCAAGCTGGTCACATGTG-3′ |
| 36B4 F | 5′-AGATTCGGGATATGCTGTTGG-3′ |
| 36B4 R | 5′-TCGGGTCCTAGACCAGTGTTC-3′ |
The endogenous CrT primers were 100% complementary to mouse CrT mRNA, but had 4–5 mismatches to the transgenic CrT, which is of rabbit origin. The primer specificity was validated by RT-PCR analysis of mouse vs. rabbit heart RNA, and mouse CrT vs. rabbit CrT plasmid DNA, which showed that mouse CrT was preferentially amplified (approximately 100-fold higher amplification efficiency). The transgenic CrT R primer hybridized with the c-myc epitope tag of the CrT transgene, which ensured transgene specificity. The total CrT primers hybridized with regions conserved between the mouse and rabbit CrT genes. RNA was extracted from frozen left ventricular tissue from GAMT−/− (n = 8) and WT (n = 8) mice using the RNeasy Kit (Qiagen, UK), involving treatment with proteinase K and DNase I and used in RT-PCR (Qiagen Quantitect SYBR Green RT-PCR kit, Qiagen, UK) using the Rotor-Gene system (Corbett Research Ltd., UK).
2.4. Western blotting
Approximately 10 mg of frozen, grounded left ventricular tissue was mixed with 500 μL of extraction buffer (50 mM TRIS, 150 mM NaCl, 2% SDS, 1 mM DTT, 1 mM PMSF and 1 pellet of protease inhibitor cocktail (Roche, UK) per 100 mL) and homogenised in a glass tissue homogeniser for 30 s, followed by 1 h incubation at 4 °C with constant agitation. The extract was centrifuged at 15,400 g for 10min at 4 °C and the supernatant was analysed for protein concentration using the BCA protein assay (Pierce, UK). Equal amounts of protein (40 μg) were loaded onto SDS-PAGE gels (4% stacking gel, 12% separating gel) which were run at − 200 V for 1 h followed by transfer onto a PVDF membrane (GE Health Care, UK) at 200 mA overnight at 4 °C. The membrane was stained with appropriate antibodies: either anti-MLC2v (Synaptic Systems), rabbit anti-SGK1 (Abcam, UK), or rabbit anti-phospho-SGK1 (Abcam, UK) followed by goat anti-rabbit-HRP (Santa Cruz, UK). To confirm equal loading of protein, the membrane was stripped of antibodies by incubating for 30 min in stripping buffer (2% SDS, 62.5 mM TRIS–HCl pH 6.8, 100 mM 2-mercaptoethanol) at 55 °C, followed by staining with mouse anti-mouse α-actinin (Sigma-Aldrich, UK) and goat anti-mouse-HRP (Promega, UK), or rabbit anti-β-tubulin (Abcam, UK) and goat anti-rabbit-HRP (Santa Cruz, UK). The antibodies were detected with the ECL Advance chemiluminescence kit (GE Health Care, UK), and the FluorChem 8800 imager. SGK1 was measured in hearts from GAMT−/− (n = 4) and WT (n = 4) mice, phospho-SGK1 in hearts from GAMT−/− (n = 3) and WT (n = 2) mice, and MLC2v was measured in hearts from CrT-OE (n = 2) and WT (n = 2) mice.
2.5. Biochemical measurements
In CrT-OE mice, transgene expression is cardiac specific. Therefore, any extra-cardiac changes in creatine metabolism are not expected. However, to exclude changes in extracellular creatine levels potentially affecting in vivo myocardial creatine uptake we measured plasma creatine levels in CrT-OE (n = 9) and WT (n = 4) mice. Blood samples were centrifuged at 1800 g for 10min. The supernatant was used for the determination of creatine plasma concentration by HPLC. Creatine content in left and right ventricles of CrT-OE (n = 9) and WT (n = 6) hearts was also determined by HPLC as described previously [13]. Due to the radioactive nature of the creatine uptake experiments, creatine content was measured in a separate set of hearts. Total left ventricular creatine content was also quantified by HPLC in the cohort of CrT-OE mice used for CrT mRNA measurements (n = 31).
2.6. Calculation of ΔGCrT and reverse potential
Assuming a stoichiometry of 2:1 for Na+:Cr, the free energy of creatine transport through the CrT (ΔGCrT) was calculated from
and assuming a stoichiometry of 2:1:1 for Na+:Cr:Cl− from
where ΔGNa+ = RT ln[Na+]i / [Na+]e + zFVm, ΔGCl− = RT ln[Cl−]i / [Cl−]e + zFVm, ΔGCr = RT ln[Cr]i / [Cr]e, the gas constant (R) is 8.31J mol−1 K−1, the temperature (T) is 310 K, the intra- and extracellular Na+ concentrations ([Na+]i and [Na+]e) are 10 mM and 146 mM, respectively, z is the valence of the ion, Faraday's constant (F) is 9.65 × 104J V−1 mol−1, Vm is the membrane potential, the intra- and extracellular Cl− concentrations ([Cl−]i and [Cl−]e) are 30 mM and 127 mM, respectively, the extracellular creatine concentration ([Cr]e) is measured in plasma and the intracellular creatine concentration ([Cr]i) is calculated assuming total heart protein content to be 0.17 g protein per g wet weight and intracellular volume to be 0.5 mL/g wet weight. The reverse potential is Vm when ΔGCrT = 0.
2.7. Statistical analysis
Creatine uptake in GAMT−/− and WT hearts was fitted according to Michaelis–Menten kinetics (uptake = (Vmax × [Cr]e)/(Km + [Cr]e)) using non-linear regression. Statistical significance was assessed using analysis of variance or a Student's t-test, where appropriate. Statistical significance of differences in the CrT mRNA expression was assessed with the non-parametric Kruskal–Wallis test with Dunn's correction for multiple comparisons, as the CrT expression in the CrT-OE group did not follow Gaussian distribution. Correlation between the expression of the endogenous and of the transgenic CrT vs. total creatine was assessed using Pearson's correlation, and results were considered significant at p < 0.05. Data are mean ± standard deviation. Differences were considered significant at p < 0.05.
3. Results
3.1. Creatine uptake
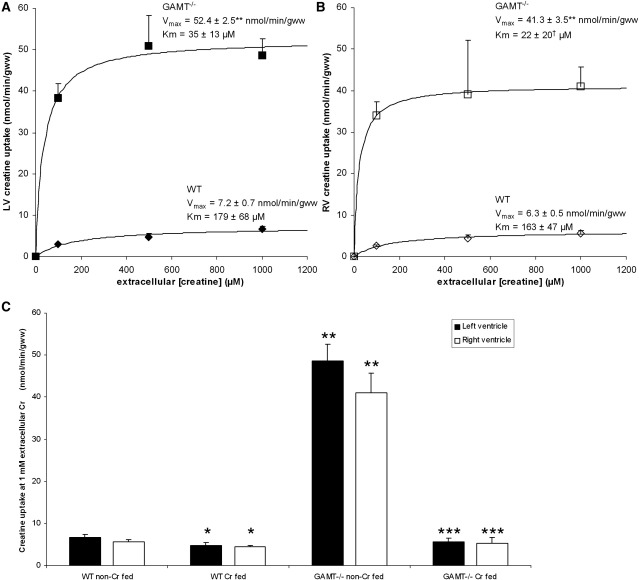
Left ventricular creatine uptake kinetics in WT hearts followed Michaelis–Menten kinetics with a Vmax of 7.2 ± 0.7 nmol/min/gww and a Km of 179 ± 68 μmol/L, in line with data on creatine uptake in isolated rat hearts [5]. In contrast, GAMT−/− mouse left ventricles, with undetectable creatine levels, showed a massive increase in creatine uptake capacity. As shown in Fig. 1A, apparent Vmax values increased from 7.2 ± 0.7 in WT to 52.4 ± 2.5 nmol/min/gww in GAMT−/− (p < 0.001). Apparent Km values were reduced from 179 ± 68 in WT mice to 35 ± 13 μmol/L in GAMT−/− mice although the data did not fit Michaelis–Menten kinetics adequately for the latter to be significant. Right ventricular creatine uptake kinetics were similar to left ventricular uptake kinetics, but in GAMT−/−, right ventricular uptake was generally 10–15% lower (p < 0.05 vs GAMT−/− LV), as shown in Fig. 1B. Right ventricular apparent Vmax values amounted to 6.3 ± 0.5 in WT and 41.3 ± 3.5 nmol/min/gww in GAMT−/− (p < 0.001) and apparent Km values amounted to 22 ± 20 in WT and 163 ± 47 in GAMT−/− (p < 0.05). Contractile function was similar in both groups and the rate pressure product amounted to 23 ± 9 × 103 and 22 ± 12 × 103 mmHg/min in hearts from WT and GAMT−/− mice, respectively.
Fig. 1.
Left (A) and right (B) ventricular creatine uptake kinetics in hearts from wildtype (WT) and GAMT−/− mice, using different extracellular creatine concentrations (in both groups n = 4 or 5 per [Cr]e). (C) Creatine uptake levels in hearts from WT and GAMT−/− mice fed with (Cr fed) or without creatine (non-Cr fed) (5 g /kg) supplemented to their diet using 1 mmol/L extracellular creatine. ⁎p < 0.05 vs WT-Cr; ⁎⁎p < 0.001 vs WT-Cr; ⁎⁎⁎p < 0.05 vs GAMT−/−Cr; †p < 0.05 vs WT.
When mice were fed a creatine supplemented diet, the difference in creatine uptake between WT and GAMT−/− mice essentially disappeared. As shown in Fig. 1C, cardiac creatine uptake was significantly lower in both ventricles of creatine fed mice compared to those with a creatine-free diet for both GAMT−/− (p < 0.001 for both ventricles)—and WT mice (p < 0.05 for both ventricles). Left ventricular creatine uptake in the presence of 1 mmol/L creatine amounted to 4.8 ± 0.6 and 5.6 ± 0.9 nmol/min/gww in hearts from creatine fed WT and creatine fed GAMT−/− mice (NS), respectively. Right ventricular creatine uptake amounted to 4.5 ±0.4 and 5.3 ± 1.3 nmol/min/gww in creatine fed WT and creatine fed GAMT−/− hearts (NS), respectively.
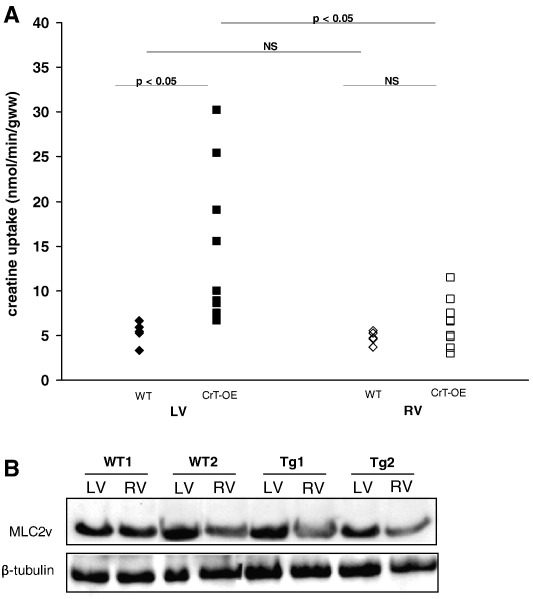
As expected, CrT-OE mice also showed increased left ventricular creatine uptake levels. As seen in Fig. 2A, creatine uptake in CrT-OE showed considerable variation between hearts. Average left ventricular creatine uptake levels were 5.3 ± 1.2 and 14.6 ± 8.5 nmol/min/gww in WT and CrT-OE hearts, respectively (p < 0.001). Right ventricular creatine uptake levels did not differ significantly between groups and amounted to 4.8 ± 0.7 and 6.4 ± 2.7 nmol/min/gww in WT and CrT-OE hearts (p = 0.06), respectively. In CrT-OE hearts, right ventricular creatine uptake was significantly lower than left ventricular creatine uptake (p < 0.05). As shown in Fig. 2B, the expression of MLC2v protein whose promoter drives the CrT-myc transgene expression, was also lower (approximately 25%, p < 0.05) in the right ventricle than it was in the left ventricle in both CrT-OE and WT mice. Contractile function was similar in both groups with rate pressure products of 25 ± 7 × 103 and 24 ± 11 × 103 mmHg/min in hearts from WT and CrT-OE mice, respectively.
Fig. 2.
(A) Left and right ventricular creatine uptake levels in hearts from wildtype (WT) mice and from mice overexpressing the cardiac creatine transporter (CrT-OE) using 1 mmol/L extracellular creatine. The average left ventricular creatine uptake levels amounted to 5.3 ± 1.2 and 14.6 ± 8.5 nmol/min/gww in wildtype (n = 5) and CrT-OE (n = 9) hearts, respectively (p < 0.001). The average right ventricular creatine uptake levels were 4.8 ± 0.7 and 6.4 ± 2.7 nmol/min/gww in wildtype (n = 5) and CrT-OE (n = 9) hearts, respectively (NS). (B) Murine ventricular myosin light chain 2 (MLC2v) protein levels were approximately 25% lower in right ventricle than in left ventricle in both wildtype and creatine transporter overexpressing (CrT-OE) hearts.
3.2. CrT mRNA levels
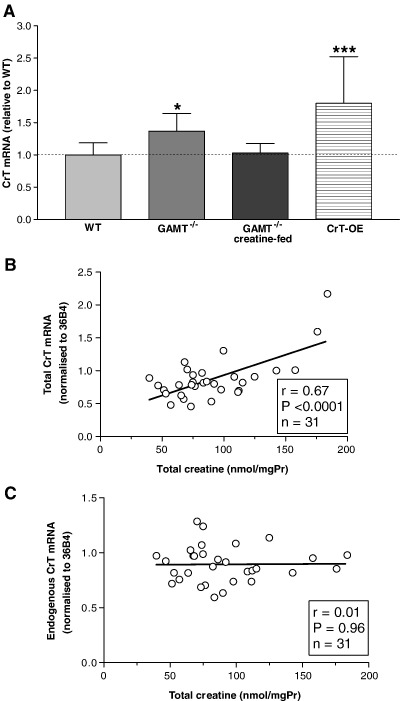
As shown in Fig. 3A, the expression of total CrT mRNA was elevated by approximately 40% in creatine-free GAMT−/− hearts relative to WT. However, when the GAMT−/− mice were fed creatine from birth, the difference between the WT and GAMT−/− CrT mRNA was abolished. Some WT mice were also fed creatine, but since their CrT mRNA levels (0.77 ± 0.09; n = 4) did not deviate from those in WT control mice (0.73 ± 0.15; n = 8; p = 0.69), the two data sets were pooled.
Fig. 3.
(A) Total CrT mRNA levels in hearts from wildtype mice (WT; n = 16), from creatine-free GAMT−/− mice (n = 11), from GAMT−/− mice fed a creatine supplemented diet (5 g/kg; n = 5) and from mice overexpressing the cardiac creatine transporter (CrT-OE; n = 31). Some WT mice were also fed creatine, but since their CrT mRNA levels did not deviate from those in WT control mice, the two data sets were pooled. ⁎p < 0.05; ⁎⁎⁎p < 0.001 vs WT. In hearts from CrT-OE mice creatine levels correlate well with total CrT mRNA (B) but not with endogenous CrT mRNA (C).
In hearts from CrT-OE mice, total (endogenous plus CrT-myc) CrT mRNA levels correlate well with total creatine levels (r = 0.67; Fig. 3B), which is in line with our previously published data showing that CrT-myc mRNA levels correlate with creatine levels [11]. However, as shown in Fig. 3C, mRNA levels of the endogenous CrT in these hearts were not affected by increased total creatine levels (r = 0.01).
3.3. SGK1 levels
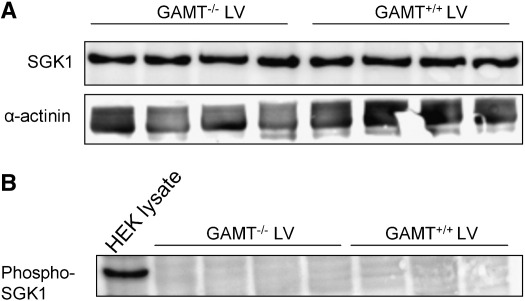
As can be seen in Fig. 4A, the levels of SGK1, a candidate CrT regulator, in GAMT−/− were unchanged compared to WT controls, and SGK1/α-actinin levels amounted to 0.21 ± 0.06 in WT and 0.19 ± 0.02 in GAMT−/− (NS). The phosphorylated, and thus active, form of SGK1 was undetectable in hearts from mice with both genotypes (GAMT−/− and WT) (Fig. 4B), suggesting that the WT and GAMT−/− hearts do not contain phospho-SGK1 or that its level is below the threshold for detection.
Fig. 4.
(A) Serum and glucocorticoid inducible kinase 1 (SGK1) protein levels analysed by Western blotting hearts from four GAMT−/− and four wildtype (GAMT+/+) mice. SGK1 levels did not differ between both groups. Equal loading of lanes was confirmed by stripping the blot, followed by staining for α-actinin. The results were reproduced in a separate experiment. (B) The phosphorylated, and therefore, active fraction, of SGK1 (phospho-SGK1) was undetectable in the left ventricles of either mouse strain.
3.4. Creatine levels
Plasma creatine levels were unaltered in CrT-OE mice and were 64 ± 15 nmol/L in WT controls (n = 4) and 56 ± 14 nmol/L in CrT-OE (n = 9; NS). Left ventricular creatine levels were 74 ± 5 and 133 ± 43 nmol/mg protein in WT controls (n = 6) and CrT-OE mice (n = 9; p < 0.01 vs WT), respectively. In both strains, right ventricular creatine levels tended to be lower (by about 7%) than left ventricular levels, a finding that was significant in WT controls (69 ± 7 nmol/mg protein; p < 0.01 vs LV) but not in CrT-OE (125 ± 46 nmol/mg protein; NS vs CrT-OE LV; p < 0.01 vs WT RV).
3.5. Free energy change of creatine transport
Using the measured creatine levels we calculated the reverse potential (i.e. Vm when ΔGCrT = 0) for creatine transport in WT and CrT-OE left ventricles. Assuming a stoichiometry of 2:1 for Na+:Cr we calculated a reverse potential of 3 mV in WT hearts (74 nmol/mg protein creatine), and of − 26 mV in CrT-OE hearts with very high creatine levels (4-fold normal levels, i.e. 296 nmol/mg protein). When a stoichiometry of 2:1:1 for Na+:Cr:Cl− was assumed, we found a reverse potential of 45 mV in WT and of − 14 mV in hearts with very high creatine levels.
4. Discussion
We have demonstrated that in creatine-free GAMT−/− hearts, the creatine uptake capacity is massively increased to more than 7-fold that of WT values, while CrT mRNA levels are increased only 1.4-fold, and that feeding these mice a creatine supplemented diet essentially abolishes these changes. In addition, we have shown that in hearts with increased creatine levels due to overexpression of the CrT, the creatine uptake capacity is increased, but endogenous CrT mRNA levels do not change with increased intracellular creatine content. Together, these results suggest that CrT activity can be regulated by CrT gene expression but that transcriptional control is very limited and other mechanisms are likely to dominate the regulation of CrT activity in (patho-) physiological conditions.
There are several lines of evidence to suggest that intra- and extracellular creatine concentrations alter CrT activity. For example, chronic increases in plasma creatine concentrations due to creatine feeding result in a decrease in creatine uptake kinetics and unaltered myocardial intracellular creatine levels [2]. This finding is confirmed by our current observation that creatine uptake in hearts from WT mice is reduced when mice are fed a creatine supplemented diet. Similarly, intracellular creatine depletion by feeding the creatine analogue β-GPA results in an increased creatine uptake capacity [2]. In human muscle cells as well as in G8 myoblasts high extracellular creatine concentrations reduced creatine uptake whereas low extracellular creatine concentrations resulted in increased creatine uptake [14]. However, the mechanism whereby creatine concentrations alter CrT activity is poorly understood and literature data regarding CrT regulation should be interpreted with caution since anti-CrT antibodies used in many studies have since been found to cross react with the pyruvate dehydrogenase complex [15].
Regulation of CrT activity could take place at different levels. Sandoval et al. [16] characterized the human CrT promoter, and suggested that its features were compatible with that of a housekeeping gene. However, the increase in CrT mRNA levels in hearts from creatine-free GAMT−/− mice, and the reversal of this increase upon creatine feeding, shows for the first time that (in the mouse at least) the gene is susceptible to transcriptional regulation in response to a changing physiological environment. And yet an increase of only 40% in CrT mRNA in these hearts is relatively small compared to the 7-fold increase in creatine uptake, especially considering the extreme stimulus of zero creatine, and suggests that alterations in gene expression play only a minor role in regulation of CrT activity. In support of this conclusion is our finding that endogenous CrT mRNA is not downregulated by increased intracellular creatine concentrations in hearts from CrT-OE mice, and by the fact that we did not find any reduction in CrT mRNA levels after feeding WT mice a creatine supplemented diet, despite CrT activity being reduced in these hearts. Finally, the finding that total CrT mRNA levels in hearts from CrT-OE mice are higher than those in hearts from creatine-free GAMT−/− mice despite the much higher creatine uptake capacity in the GAMT−/− hearts also indicates that CrT activity is primarily subject to post-transcriptional, rather than transcriptional, regulation.
Such post-transcriptional regulation could occur via CrT protein modification such as phosphorylation since the CrT protein has putative phosphorylation sites for PKC [17]. In addition, activity of the closely related transmembrane dopamine transporter is known to be downregulated by translocation of intracellular dopamine transporter protein from the sarcolemma to intracellular pools [18], and it is conceivable that a similar regulating mechanism exists for the CrT.
In Xenopus oocytes expressing the CrT, coexpression of SGK1 resulted in an increase in creatine uptake, showing that SGK1 stimulates CrT activity [7]. However, the unaltered SGK1 levels in GAMT−/− mice do not suggest that SGK1 is involved in the upregulation in creatine uptake capacity in these hearts, although we cannot exclude a difference in SGK1 phosphorylation state in GAMT−/− hearts, which may have been undetectable with the methodology available to us.
In hearts from mice overexpressing the cardiac CrT we observed elevated creatine uptake with a high degree of variation between mice. This is in line with the variation in creatine concentration and CrT-myc mRNA levels in this, and our previous study [11]. It is interesting to note that right ventricular (RV) creatine uptake in the CrT-OE hearts was, on average, 50% lower than left ventricular (LV) creatine uptake. Transgenic CrT-myc expression is under control of the MLC2v promoter, which is normally expressed in both ventricles, but differences between ventricular expression levels for a transgene under the control of the MLC2v promoter have been reported before [19]. This, and the lower levels of MLC2v in the RV measured here, probably explains the lower creatine uptake levels in the right ventricle.
In contrast to rat myocardium where RV and LV creatine levels were found to be the same [20], RV creatine content was significantly lower than LV creatine content in mouse hearts. However, while creatine uptake was 50% lower in the RV of CrT-OE mice compared to LV, the actual creatine concentration was only 6% lower in RV. This discrepancy can mainly be attributed to hearts with very high LV creatine uptake, since those hearts showed the greatest difference between right and left ventricular creatine uptake. A further discrepancy is that creatine uptake has been measured at up to six times higher than normal values, while LV creatine concentrations have never been observed at more than 3–4 times higher than WT levels, in this or our previous study [11]. Altogether, this suggests that when the creatine uptake capacity exceeds three to four times the normal levels, other factors start to play a significant role in determining the myocardial creatine concentration. One potential factor is whether the CrT can ever operate in reverse to transport creatine out of the cell. Therefore, we calculated the reverse potential at which creatine transport out of the cell becomes thermodynamically possible. Our results suggest that reverse transport is not possible under normal conditions. However, in hearts with very high creatine levels the reverse potential will be reduced to such an extent that during the plateau phase of the action potential ‘reverse creatine transport’ is thermodynamically possible. From previous (unpublished) conscious ECG data in these CrT-OE mice we calculate that the Q-T interval, which reflects action potential duration, can take up to 30% of the cardiac cycle. Thus, it is theoretically possible that reverse creatine transport could take place during a substantial fraction of the time in hearts with very high creatine levels.
Although we do not provide direct evidence of CrT reversal, this theory may well explain our findings. Thus, we hypothesize that in hearts with very high LV expression of transgenic CrT, reverse creatine transport will occur during systole, acting to lower the intracellular creatine concentration below expected values. This could explain the dissociation between LV creatine uptake levels and creatine content in these hearts, as well as the relatively small difference in creatine concentrations between ventricles despite large differences in creatine uptake.
GAMT−/− mice accumulate the creatine precursor guanidinoacetate, which, although it is capable of being phosphorylated in the creatine kinase reaction, is a very poor substrate [10]. This implies that GAMT−/− hearts will have a very high capacity to phosphorylate creatine, rapidly converting to PCr the instant it is transported into the cell. This will act to keep the intracellular free creatine concentration particularly low, facilitating further creatine uptake during the experiment. One limitation of this study is that we cannot exclude 14C accumulation in myocardium through processes other than CrT mediated uptake. However, residual extracellular 14C-creatine is unlikely to be a confounding factor as we used a 15 min washout period, when in a previous study using rat hearts, no 14C could be detected in the coronary effluent after 10 min of washout [2]. Regardless, residual extracellular 14C would be similar in absolute terms for all groups and would therefore not affect relative differences between groups, or at worst result in an underestimation. In creatine-free hearts from GAMT−/− mice, the lack of intracellular creatine means that the transsarcolemmal creatine gradient is reversed during the experiment and passive diffusion of creatine into the cells could potentially contribute to 14C accumulation in these hearts. However, the high creatine uptake rates in these hearts imply that this gradient rapidly disappears during the experiment and, therefore, is unlikely to substantially contribute to the 14C-creatine accumulation.
The large increase in creatine uptake levels in hearts from GAMT−/− mice show that the heart has an enormous capacity to regulate its creatine uptake capacity, over a much larger range than known before. Previously reported changes in creatine uptake capacity are generally smaller than 50%, in contrast to the > 7-fold increase reported here. The increase is particularly large when compared to the increase in creatine uptake in hearts from rats fed with β-GPA. In these hearts intracellular creatine levels were reduced to only 20% of control, while the Vmax for creatine uptake was increased by 63%. This suggests that the removal of the last 20% of creatine provides a very strong signal to increase CrT activity. Our results show that the heart has the ability to upregulate its creatine uptake capacity to an extent that is more than required to reverse the reduction in creatine uptake capacity that is observed in the failing heart. Therefore, it is worthwhile to unravel the molecular signalling mechanisms activated by a lack of creatine since it may lead to novel molecular targets for pharmacological manipulation and restoration of myocardial creatine content in the failing heart.
Acknowledgment
This study was supported by the British Heart Foundation.
References
- 1.Wyss M., Kaddurah-Daouk R. Creatine and creatinine metabolism. Physiol. Rev. 2000 Jul;80(3):1107–1213. doi: 10.1152/physrev.2000.80.3.1107. [DOI] [PubMed] [Google Scholar]
- 2.Boehm E., Chan S., Monfared M., Wallimann T., Clarke K., Neubauer S. Creatine transporter activity and content in the rat heart supplemented by and depleted of creatine. Am. J. Physiol. 2003 Feb;284(2):E399–E406. doi: 10.1152/ajpendo.00259.2002. [DOI] [PubMed] [Google Scholar]
- 3.Neubauer S. The failing heart — an engine out of fuel. N. Engl. J. Med. 2007 Mar 15;356(11):1140–1151. doi: 10.1056/NEJMra063052. [DOI] [PubMed] [Google Scholar]
- 4.Ingwall J.S., Weiss R.G. Is the failing heart energy starved? On using chemical energy to support cardiac function. Circ. Res. 2004 Jul 23;95(2):135–145. doi: 10.1161/01.RES.0000137170.41939.d9. [DOI] [PubMed] [Google Scholar]
- 5.Ten Hove M., Chan S., Lygate C., Monfared M., Boehm E., Hulbert K. Mechanisms of creatine depletion in chronically failing rat heart. J. Mol. Cell. Cardiol. 2005 Feb;38(2):309–313. doi: 10.1016/j.yjmcc.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Lygate C.A., Fischer A., Sebag-Montefiore L., Wallis J., Ten Hove M., Neubauer S. The creatine kinase energy transport system in the failing mouse heart. J. Mol. Cell. Cardiol. 2007 Mar 27;42(6):1129–1136. doi: 10.1016/j.yjmcc.2007.03.899. [DOI] [PubMed] [Google Scholar]
- 7.Shojaiefard M., Christie D.L., Lang F. Stimulation of the creatine transporter SLC6A8 by the protein kinases SGK1 and SGK3. Biochem. Biophys. Res. Commun. 2005 Sep 2;334(3):742–746. doi: 10.1016/j.bbrc.2005.06.164. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt A., Marescau B., Boehm E.A., Renema W.K., Peco R., Das A. Severely altered guanidino compound levels, disturbed body weight homeostasis and impaired fertility in a mouse model of guanidinoacetate N-methyltransferase (GAMT) deficiency. Hum. Mol. Genet. 2004 May 1;13(9):905–921. doi: 10.1093/hmg/ddh112. [DOI] [PubMed] [Google Scholar]
- 9.Ten Hove M., Lygate C.A., Fischer A., Schneider J.E., Sang A.E., Hulbert K. Reduced inotropic reserve and increased susceptibility to cardiac ischemia/reperfusion injury in phosphocreatine-deficient guanidinoacetate-N-methyltransferase-knockout mice. Circulation. 2005 May 17;111(19):2477–2485. doi: 10.1161/01.CIR.0000165147.99592.01. [DOI] [PubMed] [Google Scholar]
- 10.Boehm E.A., Radda G.K., Tomlin H., Clark J.F. The utilisation of creatine and its analogues by cytosolic and mitochondrial creatine kinase. Biochim. Biophys. Acta. 1996;1274(3):119–128. doi: 10.1016/0005-2728(96)00018-7. [DOI] [PubMed] [Google Scholar]
- 11.Wallis J., Lygate C.A., Fischer A., Ten Hove M., Schneider J.E., Sebag-Montefiore L. Supra-normal myocardial creatine and phosphocreatine concentrations lead to cardiac hypertrophy and heart failure — insights from creatine transporter over-expression transgenic mice. Circulation. 2005 15/11/2005;112(20):3131–3139. doi: 10.1161/CIRCULATIONAHA.105.572990. [DOI] [PubMed] [Google Scholar]
- 12.Wang X., Seed B. A PCR primer bank for quantitative gene expression analysis. Nucleic. Acids. Res. 2003 Dec 15;31(24):e154. doi: 10.1093/nar/gng154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neubauer S., Horn M., Naumann A., Tian R., Hu K., Laser M. Impairment of energy metabolism in intact residual myocardium of rat hearts with chronic myocardial infarction. J. Clin. Invest. 1995;95(3):1092–1100. doi: 10.1172/JCI117756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Loike J.D., Zalutsky D.L., Kaback E., Miranda A.F., Silverstein S.C. Extracellular creatine regulates creatine transport in rat and human-muscle cells. Proc. Nat. Acad. Sci. U. S. A. 1988;85(3):807–811. doi: 10.1073/pnas.85.3.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Speer O., Neukomm L.J., Murphy R.M., Zanolla E., Schlattner U., Henry H. Creatine transporters: a reappraisal. Mol. Cell. Biochem. 2004 Jan-Feb;256–257(1–2):407–424. doi: 10.1023/b:mcbi.0000009886.98508.e7. [DOI] [PubMed] [Google Scholar]
- 16.Sandoval N., Bauer D., Brenner V., Coy J.F., Drescher B., Kioschis P. The genomic organization of a human creatine transporter (CRTR) gene located in Xq28. Genomics. 1996 Jul 15;35(2):383–385. doi: 10.1006/geno.1996.0373. [DOI] [PubMed] [Google Scholar]
- 17.Nash S.R., Giros B., Kingsmore S.F., Rochelle J.M., Suter S.T., Gregor P. Cloning, pharmacological characterization, and genomic localization of the human creatine transporter. Receptors Channels. 1994;2:165–174. [PubMed] [Google Scholar]
- 18.Daniels G.M., Amara S.G. Regulated trafficking of the human dopamine transporter. Clathrin-mediated internalization and lysosomal degradation in response to phorbol esters. J. Biol. Chem. 1999 Dec 10;274(50):35794–35801. doi: 10.1074/jbc.274.50.35794. [DOI] [PubMed] [Google Scholar]
- 19.Lee K.J., Ross R.S., Rockman H.A., Harris A.N., O'Brien T.X., van Bilsen M. Myosin light chain-2 luciferase transgenic mice reveal distinct regulatory programs for cardiac and skeletal muscle-specific expression of a single contractile protein gene. J. Biol. Chem. 1992 Aug 5;267(22):15875–15885. [PubMed] [Google Scholar]
- 20.Laser A., Ingwall J.S., Tian R., Reis I., Hu K., Gaudron P. Regional biochemical remodeling in non-infarcted tissue of rat heart post-myocardial infarction. J. Mol. Cell. Cardiol. 1996 Jul;28(7):1531–1538. doi: 10.1006/jmcc.1996.0143. [DOI] [PubMed] [Google Scholar]