Abstract
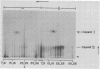
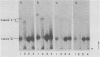
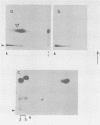
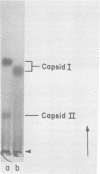
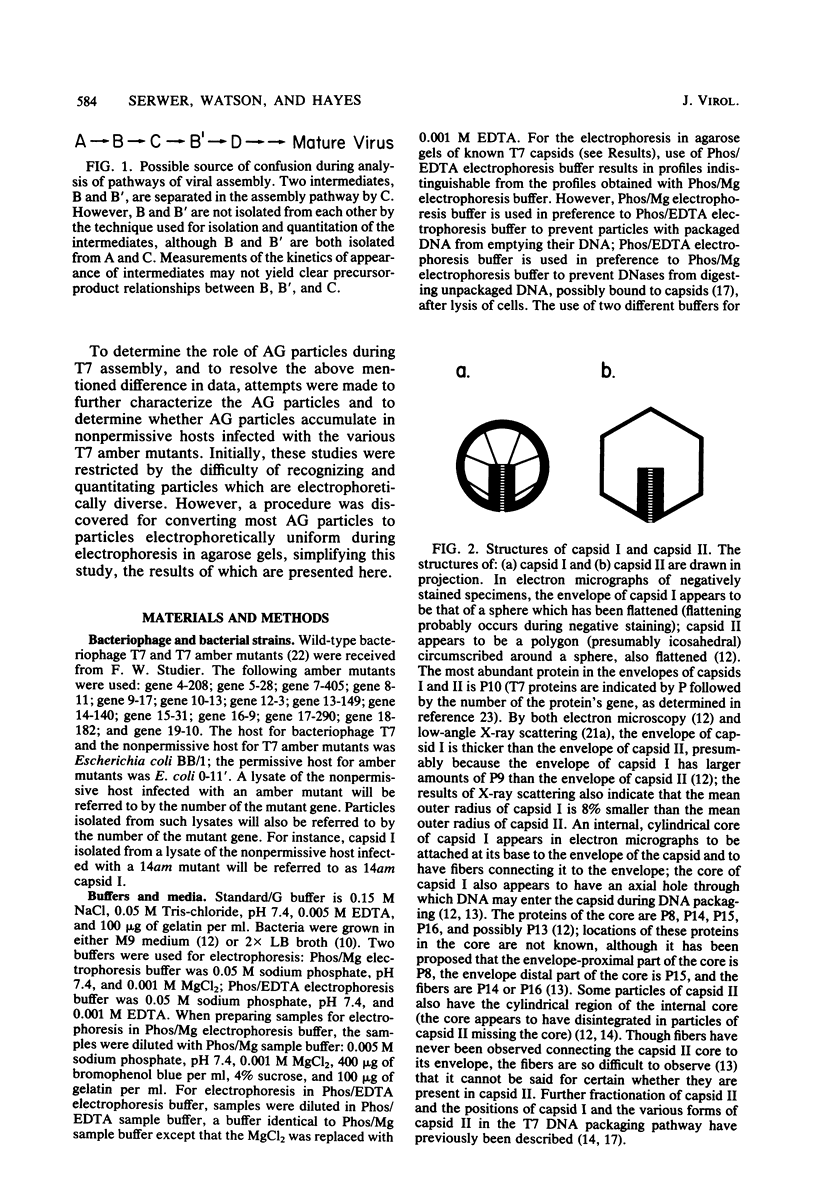
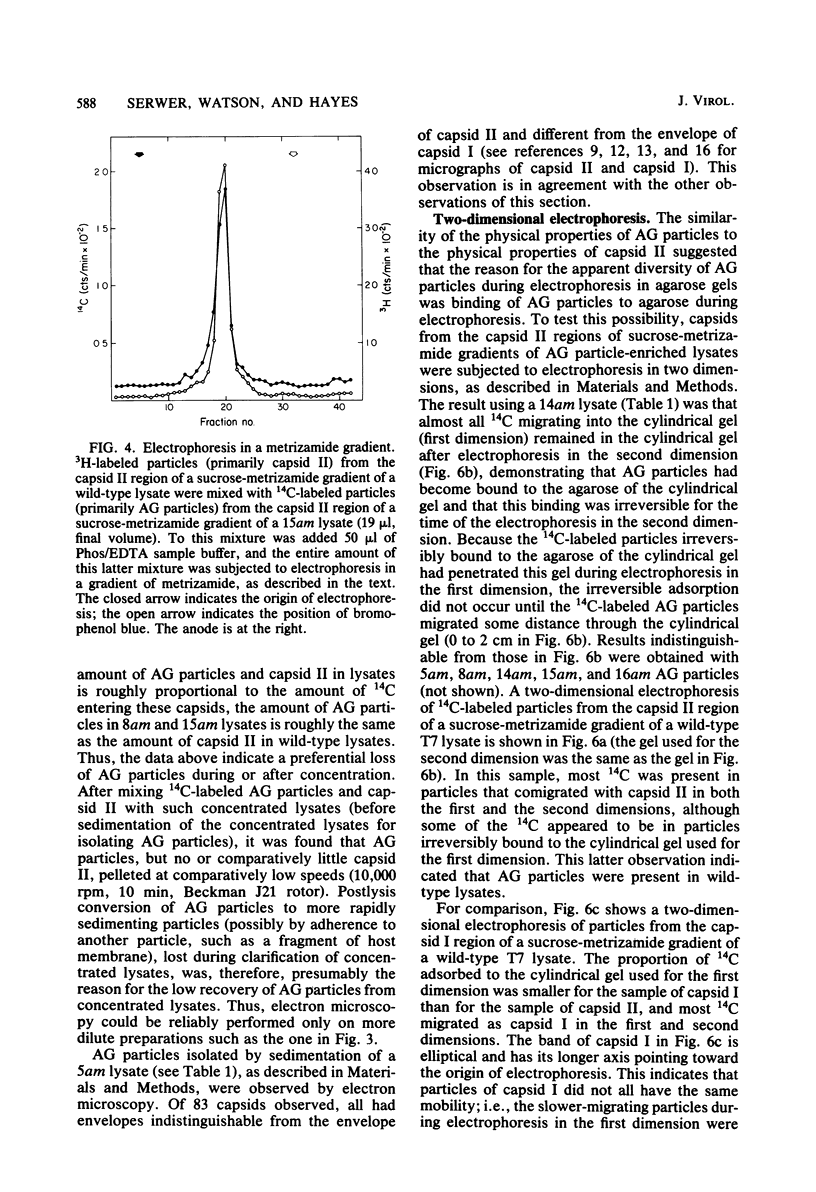
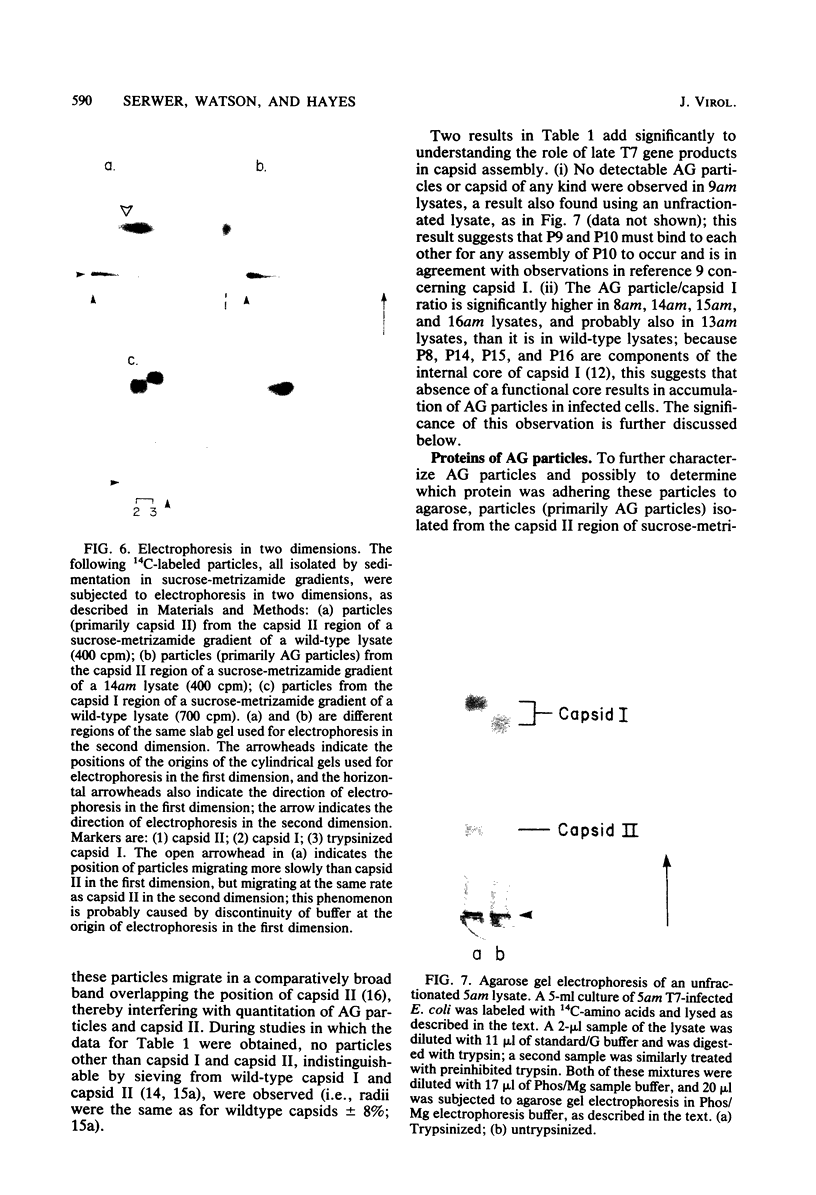
It has previously been shown that: (i) during infection of its host, the DNA bacteriophage T7 assembles a DNA-free procapsid (capsid I), a capsid with an envelope differing physically and chemically from the capsid of the mature bacteriophage, and (ii) capsid I converts to a capsid (capsid II) with a bacteriophage-like envelope as it packages DNA. Lysates of phage T7-infected Escherichia coli contained a particle (AG particle) which copurified with capsid II during buoyant density sedimentation, velocity sedimentation, and solid support-free electrophoresis, but was distinguished from capsid II by its apparent diversity during electrophoresis in agarose gels. Treatment of AG particles with trypsin converted most of them to particles that comigrated with trypsin-treated capsid II during electrophoresis in agarose gels. Irreversible binding of AG particles to agarose gels was shown to contribute to the apparent diversity of AG particles during agarose gel electrophoresis. The results of quantitation of AG particles and of capsid I and capsid II in lysates of a nonpermissive host infected with T7 amber mutants suggested that, in site of their capsid II-like properties, most AG particles were produced during assembly of capsid I and not during DNA packaging. The presence of AG particles in T7 lysates explains contradictions in previous data concerning the pathway of T7 assembly.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Chase T., Jr, Shaw E. p-Nitrophenyl-p'-guanidinobenzoate HCl: a new active site titrant for trypsin. Biochem Biophys Res Commun. 1967 Nov 30;29(4):508–514. doi: 10.1016/0006-291x(67)90513-x. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Krüger D. H., Schroeder C. Bacteriophage T3 and bacteriophage T7 virus-host cell interactions. Microbiol Rev. 1981 Mar;45(1):9–51. doi: 10.1128/mr.45.1.9-51.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laskey R. A., Mills A. D. Quantitative film detection of 3H and 14C in polyacrylamide gels by fluorography. Eur J Biochem. 1975 Aug 15;56(2):335–341. doi: 10.1111/j.1432-1033.1975.tb02238.x. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Unified theory for gel electrophoresis and gel filtration. Proc Natl Acad Sci U S A. 1970 Apr;65(4):970–977. doi: 10.1073/pnas.65.4.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Sadowski P., McGeer A., Becker A. Terminal cross-linking of DNA catalyzed by an enzyme system containing DNA ligase, DNA polymerase, and exonuclease of bacteriophage T7. Can J Biochem. 1974 Jun;52(6):525–535. doi: 10.1139/o74-077. [DOI] [PubMed] [Google Scholar]
- Serwer P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J Mol Biol. 1980 Mar 25;138(1):65–91. doi: 10.1016/s0022-2836(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Serwer P. A technique for electrophoresis in multiple-concentration agarose gels. Anal Biochem. 1980 Jan 1;101(1):154–159. doi: 10.1016/0003-2697(80)90054-8. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fibrous projections from the core of a bacteriophage T7 procapsid. J Supramol Struct. 1979;11(3):321–326. doi: 10.1002/jss.400110307. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Serwer P., Pichler M. E. Electrophoresis of bacteriophage T7 and T7 capsids in agarose gels. J Virol. 1978 Dec;28(3):917–928. doi: 10.1128/jvi.28.3.917-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Capsid-DNA complexes in the DNA packaging pathway of bacteriophage T7: characterization of the capsids bound to monomeric and concatemeric DNA. Virology. 1981 Jan 15;108(1):164–176. doi: 10.1016/0042-6822(81)90536-5. [DOI] [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Detection of viral capsid-DNA complexes. Prog Clin Biol Res. 1981;64:231–238. [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Electrophoresis in density gradients of metrizamide. Anal Biochem. 1981 Jul 1;114(2):342–348. doi: 10.1016/0003-2697(81)90491-7. [DOI] [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Function of an internal bacteriophage T7 core during assembly of a T7 procapsid. J Virol. 1982 May;42(2):595–601. doi: 10.1128/jvi.42.2.595-601.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroud R. M., Serwer P., Ross M. J. Assembly of bacteriophage T7. Dimensions of the bacteriophage and its capsids. Biophys J. 1981 Dec;36(3):743–757. doi: 10.1016/S0006-3495(81)84763-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]