Abstract
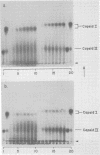
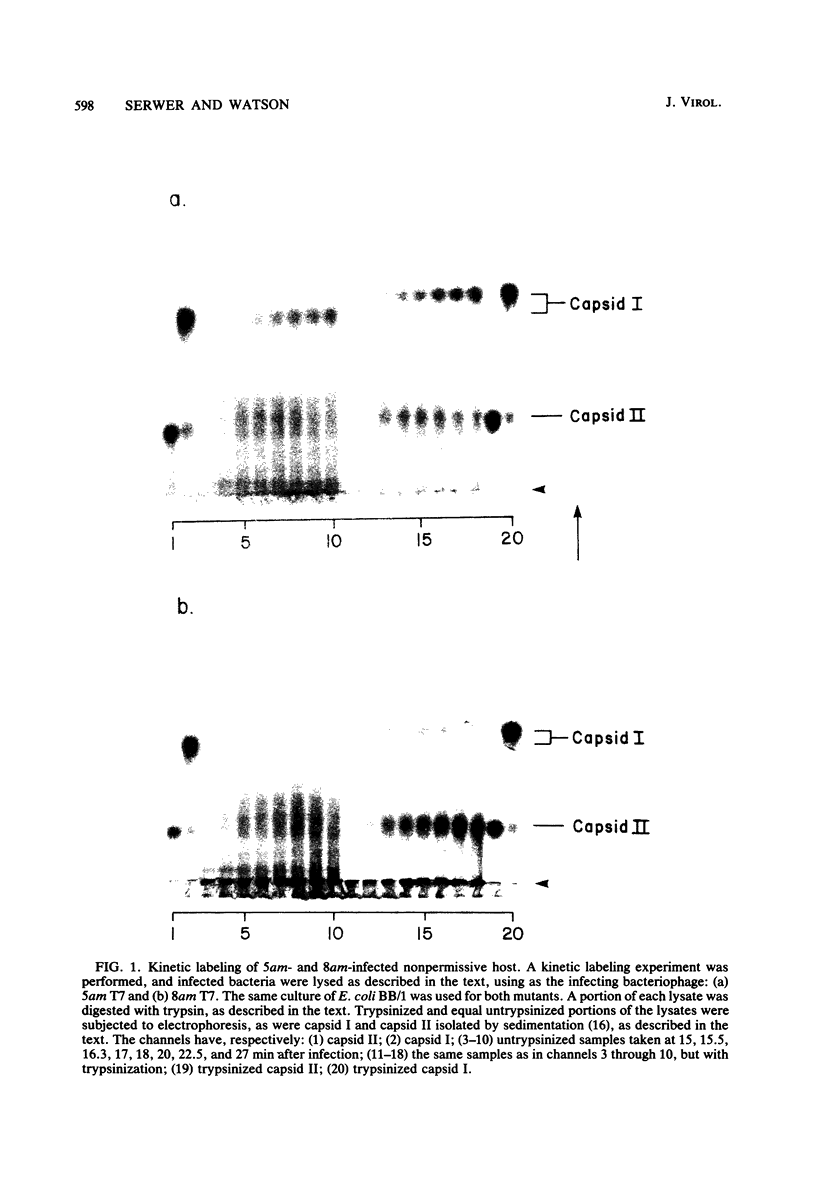
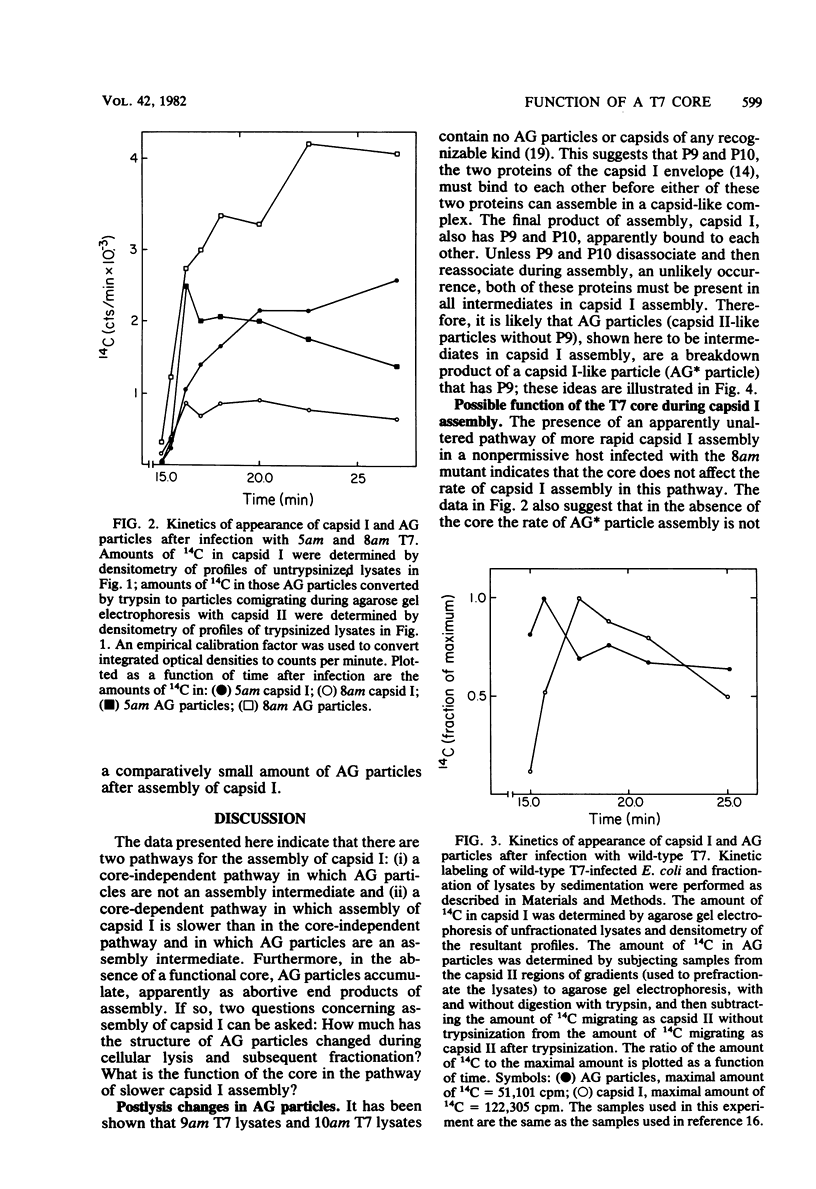
A DNA-free, proteinaceous procapsid of bacteriophage T7 (capsid I) has been shown in previous studies to consist of an external, spherical shell (envelope) and an internal, cylindrical core with fibrous projections that connect the core to the envelope. To determine the role of the core in assembly of the envelope of capsid I, the kinetics of appearance of capsid I and possible intermediates in capsid I assembly (AG particles) were determined in the presence and absence of the core. For obtaining these data, agarose gel electrophoresis was used and appeared to be a technique more accurate and efficient than techniques used for obtaining similar data in the past. The results of these experiments were: (i) in the presence of the core, AG particles behaved kinetically as intermediates in the assembly of capsid I; (ii) in the absence of the core, assembly of capsid I terminated prematurely and AG particles accumulated. These and other data have been interpreted by assuming that: AG particles are breakdown products of precursors of capsid I; these precursors have uncorrected errors in the assembly of their envelope; and a function of the core is to correct these errors.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. P22 morphogenesis. I: Catalytic scaffolding protein in capsid assembly. J Supramol Struct. 1974;2(2-4):202–224. doi: 10.1002/jss.400020215. [DOI] [PubMed] [Google Scholar]
- Casjens S., King J. Virus assembly. Annu Rev Biochem. 1975;44:555–611. doi: 10.1146/annurev.bi.44.070175.003011. [DOI] [PubMed] [Google Scholar]
- Caspar D. L. Movement and self-control in protein assemblies. Quasi-equivalence revisited. Biophys J. 1980 Oct;32(1):103–138. doi: 10.1016/S0006-3495(80)84929-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowther R. A., Klug A. Structural analysis of macromolecular assemblies by image reconstruction from electron micrographs. Annu Rev Biochem. 1975;44:161–182. doi: 10.1146/annurev.bi.44.070175.001113. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Casjens S. R. DNA packaging by the double-stranded DNA bacteriophages. Cell. 1980 Sep;21(2):319–331. doi: 10.1016/0092-8674(80)90468-7. [DOI] [PubMed] [Google Scholar]
- Fuller M. T., King J. Regulation of coat protein polymerization by the scaffolding protein of bacteriophage P22. Biophys J. 1980 Oct;32(1):381–401. doi: 10.1016/S0006-3495(80)84963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harrison S. C. Protein interfaces and intersubunit bonding. The case of tomato bushy stunt virus. Biophys J. 1980 Oct;32(1):139–153. doi: 10.1016/S0006-3495(80)84930-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn T., Katsura I. Structure and assembly of bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:69–110. doi: 10.1007/978-3-642-66800-5_3. [DOI] [PubMed] [Google Scholar]
- Murialdo H., Becker A. Head morphogenesis of complex double-stranded deoxyribonucleic acid bacteriophages. Microbiol Rev. 1978 Sep;42(3):529–576. doi: 10.1128/mr.42.3.529-576.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeder G. S., Sadowski P. D. Bacteriophage T7 morphogenesis: phage-related particles in cells infected with wild-type and mutant T7 phage. Virology. 1977 Jan;76(1):263–285. doi: 10.1016/0042-6822(77)90302-6. [DOI] [PubMed] [Google Scholar]
- Serwer P. A metrizamide-impermeable capsid in the DNA packaging pathway of bacteriophage T7. J Mol Biol. 1980 Mar 25;138(1):65–91. doi: 10.1016/s0022-2836(80)80005-2. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fast sedimenting bacteriophage T7 DNA from T7-infected Escherichia coli. Virology. 1974 May;59(1):70–88. doi: 10.1016/0042-6822(74)90207-4. [DOI] [PubMed] [Google Scholar]
- Serwer P. Fibrous projections from the core of a bacteriophage T7 procapsid. J Supramol Struct. 1979;11(3):321–326. doi: 10.1002/jss.400110307. [DOI] [PubMed] [Google Scholar]
- Serwer P. Internal proteins of bacteriophage T7. J Mol Biol. 1976 Nov 5;107(3):271–291. doi: 10.1016/s0022-2836(76)80005-8. [DOI] [PubMed] [Google Scholar]
- Serwer P., Pichler M. E. Electrophoresis of bacteriophage T7 and T7 capsids in agarose gels. J Virol. 1978 Dec;28(3):917–928. doi: 10.1128/jvi.28.3.917-928.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serwer P., Watson R. H. Capsid-DNA complexes in the DNA packaging pathway of bacteriophage T7: characterization of the capsids bound to monomeric and concatemeric DNA. Virology. 1981 Jan 15;108(1):164–176. doi: 10.1016/0042-6822(81)90536-5. [DOI] [PubMed] [Google Scholar]
- Serwer P., Watson R. H., Hayes S. J. Detection and characterization of agarose-binding, capsid-like particles produced during assembly of a bacteriophage T7 procapsid. J Virol. 1982 May;42(2):583–594. doi: 10.1128/jvi.42.2.583-594.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Bacteriophage T7. Science. 1972 Apr 28;176(4033):367–376. doi: 10.1126/science.176.4033.367. [DOI] [PubMed] [Google Scholar]
- Studier F. W. The genetics and physiology of bacteriophage T7. Virology. 1969 Nov;39(3):562–574. doi: 10.1016/0042-6822(69)90104-4. [DOI] [PubMed] [Google Scholar]